Abstract
Background
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is an inherited cardiomyopathy characterized histologically by the replacement of ventricular myocardium with fibrous and fatty tissue, and clinically by ventricular tachycardia arrhythmias primarily of right ventricular (RV) origin. Implantable cardioverter defibrillator (ICD) is the only proven therapy to reduce mortality in ARVC/D patients. However, it has the risk of inappropriate anti-tachycardia pacing (ATP) or shocks. This study aimed to assess the occurrence of appropriate and inappropriate ICD therapies in ARVC/D patients who underwent ICD implantation in a single Cardiac Centre.
Methods
Retrospective analysis of the data of patients with the diagnosis of ARVC/D based on the 2010 revised Task Force Criteria, who underwent ICD implantation in the Heart Centre, at King Faisal Specialist Hospital and Research Center (KFSH&RC), Riyadh between January 1997 and May 2016. The clinical data and information about appropriate and inappropriate ICD therapies were obtained from medical records with the review of the available intra-cardiac electrograms (EGMs).
Results
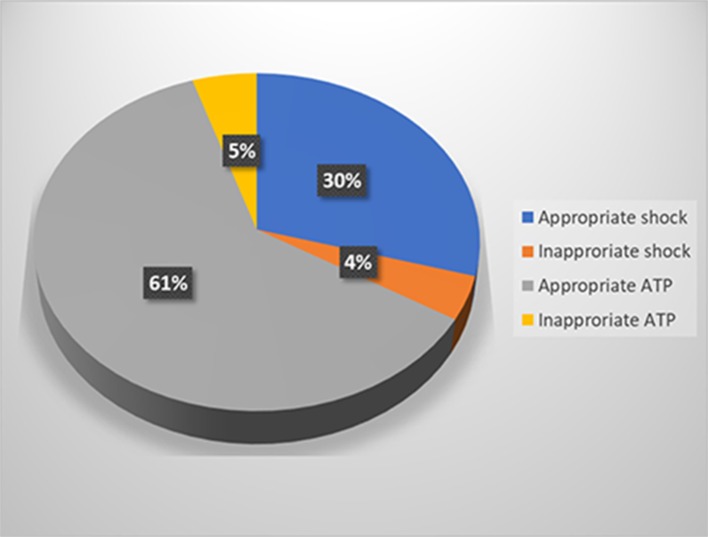
Twenty-two ARVC/D patients with ICD implantation (20 males (91%), mean age at ICD implantation: 32 ± 14 years). ICD was implanted for secondary prevention of sudden cardiac death (SCD) in 15 patients (68.2%), and for primary prevention in 7 patients (31.8%). At mean follow-up of 9.4 ± 4.8 years, 11 patients (50%) had appropriate ICD therapies, and five patients (22.7%) had inappropriate ICD therapies. Out of 950 ICD therapies, 865 (91%) were appropriate (586 episodes of VT/VF treated with ATP (61.3%), and 279 episodes treated with shocks (29.37%)) and 85 (9.4%) were inappropriate (45 episodes treated with ATP (4.73%), and 40 treated with shocks (4.21%)).
Conclusion
ARVC/D patients are at risk of VT/VF arrhythmias. ICD therapy is the only proven life-saving therapy in those patients. Most of ICD therapies in our patient’s population are appropriate, and ATP therapy is effective in terminating most of VT episodes. Although we do not have any patient with subcutaneous ICD, the high success rate of ATP suggests that transvenous ICD would be more appropriate in ARVC/D patients.
Keywords: Implantable cardioverter defibrillator, Arrhythmogenic right ventricular cardiomyopathy, Shocks, Anti-tachycardia pacing
Introduction
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is an inherited cardiomyopathy characterized histologically by the replacement of ventricular myocardium with fibrous and fatty tissue, and clinically by ventricular arrhythmias primarily of right ventricular (RV) origin [1, 2]. The diagnosis of ARVC/D is based on a combination of diagnostic criteria proposed by the International Task Force for Cardiomyopathy which has been published initially in 1994 [3] then revised in 2010 [4]. Patients with ARVC/D usually present with symptoms due to ventricular arrhythmias, such as palpitations, dizziness, chest discomfort or syncope [1, 2]. ARVC/D patients are at risk of sudden cardiac death (SCD) caused by sustained ventricular tachycardia (VT) or ventricular fibrillation (VF) which may be the initial presentation of ARVC/D [5]. Implantable cardioverter defibrillator (ICD) is the only proven therapy to reduce mortality in ARVC/D patients. However, it is not without risks that include inappropriate interventions with anti-tachycardia pacing (ATP) or shocks [6, 7].
The objective of this study is to assess the occurrence of appropriate and inappropriate ICD therapies in ARVC/D patients who underwent ICD implantation in the Heart Center, King Faisal Specialist Hospital and Research Center (KFSH&RC), Riyadh.
