Abstract
Most maternal caregiving behaviors change across lactation to match the developmental needs of the continuously aging offspring. However, it is mostly unknown whether the dams’ postpartum stage or litter age is the primary driving force of these changes. In this study, postnatal day 1 and 8 litters were cross-fostered or in-fostered to postpartum day 1 or 8 dams. Five days later, undisturbed observations of maternal caregiving behaviors were performed on the subsequent two days. We found a main effect of dams’ postpartum stage, which was driven by an interaction between postpartum stage and litter age, on the frequency that mothers spent with the pups and displayed erect postures over them (hovering over and kyphosis): early-postpartum dams were in contact with younger litters and in erect postures more often with younger litters compared to later-postpartum dams with younger litters. Additionally, there was an interaction between postpartum stage and litter age on the litter weight’s because older litters living with later-postpartum dams were heavier than older litters living with early-postpartum dams. There was also an interaction between postpartum stage and litter age on the dams’ bodyweight, with early-postpartum dams living with younger litters weighing the least and later-postpartum dams living with younger litters weighing the most. Because activity of the neuropeptide, orexin, within the medial preoptic area (mPOA) has been implicated in maternal nursing and other caregiving behaviors, we measured mPOA levels of orexin-A but it was not affected by postpartum stage or litter age (nor was there an interaction). However, high orexin-A was negatively associated with frequency of contact with pups and display of erect postures. These results indicate that changes in caregiving across lactation are driven by endogenous factors in the dams, cues they receive from offspring, and interactions between these factors.
Keywords: maternal behavior, lactation, nursing, orexin, preoptic area, reproduction
1. Introduction
For many female mammals that give birth to altricial young, maternal caregiving involves frequent and prolonged contact that is necessary for offspring survival. Mother-young interactions decline across lactation, however, as the offspring achieve greater metabolic, nutritional, and behavioral independence (Altmann, 2001; Grota and Ader, 1969; Konner, 1976; Reisbick et al., 1975). Research investigating the factors underlying this decline in maternal care has mostly focused on changes in the sensory cues from the continuously developing offspring and concludes that cues from older pups are unable to evoke strong maternal responses from the mothers. In support, early research on laboratory rats showed that the decline in mothering across lactation could be prevented if older litters are replaced with younger litters (Reisbick et al., 1975; Rosenblatt, 1969), and a similar phenomenon has been reported for brooding behavior in hens (Richard-Yris and Leboucher, 1987). This is partly due to the fact that younger pups are especially rewarding to mother rats (Wansaw et al., 2008), and even maternally inexperienced nulliparous rats are more likely to approach and act maternally to younger pups than older pups (Stern and Mackinnon, 1978). Dams can presumably distinguish among pups of different ages based on the pups’ size, fur, vocalizations, odors, suckling patterns, and general mobility (Allin and Banks, 1971; Brake et al., 1986; Bruce, 1961; Brunelli et al., 1996; Conely and Bell, 1978; Graham and Letz, 1979; Grant et al., 2012; Nitschke et al., 1975; Noirot, 1968; Okon, 1971; Oswalt and Meier, 1975; Sales, 1979), and maternal caregiving behaviors during both early and late lactation are strongly regulated by these offspring sensory cues (Fleming and Rosenblatt, 1974; Herrenkohl and Rosenberg, 1972; Smotherman et al., 1974; Stern and Johnson, 1989, 1990).
Although maternal behaviors across lactation are clearly regulated by changes in offspring sensory cues, there has been less focus on how endogenous factors within mothers influence and even interact with litter age to contribute to dams’ maternal caregiving behaviors toward younger and older litters. Studies using conditioned place preference to examine the rewarding properties of pups show that pups of any age are equally rewarding to early-postpartum dams, but that late-postpartum dams only prefer chambers associated with younger pups (Wansaw et al., 2008). Furthermore, while early work showed that interacting with young donor pups can delay dams’ natural decline in pup anogenital licking, retrieval, and nest building across lactation, the time that dams spent hovering over the litter or nursing them was apparently unaffected by litter age (Reisbick et al., 1975). On the other hand, while interacting with older donor pups does accelerate the natural decline in caregiving, it does not do so to the degree expected based on the litter’s age (Rosenblatt, 1969). Taken together, these studies suggest that elapsed postpartum time also is an important contributor to mothers’ caregiving behaviors.
While these previous studies indicate that both litter age and postpartum stage contribute to the decline in mother-offspring contact across lactation, mother-offspring interactions were observed each day immediately following temporary provisioning of test pups of varying ages (Reisbick et al., 1975; Rosenblatt, 1969). This procedure is problematic because fostering pups, and the associated dam-pup separations that must then occur (i.e. “handing”), acutely increases caregiving behaviors by postpartum laboratory rodents (Claessens et al., 2012; Darnaudéry et al., 2004; Kelly et al., 2017; Reis et al., 2014; Sherrod et al., 1974; Van der Veen et al., 2008). A recent study also demonstrated that as little as one-minute separations of dams and litters affects caregiving chronically across lactation, accelerating the natural decline in nursing and increasing dams’ time away from the nest (Reis et al., 2014). Fostering also alters the cues emitted by pups, by decreasing the number of ultrasonic vocalizations produced by young pups but increasing vocalizations by older pups (Bell et al., 1971; Darnaudéry et al., 2004). Therefore, interpreting the previous studies investigating the effects of offspring age and postpartum stage on mother-litter interactions is complicated by the unintended effects of the daily mother-offspring separations they used.
Brain sites underlying the decline in maternal behavior across lactation are almost never studied but are known to include the medial preoptic area (mPOA). The mPOA is best known for its positive role in the onset and early maintenance of maternal caregiving behaviors (Lonstein et al., 2014; Numan and Insel, 2003), but it also has a role in their termination. Pereira and Morrell (2009) have shown that inactivating the mPOA of late-postpartum dams with bupivacaine increases caregiving back to early-postpartum levels (Pereira and Morrell, 2009). Many neurochemicals released in the mPOA are probably involved in how litter age and postpartum stage affect maternal caregiving, and may include the neuropeptide orexin. Orexin (otherwise known as hypocretin) is synthesized by cells of the lateral and posterior hypothalamic areas and has two forms, orexin-A and orexin-B, that bind with differing affinities to the two known orexin receptors (Sakurai et al., 1998). In parturient rats, hypothalamic orexin-A immunoreactivity falls if the litter is removed at parturition (Sun et al., 2003), suggesting a role for offspring cues in maternal central orexin-A content. Offspring-induced changes in maternal orexin-A are likely relevant for her caregiving behaviors, as intracerebroventricular infusion of orexin-A even further increases caregiving behaviors in early-postpartum mice (D’Anna and Gammie, 2006), and mPOA infusion of orexin-A increases pup licking by late-postpartum rats (Rivas et al., 2016). Conversely, orexin-1 receptor antagonism in the mPOA decreases pup licking by both early- and late-postpartum rats (Rivas et al., 2016).
