INTRODUCTION
Fanconi anemia (FA) is an inherited DNA repair disorder with heterogeneous clinical manifestations, including congenital anomalies, progressive cytopenias to frank bone marrow failure (BMF), and both hematologic and solid malignancies [1]. With one exception (X-linked FANCB), FA is attributable to biallelic autosomal inactivation of 21 genes encoding member and associated proteins of the Fanconi complex [2–4], which collectively function to identify and repair DNA breaks during normal cellular replication or in response to radiation or DNA-crosslinking agents. In fact, it is this marked susceptibility to DNA-crosslinking agents that permits rapid diagnostic testing. Following exposure to diepoxybutane (DEB) or mitomycin C (MMC), characteristic chromosomal breaks and radial figures are observed at high frequency in lymphocytes or fibroblasts of patients with FA relative to normal controls and patients with other bone marrow failure syndromes and chromosomal fragility diseases [1].
In 1982, the International Fanconi Anemia Registry (IFAR) was established at the Rockefeller University to centralize clinical and genetic characteristics of this rare group of patients, to date enrolling nearly 1300 affected individuals. The IFAR has helped the field better understand the natural history of FA. Cytopenias and progressive BMF occur early, at a median age of 7 years, and in nearly all (90–98%) by age 40 years [5, 6]. Furthermore, hematologic malignancy occurs in 33% at a median age of 40 years [6]. Other registries, including the North American Survey of Fanconi Anemia (NAS) [7], the German Fanconi Anemia Registry (GEFA) [6] and the Italian Fanconi Anemia Registry (RIAF) [9], have also elucidated regional (potentially genetically driven) differences in natural history.
The proposed mechanism of cytopenias and BMF in FA includes intolerance to oxidative stress, hypersensitivity to pro-inflammatory cytokines, and subsequently, apoptotic contraction of the stem/progenitor cell pool [10]. Stress-induced activation of stem/progenitor cells prompts exit from the quiescent G0 cell cycle phase into G1/S/G2/M phases. Increased metabolism producing DNA damaging reactive oxygen species collides with DNA replication, a condition of replicative stress capable of producing DNA double strand breaks, γH2AX phosphorylation, and in the case of FA, increased rates of apoptosis of inefficiently repaired DNA double strand breaks or proposition of stem/progenitor cells containing mutations. The overall effect is attrition of the stem cell pool and/or malignant transformation [11, 12]. In addition to coordinating DNA-damage repair, non-canonical functions of Fanconi proteins (particularly FANCC) include promotion of somatic cell survival by inhibiting apoptotic and activating cell survival pathways [13, 14].
Despite heterogeneity in the genetic etiology of FA, genotype-phenotype associations for BMF and malignancies have been identified. FANCC patients with an intron 4 (IVS4+4A>T) or exon 14 (R548X or L554P) mutation demonstrate a higher incidence and more rapid emergence of BMF compared to other groups (HR 1.5, 95% CI, 1.1–2.0) at a median age of 2.7 and 2.1 years, respectively) [15]. Davies et al. [16] additionally identified a glutathione S-transferase gene polymorphism (GSTM1) in FANCC portending faster progression to BMF (median of 3 years with GSTM1 vs. 7 years with alternative GST polymorphisms). Other FANCC patients, however, such as those with exon 1 322delG or Q13X mutations have hematologic disease at a median of 7 years more typical of FA patients generally [15]. In contrast to other FA groups, patients with mutations in FANCD1/BRCA2 and FANCN/PALB2 rapidly develop leukemia without preceding BMF [17–20]. FANCD1/BRCA2 develop leukemia before the age of 10 years (80%) with most before the age of 6 years and even earlier in those with mutations at IVS7 (< 3 years). In addition, FANCD1/BRCA2 is associated with very high rates of brain tumors and Wilm’s tumor.
