Abstract
Methotrexate is a dihydrofolate reductase inhibitor widely employed in curative treatment for children with acute lymphoblastic leukemia (ALL). However, methotrexate administration is also associated with persistent cognitive deficits among long-term childhood cancer survivors. Animal models of methotrexate-induced cognitive deficits have primarily utilized adult animals. The purpose of present study is to investigate the neurotoxicity of methotrexate in juvenile rats and its relevant mechanisms. The doses and schedule of systemic and intrathecal methotrexate, given from post-natal age 3 to 7 weeks, were chosen to model the effects of repeated methotrexate dosing on the developing brains of young children with ALL. This methotrexate regimen had no visible acute toxicity and no effect on growth. At 15 weeks of age (8 weeks after the last methotrexate dose) both spatial pattern memory and visual recognition memory were impaired. In addition, methotrexate-treated animals demonstrated impaired performance in the set-shifting assay, indicating decreased cognitive flexibility. Histopathological analysis demonstrated decreased cell proliferation in methotrexate-treated animals compared to controls, as well as changes in length and thickness of the corpus callosum. Moreover, methotrexate suppressed microglia activation and RANTES production. In conclusion, our study demonstrated that a clinically relevant regimen of systemic and intrathecal methotrexate induces persistent deficits in spatial pattern memory, visual recognition memory and executive function, lasting at least 8 weeks after the last injection. The mechanisms behind methotrexate-induced deficits are likely multifactorial and may relate to suppression of neurogenesis, alterations in neuroinflammation and microglial activation, and structural changes in the corpus callosum.
1. Introduction
Methotrexate is a dihydrofolate reductase inhibitor widely employed for the treatment of children with acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma, and osteosarcoma. Curative regimens for patients with these conditions typically include both systemic (oral and/or intravenous) administration, as well as repeated intrathecal doses to bypass the blood brain barrier and prevent central nervous system relapse. Because the most common age for diagnosis with ALL is 2–4 years, and treatment regimens have a two to three-year duration, children with ALL are repeatedly exposed to methotrexate during a period of ongoing brain development. As a result, as many as 50–70% of pediatric ALL survivors experience long-term irreversible deficits in attention, working memory and executive function (Hearps et al., 2017; Jacola et al., 2016b; Pierson et al., 2016; van der Plas et al., 2015). Unfortunately, these behavioral abnormalities generally persist into adulthood.
The pathophysiology of methotrexate-induced neurotoxicity is multifactorial and incompletely defined (Cole and Kamen, 2006; Vezmar et al., 2003). Animal modeling has shown promise in elucidating the mechanisms underlying cognitive dysfunction following both systemic and intrathecal administration of methotrexate (Li et al., 2010; Seigers et al., 2009; Thomsen et al., 2017). Data from these models suggest methotrexate can induce deficits through induction of oxidative stress (Caron et al., 2009), modulation of the immune system (Cutolo et al., 2001; Phillips et al., 2003; Zhang et al., 2009), inhibition of neurogenesis (Seigers et al., 2009), altered neurotransmission through the NMDA receptor (Cole et al., 2013; Vijayanathan et al., 2011) and/or induction of structural brain alterations (Seigers et al., 2009). Other studies point toward the alpha-7 nicotinic acetylcholine receptor, since positive modulators of this receptor, such as cotinine, will improve spatial memory and decrease depressive-like behavior in rats treated with methotrexate, cyclophosphamide and 5-flurouracil(Iarkov et al., 2016).
However, most animal models do not specifically address the effects of repeated systemic and intrathecal methotrexate administration in juvenile animals. Experiments in adult animals may not be relevant to understanding the impact of chemotherapy on the developing brain. The experiments described here were designed to address this gap, and further explore the mechanisms of methotrexate-induced cognitive deficits in juvenile subjects. The methotrexate regimen we employed was designed to model treatment for young children with ALL. Treatment included repeated administration of systemic and intrathecal methotrexate, at clinically relevant doses, over a five-week period extending from three to seven weeks of age. Similarly, the behavioral battery chosen to assess cognitive function was designed to probe those domains that are most frequently described as impaired among cancer survivors. The object placement test of pattern recognition and the novel object recognition test of recognition memory assess components of memory most frequently, albeit not exclusively, associated with hippocampal function (Rubin et al., 2014). A set shifting assay was designed to probe executive function and cognitive flexibility, domains associated with cortical function (Euston et al., 2012).
Finally histopathological examination at two time points was undertaken to describe biomarkers that correlate with methotrexate exposure, and may begin to explain the pathophysiologic mechanisms.
2. Materials and Methods
2.1 Animals and Reagents
Long Evans rats (evenly split between females and males) were purchased from Charles River Laboratories (Wilmington, MA) at 1 week of age and were habituated to the vivarium for one week before the experiments. Rats were housed in groups of two or three with a 12/12 hours light/dark cycle and ad lib. access to food (LabDiet 5001) and water. All experiments were approved by the Animal Institute and Use Committee of the Albert Einstein College of Medicine (Bronx, NY) and were conducted following the “Guide for the Care and Use of Laboratory Animals”. The NC3R’s ARRIVE guidelines were followed in the conduct and reporting of all experiments described here.
Methotrexate (USP grade), phosphate buffered saline (PBS) and other chemicals were purchased from Sigma (Saint Louis, MO) unless otherwise stated. Methanol and water (HPLC grade) were obtained from Fisher Scientific (Pittsburgh, PA). All injected solutions were sterilized by filtering through 0.22 μm syringe filters (Millipore, Billerica, MA).
2.2. Injection Schedule and CSF collection
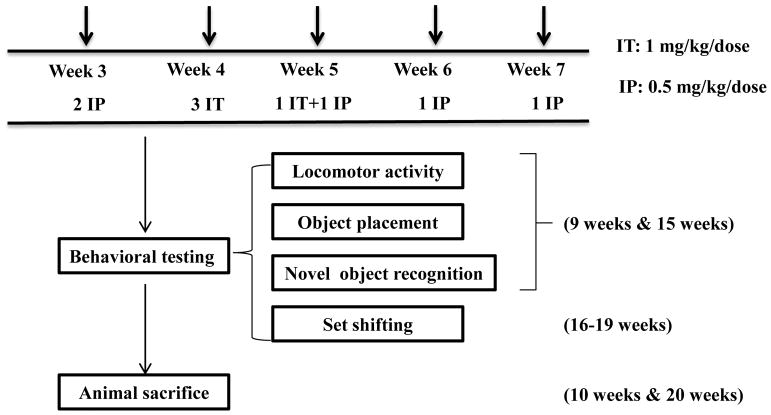
The detailed injection schedule is illustrated in Figure 1. Briefly, at 3 weeks old, rats received two IP injections (0.5 mg/kg methotrexate or PBS) one week apart. This was followed by 4 intrathecal injections within a two week period (1 mg/kg methotrexate or artificial cerebrospinal fluid [aCSF] at 4–5 weeks old, and IP injections once every week at 6 and 7 weeks of age.