To examine the independent and interacting effects of litter age and postpartum stage on maternal caregiving and mPOA orexin-A content, we permanently fostered postnatal day 1 and 8 litters within and between groups of postpartum day 1 and 8 dams before conducting analyses of maternal behavior five and six days later. This five-day acclimation period between fostering and testing avoided potential confounds due to daily fostering on maternal behavior, and generated four groups for analysis: early-postpartum dams raising younger litters (postpartum day 7/postnatal day 7 at the conclusion of testing), early-postpartum dams raising older litters (postpartum day 7/postnatal day 14 at the conclusion of testing), later-postpartum dams raising younger litters (postpartum day 14/postnatal day 7), and later-postpartum dams raising older litters (postpartum day 14/postnatal day 14). We hypothesized that caregiving behaviors would be under the control of both postpartum stage and litter age, and would be associated with orexin-A levels in the mPOA.
2. Methods
2.1 Subjects
Female Long-Evans rats were born and raised in our colony, descended from rats purchased from Harlan Laboratories (Indianapolis, IN) as described previously (Smith et al., 2012). Briefly, subjects were housed in clear polypropylene cages (48 cm × 28 cm × 16 cm) with one or two female littermates starting at weaning (21 days of age). Animals were given wood shavings for bedding, food and water ad libitum, and experienced a 12:12 light/dark cycle (0600 h lights on). Subjects were impregnated between 75–100 days old by being paired overnight with experienced male breeders in our colony during proestrus (determined by vaginal smearing). After mating, subjects were housed with another pregnant female until being singly housed starting five days before the expected day of parturition. All procedures were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee at Michigan State University.
2.2 Litter Fostering
The study used a 2 × 2 factorial design with postpartum stage (early/later) and litter age (younger/older) as factors to create four groups after in-fostering and cross-fostering the litters: 1) early-postpartum dams raising younger litters (postpartum day 7/postnatal day 7 at conclusion of testing: n = 14), 2) early-postpartum dams raising older litters (postpartum day 7/postnatal day 14: n = 11), 3) later-postpartum dams raising younger litters (postpartum day 14/postnatal day 7: n = 11), and 4) later-postpartum dams raising older litters (postpartum day 14/postnatal day 14: n = 14). All litters were culled to contain 4 males and 4 females within 24 h after birth. The day of parturition was considered postpartum day 0. For the early-postpartum subjects (i.e., postpartum day 7 dams raising younger or older litters), fostering occurred at the time of litter culling on postpartum day 1. For later-postpartum subjects (i.e., postpartum day 14 dams raising younger or older litters), fostering occurred on postpartum day 8. At the time of fostering, the subject’s litter was removed and then permanently replaced with foster pups that were either less than one day old or 8 days old. One hour later, cages were briefly checked to confirm that dams were caring for their new litters, and all of them were. All pups in all four groups survived the fostering procedure.
2.3 Observations of Maternal Behavior
Subjects’ home cage behavior was observed at 0800 and again at 1100 hr. for 30 min each on the fifth and sixth days after cross-fostering (i.e., on postpartum days 6 and 7, or postpartum days 13 and 14). Maternal behaviors (digging/nesting, licking pups, hovering over pups, and nursing in a crouched kyphotic posture or while lying supine on her side) and non-maternal behaviors (resting alone, exploring the cage, self-grooming, and eating/drinking) were recorded every 60 sec. and summed across the four observations for data analyses. Contact with the litter was calculated as the frequency that dams were in physical contact with any pup(s) during the spot checks. Maternal behaviors that occurred simultaneously at the same spot check (e.g., hovering over pups while licking them) were scored as instances of each behavior for the analyses of individuals behaviors, but were only counted for one instance of contact with the litter.
2.4 Sacrifice, Tissue Processing, and orexin-A EIA
During the afternoon following the last behavior observation (1400–1500 hr.), dams and litter were weighed, and dams were narcotized with CO2 and their brains were harvested, flash frozen, and stored at −80 °C until being sectioned into 500-μm thick sections. For the orexin-A enzyme immunoassay (EIA), two consecutive sections containing the mPOA from 8 randomly selected subjects from each of the four groups were punched using a 1.0-mm diameter Harris Micro Punch (Electron Microscopy Sciences #69034-10, Hatfield, PA) and weighed on a microbalance (Denver Instruments, Bohemia, NY; Model: APX-60). Samples were then processed using orexin EIA methods by Feng and colleagues (Feng et al., 2008; Feng et al., 2007). 0.5 M acetic acid was added to the punches at a volume 10× the weight of the tissue. Samples were placed in a boiling water bath for 10 min, then homogenized with a Fisher Scientific Sonic Dismembrator Model 100 for 10 sec. Samples were centrifuged for 5 min at 5500 rpm, the supernatants removed, transferred to a new tube, and dried in a Thermo Savant ISS110 Integrated SpeedVac concentrator for ~30 min. Dehydrated samples were stored at −80°C until reconstituting for analysis.
An orexin-A EIA kit (Phoenix Pharmaceuticals Inc., Burlingame, CA; Cat. No. EK-003-30) was used per the manufacturer’s instructions. Briefly, the dehydrated samples were reconstituted with 10 μl of 1× assay buffer, followed by 6 μl of reconstituted sample diluted with 114 μl of 1× assay buffer. Using a 96-well plate, 50 μl of all standards and diluted samples in duplicate were incubated in 25μl primary antibody specific to orexin-A and 25μl biotinylated peptide for 2 hr. After four rinses in 1× assay buffer, samples were incubated in SA-HRP solution for 1 hr. After rinsing four times in 1× assay buffer, samples were incubated in 100 μl of TMB substrate in the dark for 1 hr. The chromogenic reaction was halted by adding 100 μl of 2N HCl into each well. Finally, the 450 nm absorbances were read using an iMark microplate reader (Bio-Rad, Hercules, CA). Average absorbances were placed on the calculated standard curve, then adjusted by their dilution factor and total tissue weight to provide the final orexin-A concentration per sample.
2.5 Statistical Analyses
Data were analyzed using 2 (postpartum stage) × 2 (litter age) ANOVAs. In the case of interactions, post-hoc analyses of simple main effects were conducted. Monotonic relationships between some behaviors of particular interest and orexin-A content in the mPOA were determined using Spearman correlations, with multiple comparisons corrected using the Benjamini-Hochberg procedure, with a FDR set to 0.05 (Benjamini and Hochberg, 1995). Using Dixon’s Q outlier tests, one dam in the early-postpartum/younger litter group was an extreme outlier for dam weight and one dam in the later-postpartum/older litter group was an extreme outlier for mPOA orexin-A content; both were removed from the analyses of those variables. Statistical significance was indicated by p < .05.