To date, the only curative therapy for the hematologic aberrations of FA is allogeneic hematopoietic cell transplantation (alloHCT). However, the intrinsic FA DNA repair defect complicates alloHCT because of the general reliance on alkylating agents and radiation in pre-transplant conditioning, as well as the increased sensitivity to graft-versus-host disease (GVHD) tissue damage. Following decades of research to better understand the pathophysiology of FA and thoughtful modifications to transplant regimens, we can now more safely navigate FA patients through the conditioning and alloHCT. Further, the outcomes achieved with alternative donor (AD) stem cell sources (matched or mismatched non-sibling related donor or unrelated donor bone marrow (BM) or peripheral blood stem cells (PBSC), or umbilical cord blood (UCB)) rival those of historically superior HLA-matched sibling donor (MSD) BM. Here we review the evolution of conditioning regimens, hematopoietic stem cell (HSC) source selection and graft manipulation methods, and promising alternative therapies under development, including gene therapy, epigenetic targeting and efforts to delay or prevent BMF. Finally, we summarize the remaining challenges for FA patients after successful alloHCT, including late effects, such as endocrinopathies, growth failure, and FA-associated epithelioid malignancies.
1.0 Evolution of the Conditioning Regimens
1.1 History
While the first 30 years of advances in alloHCT for FA has been extensively reviewed elsewhere [21, 22], we briefly summarize several salient points for historical context. Gluckman et al. [23] was the first to report on the dismal outcomes for 5 FA patients undergoing HLA matched sibling donor alloHCT (MSD-HCT) with ‘standard dose’ cyclophosphamide (CY, 100–200 mg/kg), a conditioning regimen used successfully in the treatment of patients with acquired severe aplastic anemia. All 5 patients experienced severe grade III–VI acute GVHD, leading to death in 4. Following laboratory studies that demonstrated marked hypersensitivity of FA cells to alkylating agents in vitro [24, 25] and radiation in vivo [26], Gluckman proposed a 10-fold reduction in CY dose combined with a single fraction of 500 cGy thoraco-abdominal irradiation (TAI) for FA patients undergoing HLA-matched sibling donor alloHCT. Notably, survival was much improved (>80%) and graft failure was low (<10%). Thereafter, low dose CY and low dose irradiation became the ‘standard’ conditioning prior to alloHCT for FA from the mid-1980’s onward [27–29]. However, high rates of acute (25–40%) and chronic GVHD (up to 40%) remained problematic. The urgent need for GVHD preventive measures was further amplified by the association of GVHD and the development of solid tumors after transplant [30], prompting the testing of more reliable GVHD prevention methods.
1.2 Role of Fludarabine
In the mid-1990’s, graft failure was the primary limiting factor in those undergoing transplant from an unrelated donor. In 1995, prior to the addition of fludarabine (FLU) to the pre-transplant conditioning, the International Bone Marrow Transplant Registry (IBMTR, predecessor to the Center for Blood and Marrow Transplant Research [CIBMTR]) reported a graft failure rate of 24% and overall survival of 16% at 2 years for FA patients undergoing AD-HCT [27]. Similarly, poor outcomes were reported from European Bone Marrow Transplant (EBMT) registry data in 2000 [31]. Emerging evidence suggested that FLU was a potent immunosuppressive agent that could be safely administered to patients with FA [32]. As a result, Wagner and MacMillan at the University of Minnesota [33] investigated the safety and efficacy of FLU, first in patients with HLA matched sibling and then HLA matched and mismatched unrelated donors for whom the BM was T cell depleted (TCD) prior to infusion. FLU proved to be a powerful alternative to escalated doses of TBI or CY, reducing the deleterious effects of HLA mismatched grafts and recipient T cell mosaicism [34] on risks of graft failure.
