Abstract
Clostridium difficile infection (CDI) continues to possess a significant disease burden in the United States (US) as well as all over the world. Given the increase in severity and recurrence rate, the decrease in cure rate, and the fact that the virulent ribotype 027 strain remains one of the most commonly identified strains in the US, the Infectious Diseases Society of America (IDSA) published a clinical practice guideline in February 2018 moving away from metronidazole as the first-line treatment for initial CDI and recommending either oral vancomycin or fidaxomicin. The aim of this study is to evaluate the clinical data available comparing the efficacy of primary treatment of CDI between those two antibiotics. We performed a PubMed, PubMed Central, and ScienceDirect database search without restriction to regions, publication types, or languages. A comprehensive literature search was performed from January 1, 1980 up to March 20, 2018. We used the following keywords in different combinations: Clostridium difficile, Clostridium difficile infection, CDI, C. diff, C. difficile, fidaxomicin, vancomycin, pseudomembranous colitis, and antibiotic-associated colitis. The search was limited to human studies. Data were independently extracted by two reviewers with disagreements resolved by a third author. We pooled an odds ratio (OR) on two primary outcomes: Clinical cure rate and rate of recurrence during the follow-up period. The computer search was also supplemented with manual searches by the authors of the retrieved review articles and primary studies. The search phrase “((Clostridium difficile) AND vancomycin) AND fidaxomicin” had the highest yield results. We identified four observational studies with a total of 2,303 patients with CDI that met our inclusion criteria. Compared with vancomycin, fidaxomicin use was associated with a significantly lower recurrence of CDI with a pooled OR of 0.47 (95% confidence interval (CI), 0.37 - 0.60, I2 = 0). On the other hand, there was no significant association of fidaxomicin use with CDI cure rate compared to vancomycin with a pooled OR of 1.22 (95% CI, 0.93 - 1.60, I2 = 0). In light of the recently updated clinical practice guidelines by the IDSA, our review suggests that fidaxomicin has a more sustained clinical response with a statistically significant lower recurrence rate. Although fidaxomicin appears to be the better drug with statistical significance, its cost-effectiveness continues to be an ongoing controversy. More randomized clinical trials are needed to shed light on this matter to assess if there is any clinical significance in fidaxomicin superiority.
Keywords: clostridium difficile, treatment of clostridium difficile, vancomycin, fidaxomicin, c. diff, c. difficile
Introduction and background
Clostridium difficile infection (CDI) continues to possess a significant disease burden in both the United States (US) and globally. There have been a reported 453,000 infections in 2011 in the US alone, with 83,000 of those experiencing at least one recurrence and 29,000 expiring within 30 days of the initial diagnosis [1]. The incidence and severity of CDI continue to trend upwards, with a reported increase in community-acquired infections of a disease that was once considered nosocomial and antibiotic-related [2-4]. A similar trend is reported in children [5-6].
Due to its severity and high rates of recurrence, lower cure rates, and ribotype 027 virulence in the US, the Infectious Diseases Society of America (IDSA) published clinical practice guidelines in February 2018 moving away from metronidazole as the first-line treatment for initial CDI and recommending, with a strong level of evidence, either oral vancomycin or oral fidaxomicin [7].
The purpose of this systematic review and meta-analysis is to analyze the available data on the comparison of oral vancomycin and oral fidaxomicin as the first-line medication treatment of CDI. The reader must bear in mind that multiple studies have attempted to prove that although fidaxomicin is more costly than the alternatives, it may prove to be the more cost-effective option.
Review
Methods
Search Strategy and Selection Criteria
We performed a PubMed, PubMed Central, and ScienceDirect database search without restriction to regions, publication types, or languages. A comprehensive literature search was performed from inception to March 20, 2018, during which time the IDSA guidelines update on changing the initial drug of choice to oral vancomycin or fidaxomicin has been set forth. The following keywords were used in different combinations: Clostridium difficile, Clostridium difficile infection, CDI, C. diff, C. difficile, fidaxomicin, vancomycin, pseudomembranous colitis, and antibiotic-associated colitis. The search was limited to human studies. The computer search was supplemented with manual searches by the authors of the retrieved review articles and primary studies. The search phrase ((Clostridium difficile) AND vancomycin) AND fidaxomicin had the highest yield results.