3. Results
3.1 Maternal caregiving is affected by postpartum stage, litter age, and their interaction
There were main effects of postpartum stage on the frequency of maternal contact with the pups (F(1, 47) = 4.13, p < 0.05, η2p = 0.08; Fig 1A) and on the dams’ display of erect postures over them (hovering over the litter and kyphosis) (F(1, 47) = 12.267, p < 0.01, η2p = 0.21; Fig 1B), with early-postpartum dams showing higher frequencies of both behaviors compared to later-postpartum dams. There was also a main effect of the litter age on the frequency that the dams were sleeping away from the nest (F(1, 47) = 7.88, p < 0.01, η2p = 0.14; Fig 1C), with dams raising older pups sleeping away from the nest more often than dams with younger pups.
Figure 1.
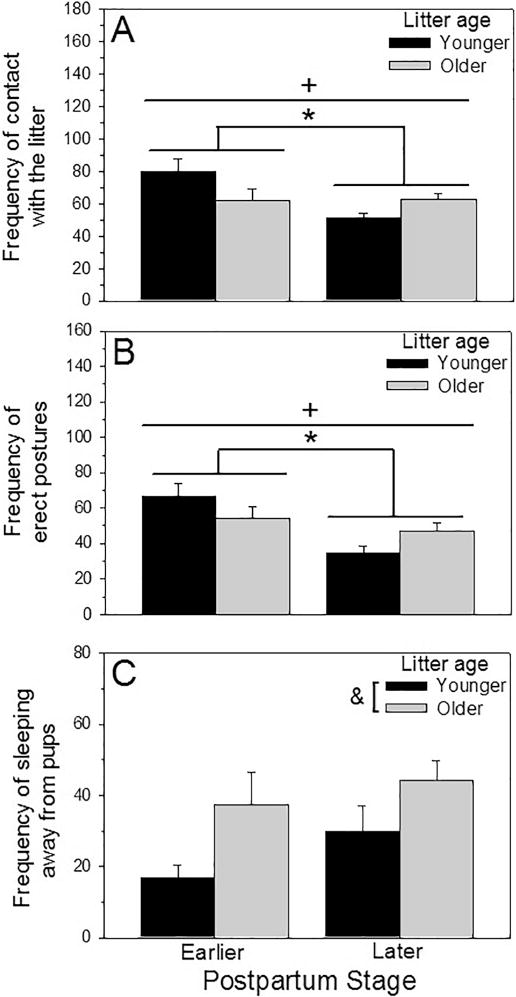
(A) Frequency of contact with the litter (Mean + SEM), (B) frequency of erect postures over the litter (kyphosis and hovering over; Mean ± SEM) and (C) frequency of sleeping away from the nest (Mean + SEM) by early-postpartum or later-postpartum dams interacting with younger or older litters. * = significant main effect of postpartum stage, p < 0.05. & = significant main effect of litter age, p < 0.05. + = significant interaction, p < 0.05.
There were also significant interactions between postpartum stage and litter age for the frequency that dams were in contact with the litter (F(1,47) = 4.34, p = 0.04, η2p = 0.09; Fig. 1A) and the frequency of erect postures over them (F(1,47) = 4.66, p = 0.04, η2p = 0.09; Fig. 1B). These interactions are driven by dams interacting with younger litters because post-hoc analysis revealed that early-postpartum dams with younger litters displayed the highest frequency of these behaviors, while later-postpartum dams with younger litters displayed the lowest frequency (frequency of contact with the litter: F(1,47) = 8.35, p = 0.01, η2p = 0.15; Erect postures: F(1,47) = 15.79, p < 0.001, η2p = 0.25). There were no significant main effects or interactions for the other caregiving behaviors assessed (see Table 1).
Table 1.
Frequency (Mean ±SEM) of maternal behaviors displayed by early-postpartum or later-postpartum dams interacting with younger or older litters.
| Postpartum Stage/Litter Age | Earlier dams/Younger litters | Earlier dams/Older litters | Later dams/Younger litters | Later dams/Older litters | p: Stage; Age; Interaction |
|---|---|---|---|---|---|
| Contact with the litter | 79.9 ± 8.0 | 62.2 ± 7.4 | 51.5 ± 2.9 | 62.5 ± 6.6 | <0.05*; 0.64; 0.04+ |
| Licking pups | 7.9 ± 2.2 | 9.6 ± 2.1 | 11.6 ± 2.1 | 7.7 ± 1.1 | 0.64; 0.60; 0.15 |
| Digging/nesting | 2.2 ± 1.3 | 5.4 ± 1.4 | 1.8 ± .5 | 2.0 ± 0.8 | 0.09; 0.13; 0.18 |
| Retrieving pups | 0.1 ± 0.1 | 0.6 ± 0.4 | 0.3 ± 0.3 | 0.5 ± 0.2 | 0.94; 0.18; 0.56 |
| Self-grooming | 6.9 ± 1.3 | 11.1 ± 2.9 | 11.7 ± 3.2 | 9.9 ± 2.4 | 0.46; 0.63; 0.23 |
| Eating/Drinking | 12.1 ± 3.8 | 20.4 ± 5.4 | 21.0 ± 6.0 | 12.2 ± 3.1 | 0.93; 0.96; 0.06 |
| Exploration | 5.3 ± 1.5 | 3.8 ± 0.8 | 6.0 ± 1.9 | 9.3 ± 2.0 | 0.08; 0.60; 0.17 |
| Hovering over the litter | 39.2 ± 9.6 | 32.7 ± 5.1 | 19.6 ± 2.5 | 31.6 ± 4.8 | 0.12; 0.68; 0.16 |
| Kyphosis | 27.8 ± 7.3 | 21.8 ± 3.5 | 14.7 ± 3.7 | 15.2 ± 3.2 | 0.06; 0.59; 0.52 |
| Erect Postures (Hovering over + Kyphosis) | 67.0 ± 6.8 | 54.6 ± 6.1 | 34.4 ± 4.3 | 46.8 ± 4.9 | <0.001*; 0.99; 0.04+ |
| Supine Nursing | 12.9 ± 5.2 | 7.6 ± 3.0 | 17.1 ± 3.8 | 12.7 ± 2.6 | 0.24; 0.23; 0.92 |
| Total Nursing (Supine + Kyphosis) | 40.6 ± 10.0 | 29.5 ± 4.9 | 31.8 ± 2.8 | 26.3 ± 3.5 | 0.36; 0.21; 0.67 |
| Sleeping away from pups | 16.9 ± 3.4 | 37.5 ± 8.9 | 29.9 ± 7.0 | 44.3 ± 5.6 | 0.12; 0.01&; 0.63 |
| Litter Weight (g) | 142.1 ± 2.7 | 211.1 ± 8.9 | 147.3 ± 4.6 | 274.3 ± 4.4 | < 0.001*; <0.001&; <0.001+ |
| Dam Weight (g) | 299.9 ± 3.3 | 310.5 ± 4.5 | 317.6 ± 5.4 | 308.6 ± 4.2 | 0.09; 0.81; 0.04+ |
Data shown are sums across the behavioral tests.