In 2007, Wagner et al. [35] updated the CIBMTR experience, reporting on effect of FLU on outcomes in 98 FA patients undergoing AD-HCT. Recipients of FLU-containing conditioning regimens had superior neutrophil (89% vs. 69%, p=0.02) and platelet recovery (74% vs. 23%, p<0.01) as well as 3 year adjusted overall survival (52% vs. 13%, p<0.001). In addition to use of FLU, age ≤10 years of age, negative CMV serostatus, and exposure to <20 blood product transfusions prior to alloHCT were factors associated with better survival. These results were corroborated by Peffault de Latour et al. [36], reporting on 795 FA patients from 1972–2009 who underwent a first transplant for pancytopenia, aplastic anemia, MDS or AML, with either MSD or HLA-matched unrelated donor (MUD) BM or PBSC. Use of FLU was consistently associated with less graft failure and improved overall survival. Age <10 years at time of transplant and absence of MDS and AML were also associated with better outcomes. The impact of FLU in FA patients undergoing alloHCT is summarized in Table 1, highlighting similar outcomes in MSD-HCT and AD-HCT with FLU in the conditioning regimen in Table 1a and quantifying the benefit of FLU on outcomes for large cohorts in Table 1b.
Table 1.
Fludarabine in conditioning improves engraftment
| Table 1a. Fludarabine Containing Regimen Experience: AD-HCT Outcomes Similar to MSD-HCT | ||||||
|---|---|---|---|---|---|---|
| Reference | n= | AlloHCT Details | % Neutrophil Engraftment (Median) | GVHD (Grade III/IV Acute, Chronic) | Overall survival | |
| MSD-HCT | Shimada 2012 [37] Japan 2001–2011 |
2 |
Indication: BMF Conditioning: FLU 150 mg/m2, CY 40 mg/kg Donor graft: MSD unmanipulated BM GVHD prophylaxis: ATG + MTX + CsA |
100% (d+16) | 0%, 0% | 100% (median follow-up 72 months) |
| Benajiba 2015 [38] France 2004–2013 |
20 |
Indication: BMF Conditioning: FLU 90 mg/m2, CY 40 mg/kg Donor graft: MSD unmanipulated (16 BM, 4 UCB) GVHD prophylaxis: CsA + MMF (+ATG in 6) |
100% (d+17) | 15%, 25% | 2 yr OS 95% | |
| AD-HCT | Chaudhury 2008 [39] Memorial-Sloan Kettering 1999–2005 |
18 |
Indication: 8 BMF, 4 MDS, 6 AML Conditioning: FLU 150 mg/m2, CY 40 mg/kg, TBI 450 cGy Donor graft: 8 mMRD, 3 MUD, 7 mMUD GCSF mobilized CD34+ selected (3 BM, 15 PBSC) GVHD prophylaxis: ATG (D-5 to −2) + tacrolimus |
100% (d+10) | 6%, 6% | 5 yr OS 72.2% (5 yr DFS 66.6%) |
| Bonfim 2012 [40] Brazil 2002–2011 |
33 |
Indication: all BMF Conditioning: FLU 125 mg/m2 + CY 60 mg/kg Donor graft: 29 MUD, 4 mMUD unmanipulated BM GVHD prophylaxis: ATG + CsA + MTX |
97% | 39% (Gr II–IV), 42% | 3 yr OS: 79% <10 yrs age: 94% MUD BMT: 86% |
|
| Shimada 2012 [37] Japan 2001–2011 |
6 |
Indiction: 5 BMF, 1 MDS Conditioning: FLU 120–180 mg/m2, CY 40 mg/kg + TBI/TLI 400–450 cGy (URD) OR TBI/TLI 200 cGy (mmRD) Donor graft: 1 MRD (maternal), 1 mMRD, 4 MUD unmanipulated BM GVHD prophylaxis: ATG + MTX + CNI |
100% (d+16) | 0%, 0% | 100% (median follow-up 72 months) | |
| Chao 2015 [41] Germany 2006–2014 |
17 |
Indication: 12 BMF, 5 MDS Conditioning: FLU 180 mg/m2, BU 2 mg/kg, CY 40 mg/kg Donor graft: 1 mMRD PBSC, 8 MUD (7 BM, 1 PBSC), 8 mMUD (5 BM, 3 PBSC), mismatched grafts CD34+ selected with addback of 1 x 106 CD3+/kg GVHD prophylaxis: CAMPATH (15) or ATG (2) + CsA |