Materials and Methods
Patients’ selection
Twenty-two ARVC/D patients were diagnosed based on 2010 task force criteria who underwent ICD implantations in our Heart Center between January 1997 and May 2017. The patients had regular follow-up in arrhythmia and device clinic every 6 months. The clinical data were collected from the patients’ medical records and the device clinic data. This study was approved by the institutional review board of the KFSH&RC.
ICDs and classification of discharges
All patients received multifunctional third- or fourth-generation ICDs. The managing cardiologist/electrophysiologist made decisions regarding ICD implantation and programming. Stored intracardiac electrograms were analyzed to classify arrhythmias responsible for precipitating defibrillator discharges, according to following definitions [8]: VF as tachycardia with a mean cycle length (CL) of ≤ 240 ms (irregular or regular); VT as a regular tachycardia with mean CL > 240 ms. Defibrillator therapies were considered appropriate versus inappropriate based on standard criteria [5]. VT storm was defined as the occurrence of VT or VF that resulted in ≥ 3 ICD interventions (shock or ATP) in a 24-h period [9]. ICDs interrogations were performed routinely 6 - 8 weeks after initial ICD implantation and then every 6 months as per device clinic routine follow-up schedule.
Definitions
Syncope was defined as a transient loss of consciousness and postural tone with spontaneous recovery. Non-sustained ventricular tachycardia (NSVT) was defined as ≥ 3 consecutive ventricular premature beats with a rate >100 beats/min, lasting < 30 s and without hemodynamic instability. NSVT was documented during exercise testing, loop monitoring or 24-h Holter monitoring.
SCD was defined as natural death from cardiac causes, heralded by abrupt loss of consciousness within 1 h of the onset of an acute change in cardiovascular status with or without pre-existing heart disease [10, 11].
Primary prevention of SCD referred to use of ICDs in individuals who are at risk for but have not yet had an episode of sustained VT, VF or cardiac arrest. Secondary prevention referred to an indication for an ICD exclusively for patients who have survived one or more cardiac arrests or sustained ventricular tachycardia [12].
Statistical analysis
Continuous data are expressed as mean ± SD and compared across groups using a Student’s t-test. Categorical variables are reported as frequency (%) and compared between groups by the chi-square or Fisher’s exact test. IBM SPSS Statistics version 20 was used for the analyses.
Results
The clinical characteristics of the patients are summarized in Table 1. The patients’ age ranged from 13 to 67 years with a mean age of 41.9 ± 17 years, and 20 patients were males (90.9%). The age at ICD implantation ranged from 12 to 54 years with a mean of 32 ± 14 years. The presenting symptoms were palpitations in 19 (86.4%), syncope in 3 (13.6%), shortness of breath in 4 (18%), chest pain in 3 (13.6%) and dizziness in 3 patients (13.6%). Thirteen patients had documented VT on initial presentation (59%), and three were survivors of SCD (13.6%).
Table 1. Clinical Characteristics of the Patients.
| Patient characteristics | ICD primary prevention N (%) | ICD secondary prevention N (%) | P value |
|---|---|---|---|
| Male (%) | 6 (85.7 %) | 14 (93.3%) | 0.563 |
| Female (%) | 1 (14.3 %) | 1 (6.7%) | 0.563 |
| Clinical presentation | |||
| Palpitations | 5 (71.4%) | 14 (93.3%) | 0.163 |
| Syncope | 1 (14.3 %) | 2 (13.3%) | 0.952 |
| Shortness of breath | 2 (28.6%) | 2 (13.3%) | 0.388 |
| Chest pain | 1 (14.3 %) | 2 (13.3%) | 0.952 |
| Dizziness | 1 (14.3 %) | 2 (13.3%) | 0.952 |
| Ventricular tachycardia | 0 (0.0%) | 12 (80%) | 0.000 |
| Sudden cardiac death | 0 (0.0%) | 3 (20.0%) | 0.203 |
| Medications | |||
| Amiodarone | 0 (0.0%) | 3 (20.0%) | 0.203 |
| Sotalol | 1 (14.0%) | 6 (40.0%) | 0.228 |
| Beta blockers | 4 (57.1%) | 7 (46.7%) | 0.647 |
| Angiotensin-converting enzyme inhibitors (ACE inhibitors) | 2 (28.6%) | 7 (46.7%) | 0.421 |
| Spironolactone | 0 (0.0%) | 2 (13.3%) | 0.311 |
| Implantable cardioverter defibrillator (ICD) therapy | |||
| Single chamber ICD | 3 (42.9%) | 10 (66.7%) | 0.296 |
| Appropriate ICD therapy | 1 (14.0%) | 10 (66.7%) | 0.022 |
| Inappropriate ICD therapy | 1 (14.0%) | 4 (26.7%) | 0.519 |
| Genetic results | |||
| Positive | 3 (13.6%) | 7 (31.8%) | 0.672 |
| Negative | 2 (9.1%) | 2 (9.1%) | |
| Not performed | 2 (9.1%) | 6 (27.3%) | |
One patient died 10.5 years after ICD implantation due to advanced heart failure and other comorbidities.
Electrocardiogram (ECG) abnormalities
ECG showed depolarization abnormalities (epsilon wave-electric potentials after the end of the QRS complex) in 6 patients (27.3%), repolarization abnormalities with T-wave inversion in V1-3 or beyond in 15 patients (68%) and T-wave inversion in V1-2 or V4, 5 or 6 in 2 patients (9%) (Fig. 1). Signal-averaged ECG was performed in three patients (13.6%), and two had at least one minor criterion for ARVC/D diagnosis (filtered QRS duration (fQRS) ≥ 114 ms, duration of terminal QRS < 40 µV (low-amplitude signal duration) ≥ 38 ms or root-mean-square voltage of terminal 40 ms ≤ 20 µV). Table 2 shows a summary of ECG changes [4] and Figure 2 shows clinical VT documentation in one of our patients.
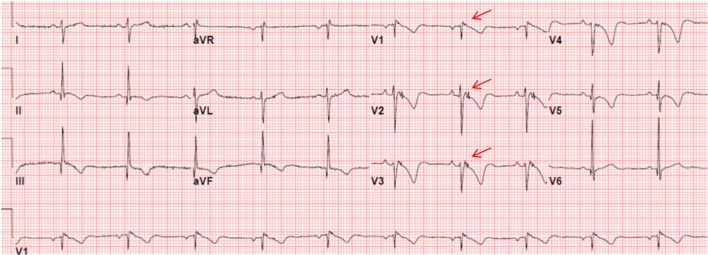
Figure 1.
ECG of a patient with ARVC/D showing the presence of an epsilon wave (electric potentials after the end of the QRS complex) (red arrows) and T-wave inversion in V1 - V5.
Table 2. Summary of ECG Changes.
| Patients | Epsilon wave (M)* | T-wave inversion in V1-3 or beyond (M)* | T-wave inversion in V1-2 or V4, 5 or 6 (m)* | RBBB + T-wave inversion in V1-4 (m)* | Terminal activation duration of QRS in V1, 2 or 3 ≥ 55 ms (m)* |
|---|---|---|---|---|---|
| 1 | + | + | - | - | + |
| 2 | - | + | - | - | - |
| 3 | - | + | - | - | - |
| 4 | - | + | - | - | - |
| 5 | + | + | - | - | - |
| 6 | - | + | - | - | - |
| 7 | - | + | - | - | - |
| 8 | + | + | - | - | - |
| 9 | - | - | - | - | - |
| 10 | + | + | - | - | - |
| 11 | - | + | - | - | - |
| 12 | - | - | - | - | - |
| 13 | - | - | + | - | + |
| 14 | - | - | - | - | - |
| 15 | + | + | - | - | + |
| 16 | - | - | + | - | + |
| 17 | - | + | - | - | - |
| 18 | - | + | - | - | + |
| 19 | + | + | - | - | - |
| 20 | - | + | - | - | - |
| 21 | - | - | - | - | - |
| 22 | - | - | - | - | + |
*M indicates major, and m indicates minor diagnostic criterion based on 2010 ARVC/D Task Force Criteria [4]. RBBB: right bundle-branch block. -: absent; +: present.
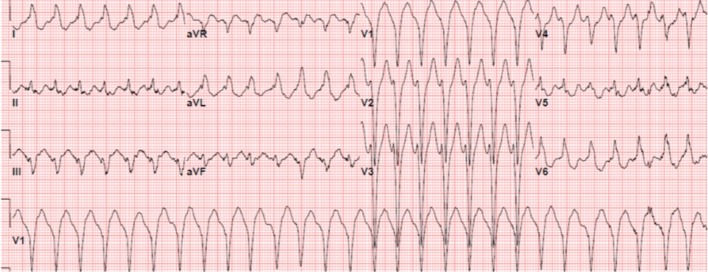
Figure 2.
ECG in a patient with ARVC/D showing ventricular tachycardia with a left bundle branch block (LBBB) morphology and superior axis suggesting a RV inferior wall origin.
Holter monitoring was performed in 11 patients (50%), and it showed > 500 ventricular extrasystoles per 24 h in six patients (54.5 %), and non-sustained VT in four patients (36.4%).
Cardiac imaging abnormalities
By 2D echocardiogram; 10 patients (45.5%) fulfill major criteria, and 4 patients (18%) fulfill minor criteria for the ARVC/D diagnosis based on 2010 task force criteria [4]. RV function was normal in nine patients (41%), mildly reduced in four patients (18.2%), mildly to moderately reduced or moderately reduced in two patients (9%), and moderately to severely reduced or severely reduced in seven patients (31.8%). Left ventricular ejection fraction (LVEF) was normal (EF > 50%) in 16 patients (72.7%), mildly reduced (EF: 40-49%) in 4 patients (18.2%), moderately reduced (EF: 30-39%) in one patient (4.5%), and severely reduced (EF < 30%) in one patient (4.5%) (Fig. 3 [13]). The echocardiogram changes are summarized in Table 3 [4].
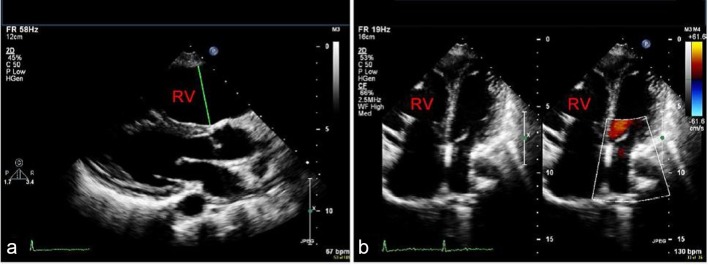
Figure 3.
Parasternal long axis (a) and apical four-chamber (b) echocardiographic views showing RV dilatation [13].
Table 3. Summary of Echocardiogram Changes.
| Patients no. | LVEF | RV systolic function | PLAX RVOT (corrected for body size (PLAX/BSA)) | PSAX RVOT (corrected for body size (PSAX/BSA)) | Other abnormalities |
|---|---|---|---|---|---|
| 1 | > 55% | Mildly reduced | 43 mm (21.5 mm/m2) | 46 mm (23 mm/m2) | |
| 2 | 45-50% (47.5%) | Moderately to severely reduced | 51 mm (24.3 m/m2) | 48 mm (29 mm/m2) | RV apical trabeculations and small aneurysms |
| 3 | 50-55% (52.5%) | Mildly to moderately reduced | 33 mm (17.3 mm/m2) | 32 mm (16.8 mm/m2) | RV wall thinning, multiple microaneurysms and hyertrabeculations. Mild TR |
| 4 | > 55% | Moderately reduced | 34 mm (16 mm/m2) | 41 mm (19 mm/m2) | RV thinned and akinetic wall, multiple microaneurysms and prominent trabeculations Moderate TR |
| 5 | > 55% | Mildly reduced | 42 mm (23.3 mm/m2) | 45 mm (25 mm/m2) | RV regional hypokinesia Mild TR |
| 6 | > 50% | Severely reduced | 34 (22.7 mm/m2) | 36 mm (24 mm/m2) | Severe TR |
| 7 | > 55% | Mildly reduced | 28 mm (14 mm/m2) | 31 mm (15.5 mm/m2) | RV thin wall with multiple microaneurysms in the apex and RVOT prominent, disarrayed trabeculations and dyskinetic anterior wall of RVOT |
| 8 | 50-55% | Moderately to severely reduced | 30 mm (15.8 mm/m2) | 35 mm (18.4 mm/m2) | Thin and fibrosed RV wall with prominent trabeculations Moderate TR SPAP 35 - 40 mm Hg. |
| 9 | > 55% | Normal | 20 mm (10 mm/m2) | 24 mm (12 mm/m2) | |
| 10 | 30-35% | Severely reduced | 41 mm (34.2 mm/m2) | 40 mm (33.3 mm/m2) | Hypertrabeculated RV Severe TR Mild-to-moderate PR |
| 11 | 45 % | Severely reduced | 33 mm (19.4 mm/m2) | 33 mm (19.4 mm/m2) | Thinning of the RV free wall |
| 12 | > 55% | Normal | 30 mm (18.7 mm/m2) | 34 mm (21.25 mm/m2) | |
| 13 | > 55% | Low normal | 29 mm (15.26 mm/m2) | 28 mm (14.7 mm/m2) | Thinning of the apical RV wall |
| 14 | 51% | Normal | 23 mm (11.5 mm/m2) | 30 mm (15 mm/m2) | RV systolic pressure 30 - 40 mm Hg |
| 15 | 44% | Normal | 32 mm (20 mm/m2) | 39 mm (24.4 mm/m2) | |
| 16 | 45% | Normal | 29 mm (16 mm/m2) | 31 mm (17 mm/m2) | RV systolic pressure 30- 35 mmHg |
| 17 | > 55% | Normal | 28 mm (18.7 mm/m2) | 30 mm (20 mm/m2) | |
| 18 | > 55% | Moderately to severely reduced | 36 mm (24 mm/m2) | 36 mm (24 mm/m2) | Mild TR |
| 19 | > 55% | Mildly reduced | 23 mm (13.5 mm/m2) | 28 mm (16.5 mm/m2) | |
| 20 | < 25% | Severely reduced | 47 mm (29.4 mm/m2) | 48 mm (30 mm/m2) | Markedly trabeculated RV |
| 21 | > 55% | Normal | 27 mm (14 mm/m2) | 24 mm (12.6 mm/m2) | |
| 22 | > 55% | Normal | 21 mm (17.5 mm/m2) | 15 mm (12.5 mm/m2) |
BSA: body surface area; LVEF: Left ventricular ejection fraction; mm: millimeters; m2: square meter; PLAX: parasternal long-axis view; PSAX: parasternal short-axis view; PR: pulmonic valvular regurgitation; RV: right ventricle; RVOT: RV outflow tract; SPAP: systolic pulmonary artery pressure; TR: tricuspid regurgitation. For the 2D echo major and minor criteria values, see reference [4].
Cardiac magnetic resonance imaging (MRI) was performed in 12 patients (54.5%); major diagnostic criteria were present in four patients (33.3 %), and minor diagnostic criteria were present in two patients (16.7%) (Fig. 4 [13]). The cardiac MRI changes are summarized in Table 4 [4].
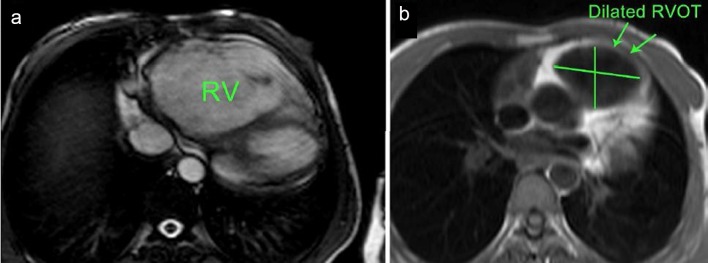
Figure 4.
(a) Axial cine SSFP (steady-state free precession) MRI showing significant RV dilatation and (b) black-blood-prepared HASTE (half-Fourier acquired single-shot turbo spin echo) axial slice MRI at level of RVOT showing RVOT dilatation [13].
Table 4. Summary of Cardiac Magnetic Resonance Imaging (MRI) Changes.
| Study no./gender | Left ventricle (LV) | Right ventricle (RV) |
|---|---|---|
| 1/M | Normal with EF 55% | EDV 151 mL (79.5 mL/m2)* ESV 83 mL (43.7 mL/m2) RVEF 45% Moderately to severely dilated with dyskinetic motion in the free wall and basal aneurysm T1-weighted images show area of severe thinning on the free wall of right ventricle |
| 2/M | Normal EDV 153 mL ESV 56 mL EF 63% |
EDV 178 mL (93.7 mL/m2) ESV 121 mL (63.7 mL/m2) EF 32% Moderate dilatation with generalized hypokinesis. There are localized RV areas of micro-aneurysms, mainly at the RVOT and the RV apex Prominent papillary muscle and the trabeculation |
| 3/M | EF > 55% | EDV 100 mL (50 mL/m2) ESV 48 mL (24 mL/m2) EF 52% Thinned of the free wall with a small area of severe thinning, nears the RV apex with dyskinesis and diastolic bulging fulfilling the criterion of aneurysm |
| 4/F | Normal systolic function | EDV 249 mL (207 mL/m2) ESV 212 mL (176.7 mL/m2) EF 15% Stroke volume 36.2 mL Severely dilated and hypertrabiculated Very thinned out RV especially apical regions with evidence of microaneurysm and diastolic bulge and segmental dilatation of RV The RVOT is dilated with hypokinesia. Evidence of at least mild-to-moderate TR regurgitation |
| 5/F | EDV 83 mL ESV 41 mL EF 50% Mild global hypokinesis and akinesis of the distal septum |
EDV 120 mL (70.6 mL/m2) RV ESV 83 mL (48.8 mL/m2) EF 31% Stroke volume 37 mL Severely dilated with severe global hypokinesis and dyskinesis of the RV apex and severe thinning noted in both cine and dark blood imaging Delayed myocardial enhancement demonstrates severe thinning of the free wall of the right ventricle |
| 6/M | EDV 99 mL ESV 39 mL EF 60% Stroke volume 59 mL |
EDV 81 mL (50.6 mL/m2) ESV 52 mL (32.4 mL/m2) RVEF 36% Mildly-to-moderately dilated with dyskinesia and akinesis of the free wall adjacent to the right ventricular apex T1, T2 and delayed gadolinium enhancement demonstrate two focal aneurysms in the free wall of RV |
| 7/M | Normal EF 58% | EDV 171 mL (90 mL/m2) ESV 93 mL (49 mL/m2) EF 49% Stroke volume 76 mL( 40 mL/m2) Focal dyskinesia in the RVOT Small patent ductus arteriosus measuring about 5 mm. Main pulmonary artery (MPA) not enlarged measuring 26 mm |
| 8/M | Normal EF 57% | EDV 87 mL (43.5 mL/m2) ESV 47 mL (23.5 mL/m2) EF 46% Mildly dilated with mild global hypokinesis Focal aneurysm noted in RV free wall or RVOT |
| 9/M | Normal LV | Moderately dilated RV and reduced RV function (CMRI was performed in another cardiac center and full report no available) |
| 10/M | EDV 110.5 mL (65 mL/m2) ESV 49.3 mL (29 mL/m2) EF 54% |
EDV187.5 mL (105 mL/m2) ESV 100 mL(59 mL/m2) EF 44% |
| 11/M | EF 45-50% | Quantification was not apparent at this point due to the poor ECG gating Dilated at the RVOT, there is evidence of macroaneurysm with small microaneurysm noted at the RV apex with a large macroaneurysm noted at the RVOT and RV free wall with dyskinesis Overall RV systolic function appeared to be mildly reduced |
| 12/M | EDV 80 mL ESV 25 mL EF 68% |
EDV 74 mL ESV 26 mL EF 64% Late gadolinium enhancement in mid myocardium at the septum |
*Between practices values corrected for body size (value/body surface area (BSA)). EF: ejection fraction; EDV: end-diastolic volume; ESV: end-systolic volume mm millimeters; m2: square meter, TR: tricuspid valve. For the cardiac MRI major and minor criteria values, see reference [4].
ICD therapy
The ICD was implanted for secondary prevention of SCD in 15 patients (68.2 %), and for primary prevention in 7 patients (31.8 %). Thirteen patients had a single chamber (59 %), and nine patients had a dual chamber ICD (41%).
Over the follow-up period of 9.4 ± 4.8 years (2 - 20.9 years); 11 patients (50%) had appropriate ICD therapies, and five patients (22.7%) had inappropriate ICD therapies. Out of 950 ICD therapies, 865 (91%) were appropriate (586 episodes of VT/VF were treated with ATP (61.3%), and 279 episodes treated with shocks (29.37%) and overall 85 episodes (9.4%) were inappropriate (45 episodes treated with ATP (4.73 %), and 40 treated with shocks (4.21%)) (Fig. 5).
Figure 5.
Implantable cardioverter defibrillator (ICD) therapy classification. ATP: antitachycardia pacing.
In the primary prevention patients, one had appropriate (14.3%), and one had inappropriate ICD therapy (14.3%). In the secondary prevention patients, 10 had appropriate (66.7%), and 4 had inappropriate ICD therapy (26.7%). Nine patients suffered at least one electrical storm (41%), all but one were due to VT/VF episodes. The time to first appropriate ICD therapy ranged from 3 days to 2 years and 9 months (mean: 6 ± 1.4 months).
When comparing patients with appropriate ICD therapy to those with inappropriate ICD therapy, only secondary prevention was a predictor of appropriate therapy (Table 5).
Table 5. Comparison Between Patients With Appropriate and Inappropriate Implantable Cardioverter Defibrillator (ICD) Therapy.
| Patient characteristics | Appropriate therapy N (%), 11 (50%) |
Inappropriate therapy N (%), 3 (13.6%) |
P value |
|---|---|---|---|
| Male (%) | 10 (90.9 %) | 3 (100%) | 0.814 |
| Female (%) | 1 (9.1 %) | 0 (0.0%) | 0.814 |
| Clinical presentation | |||
| Palpitations | 10 (90.9 %) | 3 (100%) | 0.462 |
| Syncope | 1 (9.1 %) | 1 (33.3%) | 0.552 |
| Shortness of breath | 2 (18.2%) | 1 (33.3%) | 0.727 |
| Chest pain | 2 (18.2%) | 1 (33.3%) | 0.295 |
| Dizziness | 1(9.1 %) | 1(33.3%) | 0.552 |
| Ventricular tachycardia | 8 (72.7 %) | 1 (33.3%) | 0.229 |
| Sudden cardiac death | 3(27.3%) | 0 (0.0%) | 0.176 |
| Medications | |||
| Amiodarone | 2 (18.2%) | 0 (0.0%) | 0.713 |
| Sotalol | 2 (18.2%) | 0 (0.0%) | 0.713 |
| Beta blockers | 4 (36.4%) | 1 (33.3%) | 0.870 |
| ACE inhibitors | 6 (54.5%) | 2 (66.7%) | 0.630 |
| Spironolactone | 6 (54.5%) | 1 (33.3%) | 0.416 |
| ICD type | |||
| Single ICD | 2 (18.2%) | 0 (0.0%) | 0.333 |
| Dual ICD | 9 (81.8%) | 3 (100%) | |
| Genetic results | |||
| Positive | 6 (54.5%) | 2 (66.7%) | 0.323 |
| Negative | 1 (9.1 %) | 1 (33.3%) | 0.516 |
| Not performed | 4 (36.4%) | 0 (0.0%) | 0.308 |
| Prevention type | |||
| Primary | 1 (9.1 %) | 1 (33.3%) | 0.048 |
| Secondary | 10 (90.9%) | 2 (66.7%) | |
ACE: angiotensin-converting enzyme; ICD: implantable cardioverter defibrillator.
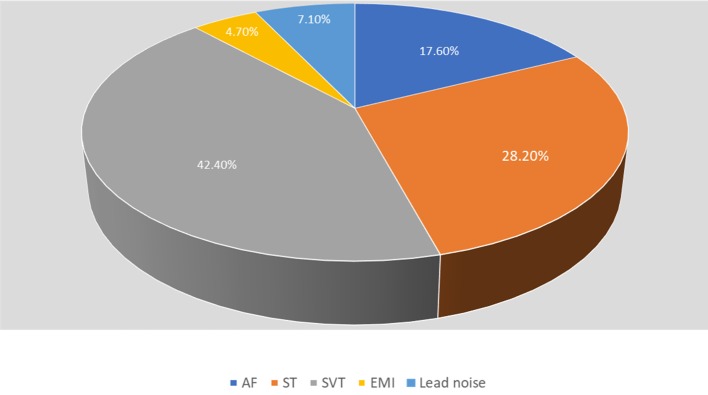
The causes of inappropriate ICD therapy were atrial fibrillation (15 episodes (17.6%)), sinus tachycardia (ST) (24 episodes (28.2%)), supraventricular tachycardia (SVT) (36 episodes (42.2%)), lead noise (6 episodes (7.1%)) and electromagnetic interference (4 episodes (4.7%)) (Fig. 6).
Figure 6.
Causes of inappropriate implantable cardioverter defibrillator (ICD) therapy. AF: atrial fibrillation; EMI: elecromanganic interference; ST: sinus tachycardia; SVT: supraventricular tachycardia.
Regarding ICD-related complications, one patient had ICD lead fracture after 6.5 years, and a new ICD lead was implanted. Another patient underwent a new ICD lead implantation at the time of ICD generator change 7 years after initial implantation prophylactically as his ICD lead was a recalled lead (Medtronic Sprint Fidelis). There was no ICD system-related infection.
Medical therapy
Almost all patients were on antiarrhythmic medications. Seven patients were on sotalol (31.8%), three on amiodarone (13.6%), and eleven on beta-blockers (50%). Nine patients were on angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEI/ARBs) (41%), and two on spironolactone (9%).
Electrophysiology study and ablation
Eight patients underwent electrophysiology study (EPS); five patients underwent VT ablation, two patients had premature ventricular contraction (PVC)’s ablation and one patient had a non-inducible VT. Three patients had EPS with SVT ablation (two atrioventricular nodal re-entry tachycardia and one atrial tachycardia).
Genetic testing
Genetic testing was performed in 14 patients (five primary prevention and nine secondary prevention patients). In the primary prevention patients; three were positive (all plakophilin-2 (PKP2) gene), and two were negative. In the secondary prevention patients; seven were positive (five PKP2, one desmoplakin (DSP) and one desmocollin-2 (DSC2) genes), and two were negative.
Discussion
The ICD therapy is the only proven therapy for SCD prevention in high-risk ARVC/D patients. Nearly two-thirds of our patients experienced appropriate ICD therapy during their follow-up indicating a high prevalence of VT/VF in ARVC/D patients. The average appropriate ICD therapy was 4.8% per patient/year, and inappropriate ICD therapy or intervention occurred in about one-fourth of the patients with an average of 1.5% per patient/year. In a recent meta-analysis, it is found that the annualized appropriate ICD intervention rate in ARVC/D patients was 9.5%, and the annualized inappropriate ICD intervention rate was 3.7% [14].
Fortunately, ATP therapy was very effective in terminating most of the VT episodes in our patients. This is compatible with what has been found in previous studies where ATP attempt has been shown to be effective in terminating about 84.4% of VT episodes [15].
Patients with secondary prevention indication had more appropriate ICD therapy compared to those with primary prevention indication (66.7% versus 14%), and there was no significant difference in appropriate and inappropriate ICD therapies in those with positive or negative genetic testing. In a large cohort of ARVC/D patients and family members, appropriate ICD therapy in secondary prevention patients was 84% and 71% in those with and without genetic mutation respectively [7]. On the other hand, appropriate ICD therapy in primary prevention patients was 16% and 29% in the two groups, respectively [7].
The most common cause of inappropriate ICD therapy in this series is SVT, followed by ST and both accounts for about 70% of inappropriate ICD therapies. This is likely due to the young age at implantation. Atrial fibrillation accounts for 17.6% of these therapies. This is compatible with what has been reported before [14].
In this small cohort, we did not find significant difference in the incidence of inappropriate ICD therapy between single and dual chamber ICD. Currently, there is no clear evidence to determine whether a single chamber or a dual chamber ICD is preferable for patients with ARVD/C [16].
Although subcutaneous ICD (S-ICD) has been used in patients with ARVC/D, the lack of ATP therapy at current S-ICD devices makes it less attractive to this group of patients. Furthermore, the negative T-waves (NTWs) in the right precordial leads may partially or completely revert with exercise in most patients with ARVC/D [17], and this may result in the lack of consistency of an appropriate sensing vector both at resting and during exercise [18], or inappropriate ICD detection and therapy [19]. However, a software update to improve the sensing of the device may minimize this problem [19].