Significant main effect of postpartum stage.
Significant main effect of litter age.
Significant interaction between postpartum stage and litter age.
Litter weights were affected by the dams’ postpartum stage (F(1, 47) = 41.92, p < 0.001, η2p = 0.47; Fig 2A), with litters being raised by later-postpartum dams weighing more than litters being raised by early-postpartum dams. Not surprisingly, older litters weighed more than younger litters (F(1, 47) = 344.99, p < 0.001, η2p = 0.88; Fig 2A). There was a significant interaction between the factors on litter weight (F(1, 47) = 30.28, p < 0.001, η2p = 0.39; Fig 2A), due to younger litters weighing the same if they were raised by early- or later-postpartum dams, but older litters raised by early-postpartum dams weighing less than older litters raised by later-postpartum dams (F(1,47) = 70.81, p < 0.001, η2p = 0.61).
Figure 2.
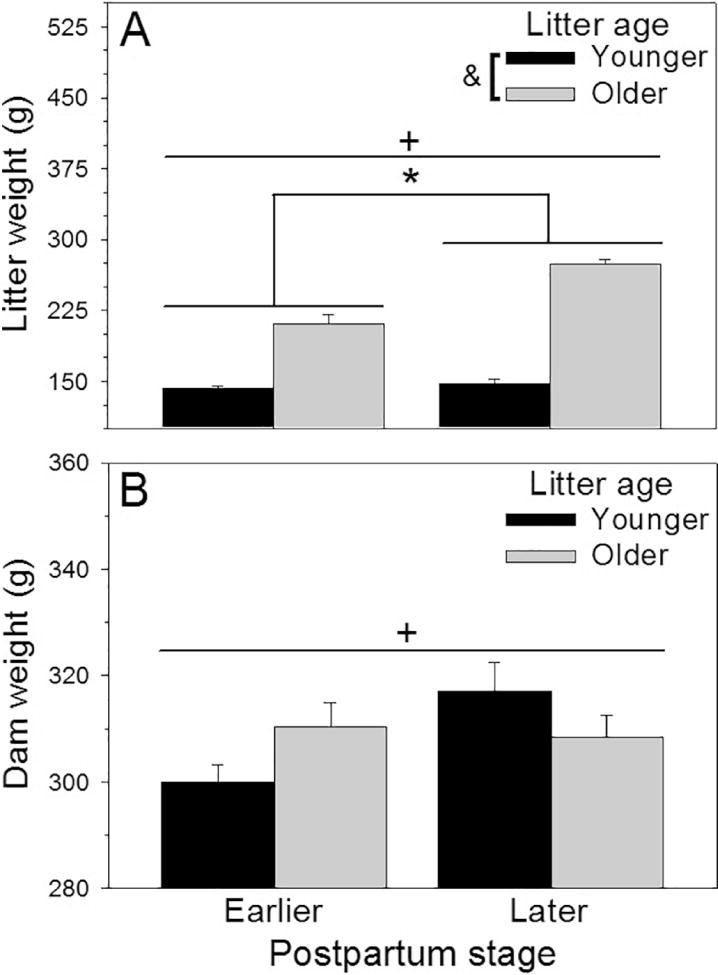
(A) Weight of younger or older litters and early-postpartum or later-postpartum dams (B) at sacrifice (Mean + SEM). * = significant main effect of postpartum stage, p < 0.05. & = significant main effect of litter age, p < 0.05. + = significant interaction, p < 0.05.
Dams’ body weights also differed among groups, with a significant interaction between postpartum stage and litter age (F(1, 45) = 4.66, p = 0.04, η2p = 0.09; Fig 2B); all dams raising older litters had similar weights but early-postpartum dams raising younger litters weighed less than the later-postpartum dams raising younger litters (F(1, 45) = 7.49, p < 0.01, η2p = 0.14). There were no main effects of postpartum stage (F(1, 45) = 3.04, p = 0.09, η2p = 0.06; Fig 2B) or litter age (F(1, 45) = 0.06, p = 0.81, η2p = 0.001; Fig 2B) on the dams’ body weights.
3.2 Orexin-A in the mPOA is not affected by postpartum stage or litter age, but high orexin-A is associated with maternal caregiving
Orexin-A in the mPOA was not significantly affected by postpartum stage (F(1, 27) = 2.54, p = 0.12, η2p = 0.09; Fig. 3) or litter age (F(1, 27) = 3.11, p = 0.09, η2p = 0.10; Fig. 3), nor was there a significant interaction between the factors (F(1, 27) = 0.12, p = 0.73, η2p = 0.001; Fig. 3). Orexin-A in the mPOA was not significantly correlated with the frequency of any behavior recorded, but when all four groups were combined and separated by a median split of their orexin-A levels (note that all groups were represented in the low and high orexin-A groups), the high orexin-A dams showed significant negative correlations between their orexin-A levels and their frequencies of contact with the litter (r15 = −0.61, p = 0.02; Fig. 4B) and in the frequency they displayed erect postures over the litter (r15 = −0.70, p < 0.01; Fig. 4D). For the low orexin half of the dams there were no significant correlations between mPOA orexin-A and any behavior.
Figure 3.
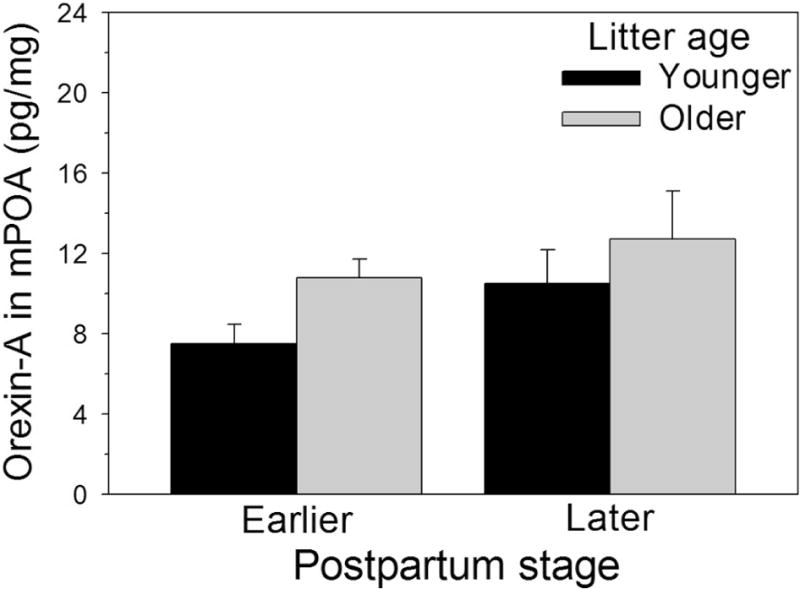
Orexin-A in the medial preoptic area (Mean + SEM) of early-postpartum or later-postpartum dams interacting with younger or older litters.