100% (d+12) | 0%, 0% | 2 yr OS 88% | |
| Table 1b. Fludarabine vs. non-Fludarabine Containing Regimen Experience | |||||
|---|---|---|---|---|---|
| Reference | n= | AlloHCT Details | Neutrophil Engraftment (FLU vs. non-FLU) | Overall survival (FLU vs. non-FLU) | Other factors influencing OS in multivariate analysis |
| Locatelli 2007 [42] Italian Registry (AIEOP) 1989–2005 |
64 |
Indication: BMF Donor graft: 31 MSD-HCT, 33 AD-HCT Conditioning regimen: FLU (total 120 mg/m2), n=25 Non-FLU, n=39 |
94% vs. 94% Multivariate analysis: No impact of FLU on engraftment (values not reported) |
8 yr OS: 86% vs. 59% (LR p=0.04) Multivariate analysis: FLU associated with decreased mortality (RR 0.16, p=0.05) |
Increased mortality: Donor type URD (RR 7.65, p=0.03) |
| Wagner 2007 [35] CIBMTR 1990–2003 |
98 |
Indication: 75 BMF, 14 MDS, 7 AML, 2 unknown Donor graft: AD-HCT Conditioning regimen: FLU (dose not described), n=46 Non-FLU, n=52 |
89% vs. 69% (LR p=0.02) Multivariate analysis: DEB mosaicism with lower engraftment in non-FLU regimen (OR 0.09, p=0.004) |
3 yr OS: 52% vs.13% (p<0.001) |
Increased mortality: >20 pRBC transfusions prior to HCT (RR 2.49, p=0.004) CMV seropositivity: D+/R+ (RR 4.52, p<0.001) |
| Gluckman 2007 [43] EBMT 1994–2005 |
93 |
Indication: 81 BMF, 8 MDS, 4 Acute leukemia Donor graft: AD-HCT (UCBT) Conditioning regimen: FLU (dose not described), n=57 Non-FLU containing, n=36 |
72% vs. 42% (LR p=0.02) Multivariate analysis: FLU associated with improved engraftment (HR 1.86, p=0.05) |
3 yr OS: 50% vs.25% (LR p=0.01) Multivariate analysis: FLU associated with increased OS (HR 1.79, p=0.04) |
Increased survival: Recipient CMV seronegative (HR 2.82, p<0.001) TNC infused >/= 4.9 x 107/kg (HR 1.75, p=0.05) |
| Stepensky 2011 [44] 3 centers: Israel x2, Russia 1993–2007 |
41 |
Indication: 35 BMF, 3 MDS, 3 AML Donor graft: 26 MSD-HCT, 15 AD-HCT Conditioning regimen: FLU (150–180 mg/m2), n=24 Non-FLU, n=17 |
92% vs.100% (LR p=0.005) | 9 yr OS: 83% vs. 35% (LR p=0.02) | No multivariate analysis completed |
| Peffault de Latour 2013 [36] EBMT 1972–2010 |
795 |
Indication: 737 BMF, 58 MDS/AML Donor graft: 471 MSD, 324 MUD Conditioning regimen: FLU (dose not described), n=233 Non-FLU, n=492 |
Overall 92% Multivariate analysis: FLU associated with decreased graft failure (HR 0.31, p=0.013) |
5 yr OS: Overall 65% Multivariate analysis: FLU associated with decreased mortality (HR 0.40, p<0.001) |
Increased mortality: Age >10 yrs 20–50 yrs (HR 1.92, p=0.003) CMV serology D−/R+ (HR 2.11, p=0.001) D+/R+ (HR 1.68, p=0.16) Time from dx to HCT >12 mon (HR 1.55, p=0.005) Indication: MDS/AML (HR 2.10, p=0.0002) Irradiation exposure >5 yrs post-HCT (HR 5.29, p=0.011) Chronic GVHD (HR 3.10, p<0.001) 2ry malignancy (HR 23, p<0.001) |
| MacMillan 2015 [45] Univ of Minnesota 1995–2012 |
130 |
Indication: 120 BMF/early MDS, 10 late MDS Donor graft: AD-HCT Conditioning regimen: FLU (140 mg/m2), n=107 Non-FLU, n=23 |
96% vs. 60% (LR p<0.01) Multivariate analysis: FLU + TBI associated with improved engraftment (450 cGy: RR 2.6, p<0.01; 300 cGy: RR 2.9, p<0.01) |
5 yr OS: Overall 58% Multivariate analysis: FLU + TBI associated with decreased mortality (450 cGy: RR 0.3, p<0.01; 300 cGy: RR 0.1, p<0.01) |