Study limitations
The main limitations of this study are the retrospective nature and the small sample size. However, the ARVC/D is a rare disease and the mean follow-up in this study is longer than previous studies with a mean follow-up of about nine and half years.
Conclusions
ARVC/D patients are at high risk of VT/VF, and ICD therapy is an effective management strategy in terminating these arrhythmias. Most of ICD therapies in ARVC/D patients are appropriate. Appropriate ICD programming and use of ATP will reduce the risk of having appropriate and inappropriate ICD shocks. Although we do not have any patient with subcutaneous ICD in this study, the high success rate of ATP suggests that transvenous ICD would be more appropriate in ARVC/D patients.
Conflict of Interest
The authors declare no conflict of interest related to this article.
Financial Support
No source of funding to declare.
References
- 1.Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65(2):384–398. doi: 10.1161/01.CIR.65.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A. et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30(6):1512–1520. doi: 10.1016/S0735-1097(97)00332-X. [DOI] [PubMed] [Google Scholar]
- 3.McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71(3):215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31(7):806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318(3):129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 6.Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, Salerno JU. et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108(25):3084–3091. doi: 10.1161/01.CIR.0000103130.33451.D2. [DOI] [PubMed] [Google Scholar]
- 7.Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, Murray B. et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet. 2015;8(3):437–446. doi: 10.1161/CIRCGENETICS.114.001003. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, Bardy GH. et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342(6):365–373. doi: 10.1056/NEJM200002103420601. [DOI] [PubMed] [Google Scholar]
- 9.Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol. 1998;32(7):1909–1915. doi: 10.1016/S0735-1097(98)00495-1. [DOI] [PubMed] [Google Scholar]
- 10.Myerburg RJ, Castellanos A. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Bonow RO, Mann DL, Zipes DP, Libby P, Braunwald E, editors. Elsevier Saunders; Philadelphia: 2012. Cardiac Arrest and Sudden Cardiac Death; pp. 845–884. [DOI] [Google Scholar]
- 11.Chugh SS, Teodorescu C, Evanado A, Reiner K. Sudden Unexplained Death in the Community. In: Brugada R, Brugada J, Brugada P, Eds, Clinical Approach to Sudden Cardiac Death Syndromes, Springer Verlag, London. 2010:1–6. doi: 10.1007/978-1-84882-927-5_1. [DOI] [Google Scholar]
- 12.Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S. et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61(12):1318–1368. doi: 10.1016/j.jacc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 13. Bandar Al-Ghamdi (April 12th 2017). Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia, Cardiomyopathies Kaan Kırali, IntechOpen. Available from: https://www.intechopen.com/books/cardiomyopathies-types-and-treatments/arrhythmogenic-right-ventricular-cardiomyopathy-dysplasia.
- 14.Schinkel AF. Implantable cardioverter defibrillators in arrhythmogenic right ventricular dysplasia/cardiomyopathy: patient outcomes, incidence of appropriate and inappropriate interventions, and complications. Circ Arrhythm Electrophysiol. 2013;6(3):562–568. doi: 10.1161/CIRCEP.113.000392. [DOI] [PubMed] [Google Scholar]
- 15.Gulizia MM, Piraino L, Scherillo M, Puntrello C, Vasco C, Scianaro MC, Mascia F. et al. A randomized study to compare ramp versus burst antitachycardia pacing therapies to treat fast ventricular tachyarrhythmias in patients with implantable cardioverter defibrillators: the PITAGORA ICD trial. Circ Arrhythm Electrophysiol. 2009;2(2):146–153. doi: 10.1161/CIRCEP.108.804211. [DOI] [PubMed] [Google Scholar]
- 16.Roguin A, Bomma CS, Nasir K, Tandri H, Tichnell C, James C, Rutberg J. et al. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2004;43(10):1843–1852. doi: 10.1016/j.jacc.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Zorzi A, ElMaghawry M, Rigato I, Cardoso Bianchini F, Crespi Ponta G, Michieli P, Migliore F. et al. Exercise-induced normalization of right precordial negative T waves in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2013;112(3):411–415. doi: 10.1016/j.amjcard.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Migliore F, Bertaglia E, Zorzi A, Corrado D. Subcutaneous Implantable Cardioverter-Defibrillator and Arrhythmogenic Right Ventricular Cardiomyopathy: The Importance of Repeat ECG Screening During Exercise Test. JACC Clin Electrophysiol. 2017;3(7):785–786. doi: 10.1016/j.jacep.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Allocca G, Sitta N, Turiano G. Inappropriate shocks by subcutaneous defibrillator in a patient with arrhythmogenic right ventricular cardiomyopathy: problem fixed. Europace. 2015;17(7):1067. doi: 10.1093/europace/euv086. [DOI] [PubMed] [Google Scholar]