Figure 4.
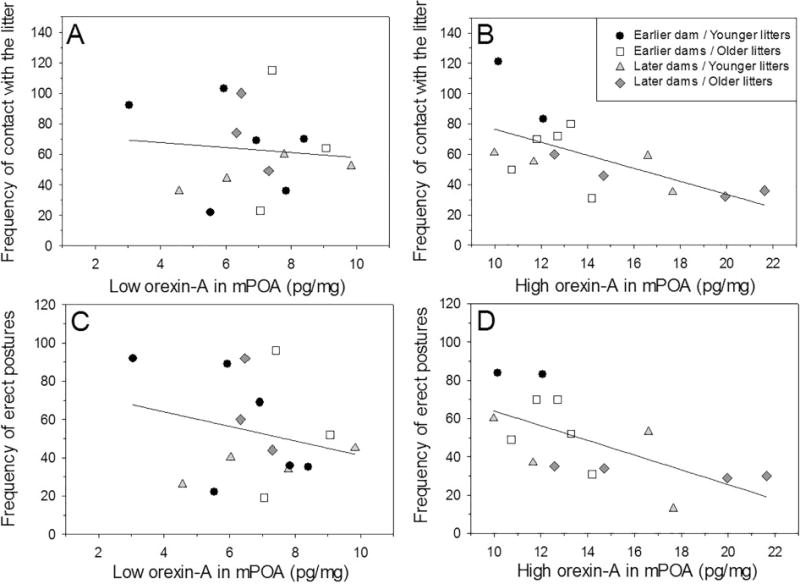
Correlations between orexin-A in the medial preoptic area of dams with the lowest orexin-A levels (based on median split) and (A) frequency of contact with the litter and (C) frequency of erect postures (kyphosis and hovering over in the nest). Correlations between orexin-A in the medial preoptic area of dams with the highest orexin-A levels (based on median split) and (B) frequency of contact with the litter and (D) frequency of erect postures. Different symbols indicate dams’ original group designation.
4 Discussion
The present study found that early-postpartum dams had a higher frequency of contact with their litters and displayed more erect postures (hovering over and kyphosis) compared to late-postpartum dams. However, these effects appear to be mostly driven by the interaction between postpartum stage and litter age, such that early-postpartum dams with younger litters had a higher frequency of contact with their litters and displayed more erect postures compared to later-postpartum dams with younger litters. This is consistent with the higher frequency and duration of hovering over the litter and kyphosis by early-postpartum dams compared to later-postpartum dams found previously (Stern and Keer, 2002). Maternal experience with the typical offspring sensory cues probably played a role, and postpartum rats will not show kyphosis while interacting with a litter in the absence of suckling, but virgins that have never been suckled will readily adopt this posture without that input (Lonstein et al., 1999). Additionally, the natural progression from dam-initiated caregiving to pup-initiated caregiving as a result of older pups’ increased mobility that becomes clear by PPD 14 (Stern and Johnson, 1989). Therefore, our younger litters were less capable than our older litters to initiate contact with the later-postpartum dams, resulting in less mother-litter contact and opportunities for dams to display erect postures over them. Additionally, less mobile younger pups would also be unable to remove themselves from contact with dams (Grant et al., 2012), which may have further contributed to dams with younger litters spending more time with those litters. On the other hand, the higher frequency of dam-litter interactions between early-postpartum dams with younger litters may be due to the fact that suckling stimulation keeps dams in the nest with the litter (Stern et al., 1992; Stern and Johnson, 1990), and while older pups disengage from the teats after satiation, younger pups do not (Hall and Rosenblatt, 1977; Lorenz et al., 1982).
Not all the results from our study are consistent with previous research investigating the interaction between postpartum stage and litter age on maternal behaviors. Previous work found that younger litters could delay the natural decline in dams’ pup retrieval and anogenital licking across lactation, while not affecting total time in contact with the pups or total time nursing (Reisbick et al., 1975). Furthermore, older litters were shown to hasten the natural decline in pup retrieval across lactation (Rosenblatt, 1969). Conversely, we found effects on the frequency that dams were in contact with the litter and displayed erect postures over them, while observing no effects on licking or carrying the pups. Differences in methods among the studies can explain these apparent discrepancies. The previous research analyzed caregiving following daily removal of litters from their home cages and introduction of test litters of different ages (Reisbick et al., 1975; Rosenblatt, 1969). This procedure allowed the experimenters to test maternal retrieval of the displaced pups each day, whereas our undisturbed observations rarely included maternal carrying of pups because it is not necessary/observed under undisturbed conditions (Brewster and Leon, 1980). As mentioned earlier, daily fostering and the associated separation between dams and litters also acutely increases caregiving behaviors (Claessens et al., 2012; Darnaudéry et al., 2004; Kelly et al., 2017; Reis et al., 2014; Sherrod et al., 1974). Even more important for our study is that mother-litter separations affect caregiving behaviors and offspring sensory cues persistently across lactation (Darnaudéry et al., 2004; Reis et al., 2014). Given that many caregiving behaviors were unaffected by either postpartum stage or litter age (or an interaction between them) in our study that involved a five-day acclimation period before observations commenced, some of the findings from previous work on this topic are probably due to the unintended effects of the daily fostering procedure itself. This possibility is further supported by the fact that mother-offspring interactions are modified through learning processes that promote proper caregiving, and that learning is probably disturbed by the daily dyadic disruption from fostering (Barrett and Fleming, 2011; Lucion and Bortolini, 2014).