Figure 1.
Injection and behavioral test schedule.
Intrathecal injections were carried out as previously described (Li et al., 2010) by transcutaneous cisterna magna puncture with a 25 gauge butterfly needle, with inhaled isofluorane anesthesia (2–5%). Correct insertion of the needle was verified by outflow of CSF, which was collected in isovolumetric amounts (i.e., volume of CSF removed was equivalent to volume of drug to be administered) prior to IT injection. All animals were monitored for signs of acute toxicity under direct visualization for one hour after injection and subsequently on a daily basis for evidence of abnormal behaviors. Intrathecal injection of methotrexate or aCSF (aCSF, Na+ 150 mM, K+ 3 mM, Ca2+ 1.4 mM, Mg2+ 0.8 mM, P 1.0 mM, and Cl− 155mM, in double distilled water) was conducted after CSF collection from cisternae magna. CSF with visible contamination by blood (approximately 10% of samples) was discarded. CSF samples were centrifuged, cell pellets were discarded and the supernatants were stored at −80 °C until analysis.
2.3. Behavioral testing schedule
Behavioral assessments of cognitive function were conducted at two time points (Figure 1) in order to assess acute effects (9 weeks of age; 1 week after the last methotrexate exposure and persistent effects (15 weeks of age; 8 weeks after the last methotrexate exposure). The battery included the following: open field (OF), object placement (OP) (a.k.a object location) and novel object recognition (NOR)(Ennaceur and Meliani, 1992), conducted as previously published (Li et al., 2010; Thomsen et al., 2017) and described briefly below. A modified set shifting assay, described below, was done at a single time point (16–19 weeks of age; 9–12 weeks following the last methotrexate exposure).
2.3.1 Open field
The open field test was used to evaluate locomotor activity and thigmotaxis, an indicator of anxiety-like behavior (Treit and Fundytus, 1988). The assay was carried out in an arena (69 × 69 × 69 cm) with visual cues for 6 min. Total track length, center track length, center time, and center entries, were recorded and analyzed by Viewer III software (Biobserve, Bonn, Germany).
2.3.2 Object placement (OP) and object recognition (NOR)
Both the OP and NOR test rely on the innate preference of rodents to preferentially explore novel environmental stimuli. Intact pattern recognition in the object placement test is indicated by a preference for an object that has been moved to a novel location. Intact recognition memory in the novel object recognition test is indicated by a preference for a novel object over the familiar one previously encountered. Briefly, during training, animals are exposed to a pair of identical objects. After a defined retention interval in their home cages (20–180 minutes depending on the task), rats were presented with one unmoved and one relocated object (OP) or one old and one novel object (NOR) in a testing trial. Total activity, assessed by track length, and total object exploration times were recorded manually in seconds, using stopwatches. Exploration was defined as any physical contact with the object (sniffing, whisking, or touching). Data from subjects with less than 4 seconds of total exploration time were excluded from analysis of preference scores (less than 2% of subjects). A preference score was determined by the ratio of time exploring the relocated or novel object to total exploration time during the testing trial, and recorded as a percentage. For each cohort of identically treated animals, intact memory was demonstrated by a group mean preference score significantly higher than 53%. Our previously published data demonstrate that animals with preference scores above 53% consistently demonstrate novel object preferences when retested (Li et al., 2010). All animals were exposed to equal conditions regarding time of training, testing, and period of retention interval. The tester was blinded to treatment conditions. The objects were previously validated for similar valance and exploration.
2.3.3 Set shifting assay
The set shifting assay was used to evaluate cognitive flexibility and executive function and was carried out for 6 days (defined as days 0 to 5). Rats were first food deprived overnight (Day 0) and then fed with 1–2 g of food everyday for 3–4 days (Day 1-Day 4). Animals were weighed daily and food was adjusted to target weight loss of approximately 2–4% per day. The accumulated weight loss was between 10–15% on the day of the set-shifting test. On Day 1, we habituated animals in the arena (23 cm × 23 cm × 23 cm) for 6 minutes three times. On Day 2 and 3, shaping trials were conducted by delivering rewards (sunflower seeds) in the target zone until the subject immediately went to the target zone upon being placed into the arena. On Day 3, animals were only rewarded when performing the target behavior (rear to the platform and nose poke into the target zone) to get the sunflower seed. On Day 4 or 5, animals that had achieved the target weight loss and reliably demonstrated target behaviors during shaping were chosen for set shifting assay. The set shifting assay was divided into 4 stages as illustrated in Table 1: SD (single discrimination, which tests the animals’ ability to discriminate between 2 textures), CD (compound discrimination, which tests the animals’ ability discriminate between 2 textures, when 2 odors were used as interference factors), CD_Rev (Compound discrimination reversal, which tests rodents ability to reverse the “correct” texture, previously learned) and ED (extradimensional shift, which tests the animals’ ability to now learn that the relevant cue is a different modality – i.e. an odor, when textures were used as interference factors.). Counterbalancing of the target stimuli (textures and odors) was used both within and between experimental groups.
Table 1. Set shifting assay test paradigm.
For each discrimination phase, the relevant and irrelevant dimension is indicated along with the order of exemplar exposure. Specific textures (T) included the following: T1: deep-pile carpet; T2: sand paper; T3: silicone sheet; T4: polyurethane foam. Odors included the following: O1: Geranium; O2: Bergamot; O3:Jasmin; O4:Cinnamon. The exemplar indicated in bold font was considered the “correct” stimulus, paired with the food reward.
| Discrimination Task | Dimension | Reward Present | ||
|---|---|---|---|---|
| Relevant | Irrelevant | + | − | |
| Simple Discrimination (SD) | Texture | -- | T1 | T2 |
| Compound Discrimination (CD) | Texture | Odor | T1/O1 | T2/O2 |
| T1/O2 | T2/O1 | |||
| Reversal Compound Discrimation (CD Rev) | Texture | Odor | T2/O1 | T1/O2 |
| T2/O2 | T1/O1 | |||
| Extradimensional Shift (ED) | Odor | Texture | O3/T3 | O4/T4 |
| O3/T4 | O4/T3 | |||
Each stage evaluates a distinct component of executive function and cognitive flexibility. Specifically, SD examines rule learning, while CD would be the component most related to attention, distractibility. CD_Rev examines rule learning, reversal learning and behavioral perseverance. ED shift evaluates more complicated rule learning including strategy switching and the ability to apply a general rule to a new circumstance. The standard criterion for animals to pass each stage was that they could perform 7 consecutive correct trials. The total number of trials each animal required to reach this criterion was recorded.