Increased mortality: Age >10 yrs 10–17 yrs (RR 2.2, p=0.33) ≥18 yrs (RR 2.7, p=0.01) Pre-HCT opportunistic infection (RR 3.5, p<0.01) Recipient CMV seropositivity (RR 2.3, p=0.02) |
AD-HCT, alternative donor hematopoietic cell transplant; MSD, matched sibling donor; alloHCT, allogeneic hematopoietic cell transplant; GVHD, graft-versus-host disease; BMF, bone marrow failure; FLU, fludarabine; CY, cyclophosphamide; BM, bone marrow; ATG, anti-thymocyte globulin; MTX, methotrexate; CsA, cyclosporine A; UCB, umbilical cord blood; MMF, mycophenylate mofetil; OS, overall survival; MDS, myelodysplastic disorder; AML, acute myeloid leukemia; TBI, total body irradiation; mMRD, mismatched related donor; mMUD mismatched unrelated donor; GCSF, granulocyte colony stimulating factor; PBSC, peripheral blood stem cell; DFS, disease free survival; MUD, matched unrelated donor; TLI, total lymphoid irradiation; URD, unrelated donor; CNI, calcineurin inhibitor; BU, busulfan; LR, log rank; RR, relative risk; OR, odds ratio; pRBC, packed red blood cell; HR, hazard ratio; TNC, total nucleated cell; NS, non-significant
1.3 Role of Irradiation
Irradiation is a highly effective means of recipient lymphodepletion historically used to immune suppress the host to prevent graft rejection as well as efficiently eradicate the diseased marrow. In FA, however, use of radiation and other DNA cross-linking agents have been particularly challenging. Because of the known late effects of radiation generally, there has been an effort to reduce or eliminate radiation from the conditioning regimen in patients with FA..
Bonfim et al. [46] were among the first to investigate a chemotherapy-only conditioning regimen in part due to the limited accessibility of radiation therapy in Brazil. Therefore, higher dose CY was investigated. Using CY 60 mg/kg as the conditioning regimen with cyclosporine A and methotrexate immunoprophylaxis (CsA/MTX), engraftment occurred in 37 of 42 (88%) recipients of T-replete MSD BM (n=37) with 1 early and 4 late graft failures. The incidence of acute (grade III–VI) and chronic GVHD was 2% and 29%, respectively. At a median follow-up of 3.7 (range 0.6–7.9) years, survival was 93%.
In order to reduce the risk of CY-related toxicities, Benajiba et al. [38] explored the effectiveness of lower dose CY (40 mg/kg) in combination with FLU 90 mg/m2 (with 6 also receiving ATG) in FA patients with BMF and a MSD. Neutrophil engraftment was achieved in 20 of 20 patients. Using CsA and mycophenolate mofetil (MMF) immunoprophylaxis, the incidence of acute (grade III/IV) and chronic GVHD was 15% and 25%, respectively, with a 2 year overall survival of 95%.
On behalf of the CIBMTR, Pasquini et al. [47] compared outcomes of irradiation containing (n=77, irradiation + CY +/− ATG) to non-irradiation containing (n=71, CY alone, CY + ATG, Busulfan + ATG, or FLU + CY) conditioning regimens prior to MSD-HCT for FA. Notably, there was a similar 5-year overall survival (78% vs. 81%, p=0.61), day 28 neutrophil engraftment (94% vs. 89%, p=0.35), day 100 grade III/IV acute GVHD (6% vs. 10%, p=0.46) and 5 year chronic GVHD (18% vs. 24%, p=0.40). Older recipient age >10 years, pre-alloHCT androgen use, and donor and/or recipient CMV seropositivity, however, were associated with poorer survival.