We also found that postpartum stage, litter age, and their interaction affected litter weights at sacrifice, with younger litters weighing the same if they were raised by early- or later-postpartum dams, but older litters weighed less if they were raised by early-postpartum dams than by later-postpartum dams. The more constant weight of the younger litters, despite later-postpartum dams spending less time with them, could be because the more maternally experienced later-postpartum dams are more efficient in their caregiving (Lonstein et al., 1999; Uriarte et al., 2008). It may also be the case that the effect found on the older litters is due to their inability to obtain sufficient milk from early-postpartum dams, even though they suckle more strongly than younger litters (Brake et al., 1986), and under some circumstances can increase dams’ typical milk production (Lau and Henning, 1984). Changes in milk content across lactation probably also affected litter weights, as milk from later lactation has more protein than milk from early lactation (Chalk and Bailey, 1979; Keen et al., 1981), and experimentally decreasing milk protein with a protein-restricted diet does reduce litter weight gains (Passos et al., 2000). It is reasonable to suggest that our findings related to litter weights are not independent of the effects of postpartum stage and litter age on maternal caregiving behavior. That is, our significant behavioral findings are probably partly a consequence of the atypical development of the pups (and vice-versa). Indeed, a protein-restricted diet not only reduces litter weights, but also pup movement and feeding independence, resulting in a corresponding increase in the time that dams spend in the nest (Massaro et al., 1974; Morgane et al., 1978; Wiener et al., 1977).
Body weight of lactating rats under typical laboratory conditions increases ~0.4–2.0 g/day (Ota and Yokoyama, 1967; Rolls and Rowe, 1982; Rolls et al., 1984). The dams in our later-postpartum/younger litter group did weigh ~9 grams more than the dams in our early-postpartum/younger litter group, despite there being no significant main effect of postpartum stage. However, this main effect of postpartum stages on the dams’ body weights was diluted by the interaction between postpartum stage and litter age. All dams raising older litters had similar weights, but for those raising younger litters, early-postpartum dams weighed less than later-postpartum dams. Daily caloric intake in the postpartum rat is 2–3 times higher than before reproduction (Strubbe and Gorissen, 1980; Woodside, 2007) and is driven by offspring suckling (Denis et al., 2003; Fleming, 1976). Given that older pups suckle longer and stronger than younger pups (Brake et al., 1986; Bruce, 1961), early-postpartum dams raising older litters may eat more because they receive abnormally high amounts of suckling. On the other hand, the relatively lower weight for the later-postpartum dams with younger litters is probably the results of body weight being related to both caloric intake and lactational demand (Woodside, 2007). Older pups ingest up to 5-fold more milk compared to younger pups (Babicky et al., 1970; Hall and Rosenblatt, 1977), so our lighter later-postpartum dams with younger litters had substantially less lactational demand.
The postpartum orexin system is sensitive to pup cues and permanent removal of the litter soon after parturition decreases hypothalamic orexin-A immunoreactivity (Sun et al., 2003). We did not, however, find main effects of postpartum stage, pup age, or an interaction between them in mPOA orexin-A levels. This suggests that offspring-related changes to hypothalamic orexin-A may not extend to the mPOA, and/or that while orexin-A is sensitive to litter presence it not sensitive to age-related changes in the pups. Although we found no significant differences among our groups in mPOA orexin-A levels, we did find that dams with high orexin-A levels (collapsed across groups and based on a median split) showed a negative correlation between orexin-A and the frequency of contact with the litter or the display of erect postures. There were no such relationships found for dams with the lower half of mPOA orexin-A levels. Intracerebroventricular orexin-A dose-dependently decreases nursing and increases pup licking behavior and in mice (D’Anna and Gammie, 2006), and in later-postpartum laboratory rats mPOA infusion of orexin-A increases pup licking (Rivas et al., 2016). While our results could suggest that mPOA orexin-A needs to be above a certain threshold for its caregiving effects, endogenous orexin-A signaling at any level is still relevant, because peripheral or central blockade of orexin-1 receptors increases kyphotic nursing and decreases pup licking in rodent mothers as a group (D’Anna and Gammie, 2006; Rivas et al., 2016). Given the arousing effect of orexin-A signaling in the mPOA (Berridge et al., 2010; Espana et al., 2001; Thakkar et al., 2001), and the importance of slow-wave sleep for nursing and milk letdown (Lincoln et al., 1980; Voloschin and Tramezzani, 1979), high orexin-A in the mPOA could interfere with sleep-dependent nursing by promoting arousal.
In sum, this study supports previous research demonstrating that postpartum stage and offspring age interact to influence maternal caregiving behaviors at different stages of lactation. These influences were also found to be associated with litter growth and maternal body weight. Because high orexin-A levels in the mPOA were related to some aspects of maternal caregiving more generally and not significantly affected by postpartum stage or litter age (or their interaction), the neurochemical factors in mothers that change strictly according to postpartum time or that are malleable in response to sensory cues emanating from differently aged pups remain to be determined.
Highlights.
Postpartum stage and offspring age interact to affect dams’ contact with pups
These factors also interact to affect litter and dam body weights
High maternal mPOA orexin-A is associated with less time contacting pups
Acknowledgments
This work was supported by NIH grant RO1HD057962 to J.S. Lonstein. The authors would like thank Olivia Spagnuolo and Michael Donlin for assistance conducting behavioral observations, and Katrina D. Linning for her help with the orexin-A EIA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allin JT, Banks EM. Effects of temperature on ultrasound production by infant albino rats. Dev Psychobiol. 1971;4(2):149–156. doi: 10.1002/dev.420040206. [DOI] [PubMed] [Google Scholar]
- Altmann J. Baboon mothers and infants. University of Chicago Press; 2001. [Google Scholar]
- Babicky A, Ostadalova I, Parizek J, Kolar J, Bibr B. Use of radioisotope techniques for determining the weaning period in experimental animals. Physiol Bohemoslov. 1970;19:457–467. [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual research review: All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry. 2011;52(4):368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Bell RW, Nitschke W, Gorry TH, Zachman TA. Infantile stimulation and ultrasonic signaling: a possible mediator of early handling phenomena. Dev Psychobiol. 1971;4(2):181–191. doi: 10.1002/dev.420040209. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological) 1995:289–300. [Google Scholar]
- Berridge CW, España RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake SC, Tavana S, Myers MM. A method for recording and analyzing intra-oral negative pressure in suckling rat pups. Physiol Behav. 1986;36(3):575–578. doi: 10.1016/0031-9384(86)90333-1. [DOI] [PubMed] [Google Scholar]
- Brewster J, Leon M. Relocation of the site of mother–young contact: Maternal transport behavior in Norway rats. J Comp Physiol Psychol. 1980;94(1):69. [Google Scholar]
- Bruce HM. Observations on the suckling stimulus and lactation in the rat. J Reprod Fertil. 1961;2(1):17–34. [Google Scholar]
- Brunelli SA, Keating CC, Hamilton NA, Hofer MA. Development of ultrasonic vocalization responses in genetically heterogeneous National Institute of Health (N: NIH) rats. I. Influence of age, testing experience, and associated factors. Dev Psychobiol. 1996;29(6):507–516. doi: 10.1002/(SICI)1098-2302(199609)29:6<507::AID-DEV3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Chalk PA, Bailey E. Changes in the yield, and carbohydrate, lipid and protein content of milk during lactation in the rat. J Dev Physiol. 1979;1(1):61–79. [PubMed] [Google Scholar]
- Claessens SE, Daskalakis NP, Oitzl MS, de Kloet ER. Early handling modulates outcome of neonatal dexamethasone exposure. Horm Behav. 2012;62(4):433–441. doi: 10.1016/j.yhbeh.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Conely L, Bell RW. Neonatal ultrasounds elicited by odor cues. Dev Psychobiol. 1978;11(3):193–197. doi: 10.1002/dev.420110302. [DOI] [PubMed] [Google Scholar]
- D’Anna KL, Gammie SC. Hypocretin- 1 Dose- Dependently Modulates Maternal Behaviour in Mice. J Neuroendocrinol. 2006;18(8):553–566. doi: 10.1111/j.1365-2826.2006.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudéry M, Koehl M, Barbazanges A, Cabib S, Le Moal M, Maccari S. Early and later adoptions differently modify mother-pup interactions. Behav neuroscience. 2004;118(3):590. doi: 10.1037/0735-7044.118.3.590. [DOI] [PubMed] [Google Scholar]
- Denis R, Williams G, Vernon R. Regulation of serum leptin and its role in the hyperphagia of lactation in the rat. J Endocrinol. 2003;176(2):193–203. doi: 10.1677/joe.0.1760193. [DOI] [PubMed] [Google Scholar]
- Espana R, Baldo B, Kelley A, Berridge C. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106(4):699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Hu Y, Strohl K. Changes in brain orexin levels in a rat model of depression induced by neonatal administration of clomipramine. J Psychopharmacol. 2008;22(7):784–791. doi: 10.1177/0269881106082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Strohl KP. Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res. 2007;1154:163–172. doi: 10.1016/j.brainres.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Fleming AS. Control of food intake in the lactating rat: role of suckling and hormones. Physiol Behav. 1976;17(5):841–848. doi: 10.1016/0031-9384(76)90051-2. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats: I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol. 1974;86(2):221. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Consiglio AR, Barros HMT, Lucion AB. Pup age and aggressive behavior in lactating rats. Braz J Med Biol Res. 2000;33(9):1083–1088. doi: 10.1590/s0100-879x2000000900015. [DOI] [PubMed] [Google Scholar]
- Graham M, Letz R. Within- species variation in the development of ultrasonic signaling of preweanling rats. Dev Psychobiol. 1979;12(2):129–136. doi: 10.1002/dev.420120205. [DOI] [PubMed] [Google Scholar]
- Grant RA, Mitchinson B, Prescott TJ. The development of whisker control in rats in relation to locomotion. Dev Psychobiol. 2012;54(2):151–168. doi: 10.1002/dev.20591. [DOI] [PubMed] [Google Scholar]
- Grota LJ, Ader R. Continuous recording of maternal behaviour in Rattus norvegicus. Animal Behav. 1969;17(4):722–729. doi: 10.1016/0003-3472(70)90083-7. [DOI] [PubMed] [Google Scholar]
- Hall WG, Rosenblatt JS. Suckling behavior and intake control in the developing rat pup. J Comp Physiol Psychol. 1977;91(6):1232. [Google Scholar]
- Herrenkohl LR, Rosenberg P. Exteroceptive stimulation of maternal behavior in the naive rat. Physiol Behav. 1972;8(4):595–598. doi: 10.1016/0031-9384(72)90080-7. [DOI] [PubMed] [Google Scholar]
- Keen CL, Lönnerdal B, Clegg M, Hurley LS. Developmental changes in composition of rat milk: trace elements, minerals, protein, carbohydrate and fat. J Nutr. 1981;111(2):226–236. doi: 10.1093/jn/111.2.226. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hiura LC, Saunders AG, Ophir AG. Oxytocin neurons exhibit extensive functional plasticity due to offspring age in mothers and fathers. Integr Comp Biol. 2017;57(3):603–618. doi: 10.1093/icb/icx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner MJ. Maternal care infant behavior and development among the Kung 1976 [Google Scholar]
- Lau C, Henning SJ. Regulation of milk ingestion in the infant rat. Physiol Behav. 1984;33(5):809–815. doi: 10.1016/0031-9384(84)90052-0. [DOI] [PubMed] [Google Scholar]
- Lincoln D, Hentzen K, Hin T, Van der Schoot P, Clarke G, Summerlee A. Sleep: a prerequisite for reflex milk ejection in the rat. Exp Brain Res. 1980;38(2):151–162. doi: 10.1007/BF00236736. [DOI] [PubMed] [Google Scholar]
- Lonstein J, Pereira M, Marler C, Morrell J. Knobil and Neill’s Physiology of Reproduction. 4th. Elsevier; New York: 2014. Parental behavior; pp. 2371–2438. [Google Scholar]
- Lorenz DN, Ellis SB, Epstein AN. Differential effects of upper gastrointestinal fill on milk ingestion and nipple attachment in the suckling rat. Dev Psychobiol. 1982;15(4):309–330. doi: 10.1002/dev.420150404. [DOI] [PubMed] [Google Scholar]
- Lucion AB, Bortolini MC. Mother–pup interactions: rodents and humans. Front endocrinol. 2014;5:17. doi: 10.3389/fendo.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro TF, Levitsky DA, Barnes RH. Protein malnutrition in the rat: Its effects on maternal behavior and pup development. Dev Psychobiol. 1974;7(6):551–561. doi: 10.1002/dev.420070607. [DOI] [PubMed] [Google Scholar]
- Morgane P, Miller M, Kemper T, Stern W, Forbes W, Hall R, Resnick O. The effects of protein malnutrition on the developing central nervous system in the rat. Neurosci Biobehav Rev. 1978;2(3):137–230. doi: 10.1016/0149-7634(79)90011-3. [DOI] [PubMed] [Google Scholar]
- Nitschke W, Bell RW, Bell NJ, Zachman T. The ontogeny of ultrasounds in two strains of Rattus norvegicus. Exp Aging Res. 1975;1(2):229–242. doi: 10.1080/03610737508257962. [DOI] [PubMed] [Google Scholar]
- Noirot E. Ultrasounds in young rodents. II. Changes with age in albino rats. Animal Behav. 1968;16(1):129–134. doi: 10.1016/0003-3472(68)90123-1. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. Motivational models of the onset and maintenance of maternal behavior and maternal aggression. The neurobiology of parental behavior. 2003:69–106. [Google Scholar]
- Okon EE. The temperature relations of vocalization in infant golden hamsters and Wistar rats. J Zool. 1971;164(2):227–237. [Google Scholar]
- Oswalt GL, Meier GW. Olfactory, thermal, and tactual influences on infantile ultrasonic vocalization in rats. Dev Psychobiol. 1975;8(2):129–135. doi: 10.1002/dev.420080205. [DOI] [PubMed] [Google Scholar]
- Ota K, Yokoyama A. Body weight and food consumption of lactating rats nursing various sizes of litters. J Endocrinol. 1967;38(3):263–268. doi: 10.1677/joe.0.0380263. [DOI] [PubMed] [Google Scholar]
- Passos M, Ramos C, Moura E. Short and long term effects of malnutrition in rats during lactation on the body weight of offspring. Nutr Res. 2000;20(11):1603–1612. [Google Scholar]
- Pereira M, Morrell JI. The changing role of the medial preoptic area in the regulation of maternal behavior across the postpartum period: facilitation followed by inhibition. Behav Brain Res. 2009;205(1):238–248. doi: 10.1016/j.bbr.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A, de Azevedo M, de Souza M, Lutz M, Alves M, Izquierdo I, Lucion A. Neonatal handling alters the structure of maternal behavior and affects mother–pup bonding. Behav Brain Res. 2014;265:216–228. doi: 10.1016/j.bbr.2014.02.036. [DOI] [PubMed] [Google Scholar]
- Reisbick S, Rosenblatt JS, Mayer AD. Decline of maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1975;89(7):722. doi: 10.1037/h0077059. [DOI] [PubMed] [Google Scholar]
- Richard-Yris MA, Leboucher G. Effects of exposure to chicks on maternal behaviour in domestic chickens. Bird Behavior. 1987;7(1):31–36. [Google Scholar]
- Rivas M, Torterolo P, Ferreira A, Benedetto L. Hypocretinergic system in the medial preoptic area promotes maternal behavior in lactating rats. Peptides. 2016;81:9–14. doi: 10.1016/j.peptides.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rowe EA. Pregnancy and lactation in the obese rat: effects on maternal and pup weights. Physiol Behav. 1982;28(3):393–400. doi: 10.1016/0031-9384(82)90130-5. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Van Duijvenvoorde P, Rowe EA. Effects of diet and obesity on body weight regulation during pregnancy and lactation in the rat. Physiol Behav. 1984;32(2):161–168. doi: 10.1016/0031-9384(84)90124-0. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. The development of maternal responsiveness in the rat. Am J Orthopsychiatry. 1969;39(1):36. doi: 10.1111/j.1939-0025.1969.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Rowell TE. On the retrieving of young and other behaviour in lactating golden hamsters. J Zool. 1960;135(2):265–282. [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Wilson S. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sales GD. Strain differences in the ultrasonic behavior of rats (Rattus norvegicus) Am Zool. 1979;19(2):513–527. [Google Scholar]
- Sherrod KB, Connor WH, Meier GW. Transient and enduring effects of handling on infant and maternal behavior in mice. Dev Psychobiol. 1974;7(1):31–37. doi: 10.1002/dev.420070106. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12(1):55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- Stern JM, Dix L, Bellomo C, Thramann C. Ventral trunk somatosensory determinants of nursing behavior in Norway rats: 2. Role of nipple and surrounding sensations. Psychobiology. 1992;20(1):71–80. [Google Scholar]
- Stern JM, Johnson SK. Perioral somatosensory determinants of nursing behavior in Norway rats (Rattus norvegicus) J Comp Psychol. 1989;103(3):269. doi: 10.1037/0735-7036.103.3.269. [DOI] [PubMed] [Google Scholar]
- Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in Norway rats. I. Effects of variations in the quality and quantity of pup stimuli. Physiol Behav. 1990;47(5):993–1011. doi: 10.1016/0031-9384(90)90026-z. [DOI] [PubMed] [Google Scholar]
- Stern JM, Keer SE. Acute hunger of rat pups elicits increased kyphotic nursing and shorter intervals between nursing bouts: Implications for changes in nursing with time postpartum. J Comp Psychol. 2002;116(1):83. doi: 10.1037/0735-7036.116.1.83. [DOI] [PubMed] [Google Scholar]
- Stern JM, Mackinnon DA. Sensory regulation of maternal behavior in rats: effects of pup age. Dev Psychobiol. 1978;11(6):579–586. doi: 10.1002/dev.420110607. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Gorissen J. Meal patterning in the lactating rat. Physiol Behav. 1980;25(5):775–777. doi: 10.1016/0031-9384(80)90383-2. [DOI] [PubMed] [Google Scholar]
- Sun G, Narita K, Murata T, Honda K, Higuchi T. Orexin- A Immunoreactivity and Prepro- Orexin mRNA Expression in Hyperphagic Rats Induced By Hypothalamic Lesions and Lactation. J Neuroendocrinol. 2003;15(1):51–60. doi: 10.1046/j.1365-2826.2003.00862.x. [DOI] [PubMed] [Google Scholar]
- Thakkar M, Ramesh V, Strecker R, McCarley R. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139(3):313–328. [PubMed] [Google Scholar]
- Uriarte N, Ferreira A, Rosa XF, Sebben V, Lucion AB. Overlapping litters in rats: effects on maternal behavior and offspring emotionality. Physiol Behav. 2008;93(4–5):1061–1070. doi: 10.1016/j.physbeh.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Van der Veen R, Abrous D, Ronald de Kloet E, Piazza P, Koehl M. Impact of intra- and interstrain cross- fostering on mouse maternal care. Genes Brain Behav. 2008;7(2):184–192. doi: 10.1111/j.1601-183X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Voloschin L, Tramezzani J. Milk Ejection Reflex Linked to Slow Wave Sleep in Nursing Rats*. Endocrinology. 1979;105(5):1202–1207. doi: 10.1210/endo-105-5-1202. [DOI] [PubMed] [Google Scholar]
- Wansaw MP, Pereira M, Morrell JI. Characterization of maternal motivation in the lactating rat: Contrasts between early and late postpartum responses. Horm Behav. 2008;54(2):294–301. doi: 10.1016/j.yhbeh.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener SG, Fitzpatrick KM, Levin R, Smotherman WP, Levine S. Alternations in the maternal behavior of rats rearing malnourished offspring. Dev Psychobiol. 1977;10(3):243–254. doi: 10.1002/dev.420100308. [DOI] [PubMed] [Google Scholar]
- Woodside B. Prolactin and the hyperphagia of lactation. Physiol Behav. 2007;91(4):375–382. doi: 10.1016/j.physbeh.2007.04.015. [DOI] [PubMed] [Google Scholar]