Data Extraction and Quality Assessment
Studies were included if they met the following: 1) used a well-defined case-control or cohort design and 2) presented an odds ratio (OR) for our main outcome with a 95% confidence interval (CI) or reported sufficient data to calculate these parameters. Exclusion criteria were: 1) case reports, case series, and review articles and 2) insufficient information concerning evaluation rates. Two authors screened all abstracts independently obtained from the initial literature search and removed those not fulfilling the inclusion criteria. The data were abstracted from all included studies into a standardized table. A third investigator reviewed the data for accuracy prior to the data query. The inclusion/exclusion decisions were made after consultation with the other authors.
Outcome Definition
In comparing the efficacy of treatment of initial CDI with vancomycin versus fidaxomicin, two endpoints were used: 1) achieving clinical cure with a resolution of symptoms without the need for further treatment and 2) recurrence of infection during the follow-up period, which was at least three weeks and up to four weeks following a 10-day course of antibiotics. This included relapse and reinfection. An overall OR was used for both of these endpoints.
Statistical Analysis
All statistical analyses were performed using the Comprehensive Meta-Analysis (CMA), Version 3 software (BioStat, Inc., Eaglewood, NJ). The pooled risk ratios of Clostridium difficile cure and recurrence in patients treated with fidaxomicin in comparison to those treated with vancomycin were calculated using the generic inverse method of DerSimonian and Laird [8]. A random effect model was used, given the high likelihood of between-study variance due to the difference in underlying population and methodology. Cochran's Q-test, which was supplemented by I2 statistic, was used to evaluate the statistical heterogeneity. This I2 statistic quantifies the proportion of the total variation across studies, that is, due to true heterogeneity rather than chance. A value of I2 of 0% to 25% denotes trivial heterogeneity, greater than 25% but ≤ 50% denotes low heterogeneity, greater than 50% but ≤ 75% denotes moderate heterogeneity, and greater than 75% represents high heterogeneity [9].
Results
Search Results
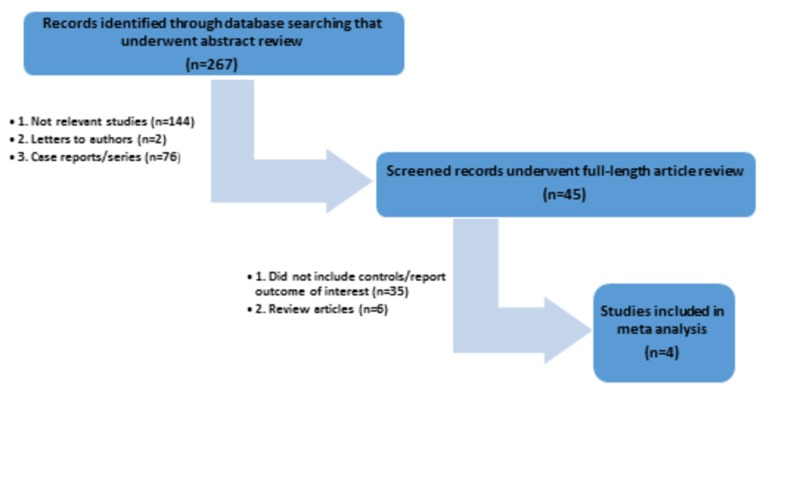
The initial search yielded 267 citations, all of which underwent title and abstract review. The majority of them were excluded at this step, including those that were case reports, letters to editor, review articles, or interventional studies. A total of 45 studies underwent full-length article review, and 41 of them were excluded as they did not include controls, did not report the outcome of interest, or were review articles. Therefore, a total of four studies [10-13] met our inclusion criteria and were included in the meta-analysis. Figure 1 outlines our search methodology and selection process. Baseline characteristics of the included studies are summarized in Table 1.
Figure 1. A flow diagram demonstrates the search methodology and selection process for this meta-analysis.
n: number
Table 1. Total Studies Utilized in This Meta-analysis.
BMI: body mass index; CDI: Clostridium difficile infection; NAP1: North American pulsed-field gel electrophoresis type 1; OR: odds ratio; UK: United Kingdom; US: United States; WBC: white blood cells
| Louie et al. [12] | Cornely et al. [13] | Loui et al. [10] | Houseman et al. [11] | |
| Country | US and Canada | US, Canada, France, Spain, Belgium, Germany, UK, Italy, Sweden | US, Canada, France, Spain, Belgium,Germany, UK, Italy, Sweden | US |
| Study design | Clinical trial | Clinical trial | Clinical trial | Clinical trial |
| Year | 2011 | 2012 | 2013 | 2016 |
| Number of participants enrolled | 629 | 535 | 1,105 | 34 |
| Number of participants enrolled/Fidaxomicin | 302 | 271 | 539 | 18 |
| Number of participants enrolled/Vancomycin | 327 | 264 | 566 | 16 |
| Overall participants analyzed | 596 | 509 | 794 | 24 |
| Overall participants analyzed/Fidaxomicin | 287 | 253 | 391 | 12 |
| Overall participants analayzed/Vancomycin | 309 | 256 | 403 | 12 |
| Mean age of participants in years | 61.9 | 63.4 | Fidaxomicin: 63.3 Vancomycin: 62.3 | Fidaxomicin: 69 Vancomycin: 66 |
| Median follow up duration | 21 days | 21 days | 21 days | 28 days |
| OR: Cure rate, Fidaxomicin vs Vancomycin | 1.24 (0.77 - 2.00) | 1.09 (0.65 - 1.83) | 1.24 (0.8 - 1.92) | 2.5 (0.46 - 13.52) |
| OR: Recurrence rate, Fidaxomicin vs Vancomycin | 0.54 (0.35 - 0.84) | 0.39 (0.24 - 0.64) | 0.46 (0.32 - 0.67) | 0.85 (0.1 - 7.04) |
| Confounder adjustment | Age, Sex, Inpatient status, NAP1 strain | Age, Sex, Inpatient status, NAP1 strain | Age, Sex, Inpatient status, NAP1 strain, BMI, WBC, serum albumin, serum creatinine, concomitant antibiotic therapy | Age, Sex, Community acquired CDI, NAPI strain |
| Quality assessment (Newcastle-Ottawa scale) | Good | Good | Good | Good |
Meta-analysis results
Recurrence Rate
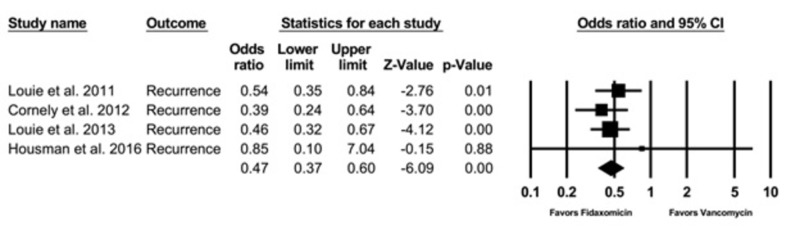
Four observational studies with a total of 2,303 patient with CDI were enrolled. Compared with vancomycin, fidaxomicin use was associated with a significantly lower recurrence with a pooled OR of 0.47 (95% CI, 0.37 - 0.60, I2 = 0) (Figure 2).
Figure 2. Recurrence rate.
CI: confidence interval
Cure Rate
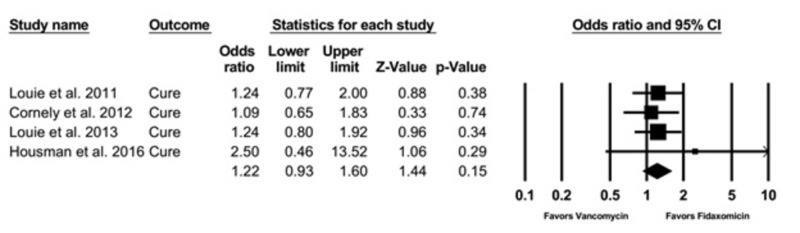
There was no significant association of fidaxomicin use with CDI cure rate compared to vancomycin with a pooled OR of 1.22 (95% CI, 0.93 - 1.60, I2 = 0) (Figure 3).