With increasing evidence associating higher cancer risk in FA patients with a history of GVHD after alloHCT, T cell depletion of the graft was considered. Whether a chemotherapy-only regimen would be sufficient in the context of T cell depletion was unknown. Tan et al. [33] first reported results in FA patients conditioned with FLU 175 mg/m2, cyclophosphamide 20 mg/kg, and ATG prior to the transplantation of T cell depleted sibling donor BM. Engraftment occurred in all patients with no patient having acute or chronic GVHD. Survival was 100% in the first 9 patients. Today, 20 patients with FA have been treated. Engraftment was observed in all 20, acute GVHD occurred in 1, chronic GVHD occurred in none and 2-year survival is 95% (unpublished). These results suggested that in recipients of HLA-matched sibling donor HSCs, addition of FLU to conditioning allowed for consistent engraftment despite reduced CY dosing and infusion of T cell depleted BM to abrogate the risk of acute and chronic GVHD.
The next question was whether TBI could be reduced or eliminated in recipients of T cell depleted AD-HCT or unmanipulated UCB given the increased risk of graft rejection. Mehta et al. [48] evaluated the safety and efficacy of a busulfan (BU) based regimen in place of TBI when used in combination with CY 40 mg/kg, FLU 140 mg/m2 and rabbit ATG 10 mg/kg and in recipients of T cell depleted AD-HCT. BU was initially administered at 0.8–1.0 mg/kg/dose IV every 12 hours x 4 doses (n=25) and subsequently at 0.6–0.8 mg/kg/dose to reduce the regimen related toxicity. Importantly, for the entire cohort, neutrophil engraftment occurred in 96% at a median of 9 days after T cell depleted AD-HCT with a 1-year overall and disease free survival of 79.2% and 76.7%, respectively.
At the University of Minnesota, a TBI dose de-escalation trial was initiated in 1999 in order to determine the minimum dose that would permit consistent engraftment in recipients of T cell depleted BM and unmanipulated UCB [49]. All patients received CY 40 mg/kg, FLU 140 mg/kg and ATG 150 mg/kg with TBI (planned doses 300 cGy to 150 cGy to 0 cGy). Relative to recipients of TBI 450 cGy in whom engraftment was 95% (n=21), engraftment remained unchanged in recipients of TBI 300 cGy (n=17). However, at a TBI dose of 150 cGy, 2 of 2 recipients had secondary graft failure, halting the trial. All subsequent patients received TBI 300 cGy, incorporating thymic shielding in 2006 [50] to limit damage to the thymic epithelium and enhance T cell recovery. Of note, children undergoing AD-HCT after TBI 300 cGy with thymic shielding demonstrate have high survival with 94% alive at 5 years [45]. MacMillan et al. 2015 [45] summarizes the results of these sequential trials.
In the context of T replete grafts, engraftment rates are excellent in the context of FLU regardless of the use of lose dose irradiation or non-radiation based conditioning. Yabe et al. [51] reported 94% sustained engraftment after AD-HCT with 100% alive at 1 year (n=16) after conditioning with FLU (150–180 mg/m2), CY 40 mg/kg, ATG 5–10 mg/kg and low dose TAI or TBI (300 cGy), using MTX + tacrolimus (+MMF in 3 patients) as immunoprophylaxis without in vivo or ex vivo T cell depletion. Importantly, acute (grade III-IV) and chronic GVHD was 6% and 31%, respectively. Motwani et al. [52] used FLU 125–150 mg/m2, CY 20–30 mg/kg, and ATG prior to transplant. While engraftment occurred in the 3 recipients of 6/6 HLA matched unrelated UCB and 2 recipients of T replete 8/8 HLA matched peripheral blood, graft failure occurred in 2 or 2 recipients of T cell depleted haploidentical peripheral blood. Chao et al. [41] reported results in 17 FA with BMF conditioned with BU 4 mg/kg, FLU 180 mg/m2, CY 40 mg/kg and CAMPATH 35 mg/m2. Grafts from an HLA matched unrelated donor (n=8) were unmanipulated and those from an HLA-mismatched related or (n=1) or unrelated donor (n=8) were T cell depleted by CD34+ selection with a fixed add back of 1 x 106 CD3+ cells/kg recipient weight, with CsA immunoprophylaxis. All exhibited neutrophil engraftment with one requiring a stem cell boost. None had grade II–IV acute or chronic GHVD. Two year overall survival was 88%. Together these results suggest that consistent engraftment can be achieved even in the presence of T cell depleted HLA mismatched unrelated donor grafts if low dose TBI or moderately dosed BU is incorporated into the FLU-CY based conditioning. In recipients of HLA matched sibling donor grafts with or without T cell depletion, FLU-CY alone appears to be sufficient.
1.4 Graft T-cell depletion
While rates of acute and chronic GVHD in FA patients are similar to those observed in other young patient populations, the apparent association between GVHD and risk of epithelioid malignancies [30, 36, 53] has led to a greater interest in the application of ex vivo and in vivo T cell depletion. In vivo T cell depletion typically relies on monoclonal antibodies directed at lymphocyte populations (e.g. ATG, CAMPATH). While relatively easy to administer as a mechanism of T cell depletion, these monoclonal antibodies can induce cytokine release from rapid cell death in addition to eliminating the T cells destined to contribute to graft-versus-leukemia, support engraftment or participate in immune recovery. More recently, carefully timed dosing of cyclophosphamide after infusion of donor hematopoietic cells (days +3 and 4) to selectively target rapidly dividing alloreactive T cells have been employed as a method of in vivo T cell depletion, successfully allowing safe use of even haploidentical donor grafts. While this method of in vivo T cell depletion allows for persistence beneficial homeostatically proliferating T cells, adaptation of the post-transplant cyclophosphamide strategy to the FA population remains to be optimized given the risk of alkylator exposure in this population.
Early ex vivo TCD methods included sheep erythrocyte resetting, soybean lectin agglutination, and counterflow centrifugal elutriation [54, 55]. However, more precise CD34+ enrichment techniques developed in recent years allow for highly purified cell population selection and administration [56]. Wagner and MacMillan incorporated a T cell add-back from the CD34-negative fraction fixing the number of T cells to 1 x 105 CD3+ cells/kg recipient weight in an attempt to minimize the risk of graft rejection and relapse in those with hematological malignancy. T cell depletion with newer techniques, such as TCR-αβ depletion may allow for retention of specific T cell subpopulations (e.g. TCR-γδ) with enhanced graft protective and graft-versus-leukemia (GVL) effects [57]. While T cell depletion could have interfered with engraftment, FLU has reduced the risk of this complication. In the setting of HCT with an HLA matched related donor, acute (grade III/IV) and chronic GVHD rates decreased from 30–40% range (25) to <5% [32]. Similar improvements have been observed in recipients of AD-HCT with grade III/IV acute GVHD and chronic GVHD rates at 0 and 9%, respectively, at the University of Minnesota.
2.0 Stem Cell Source
2.1 HLA matched sibling BM / UCB
When available, a HLA matched sibling graft is the hematopoietic stem cell source of choice. However, there have been some settings in which this option may not be appropriate (e.g. health of the donor, unknown FA status, or absence of consent or assent to donate). Historically, parents of children with FA attempted deliberate conception to produce an unaffected HLA matched sibling donor for the child with FA. In a report by Auerbach [58] who tracked the outcomes in 32 pregnancies, 30 children were born with 5 (17%) being HLA matched. Importantly, two fetuses were aborted when found prenatally not to be HLA matched to the living child with FA. Reproductive technology now offers the possibility of pre-implantation genetic diagnosis (PGD) to select an embryo from in vitro fertilization (IVF) that fulfills these criteria. Such “savior siblings” for FA were first reported in 2001 [59, 60]. However, a recent survey of US and Canadian FA support groups members found 63% of families were aware of this IVF/PGD with only 34% being offered this option by their health care provider [61]. However, considering recent improvements in outcomes with AD-HCT and the cost, variable success and ethical concerns of IVF/PGD, use of these reproductive technologies may be less important.
At the University of Minnesota, 20 patients have had a HLA matched sibling donor between June 2001 and May 2015. Nine had an unmanipulated UCB graft while 11 received T cell depleted sibling donor BM. Neutrophil engraftment occurred in 100% with slightly faster engraftment in recipients of BM recipients (10 vs. 14 days for UCB). Rates of grade III–IV acute GVHD by day +100 were 0% and 11% for BM and UCB recipients, respectively (LR 1.222, p=0.27). No patients had chronic GVHD. There was no statistically significant difference in 3 year overall survival, 100% for BM and 89% for UCB recipients (LR 1.22, p=0.27).
In summary, bone marrow from an HLA matched sibling is the graft of choice when it is available. However, in the setting of UCB, there are times the cell dose is <2 x 107 nucleated cells per kilogram recipient weight; in these cases, supplemental BM may be added to the graft.
2.2 Alternative donor stem cell sources
For FA patients without an HLA matched sibling donor, extended family searches have occasionally identified a HLA matched or 1-antigen mismatched unrelated donor within the family. While a reasonable donor choice, the largest experience is with an allele level HLA matched unrelated donor at HLA A, B, C and DRB1. Today, survival is similar to that observed with an HLA matched sibling donor, likely reflecting improved donor selection (e.g. more accurate allele level HLA-typing and considerations of CMV serology, donor age and gender), optimized conditioning regimens and post transplant immune suppressive therapies. Therefore, an HLA matched unrelated donor is the second best option.
For those without an HLA matched unrelated marrow donor, there is no clear next best choice. At the University of Minnesota, an adequately dosed HLA 5–6/6 matched UCB (based on antigen level typing for HLA A and B, and allele level typing for HLA DRB1) is often chosen over an HLA mismatched unrelated marrow donor in part due to its rapid availability but also institutional preference. In the absence of a 5–6/6 matched UCB unit or 7/8 matched marrow donor, a 4/6 matched UCB unit may be considered if there are no other treatment options. As of May 2015, twenty patients with FA were transplanted with unrelated donor UCB (two 4/6 HLA-matched, fifteen 5/6, three 6/6). Engraftment occurred in 84% at a median of 19 days. Grade III–IV acute GHVD and chronic GVHD occurred in 10% and 12%, respectively, with a 3 year overall survival of 71%.
While relatively uncommon, haploidentical transplants have also been used in patients with FA. Graft manipulation to control donor T cell content with additional agents, such as ATG or CAMPATH, have been explored (Table 2). Zecca et al. [65] reported results in 12 FA patients who received CD34+ selected haploidentical PBSCs in FA. While graft failure occurred in 25% and chronic GVHD in 35%, grade III/IV acute GVHD was not observed and overall survival was 83% at 5 years. More recently, Locatelli reported results of haploidentical AD-HCT using TCR αβ depletion to prevent GVHD [66, 70]. In the 4 FA patients, neutrophil engraftment occurred in all 4, none had grade III/IV acute or chronic GVHD, and all 4 were alive at the time of the report with a median survival of 18 months after transplant. Lastly, post-transplant CY has also been explored as a relatively simple alternative to ex vivo T cell depletion. While this alkylator-based approach to haploidentical alloHCT is particularly challenging for FA patients because of the patient’s underlying hypersensitivity to DNA cross-linking agents relative to the insensitive donor alloreactive T cells, there has been some success. In 3 patients, Thacker et al. [67] have used haploidentical donor grafts followed by PTCy at 25 mg/kg on days +3 and +4 (rather than 50 mg/kg x 2 in other patient populations). All 3 patients engrafted with one dying at day +27 of disseminated toxoplasmosis and CMV. Of the 2 surviving patients, one had severe acute GVHD and chronic GVHD. Modifications in this approach are currently being explored.
Table 2.
Haploidentical donor transplant for FA
| Ref | Alloreactive donor T cell Reduction method | n= | Neutrophil Engraftment | