Abstract
Background
The prognostic impact of different distant metastases pattern in liver cancer is unexplored still now. The aim of this study is to analyze the metastasis patterns and prognosis differences for patients with stage IV liver cancers.
Methods
A SEER analysis was performed. Overall survival and cancer-specific survival were calculated by the Kaplan-Meier method. Multivariable Cox regression models were used to further analyze survival outcome and other prognostic factors.
Results
A total of 37526 eligible cases were retrieved in the Surveillance, Epidemiology, and End Results database. Among these patients, stage of IV liver cancer accounted for 14.80% (5555/37526) at initial diagnosis. Patients who suffered bone, brain or lung metastasis occupied 55.61% (3089/5555). Comparing with other two single metastases, the patients with brain metastasis exhibited worst overall survival whose mean of survival was 4.758 months. Multivariate analysis with Cox hazard regression model showed that metastatic site was an independent prognostic factor of overall survival and cancer-specific survival in patients with single metastasis (P<0.05). The results of univariate analysis showed that metastatic pattern was significantly correlated with overall survival (P = 0.038) and cancer-specific survival (P = 0.035) of patients with two sites.
Conclusions
Lung was the most common site of single metastasis for liver cancers. Patients with bone metastasis had best survival outcome comparing with other two distant metastases. Patients with two metastatic sites, where one of them is the lung tends to have a slight trend to a worse outcome.
Introduction
Primary liver cancer (LCa) is estimated to be one of the most common cancers worldwide, as well as one of the most common causes of cancer-related mortality. In the United States, LCa represents the fifth most common cancer deaths in men and the eighth most common deaths in women [1]. Hepatitis C virus infection is the leading cause of LCa in the United States, whereas hepatitis B virus infection is the leading cause worldwide, particularly in regions of Asia and Africa [2]. Other relevant risk factors consist of heavy alcohol drinking, tobacco, overweight, metabolic syndrome, and selected aspects of diet [3–7]. In countries without nationwide LCa surveillance programs, up to 30%-35% of patients present with macrovascular invasion and/or extrahepatic spread at initial diagnosis and the most common sites of distant metastasis are lungs, bones and adrenal glands [8–11]. The survival time of untreated metastatic LCa rarely exceed 62 days (IQR, 31–153 days) [12]. As a multi-kinase inhibitor, sorafenib, has become the standard treatment for metastatic LCa patients[13]. However, among patients with decompensated cirrhosis, the median survival benefit was 31 days, and it was not cost-effective (ICER, $224,914 per life year gained) [12]. One of the main issues related to the cost of LCa is that treatments and testing do not equate with equivalent benefit. Hence, further understanding of outcome of LCa, especially metastatic LCa, might help make suitable medical decision and save the unnecessary expend on the LCa.
However, to date little attention has been focused on the prognostic significance of distant metastatic patterns of LCa patients at the initial diagnosis. Since knowledge of prognosis of these patterns is crucial for pre-treatment evaluation, our study aimed to describe the distant metastatic site, frequency of occurrence and pattern of these metastases based on a large population using SEER database. In addition, with the considerable advances in treatment for LCa, such as surgical resection, percutaneous ablation, transcatheter arterial chemoembolization (TACE), and liver transplantation, the survival of LCa patients has improved much in recent years[14–18]. As a result, likelihood of encountering distant metastases from early stage LCa is rising. Hence, understanding the prognosis of different distant metastatic pattern in LCa patients at initial diagnosis would provide more evidence for precise medicine for metastatic LCa patients developed from early stages after diverse treatments.
Materials and methods
Database and patient selection
The Surveillance, Epidemiology, and End Results (SEER) program is a United States population-based cancer registry that began in 1973 and is supported by both the National Cancer Institute and Centers for Disease Control and Prevention. A total of 18 population-based cancer registries in the United States were included in the current SEER database. We totally choose 37526 cases according to the following criteria: (1) pathologically confirmed LCa with active follow-up and confirmed age. (2) the year of diagnosis from 2010 to 2014; (3) enrolled patients should have confirmed metastatic information of bone, brain, and lung. Patients with benign or borderline tumors, unknown age and unknown survival months were excluded. SEER*Stat software (SEER*Stat 8.2.3) was used to extract the data.
Statistical analysis
Frequency distribution of demographic and clinicalpathogical characteristics across metastatic groups were compared using Pearson’ s Chi-square tests. Primary end points include overall survival (OS; defined as the time from diagnosis till death due to any reason) and cancer-specific survival (CSS; defined as the time from diagnosis till death due to LCa). The Kaplan-Meier analyses were used to generate the survival curves and the Log Rank test was applied to analyze the differences among the curves. Adjusted HRs with 95% CIs were calculated using Cox proportional hazard regression models to estimate prognostic factors. All statistical tests were 2-sided, and P<0.05 was considered statistically significant. The statistical software SPSS 22.0 was utilized for all data analyses.
Results
Patients’ characteristics and frequency difference of different metastases pattern
A total of 37526 eligible cases were retrieved in the SEER database. Among these patients, stage of IV LCa accounted for 14.80%(5555/37526) at initial diagnosis. The SEER database only offered metastatic information of bone, brain and lung. Patients who suffered metastasis to either one of these four organs occupied 55.61%(3089/5555).
Table 1 summarised the distribution of clinical characteristics of these patients. Age at diagnosis had substantive differences across the bone metastasis and lung metastasis groups (both, P<0.05). The distribution of race, histological grade and insurance status among patients with bone metastasis and without bone metastasis was significantly distinguishing (P<0.05). Similar phenomenon was observed in lung metastasis (P<0.05) while not in brain metastasis (P>0.05). As shown in Table 1, there were a series of significant differences among the three groups of patient samples including nodal status, T-stage, and marital status (all, P<0.05).
Table 1. Clinical features and metastasis sites.
| Features | Bone metastasis (%) | P | Brain metastasis (%) | P | Lung metastasis (%) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | ||||
| Age | |||||||||
| Mean (years) | 63.60 | 64.45 | 0.011 | 63.63 | 62.63 | 0.391 | 63.72 | 62.36 | <0.001 |
| SD | 12.52 | 11.29 | 12.47 | 10.93 | 12.28 | 14.84 | |||
| Race | |||||||||
| White | 21901(95.5) | 1025(4.5) | <0.001 | 22805(99.6) | 86(0.4) | 0.340 | 21463(93.8) | 1417(6.2) | <0.001 |
| Black | 4202(94.3) | 254(5.7) | 4425(99.6) | 18(0.4) | 4082(92.1) | 351(7.9) | |||
| Other | 5030(96.6) | 177(3.4) | 5182(99.7) | 13(0.3) | 4826(92.8) | 372(7.2) | |||
| Nodal status | |||||||||
| Nx | 1921(88.6) | 246(11.4) | <0.001 | 2124(99.0) | 22(1.0) | <0.001 | 1809(84.1) | 343(15.9) | <0.001 |
| N0 | 24731(97.0) | 778(3.0) | 25428(99.7) | 64(0.3) | 24390(95.8) | 1072(4.2) | |||
| N1 | 1846(88.4) | 242(11.6) | 2070(99.5) | 11(0.5) | 1738(83.4) | 347(6.6) | |||
| T-stage | |||||||||
| Tx | 2462(87.8) | 341(12.2) | <0.001 | 2753(99.1) | 26(0.9) | <0.001 | 2397(85.9) | 395(14.1) | <0.001 |
| T1 | 12401(97.9) | 264(2.1) | 12632(99.8) | 24(0.2) | 12275(97.1) | 369(2.9) | |||
| T2 | 6157(97.7) | 148(2.3) | 6287(99.8) | 14(0.2) | 6133(97.4) | 162(2.6) | |||