2.4 Organ and sample collection
Animals were sacrificed at 10 and 20 weeks of age, respectively. Animals were first anesthetized with inhaled isoflurane, 5%. Blood samples were obtained from the apex of the heart by butterfly needles. The heart was then exposed and animals were extensively perfused with cold PBS. Brains were dissected into right and left hemispheres. The left hemisphere of the brain was fixed in 4% paraformaldehyde for 24 hours at 4°C, then transferred into 30% sucrose for another day at 4°C and embedded in paraffin for sagittal sections. Sections were collected from the midline. The thickness of each section is 10 um for Nissl staining and 5 um for all the other staining. The right brain hemisphere was snap-frozen for tissue lysates. Blood samples were centrifuged, cell pellets were discarded and the supernatant were obtained and stored at −80 °C until analysis.
2.5 Immunohistochemistry and Immunofluorescence
For Ki67 and Iba-1 immunohistochemistry, sections were incubated with primary antibody rabbit anti-rat Ki67 (WAKO Chemicals, Mountain View, CA) or rabbit anti-rat Iba-1 (Wako), followed by a biotinylated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA) and HPR-streptavidin (Jackson Immunoresearch), and developed with 3,3′-diaminobenzidine (DAB, WAKO). For Iba-1 staining, a representative image was taken from the middle cortex (located right above hippocampus) and hippocampus in each section, and quantitation of the staining intensity in each image was performed using Image J (NIH, Bethesda, MD). For Ki67 staining, the number of Ki67 positive cells in the whole brain section was counted manually. All images were taken using a 3D Histech P250 High Capacity Slide Scanner.
For immunofluorescence staining, paraffin sections were incubated with mouse anti-rat SMI99 (myelin basic protein; MBP, Millipore, Billerica, MA) or rabbit anti-glial fibrillary acidic protein (GFAP) (Millipore), followed by the secondary antibodies donkey anti-mouse Alexa Fluor 488, or donkey anti-rabbit IgG Alexa Fluor 594, respectively (Jackson ImmunoResearch). Sections were imaged using a fluorescence microscope (Zeiss AxioObserver CLEM or a P250 Scanner). The staining intensity of SMI99 in a sagittal brain section and GFAP in the hippocampus was quantified by image J (NIH).
For Nissl staining, slides were first deparaffinized and hydrated, then stained in 0.02% crystal violet in 0.2% sodium acetate buffer for one hour. Following deionized water rinses, the slides were differentiated in 95% alcohol, dehydrated in a standard alcohol series, and cleared in xylene. The corpus callosum was divided into five identical segments from anterior to posterior (genu, anterior body, posterior body, isthmus and splenium) according to previous published guidelines (Duara et al., 1991). The length of corpus callosum and the thickness of each segment were measured by Image J (NIH). The investigator conducting the quantitation was blinded to the treatment arm of each sample.
2.6. Luminex and ELISAs
The expressions of cytokines including IL-1β, TNF, IL-6, RANTES, IL-10, IL-2, INF-γ, in the serum and CSF at 20 weeks of age were first screened by Luminex kit (Millipore). Luminex data were analyzed using Luminex Xponent Analysis software (Millipore). Then cytokines that tend to have higher expression including RANTES, IL-6 and TNF were further analyzed by corresponding ELISA kit (BD Biosciences, Billerica, MA) in a larger cohort following manufacturer’s instructions.
2.7 Statistics
Data were analyzed by D’Agostino-Pearson test to assess whether the data was normally distributed. Mean preference scores were compared with GraphPad Prism 6.07 (GraphPad Software, San Diego, CA) using two-tailed t-tests (for normally distributed data) or Mann-Whitney U test (non-normally distributed data). Contingencies were analyzed by Chi-Square Test. Biochemical data were analyzed using one-way ANOVA analysis. For all statistical tests, a probability value less than 0.05 was considered to be statistically significant.
3. Results
3.1 There were no differences in the weight, general locomotor activity and anxiety-like behavior after methotrexate treatment
Administration of methotrexate to juvenile rats at this dose and schedule did not alter weight gain relative to saline-treated controls (Supplemental Figure 1A). General locomotor activity was not altered by methotrexate treatment, as the total track length was similar between methotrexate and the control group. (Supplemental Figure 1B). methotrexate administration did not alter thigmotaxis, an anxiety behavior, as indicated by the similar center track length (Supplemental Figure 1C), center time (data not shown) and center entries (data not shown) between the two groups.
3.2 Methotrexate treated juvenile animals displayed impairment in spatial and object recognition memory
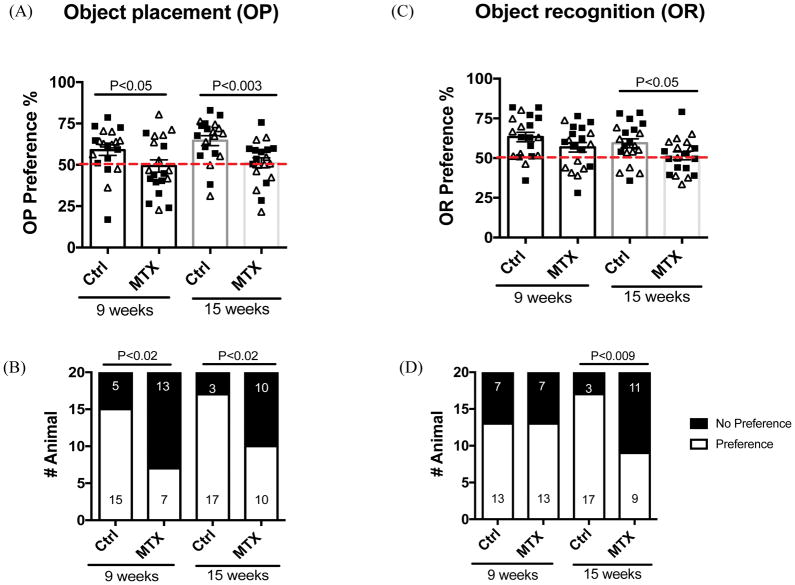
Methotrexate-treated juvenile rats displayed pattern recognition deficits in the object placement test, indicated by their mean preference score less than 53% (U=126, df=38, P<0.05) as early as 9 weeks of age (Figure 2A). This deficit persisted at 15 weeks of age, 8 weeks after the last dose of methotrexate (Figure 2A; t=3.178, df=38, P<0.003). In contrast, control animals displayed intact spatial memory (Figure 2A) at both time points. In addition, a greater proportion of methotrexate-treated animals failed (did not reliably prefer the novel, displaced object) in the object placement test at both time points compared to the control group (Figure 2B). At 9 weeks of age, there were 13 failures among 20 total methotrexate-treated animals (65%) vs. 5 failures of 20 total control animals (25%) (Figure 2B, Chi-Square=6.456, df = 1, P<0.02). At 15 weeks of age, there were 10 failures among 20 total methotrexate-treated animals (50 %) vs. 3 failures of 20 total control animals (15 %) (Figure 2B, Chi-Square=5.584, df = 1; P<0.02).