Figure 3. Cure rate.
CI: confidence interval
Evaluation for Publication Bias
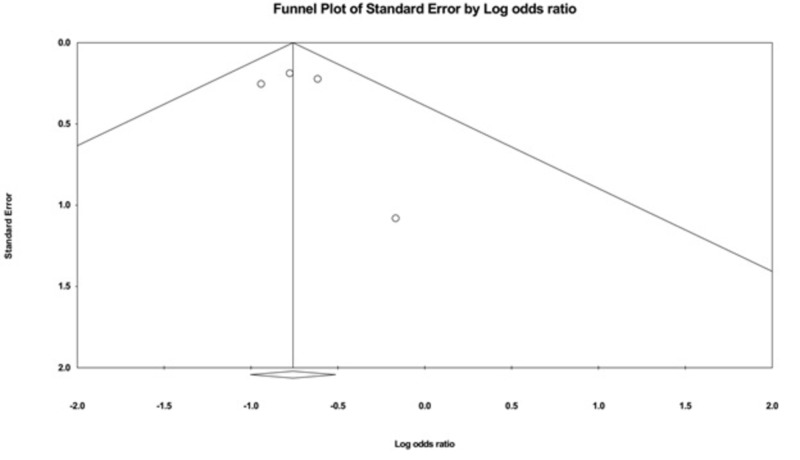
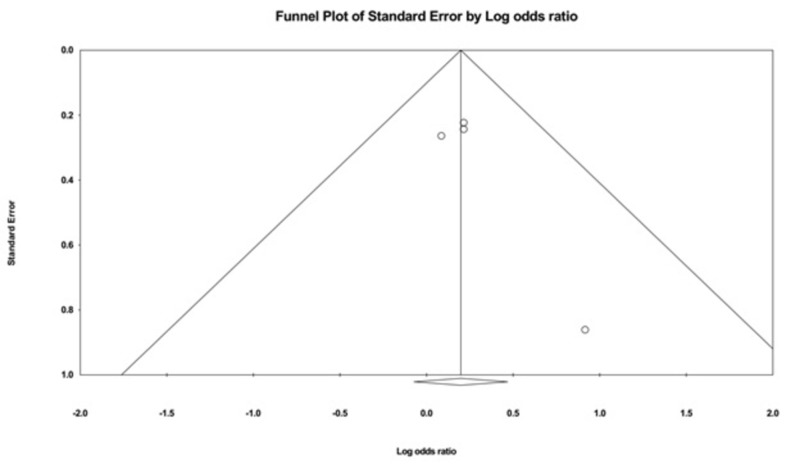
The funnel plots evaluating recurrence and cure rate are shown in Figures 4-5. They are symmetric and do not suggest the presence of publication bias in favor of a positive study for all of the outcomes. In addition, Egger’s regression asymmetry test showed no evidence of publication bias (P > 0.05 for both outcomes).
Figure 4. A funnel plot: recurrence rate.
Figure 5. A funnel plot: cure rate .
Sensitivity Analysis
Sensitivity analysis was performed by excluding one study at a time to investigate the effect of each study on the pooled odds ratio for each outcome assessed. The pooled effect estimate from this sensitivity analysis remained essentially unchanged.
Discussion
The studies we used in our meta-analysis included patients with their first episode of CDI. All of these patients had clinical symptoms suggestive of CDI, including a minimum of three episodes of unformed stool in a 24-hour period with no known or documented the prior diagnosis of CDI. The diagnosis was made by a positive C. difficile polymerase chain reaction test or toxin A, B, C assay, depending on the specific study criteria. These patients were then randomized for treatment with vancomycin, 124 mg orally four times daily, or fidaxomicin, 200 mg orally twice a day, for a total of 10 days. These patients were followed up for a minimum of three weeks and up to four weeks after completion of their antibiotic course.
In our review and meta-analysis of two endpoints (cure rate or the rate of symptoms’ resolution and the recurrence of symptoms within the follow-up period), we can show that the use of fidaxomicin was associated with a statistically significant lower recurrence. However, there was no significant difference with the cure rate when compared with that of vancomycin.
Fidaxomicin is the first macrolide antibacterial agent approved for the treatment of CDI [14]. It inhibits transcription by binding to the deoxyribonucleic acid (DNA)-template ribonucleic acid (RNA) polymerase sigma subunit and hence, prevents the initial separation of the bacterial DNA strands. Essentially, it inhibits the initiation of RNA synthesis very early on in that pathway [15-16]. This unique mechanism of action might explain fidaxomicin’s very narrow spectrum of antimicrobial coverage at low concentrations [17-19].
Multiple studies have shown fidaxomicin to have a substantially higher in vitro activity against C. difficile compared to vancomycin [20-23] with a more prolonged post-antibiotic effect [24]. Furthermore, fidaxomicin is a bactericidal drug, whereas vancomycin is bacteriostatic [25]. Fidaxomicin’s lower rates of relapse following treatment might be attributed to the fact that it causes fewer changes to the bowel microbiota of C. difficile-infected patients compared to vancomycin both during [26-27] and after treatment [28]. Fidaxomicin also has a narrower antimicrobial coverage [17], and unlike vancomycin, it inhibits sporulation. As Louie et al. [29] showed, patients treated with fidaxomicin had a 2.3 log10 lower fecal spore counts at 21 - 28 days post-therapy compared to a patient treated with vancomycin. Similar results were reported by Housman et al. years later [11]. This might at least suggest that fidaxomicin might be a better first-line option for patients at a higher risk of recurrence, like older patients or patients with cancer [10]. It is worth noting here that a study by Nerandzic et al. in 2012 showed that fidaxomicin reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in CDI patients compared to vancomycin [30].
Similar to vancomycin, fidaxomicin demonstrates minimal systemic absorption, which explains why the most common side effects are gastrointestinal in nature, like nausea and abdominal pain, both of which are likely to be part of CDI symptomatology [31-33]. This characteristic makes it a very well-tolerated drug in both adults and children [32-37].
Whenever fidaxomicin is compared with vancomycin, the cost is always an examined variable. A 10-day course has at least three times more acquisition cost than that of vancomycin [38]. However, multiple studies in high-income countries have shown the cost-effectiveness of fidaxomicin [39-41]. A recent systemic review by Burton et al. in 2017 also showed that fidaxomicin was, in fact, more cost-effective than vancomycin [42]. Prior to that, Stranges et al. performed a cost-utility analysis in the US in 2013 which showed an incremental cost-effectiveness ratio of $67,576/quality adjusted life-year (QALY) confirming the results of Sclar et al. in 2012, who also showed potential cost-effectiveness in the US health system [43-44]. Furthermore, a study with regards to hospital cost savings in 2015 showed that their hospital saved $3,047 USD (United States dollars) per patient treated with fidaxomicin compared to a patient treated with vancomycin [45]. Similar promising results were seen in patients with cancer and patients with renal impairment [41, 46]. This postulated cost-effectiveness might be explained by the decreased recurrence rate and hence, hospital readmissions that fidaxomicin provides, both of which, in addition to the length of stay, have not been well compared.
On the other hand, other studies have shown conflicting results. Reveles et al. found similar total costs comparing the two drugs [47]. Another systemic review by Le et al. in 2018 concluded that the cost-effectiveness of fidaxomicin compared to vancomycin was not definitive [48]. Costs will vary between healthcare systems as well; an increase in cost with the use of fidaxomicin has been suggested in the Canadian health care system [49].
All in all, this calls for research and strong randomized control trials to assess the cost-effectiveness of fidaxomicin compared to vancomycin in the US.
Conclusions
In light of the recently updated clinical practice guidelines by the IDSA for the treatment of C. difficile infection, we set out to compare both of the recommended first-line drugs, vancomycin and fidaxomicin. Our meta-analysis found a similar cure rate; however, fidaxomicin was found to have a more sustained clinical response with a statistically significant lower recurrence rate. Although fidaxomicin appears to be the better drug with statistical significance, its cost-effectiveness continues to be an ongoing controversy. More randomized clinical trials are needed to shed light on this matter to assess if there is any clinical significance in fidaxomicin superiority, particularly as CDI incidence, severity, and recurrence rates continue to be on an upward trajectory.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
This work has been submitted to the American College of Gastroenterology Conference 2018 in abstract form. This manuscript is not previously published or currently under consideration for publication elsewhere and, if accepted, will not be published elsewhere without written consent of the publisher
References
- 1.Burden of Clostridium difficile infection in the United States. Lessa FC, Mu Y, Bamberg WM, et al. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The changing epidemiology of Clostridium difficile infections. Freeman J, Bauer MP, Baines SD, et al. Clin Microbiol Rev. 2010;23:529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. Chitnis AS, Holzbauer SM, Belflower RM, et al. JAMA Intern Med. 2013;173:1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Khanna S, Pardi DS, Aronson SL, et al. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The epidemiology of Clostridium difficile infection in children: a population-based study. Khanna S, Baddour LM, Huskins WC, et al. Clin Infect Dis. 2013;56:1401–1406. doi: 10.1093/cid/cit075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Changing epidemiology of Clostridium difficile-associated disease in children. Benson L, Song X, Campos J, Singh N. Infect Control Hosp Epidemiol. 2007;28:1233–1235. doi: 10.1086/520732. [DOI] [PubMed] [Google Scholar]
- 7.Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) McDonald LC, Gerding DN, Johnson S, et al. Clin Infect Dis. 2018;66:0. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meta-analysis in clinical trials. DerSimonian R, Laird N. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Measuring inconsistency in meta-analyses. Higgins JP, Thompson SG, Deeks JJ, et al. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effect of age on treatment outcomes in Clostridium difficile infection. Louie TJ, Miller MA, Crook DW, et al. J Am Geriatr Soc. 2013;61:222–230. doi: 10.1111/jgs.12090. [DOI] [PubMed] [Google Scholar]
- 11.Assessment of Clostridium difficile burden in patients over time with first episode infection following fidaxomicin or vancomycin. Housman ST, Thabit AK, Kuti JL, et al. Infect Control Hosp Epidemiol. 2016;37:215–218. doi: 10.1017/ice.2015.270. [DOI] [PubMed] [Google Scholar]
- 12.Fidaxomicin versus vancomycin for Clostridium difficile infection. Louie TJ, Miller MA, Mullane KM, et al. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 13.Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non inferiority, randomised controlled trial. Cornely OA, Crook DW, Esposito R, et al. Lancet Infect Dis. 2012;12:281–289. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 14.Drug evaluation OPT-80, a narrow-spectrum macrocyclic antibiotic. Johnson AP. http://www.ncbi.nlm.nih.gov/pubmed/17328233. Curr Opin Investig Drugs. 2007;8:168–173. [PubMed] [Google Scholar]
- 15.Fidaxomicin is an inhibitor of the initiation of bacterial RNA synthesis. Artsimovitch I, Seddon J, Sears P. Clin Infect Dis. 2012;55:0–31. doi: 10.1093/cid/cis358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inhibition by lipiarmycin of bacteriophage growth in Bacillus subtilis. Osburne MS, Sonenshein AL. http://jvi.asm.org/content/33/3/945.short. J Virol. 1980;33:945–953. doi: 10.1128/jvi.33.3.945-953.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidaxomicin A novel agent for the treatment of Clostridium difficile infection. Zhanel GG, Walkty AJ, Karlowsky JA. Can J Infect Dis Med Microbiol. 2015;26:305–312. doi: 10.1155/2015/934594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antimicrobial activities of fidaxomicin. Goldstein EJ, Babakhani F, Citron DM. Clin Infect Dis. 2012;55:0–48. doi: 10.1093/cid/cis339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.OPT-80 a macrocyclic antimicrobial agent for the treatment of Clostridium difficile infections: a review. Gerber M, Ackermann G. Expert Opin Investig Drugs. 2008;17:547–553. doi: 10.1517/13543784.17.4.547. [DOI] [PubMed] [Google Scholar]
- 20.In vitro activity of OPT-80 tested against clinical isolates of toxin-producing Clostridium difficile. Karlowsky JA, Laing NM, Zhanel GG. Antimicrob Agents Chemother. 2008;52:4163–4165. doi: 10.1128/AAC.00476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.In vitro activity of OPT-80 against Clostridium difficile. Ackermann G, Löffler B, Adler D, Rodloff AC. Antimicrob Agents Chemother. 2004;48:2280–2282. doi: 10.1128/AAC.48.6.2280-2282.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Activity of OPT-80, a novel macrocycle, compared with those of eight other agents against selected anaerobic species. Credito KL, Appelbaum PC. Antimicrob Agents Chemother. 2004;48:4430–4434. doi: 10.1128/AAC.48.11.4430-4434.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Effects of inoculum, pH, and cations on the in vitro activity of fidaxomicin (OPT-80, PAR-101) against Clostridium difficile. Babakhani F, Seddon J, Robert N, et al. Antimicrob Agents Chemother. 2010;54:2674–2676. doi: 10.1128/AAC.01842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Postantibiotic effect of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. Babakhani F, Gomez A, Robert N, et al. http://55. Antimicrob Agents Chemother. 2011;55:4427–4429. doi: 10.1128/AAC.00104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Killing kinetics of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. Babakhani F, Gomez A, Robert N, Sears P. J Med Microbiol. 2011;60:1213–1217. doi: 10.1099/jmm.0.029470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Tannock GW, Munro K, Taylor C, et al. Microbiology. 2010;156:3354–3359. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 27.Successful treatment of simulated Clostridium difficile infection in a human gut model by fidaxomicin first line and after vancomycin or metronidazole failure. Chilton CH, Crowther GS, Freeman J, et al. J Antimicrob Chemother. 2014;69:451–462. doi: 10.1093/jac/dkt347. [DOI] [PubMed] [Google Scholar]
- 28.Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Louie TJ, Cannon K, Byrne B, et al. Clin Infect Dis. 2012;55:0–42. doi: 10.1093/cid/cis338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical outcomes safety, and pharmacokinetics of OPT-80 in a phase 2 trial with patients with Clostridium difficile infection. Louie T, Miller M, Donskey C, et al. Antimicrob Agents Chemother. 2009;53:223–228. doi: 10.1128/AAC.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Nerandzic MM, Mullane K, Miller MA, et al. Clin Infect Dis. 2012;55:0–26. doi: 10.1093/cid/cis440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fidaxomicin attains high fecal concentrations with minimal plasma concentrations following oral administration in patients with Clostridium difficile infection. Sears P, Crook DW, Louie TJ, et al. Clin Infect Dis. 2012;55:0–20. doi: 10.1093/cid/cis337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comparison of the safety, tolerability, and pharmacokinetics of fidaxomicin in healthy Japanese and Caucasian subjects. Oshima H, Yamazaki T, Benner L, et al. Clin Drug Investig. 2015;35:375–384. doi: 10.1007/s40261-015-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The efficacy of fidaxomicin in the treatment of Clostridium difficile infection in a real-world clinical setting: a Spanish multi-centre retrospective cohort. Fehér C, Múñez Rubio E, Merino Amador P, et al. Eur J Clin Microbiol Infect Dis. 2017;36:295–303. doi: 10.1007/s10096-016-2802-x. [DOI] [PubMed] [Google Scholar]
- 34.Safety and efficacy of fidaxomicin in the treatment of Clostridium difficile-associated diarrhea. Golan Y, Epstein L. Therap Adv Gastroenterol. 2012;5:395–402. doi: 10.1177/1756283X12461294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efficacy and safety of fidaxomicin compared with oral vancomycin for the treatment of adults with Clostridium difficile-associated diarrhea: data from the OPT-80-003 and OPT-80-004 studies. Chen LF, Anderson DJ. Future Microbiol. 2012;7:677–683. doi: 10.2217/fmb.12.44. [DOI] [PubMed] [Google Scholar]
- 36.Fidaxomicin (OPT-80) for the treatment of Clostridium difficile infection. Miller M. Expert Opin Pharmacother. 2010;11:1569–1578. doi: 10.1517/14656566.2010.485614. [DOI] [PubMed] [Google Scholar]
- 37.Safety and pharmacokinetic study of fidaxomicin in children with Clostridium difficile-associated diarrhea: a phase 2a multicenter clinical trial. O'Gorman MA, Michaels MG, Kaplan SL, et al. J Pediatric Infect Dis Soc. 2017;(Epub ahead of print) doi: 10.1093/jpids/pix037. [DOI] [PubMed] [Google Scholar]
- 38.Fidaxomicin (Dificid), a novel oral macrocyclic antibacterial agent for the treatment of Clostridium difficile-associated diarrhea in adults. Cruz MP. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3411227/ P T. 2012;37:278–281. [PMC free article] [PubMed] [Google Scholar]
- 39.Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. Nathwani D, Cornely OA, Van Engen AK, et al. J Antimicrob Chemother. 2014;69:2901–2912. doi: 10.1093/jac/dku257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cost-effectiveness analysis on the use of fidaxomicin and vancomycin to treat Clostridium difficile infection in France. Watt M, Dinh A, Le Monnier A, Tilleul P. J Med Econ. 2017;20:678–686. doi: 10.1080/13696998.2017.1302946. [DOI] [PubMed] [Google Scholar]
- 41.A cost-effectiveness and budget impact analysis of first-line fidaxomicin for patients with Clostridium difficile infection (CDI) in Germany. Watt M, McCrea C, Johal S, et al. Infection. 2016;44:599–606. doi: 10.1007/s15010-016-0894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.A systematic literature review of economic evaluations of antibiotic treatments for Clostridium difficile infection. Burton HE, Mitchell SA, Watt M. Pharmacoeconomics. 2017;35:1123–1140. doi: 10.1007/s40273-017-0540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Stranges PM, Hutton DW, Collins CD. Value Health. 2013;16:297–304. doi: 10.1016/j.jval.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Fidaxomicin for Clostridium difficile-associated diarrhoea: epidemiological method for estimation of warranted price. Sclar DA, Robison LM, Oganov AM, et al. Clin Drug Investig. 2012;32:0–24. doi: 10.1007/BF03261906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Gallagher JC, Reilly JP, Navalkele B, et al. Antimicrob Agents Chemother. 2015;59:7007–7010. doi: 10.1128/AAC.00939-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Economic assessment of fidaxomicin for the treatment of Clostridium difficile infection (CDI) in special populations (patients with cancer, concomitant antibiotic treatment or renal impairment) in Spain. Rubio-Terrés C, Cobo Reinoso J, Grau Cerrato S, et al. Eur J Clin Microbiol Infect Dis. 2015;34:2213–2223. doi: 10.1007/s10096-015-2472-0. [DOI] [PubMed] [Google Scholar]
- 47.Fidaxomicin versus vancomycin as a first-line treatment for Clostridium difficile-associated diarrhea in specific patient populations: a pharmacoeconomic evaluation. Reveles KR, Backo JL, Corvino FA, et al. Pharmacotherapy. 2017;37:1489–1497. doi: 10.1002/phar.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cost-effectiveness of competing treatment strategies for Clostridium difficile infection: a systematic review. Le P, Nghiem VT, Mullen PD, Deshpande A. Infect Control Hosp Epidemiol. 2018;39:412–424. doi: 10.1017/ice.2017.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clinical and economic consequences of vancomycin and fidaxomicin for the treatment of Clostridium difficile infection in Canada. Wagner M, Lavoie L, Goetghebeur M. Can J Infect Dis Med Microbiol. 2014;25:87–94. doi: 10.1155/2014/793532. [DOI] [PMC free article] [PubMed] [Google Scholar]