| T3 | 6502(93.9) | 426(6.1) | 6894(99.6) | 27(0.4) | 6287(90.9) | 628(9.1) | |||
| T4 | 963(92.8) | 75(7.2) | 1032(99.4) | 6(0.6) | 827(80.4) | 201(19.6) | |||
| Histological grade | |||||||||
| Well | 3250(97.5) | 84(2.5) | <0.001 | 3319(99.7) | 9(0.3) | 0.148 | 3235(97.2) | 93(2.8) | <0.001 |
| Moderate | 5095(97.2) | 143(2.8) | 5219(99.7) | 17(0.3) | 4988(95.6) | 232(4.4) | |||
| Poorly | 2615(94.5) | 151(5.5) | 2757(99.6) | 12(0.4) | 2455(88.9) | 306(11.1) | |||
| Undifferentiated | 269(95.7) | 12(4.3) | 278(98.9) | 3(1.1) | 249(88.0) | 34(12.0) | |||
| Marital status | |||||||||
| Married | 15198(95.8) | 663(4.2) | <0.001 | 15800(99.7) | 42(0.3) | 0.008 | 14843(93.8) | 983(6.2) | 0.002 |
| Single | 7559(91.4) | 715(8.6) | 15039(99.6) | 67(0.4) | 14032(92.9) | 1069(7.1) | |||
| Insurance | |||||||||
| Insured | 35901(95.5) | 1690(4.5) | 0.002 | 30411(99.7) | 102(0.3) | 0.055 | 28548(93.6) | 1941(6.4) | <0.001 |
| Uninsured | 1315(93.7) | 88(6.3) | 1392(99.4) | 9(0.6) | 1244(89.1) | 152(10.9) | |||
SD: Standard deviation
The metastatic pattern of LCa was presented in Table 2. There were 7 possible metastatic forms, including 3 single metastases and 4 combinations of metastases. Among patients with single metastasis, we found that lung was still the most common site of metastasis for LCa (30.20%), followed by bone (17.8%) and brain (0.70%) metastasis. As for two sites, the combination of bone and lung metastases occupied most achieving to 5.65%.
Table 2. Frequencies of combination metastasis.
| Features | Bladder cancer | |
|---|---|---|
| Number | Percentage (%) | |
| One site | ||
| Only bone | 987 | 17.8 |
| Only brain | 39 | 0.70 |
| Only lung | 1676 | 30.2 |
| Two sites | ||
| Lung and bone | 314 | 5.65 |
| Lung and brain | 24 | 0.43 |
| Bone and brain | 24 | 0.43 |
| Three sites | ||
| Bone and brain and lung | 18 | 0.32 |
Univariate and multivariate survival analysis of patients with four single metastases
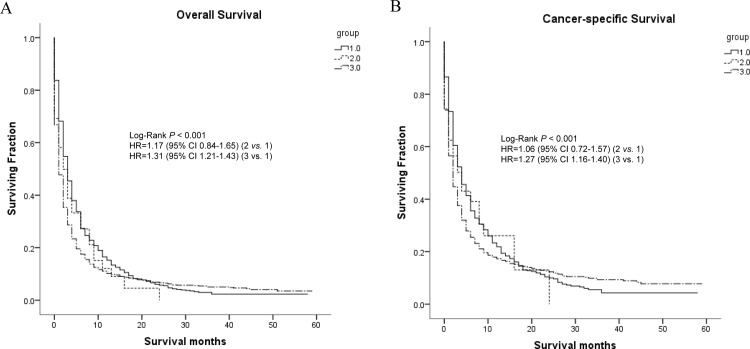
Moreover, we conducted univariate analysis (Table 3) to evaluate the impact of single metastases and baseline characteristics on OS and CCS. As was shown, metastatic sites had significant impact on OS and CCS (both, P<0.05). Comparing with other two single metastases, the patients with brain metastasis exhibited worst OS whose mean of survival was 4.758 months. As for CCS, patients with lung metastasis exhibited worst OS whose mean of survival was 7.875 months. Further multivariate analysis with Cox hazard regression model showed that metastatic site was an independent prognostic factor for both OS and CCS (P<0.05) (Table 4). Univariate analysis showed that N-classification was significantly associated with OS (P<0.05) and CCS (P<0.05) while only with OS (P<0.05) in multivariate model. In addition, other factors including T-stage, differentiated grade and marital status were all distinctly correlated with OS and CCS in both univariate and multivariate model (all, P<0.05). Fig 1 exhibited the survival curves generated by Kaplan-Meier analyses using univariate model.