Figure 2. The spatial and recognition memory deficits induced by methotrexate treatment.
Both object placement (A, B) and object recognition (C, D) tests were conducted at 9 and 15 weeks of age, respectively. (A) and (C) display individual OP and OR preference score. A dotted red line indicates a cutoff value for preference (53%). (B) and (D) illustrate the number of animals that passed or failed the indicated test. Clear triangles represent females, while solid squares represent males.
Impaired novel object recognition was not detected at the early (9 week of age) time point. Mean preference scores in novel object recognition were lower among methotrexate-treated animals than controls, although the difference was not significantly different (Figure 2C). However, at 15 weeks of age, methotrexate treated animals displayed significantly decreased preference scores compared to the control group (Figure 2C, t=2.1, df=38, P<0.05). Further, significantly more methotrexate treated animals (55%) failed to prefer the novel object compared to the controls (15%) by 15 weeks of age (Figure 2D, Chi-Square=7.033, df = 1; P<0.009).
3.3 Methotrexate-treated animals demonstrated decreased ability on set-shifting
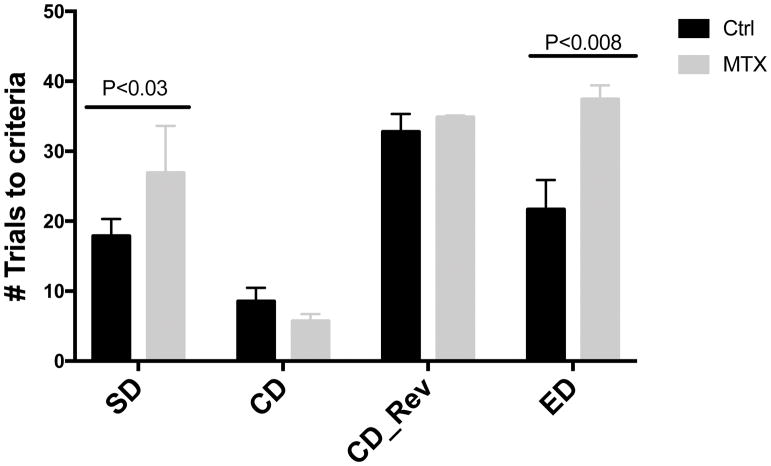
Figure 3 shows the numbers of trials that each group needed to reach criterion at different stages of the tests. Methotrexate-treated animals required significantly more trials than control animals to reach criterion in the SD (t=2.707, df=15, P<0.03) and ED stages (t=3.7465, df=15, P<0.008) of the set shifting assay, indicating impaired cognitive flexibility and executive function more than 8 weeks after the most recent dose of methotrexate.
Figure 3. Animals administered with methotrexate displayed impairment in executive function.
The set shifting task was conducted in control (n =9) and methotrexate-treated animals (n=8) at 16–19 weeks of age. In this assay, we tested the ability of single discrimination (SD), compound discrimination (CD), compound discrimination reversal (CD_Rev) and extra-dimensional (ED) shift discrimination.
3.4 Intrathecal methotrexate alters folate physiology in the CNS of juvenile animals
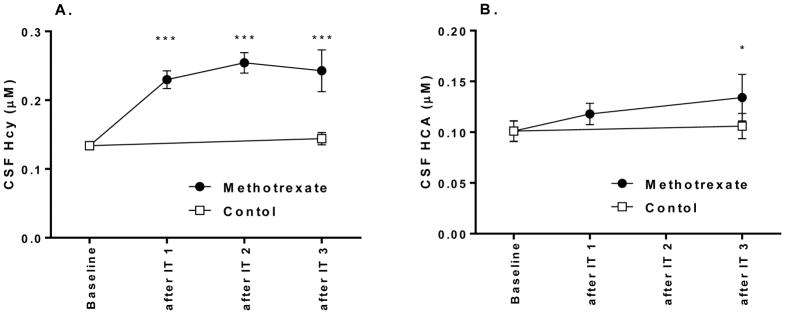
Consistent with our prior observations in adult animals(Vijayanathan et al., 2011), we observed a significant decline in mean CSF folate after intrathecal methotrexate from baseline (3.1 nM vs 28.6 nM; n = 4; P < 0.0001, two-tailed t-test). The concentrations of homocysteine and its metabolite homocysteic acid also increased after intrathecal methotrexate (Figure 4).
Figure 4. methotrexate treatment led to an increase in CSF homocysteine and the homocysteine metabolite, homocysteic acid.
CSF was collected before each of four doses of intrathecal methotrexate. Homocysteine (Hcy) and Homocysteic acid (HCA) were measured by HPLC, as previously published.(Vijayanathan et al., 2011) At each timepoint, values in CSF were compared with baseline using t-tests. *, P<0.05. ***, P<0.001.
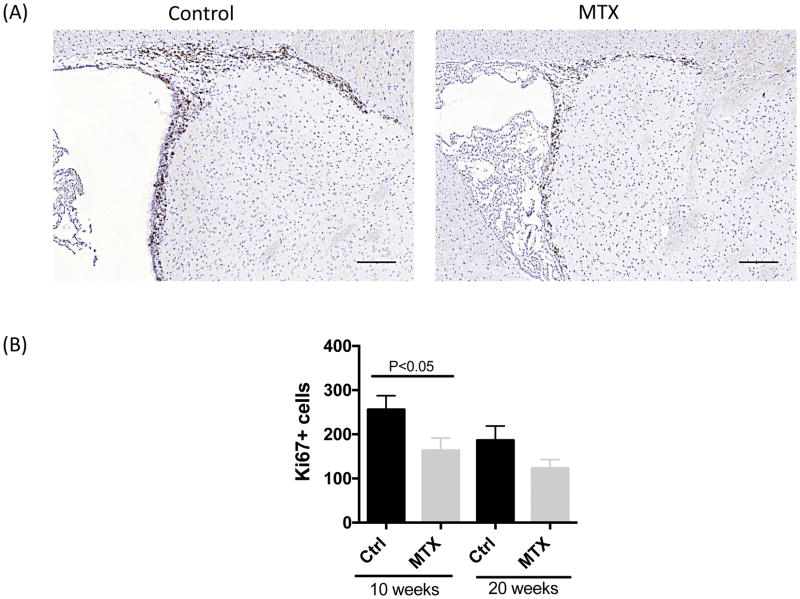
3.5 Methotrexate exposure results in decreased cellular proliferation within the brain
Among control animals, cell division, indicated by positive staining for Ki67, was observed predominantly in paraventricular areas and rostral migratory streams. At 10 weeks of age, methotrexate-treated animals exhibited fewer Ki67+ cells than controls (Figure 5A,B; t=2.191, df=18, P<0.05), primarily in the rostral migratory stream. However, at 20 weeks of age, this difference diminished (Figure 5B). In both groups, more Ki67+ cells were observed at 10 weeks of age than 20 weeks of age, although the differences were not statistically significant (Figure 5B).
Figure 5. Ki 67 staining in brain sections of control and methotrexate treated animals.
A representative image of Ki67 staining in the subventricular region of control and methotrexate treated animals is shown in (A). The quantitation of the number of Ki67+ cells in a whole brain sagittal section is shown in (B). Sections are collected from midline with equal thickness of 5 um. Ki67+ cells are located in the subventricular zone, choroid plexus and rostral migratory stream. The number of animals in each group is 10 and 10 (Ctrl and methotrexate at 10 weeks of age), 16 and 20 (Ctrl and methotrexate at 20 weeks of age), half female and half male, respectively. The scale bar refers to 100 um.
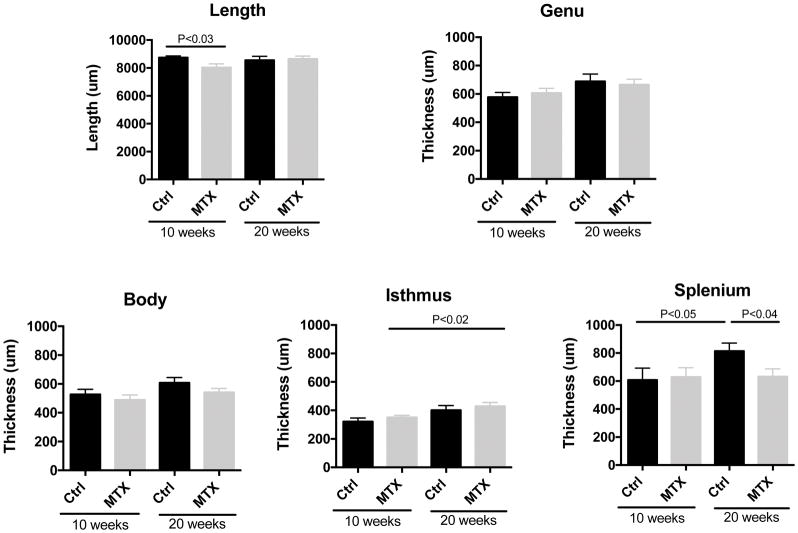
3.6 Methotrexate-treated animals showed reduced length and the thickness of corpus callosum
Methotrexate treatment altered the structure of corpus callosum at both the early (10 weeks of age) and late (20 weeks) time points. At 10 weeks of age, the corpus callosum of methotrexate-treated animals was significantly shorter (Figure 6, t=2.437, df=18, P<0.03). At 20 weeks of age, the length was similar between the two groups. However, the thickness of posterior part of corpus callosum, the splenium, was significantly decreased in methotrexate-treated animals compared to the controls (Figure 6; t=2.255, df=34, P<0.04).
Figure 6. The length and the thickness of corpus callosum after methotrexate administration.
Sections are collected from midline with equal thickness of 10 um. The length of corpus callosum was divided into five equal segments from anterior to posterior: genu, anterior body, middle body, isthmus and splenium. The length and the thickness of each segment were measured by Image J on Nissl stained slides. The number of animals in each group is 10 and 10 (Ctrl and methotrexate at 10 weeks of age), 16 and 20 (Ctrl and methotrexate at 20 weeks of age), half female and half male, respectively.
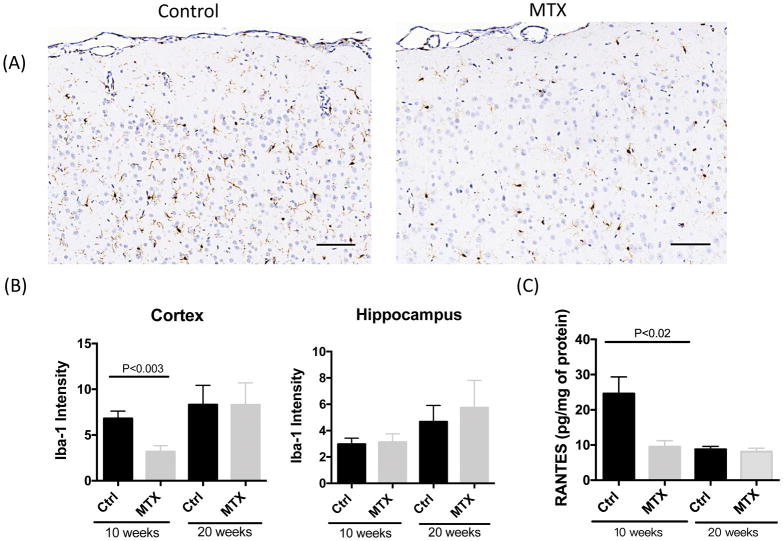
3.7 Methotrexate treatment reduced microglial activation and depressed RANTES expression
Microglia activation was significantly suppressed in the cortex after methotrexate administration at 10 weeks of age, (Figure 7A,B, t=3.506, df=17, P<0.003) while no differences were observed in Iba-1 staining at 20 weeks of age (Figure 6B). Comparable intensity of microglia staining was observed in the hippocampus between methotrexate and control groups (Figure 7B). In addition, the expression of RANTES was significantly decreased in the right hemisphere lysates at 10 weeks of age (Figure 6C, t=3.014, df=12, P<0.02) but not at 20 weeks of age (Figure 7C). No significant differences were found in the expressions of TNF and IL-6 in the serum, CSF and brain lysates, as well as RANTES in the serum and CSF at either time point (data not shown).
Figure 7. Neuroinflammation in the brain of methotrexate treated animals.
Representative images of Iba-1 staining in the middle cortex (right above the hippocampus) are shown in (A). The quantitation of staining intensity in cortex and hippocampus is indicated in (B). The expression RANTES in brain parenchyma is shown in (C). For Iba-1 staining, the number of animals in each group is 10 and 10 (Ctrl and methotrexate at 10 weeks of age, half male and half female), 16 and 20 (Ctrl and methotrexate at 20 weeks of age, half male and half female), respectively. For RANTES ELISA, the number of animals in each group is 7 and 7 (Ctrl and methotrexate at 10 weeks of age, 4 female and 3 male in each group), 3 and 7 (Ctrl and methotrexate at 20 weeks of age, 1 female and 2 male in control and 4 female and 3 male in methotrexate group), respectively.
4. Discussion
Methotrexate is a chemotherapeutic agent given to pediatric and adult patients with a wide variety of cancers, including ALL, non-Hodgkin lymphoma, osteosarcoma, head and neck cancer, and breast cancer (Bast, 2016). However, methotrexate exposure has also been associated with persistent cognitive deficits among survivors, including impairments of memory, attention, and executive functions. Previous animal studies have demonstrated that methotrexate administered IP (Yang et al., 2012), IV (Seigers et al., 2009) or intrathecal (Li et al., 2010) also induces cognitive deficits in adult animals. However, little is known regarding the effect of methotrexate on juvenile animals, which is critical for understanding the mechanisms of cognitive impairment when chemotherapy is administered within the context of brain development.
Here we demonstrate that a clinically relevant protocol of systemic and intrathecal methotrexate induces deficits in both memory and executive function which persist for at least 8 weeks after the final injection. The deficits observed after methotrexate exposure in this juvenile animal model are similar to what is observed among survivors of childhood leukemia. The animals described here exhibited focal deficits (memory and executive function) but normal growth, locomotor activity, and grooming behaviors, and an absence of anxiety-related behaviors. Similarly, childhood leukemia survivors show grossly normal function, with mean IQ in the normal range(Iyer et al., 2015; Krull et al., 2016). However, more focused testing reveals that impairments in working memory and executive function are more frequent among cancer survivors than controls(Jacola et al., 2016a; Krull et al., 2016).
As we have previously demonstrated in adult rats treated with methotrexate, juvenile rats exhibit biochemical effects of methotrexate-induced perturbations in folate physiology, including decreased folate, increased homocysteine, and presence of homocysteine metabolites within the CSF. Here we also describe histologic evidence of structural changes induced by methotrexate exposure, including decreased cellular proliferation, primarily within the rostral migratory stream, as well as structural changes in the corpus callosum. In addition we note decreased microglial activation and RANTES expression, suggesting a decreased neuroinflammatory response.
It is possible that these biomarkers are simply reflective of prior methotrexate exposure, and are unrelated to the pathophysiology of methotrexate-induced cognitive deficits. methotrexate is commonly employed as an antiinflammatory agent, so decreased chemokine concentrations could be viewed as an expected effect of exposure to this drug. Similarly, as an inhibitor of thymidine and purine synthesis, methotrexate could reasonably be expected to transiently decrease cellular proliferation. However, it is also possible that these changes reflect processes that do contribute mechanistically to the observed behavioral abnormalities.
Neurogenesis is believed to be necessary to maintain the functional stability of adult brain circuitry, and also plays a role in cognition (Apple et al., 2017a; Apple et al., 2017b). Decreased neurogenesis has been tied to impaired memory in studies of adult rats treated with other chemotherapeutic agents.(Gonçalves et al., 2016; Kitamura et al., 2015; Nokia et al., 2012; Rendeiro et al., 2016; Yang et al., 2010) In our study, juvenile animals treated with methotrexate displayed cognitive impairment accompanied by reduced number of proliferating cells, predominantly in the SVZ and RMS regions at 10 weeks of age, although no difference was observed at the later time point. Suppressed neurogenesis can be reversed pharmacologically, by antidepressant or antipsychotic drugs (Apple et al., 2017a), or can be rescued by exogenous administration of neural stem cells (Acharya et al., 2015), suggesting potential protective strategies that can be tested in our preclinical model and eventually among patients being treated for cancer.
Our observation that methotrexate led to alterations in the length and thickness of the corpus callosum echoes results in adult animals. Seigers et al, for example, have shown that single intravenous injection of methotrexate to adult rats resulted in decreased white matter density in lateral corpus callosum, lasting for 3 weeks (Seigers et al., 2009). The corpus callosum exerts a fundamental role in integrating information and mediating complex behaviors, including cognition and executive function (Hinkley et al., 2012; Kok et al., 2014). The structural changes we observed after methotrexate exposure are therefore consistent with impaired executive abilities. Although we did not directly assess processing speed in this model, decreased processing speed has been observed among childhood cancer survivors (Aukema et al.; Iyer et al., 2015; Kamdar et al., 2011; Mennes et al., 2005; Peterson et al., 2008) and has been linked to white matter changes detected on MRI (Aukema et al., 2009; Mennes et al., 2005).
The relationship between the cognitive deficits we described and changes in neuroinflammation or microglial activation could not be resolved by this study, which was designed to detect associations but not to prove causal relationships. Neuroinflammation and microglial activation are thought to contribute early in the pathogenesis of cognitive deficits associated with naturally occurring neurodegenerative conditions (Heppner et al., 2015; Rodriguez and Kern, 2011). In these experiments, we showed a decrease both in the chemokine RANTES within brain lysates, and a decrease in cerebral gliosis after methotrexate exposure, compared to control animals. RANTES (also known as chemokine with CC motif ligand 5) is responsible for recruiting T-lymphocytes and activating NK cells at sites of inflammation, and thus plays an important role in neuroinflammatory and demyelinating conditions like multiple sclerosis (Mori et al., 2016).
However, both inflammation and the resulting gliosis also contribute to repair processes after toxic or traumatic brain injury (Anderson et al., 2016; Fawcett and Asher, 1999). While glial cells contribute to neuropathy, inhibition of gliosis is not necessarily neuroprotective (Di Cesare Mannelli et al., 2014). Consequently, the net balance of detrimental and reparative effects of inflammation and gliosis on subsequent brain function is unclear. In addition, we acknowledge that decreased cerebral gliosis was inferred by decreased Iba-1 intensity; no morphologic analysis was conducted to define whether this decreased staining was due to a decrease in microglial cell number or individual cells’ activation states. Additional studies will be necessary to dissect the causal relationships among these early, transient changes and cognitive deficits that persist many weeks later.
5. Conclusion
A clinically relevant schedule of methotrexate administration to juvenile rats was sufficient to induce persistent impairments of memory and executive function that resemble deficits described among survivors of childhood cancer. In addition, we note methotrexate-induced changes in neuroinflammatory markers and cellular proliferation, along with structural changes in the corpus callosum. Further study will determine whether the observed changes are directly responsible for causing cognitive deficits, or are simply biomarkers reflective of prolonged chemotherapy exposure.
Supplementary Material
Supplemental Figure 1. methotrexate did not affect general weight loss or neuromuscular strength. (A) Growth curve of control and methotrexate treated animals. Both control and methotrexate treated animals were weighted at 2,3,4,5,9 and 16 weeks of age, respectively. The number of animals in the control and methotrexate treated groups is 17 (F), 14 (M) and 19 (F), 20 (M), respectively. Open field test were performed at 9 and 15 weeks of age. The total track length and the center track length are shown in (B) and (C), respectively. Clear triangles represent females, while solid squares represent males.
Supplemental Figure 2. GFAP and MBP staining in the brain sections of control and methotrexate treated rats. The representative image and the quantitation of GFAP and MBP staining are shown on the left (A) and right (B), respectively. The number of animals in each group is 10 and 10 (Ctrl and methotrexate at 10 weeks of age), 16 and 20 (Ctrl and methotrexate at 20 weeks of age), respectively.
Highlights.
A clinically relevant methotrexate regimen, including systemic and intrathecal dosing, administered to juvenile animals, induced cognitive deficits that persist into adulthood.
Deficits included impairment of pattern recognition, recognition memory, and executive function, suggesting methotrexate exposure impacts both hippocampal and cortical functions.
Pathologic examination demonstrated decreased neurogenesis, alterations in neuroinflammation and microglial activation, and structural changes of the corpus callosum.
Acknowledgments
This work was supported in part by NIH Grant U54 HD090260-02 to the Rose F Kennedy IDDRC; Einstein Shared Instrumentation Support #1S10OD019961-01; and NIH/NCI R01-CA182284.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya MM, Martirosian V, Chmielewski NN, Hanna N, Tran KK, Liao AC, Christie LA, Parihar VK, Limoli CL. Stem cell transplantation reverses chemotherapy-induced cognitive dysfunction. Cancer Res. 2015;75:676–686. doi: 10.1158/0008-5472.CAN-14-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017a;1655:270–276. doi: 10.1016/j.brainres.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Apple DM, Solano-Fonseca R, Kokovay E. Neurogenesis in the aging brain. Biochem Pharmacol. 2017b doi: 10.1016/j.bcp.2017.06.116. [DOI] [PubMed] [Google Scholar]
- Aukema EJ, Caan MW, Oudhuis N, Majoie CB, Vos FM, Reneman L, Last BF, Grootenhuis MA, Schouten-van Meeteren AY, Aukema EJ, Caan MWA, Oudhuis N, Majoie CBLM, Vos FM, Reneman L, Last BF, Grootenhuis MA, Schouten-van Meeteren AYN. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. International Journal of Radiation Oncology, Biology, Physics. 2009;74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- Aukema EJ, Caan MWA, Oudhuis N, Majoie CBLM, Vos FM, Reneman L, Last BF, Grootenhuis MA, Schouten-van Meeteren AYN. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. International Journal of Radiation Oncology, Biology, Physics. 74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- Bast RC. Holland-Frei cancer medicine. John Wiley & Sons, Inc; Hoboken, New Jersey: 2016. [Google Scholar]
- Caron JE, Krull KR, Hockenberry M, Jain N, Kaemingk K, Moore IM. Oxidative stress and executive function in children receiving chemotherapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53:551–556. doi: 10.1002/pbc.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PD, Kamen BA. Delayed Neurotoxicity Associated with Therapy for Patients with Childhood Leukemia. Mental Retardation and Developmental Disabilities Research Reviews. 2006;12:174–183. doi: 10.1002/mrdd.20113. [DOI] [PubMed] [Google Scholar]
- Cole PD, Vijayanathan V, Ali NF, Wagshul ME, Tanenbaum EJ, Price J, Dalal V, Gulinello ME. Memantine protects rats treated with intrathecal methotrexate from developing spatial memory deficits. Clin Cancer Res. 2013;19:4446–4454. doi: 10.1158/1078-0432.CCR-13-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Pizzorni C, Seriolo B, Straub RH. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001;60:729–735. doi: 10.1136/ard.60.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Pacini A, Micheli L, Tani A, Zanardelli M, Ghelardini C. Glial role in oxaliplatin-induced neuropathic pain. Experimental Neurology. 2014;261:22–33. doi: 10.1016/j.expneurol.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Duara R, Kushch A, Gross-Glenn K, Barker WW, Jallad B, Pascal S, Loewenstein DA, Sheldon J, Rabin M, Levin B, et al. Neuroanatomic differences between dyslexic and normal readers on magnetic resonance imaging scans. Arch Neurol. 1991;48:410–416. doi: 10.1001/archneur.1991.00530160078018. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Meliani K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav Brain Res. 1992;51:83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Gonçalves JT, Schafer ST, Gage FH. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Hearps S, Seal M, Anderson V, McCarthy M, Connellan M, Downie P, De Luca C. The relationship between cognitive and neuroimaging outcomes in children treated for acute lymphoblastic leukemia with chemotherapy only: A systematic review. Pediatric Blood & Cancer. 2017;64:225–233. doi: 10.1002/pbc.26188. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Hinkley LB, Marco EJ, Findlay AM, Honma S, Jeremy RJ, Strominger Z, Bukshpun P, Wakahiro M, Brown WS, Paul LK, Barkovich AJ, Mukherjee P, Nagarajan SS, Sherr EH. The role of corpus callosum development in functional connectivity and cognitive processing. PLoS One. 2012;7:e39804. doi: 10.1371/journal.pone.0039804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iarkov A, Appunn D, Echeverria V. Post-treatment with cotinine improved memory and decreased depressive-like behavior after chemotherapy in rats. Cancer Chemotherapy and Pharmacology. 2016;78:1033–1039. doi: 10.1007/s00280-016-3161-0. [DOI] [PubMed] [Google Scholar]
- Iyer NS, Balsamo LM, Bracken MB, Kadan-Lottick NS. Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood. 2015;126:346–353. doi: 10.1182/blood-2015-02-627414. [DOI] [PubMed] [Google Scholar]
- Jacola LM, Krull KR, Pui CH, Pei D, Cheng C, Reddick WE, Conklin HM. Longitudinal Assessment of Neurocognitive Outcomes in Survivors of Childhood Acute Lymphoblastic Leukemia Treated on a Contemporary Chemotherapy Protocol. Journal of Clinical Oncology. 2016a;34:1239–1247. doi: 10.1200/JCO.2015.64.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacola LM, Krull KR, Pui CH, Pei D, Cheng C, Reddick WE, Conklin HM. Longitudinal Assessment of Neurocognitive Outcomes in Survivors of Childhood Acute Lymphoblastic Leukemia Treated on a Contemporary Chemotherapy Protocol. J Clin Oncol. 2016b;34:1239–1247. doi: 10.1200/JCO.2015.64.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar KY, Krull KR, El-Zein RA, Brouwers P, Potter BS, Harris LL, Holm S, Dreyer Z, Scaglia F, Etzel CJ, Bondy M, Okcu MF. Folate pathway polymorphisms predict deficits in attention and processing speed after childhood leukemia therapy. Pediatric Blood & Cancer. 2011;57:454–460. doi: 10.1002/pbc.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Hattori S, Yoneda S, Watanabe S, Kanemoto E, Sugimoto M, Kawai T, Machida A, Kanzaki H, Miyazaki I, Asanuma M, Sendo T. Doxorubicin and cyclophosphamide treatment produces anxiety-like behavior and spatial cognition impairment in rats: Possible involvement of hippocampal neurogenesis via brain-derived neurotrophic factor and cyclin D1 regulation. Behavioural Brain Research. 2015;292:184–193. doi: 10.1016/j.bbr.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Kok R, Lucassen N, Bakermans-Kranenburg MJ, van IMH, Ghassabian A, Roza SJ, Govaert P, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H. Parenting, corpus callosum, and executive function in preschool children. Child Neuropsychol. 2014;20:583–606. doi: 10.1080/09297049.2013.832741. [DOI] [PubMed] [Google Scholar]
- Krull KR, Cheung YT, Liu W, Fellah S, Reddick WE, Brinkman TM, Kimberg C, Ogg R, Srivastava D, Pui CH, Robison LL, Hudson MM. Chemotherapy Pharmacodynamics and Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Journal of Clinical Oncology. 2016;34:2644–2653. doi: 10.1200/JCO.2015.65.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Vijayanathan V, Gulinello M, Cole PD. Intrathecal methotrexate induces focal cognitive deficits and increases cerebrospinal fluid homocysteine. Pharmacol Biochem Behav. 2010;95:428–433. doi: 10.1016/j.pbb.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Mennes M, Stiers P, Vandenbussche E, Vercruysse G, Uyttebroeck A, De Meyer G, Van Cool SW. Attention and information processing in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Pediatric Blood & Cancer. 2005;44:478–486. doi: 10.1002/pbc.20147. [DOI] [PubMed] [Google Scholar]
- Mori F, Nisticò R, Nicoletti CG, Zagaglia S, Mandolesi G, Piccinin S, Martino G, Finardi A, Rossini PM, Marfia GA, Furlan R, Centonze D. RANTES correlates with inflammatory activity and synaptic excitability in multiple sclerosis. Multiple Sclerosis Journal. 2016;22:1405–1412. doi: 10.1177/1352458515621796. [DOI] [PubMed] [Google Scholar]
- Nokia MS, Anderson ML, Shors TJ. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. European Journal of Neuroscience. 2012;36:3521–3530. doi: 10.1111/ejn.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CC, Johnson CE, Ramirez LY, Huestis S, Pai ALH, Demaree HA, Drotar D. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2008;51:99–104. doi: 10.1002/pbc.21544. [DOI] [PubMed] [Google Scholar]
- Phillips DC, Woollard KJ, Griffiths HR. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br J Pharmacol. 2003;138:501–511. doi: 10.1038/sj.bjp.0705054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson C, Waite E, Pyykkonen B. A meta-analysis of the neuropsychological effects of chemotherapy in the treatment of childhood cancer. Pediatr Blood Cancer. 2016;63:1998–2003. doi: 10.1002/pbc.26117. [DOI] [PubMed] [Google Scholar]
- Rendeiro C, Sheriff A, Bhattacharya TK, Gogola JV, Baxter JH, Chen H, Helferich WG, Roy EJ, Rhodes JS. Long-lasting impairments in adult neurogenesis, spatial learning and memory from a standard chemotherapy regimen used to treat breast cancer. Behavioural Brain Research. 2016;315:10–22. doi: 10.1016/j.bbr.2016.07.043. [DOI] [PubMed] [Google Scholar]
- Rodriguez JI, Kern JK. Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Biol. 2011;7:205–213. doi: 10.1017/S1740925X12000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RD, Watson PD, Duff MC, Cohen NJ. The role of the hippocampus in flexible cognition and social behavior. Front Hum Neurosci. 2014;8:742. doi: 10.3389/fnhum.2014.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Coppens CM, van der Most PJ, van Dam FS, Koolhaas JM, Buwalda B. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res. 2009;201:279–284. doi: 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Thomsen AM, Gulinello ME, Wen J, Schmiegelow K, Cole PD. Liposomal Cytarabine Induces Less Neurocognitive Dysfunction Than Intrathecal Methotrexate in an Animal Model. Journal of Pediatric Hematology/Oncology. 2017 doi: 10.1097/MPH.0000000000000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- van der Plas E, Nieman BJ, Butcher DT, Hitzler JK, Weksberg R, Ito S, Schachar R. Neurocognitive Late Effects of Chemotherapy in Survivors of Acute Lymphoblastic Leukemia: Focus on Methotrexate. J Can Acad Child Adolesc Psychiatry. 2015;24:25–32. [PMC free article] [PubMed] [Google Scholar]
- Vezmar S, Becker A, Bode U, Jaehde U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy. 2003;49:92–104. doi: 10.1159/000069773. [DOI] [PubMed] [Google Scholar]
- Vijayanathan V, Gulinello M, Ali N, Cole PD. Persistent cognitive deficits, induced by intrathecal methotrexate, are associated with elevated CSF concentrations of excitotoxic glutamate analogs and can be reversed by an NMDA antagonist. Behav Brain Res. 2011;225:491–497. doi: 10.1016/j.bbr.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, Kim JC, Wang H, Shin T, Moon C. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiology of Learning and Memory. 2010;93:487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim JS, Kim J, Jang S, Kim SH, Kim JC, Shin T, Wang H, Moon C. Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Res Bull. 2012;89:50–56. doi: 10.1016/j.brainresbull.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao P, Li A, Lv X, Gao Y, Sun H, Ding Y, Liu J. Effects of methotrexate on plasma cytokines and cardiac remodeling and function in postmyocarditis rats. Mediators Inflamm. 2009;2009:389720. doi: 10.1155/2009/389720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. methotrexate did not affect general weight loss or neuromuscular strength. (A) Growth curve of control and methotrexate treated animals. Both control and methotrexate treated animals were weighted at 2,3,4,5,9 and 16 weeks of age, respectively. The number of animals in the control and methotrexate treated groups is 17 (F), 14 (M) and 19 (F), 20 (M), respectively. Open field test were performed at 9 and 15 weeks of age. The total track length and the center track length are shown in (B) and (C), respectively. Clear triangles represent females, while solid squares represent males.
Supplemental Figure 2. GFAP and MBP staining in the brain sections of control and methotrexate treated rats. The representative image and the quantitation of GFAP and MBP staining are shown on the left (A) and right (B), respectively. The number of animals in each group is 10 and 10 (Ctrl and methotrexate at 10 weeks of age), 16 and 20 (Ctrl and methotrexate at 20 weeks of age), respectively.