Table 3. Univariate survival analysis of patients with three single metastases.
| Risk Factors | Overall Survival | Cancer-specific Survival | ||||
|---|---|---|---|---|---|---|
| Mean of survival months | 95% CI | P | Mean of survival months | 95% CI | P | |
| Metastasis site | <0.001 | <0.001 | ||||
| bone metastasis | 6.692 | (5.944, 7.441) | 8.944 | (7.796, 10.092) | ||
| brain metastasis | 4.758 | (2.715, 6.801) | 9.075 | (4.218, 13.932) | ||
| lung metastasis | 5.683 | (4.999, 6.367) | 7.875 | (6.695, 9.055) | ||
| Race | 0.808 | 0.444 | ||||
| White | 6.161 | (5.529, 6.793) | 8.703 | (7.587, 9.819) | ||
| Black | 5.424 | (4.364, 6.483) | 7.560 | (5.853, 9.267) | ||
| Other | 6.277 | (4.926, 6.544) | 7.581 | (5.788, 9.374) | ||
| N-classification | 0.002 | 0.002 | ||||
| N0 | 6.060 | (5.428, 6.693) | 9.088 | (8.013, 10.163) | ||
| N1 | 4.191 | (3.391, 4.991) | 5.941 | (4.689, 7.193) | ||
| T-stage | <0.001 | <0.001 | ||||
| T1 | 6.966 | (5.828, 8.105) | 10.277 | (8.505, 12.048) | ||
| T2 | 7.531 | (5.767, 9.294) | 10.056 | (7.669, 12.443) | ||
| T3 | 4.752 | (4.143, 5.362) | 6.646 | (5.716, 7.575) | ||
| T4 | 4.296 | (3.119, 5.473) | 5.296 | (3.811, 6.781) | ||
| Differentiated grade | <0.001 | <0.001 | ||||
| Well | 9.590 | (6.620, 12.531) | 13.786 | (9.487, 18.086) | ||
| Moderate | 8.286 | (6.519, 10.053) | 12.746 | (9.898, 15.593) | ||
| Poorly | 3.449 | (2.570, 4.328) | 5.628 | (3.786, 7.470) | ||
| Undifferentiated | 10.827 | (4.652, 17.003) | 17.239 | (8.584, 25.893) | ||
| Marital status | 0.003 | 0.002 | ||||
| Married | 6.488 | (5.583, 7.393) | 9.182 | (7.833, 10.531) | ||
| Single | 5.063 | (4.371, 5.755) | 7.110 | (6.038, 8.182) | ||
| Insurance status | 0.025 | 0.530 | ||||
| Insured | 5.793 | (5.379, 6.567) | 8.403 | (7.512, 9.293) | ||
| Uninsured | 4.389 | (2.524, 6.253) | 6.404 | (3.486, 9.322) | ||
Table 4. Multivariate survival analysis of patients with three single metastases.
| Risk Factors | Overall Survival | Cancer-specific Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Metastasis site | <0.001 | <0.001 | ||||
| bone metastasis | 1 | Ref | 1 | Ref | ||
| brain metastasis | 1.194 | (0.849, 1.680) | 0.309 | 1.068 | (0.720, 1.586) | 0.743 |
| lung metastasis | 1.391 | (1.276, 1.517) | <0.001 | 1.322 | (1.201, 1.456) | <0.001 |
| Race 0.597 | 0.870 | |||||
| White | 1 | Ref | 1 | Ref | ||
| Black | 1.052 | (0.940, 1.177) | 0.380 | 1.055 | (0.932, 1.194) | 0.400 |
| Other | 0.966 | (0.858, 1.086) | 0.561 | 1.007 | (0.885, 1.146) | 0.915 |
| N-classification | 0.039 | 0.138 | ||||
| N0 | 1 | Ref | 1 | Ref | ||
| N1 | 1.135 | (1.006, 1.281) | 0.039 | 1.107 | (0.968, 1.265) | 0.138 |
| T-stage | 0.003 | 0.003 | ||||
| T1 | 1 | Ref | 1 | Ref | ||
| T2 | 0.978 | (0.825, 1.159) | 0.794 | 1.010 | (0.836, 1.221) | 0.918 |
| T3 | 1.204 | (1.066, 1.359) | 0.003 | 1.237 | (1.080, 1.417) | 0.002 |
| T4 | 1.189 | (0.994, 1.421) | 0.058 | 1.341 | (1.105, 1.627) | 0.003 |
| Differentiated grade | <0.001 | <0.001 | ||||
| Well | 1 | Ref | 1 | Ref | ||
| Moderate | 1.037 | (0.826 1.302) | 0.756 | 1.001 | (0.774, 1.303) | 0.975 |
| Poorly | 1.644 | (1.314, 2.057) | <0.001 | 1.720 | (1.336, 2.216) | <0.001 |
| Undifferentiated | 1.291 | (0.881, 1.892) | 0.191 | 1.233 | (0.794, 1.914) | 0.351 |
| Marital status | 0.008 | 0.011 | ||||
| Marrried | 1 | Ref | 1 | Ref | ||
| Single | 1.122 | (1.031, 1.222) | 0.008 | 1.131 | (1.028, 1.244) | 0.011 |
| Insurance status | 0.016 | 0.031 | ||||
| Insured | 1 | Ref | 1 | Ref | ||
| Uninsured | 1.222 | (1.039, 1.438) | 0.016 | 1.218 | (1.019, 1.456) | 0.031 |
Fig 1.
Kaplan-Meier curves and Log-rank test for overall survival (A) and cancer-specific survival (B) according to different metastasis (only one site). Note: 1 = Bone metastasis; 2 = Brain metastasis; 3 = Lung metastasis.
Univariate and multivariate survival analysis of patients with different combinations of metastases
Patients with two metastatic sites had 3 forms, including bone with brain metastasis, bone with lung metastasis and brain with lung metastasis. The univariate analysis results showed that metastatic pattern was associated with OS (Bone and brain metastasis: 8.640 months; Bone and lung metastasis: 4.107 months; Brain and lung metastasis: 2.542 months; P = 0.038) and CCS (Bone and brain metastasis: 11.437 months; Bone and lung metastasis: 5.553 months; Brain and lung metastasis: 3.810 months; P = 0.035) of patients (S1 Table). In multivariate model analysis, the results showed that the patients with combination of brain and lung had the worse OS than combination of bone and brain (HR, 2.002; 95% CI, 1.081–3.707; P = 0.027). As for CCS, the patients with bone and brain had the best outcome in the three patterns with two sites (Bone and lung: HR, 1.730, 95% CI, 1.002–2.986, P = 0.049; Brain and lung: HR, 1.322, 95% CI, 1.117–4.508, P = 0.023) (S2 Table). Survival curves for these 3 forms of metastases using Kaplan-Meier method was shown in Fig 2.
Fig 2.
Kaplan-Meier curves and Log-rank test for overall survival (A) and cancer-specific survival (B) according to different metastasis (two sites). Note:1 = Bone and brain metastasis; 2 = Bone and lung metastasis; 3 = Brain and lung metastasis.
Discussion
In contrast to declining trends of other common cancers in mortality, death rates rose from 2010 to 2014 by almost 3% per year for LCa, particularly for metastatic patients with 5-year relative survival rates of 3.1%[1,19]. Metastatic LCa represents a heterogeneous disease; clinical outcomes are highly variable and depend on the underlying tumor biology and patient characteristics[20,21]. The prognostic influence of metastasis at initial diagnosis and factors associated with specific organ involvement have been understudied[22,23]. Nevertheless, there was still no study that focused on the prognosis of different distant metastases pattern in LCa. A better understanding of patterns of metastases would be valuable to assess prognosis, select appropriate treatments, and determine disease monitoring.
In our retrospective study, we observed that LCa predominantly metastasize to lung in single metastasis which is in line with previous studies[23–25]. Studies have already indicated that brain is the least common distant metastatic organ in LCa patients[26,27]. Consistent with these studies, our results also showed that brain also was the least common metastatic site in all LCa. As for bone metastasis, we found this pattern occupied 17.8% of all, which is lower than 25.5% to 38.5% reported by previous studies[28,29]. Referring to demographics and clinical features of patients, we found that that elder people seemed to suffer bone and lung metastases more frequently at diagnosis except for brain metastasis. We also found that black people was associated with more involvement of bone, and lung metastases than white people. Of note, we found that uninsured patients had more metastases to bone, brain, and lung than insured patients. Similar phenomenon was observed in aspect of marital status. In addition, patients with lymph node positive, high tumor stage and poorly differentiated histological grade were more inclined to suffer distant metastases.
We also made some findings in survival analysis. First, in multivariate survival analysis of patients with three single metastases, the OS and CCS of isolated bone metastases were the best. Outcome of patients with lung metastasis was worse than patients with bone metastasis. Hence, the presence of lung metastasis was an indicator of poor survival for primary LCa. The multivariate analysis revealed that patients with lymph node positive plus either one of other three metastases had worse overall survival than those with only distant organ metastases. Several studies reported that tumor cells metastasizing only to lymph nodes might specific epigenetic modifications that could prevent them spread to visceral organs[30,31]. Recent two studies from American and Austria have confirmed that tumor cells in lymph node could spread into vascular circulation and metastasize to distant organ[32,33]. This indicated that the tumor cells in lymph node of patients with both lymph node and distant organ metastases had a more aggressive phenotype[34]. Hence, we should focus on both distant organ metastases and lymph node status in clinical practice. In addition, poorer differentiated grade was associated with worse OS and CCS.
Some LCa patients developed more than one metastatic site, and few studies have reported on the combination of metastasis in these patients. In our study, metastasis to two sites most commonly involved the lung and bone achieving to 5.6%. In survival analysis of patients with combination of two metastatic sites, we found that patients with bone metastases plus either one of other two organ metastases had better OS and CCS than those without bone metastases. This implied that visceral metastases resulted in shorter survival than bone metastases, which was also confirmed in univariate and multivariate survival analysis of patients with only one metastasis. Our study also indicated that patients with two metastatic sites, where one of them is the lung tends to have a slight trend to a worse outcome. We therefore believe that it is important to classify patients with two metastatic sites involving the lung in order to improve the prognosis or treatment value in these specific patients.
Despite valuable findings above, there are several limitations in our study due to the retrospective nature. First, metastases to only the brain, bone and lung were included in the study. Metastasis to the adrenal glands or other metastasis sites may also influence the prognosis of LCa. Second, due to the absence of information on chemotherapy or targeted therapy included in the SEER database, their effects on survival could not be evaluated. This may cause a certain bias in our results. Third, some types of imaging that was done for patients might ignore potential distant metastases. The SEER database didn’t offer the information of the type of imaging, which could lead to bias.
Conclusion
Lung is the most common site of single metastasis for LCa. Importantly, our results identify that metastases to the lung alone or in combination with other organs indicated a worse outcome for patients with distant metastasis. Information on the prognostic impact of different sites of metastases would provide more evidence for precise medicine and individualized therapy.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The author would like to thank the members of the research group for useful discussions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author received no specific funding for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–9. 10.1016/j.jhep.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 3.Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Annals of oncology: official journal of the European Society for Medical Oncology. 2014;25(8):1526–35. 10.1093/annonc/mdu020 [DOI] [PubMed] [Google Scholar]
- 4.Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer letters. 2009;286(1):9–14. 10.1016/j.canlet.2008.10.040 [DOI] [PubMed] [Google Scholar]
- 5.Turati F, Trichopoulos D, Polesel J, Bravi F, Rossi M, Talamini R, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60(3):606–11. 10.1016/j.jhep.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 6.Shivappa N, Hebert JR, Polesel J, Zucchetto A, Crispo A, Montella M, et al. Inflammatory potential of diet and risk for hepatocellular cancer in a case-control study from Italy. The British journal of nutrition. 2016;115(2):324–31. 10.1017/S0007114515004419 [DOI] [PubMed] [Google Scholar]
- 7.Polesel J, Talamini R, Montella M, Maso LD, Crovatto M, Parpinel M, et al. Nutrients intake and the risk of hepatocellular carcinoma in Italy. European journal of cancer (Oxford, England: 1990). 2007;43(16):2381–7. 10.1016/j.ejca.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 8.Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216(3):698–703. 10.1148/radiology.216.3.r00se24698 [DOI] [PubMed] [Google Scholar]
- 9.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13(3):414–20. 10.3748/wjg.v13.i3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nature reviews. Gastroenterology & hepatology. 2013;10(1):34–42. 10.1038/nrgastro.2012.199 [DOI] [PubMed] [Google Scholar]
- 11.Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol. 2016;65(6):1148–54. 10.1016/j.jhep.2016.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh ND, Marshall VD, Singal AG, Nathan H, Lok AS, Balkrishnan R et al. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: An analysis of the SEER-Medicare database. Hepatology. 2017;65(1):122–33. 10.1002/hep.28881 [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359(4):378–90. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 14.Shiina S, Teratani T, Obi S, Hilgard P, Gane E, Blanc JF, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122–30. [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. The New England journal of medicine. 1996;334(11):693–9. 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 16.Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32(6):1224–9. 10.1053/jhep.2000.20456 [DOI] [PubMed] [Google Scholar]
- 17.Shiina S, Tagawa K, Niwa Y, Unuma T, Komatsu Y, Yoshiura K, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR. American journal of roentgenology. 1993;160(5):1023–8. 10.2214/ajr.160.5.7682378 [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet (London, England). 2002;359(9319):1734–9. 10.1016/s0140-6736(02)08649-x [DOI] [PubMed] [Google Scholar]
- 19.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–37. 10.1002/cncr.29936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Seminars in liver disease. 1999;19(3):271–85. 10.1055/s-2007-1007117 [DOI] [PubMed] [Google Scholar]
- 21.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29(4):502–10. 10.1111/j.1478-3231.2008.01957.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu W, He X, Andayani D, Yang L, Ye J, Li Y, et al. Pattern of distant extrahepatic metastases in primary liver cancer: a SEER based study. J Cancer. 2017;8(12):2312–8. 10.7150/jca.19056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JI, Kim JK, Kim DY, Ahn SH, Park JY, Kim SU, et al. Prognosis of hepatocellular carcinoma patients with extrahepatic metastasis and the controllability of intrahepatic lesions. Clin Exp Metastasis. 2014;31(4):475–82. 10.1007/s10585-014-9641-x [DOI] [PubMed] [Google Scholar]
- 24.Sawabe M, Nakamura T, Kanno J, Kasuga T. Analysis of morphological factors of hepatocellular carcinoma in 98 autopsy cases with respect to pulmonary metastasis. Acta pathologica japonica. 1987;37(9):1389–404. [DOI] [PubMed] [Google Scholar]
- 25.Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20(11):1781–7. 10.1111/j.1440-1746.2005.03919.x [DOI] [PubMed] [Google Scholar]
- 26.Seinfeld J, Wagner AS, Kleinschmidt-DeMasters BK. Brain metastases from hepatocellular carcinoma in US patients. J Neurooncol. 2006;76(1):93–8. 10.1007/s11060-005-4175-3 [DOI] [PubMed] [Google Scholar]
- 27.Del Ben M, Caporale A, Feole K, Alessandri C, Angelico F. Intracranial hemorrage due to brain metastases in an Italian HCV patient with hepatocellular carcinoma. Journal of experimental & clinical cancer research: CR. 2003;22(4):641–4. [PubMed] [Google Scholar]
- 28.Fukutomi M, Yokota M, Chuman H, Harada H, Zaitsu Y, Funakoshi A, et al. Increased incidence of bone metastases in hepatocellular carcinoma. European journal of gastroenterology & hepatology. 2001;13(9):1083–8. [DOI] [PubMed] [Google Scholar]
- 29.Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117(19):4475–83. 10.1002/cncr.25960 [DOI] [PubMed] [Google Scholar]
- 30.Barekati Z, Radpour R, Lu Q, Bitzer J, Zheng H, Toniolo P, et al. Methylation signature of lymph node metastases in breast cancer patients. BMC Cancer. 2012;12:244 10.1186/1471-2407-12-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briganti A, Passoni NM, Abdollah F, Nini A, Montorsi F, Karnes RJ. Treatment of lymph node-positive prostate cancer: teaching old dogmas new tricks. European urology. 2014;65(1):26–7; discussion 8. 10.1016/j.eururo.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 32.Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science (New York, N.Y.). 2018;359(6382):1403–7. 10.1126/science.aal3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science (New York, N.Y.). 2018;359(6382):1408–11. 10.1126/science.aal3662 [DOI] [PubMed] [Google Scholar]
- 34.Furuya Y, Akakura K, Akimoto S, Ito H. Prognosis of patients with prostate carcinoma presenting as nonregional lymph node metastases. Urologia internationalis. 1998;61(1):17–21. 10.1159/000030277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.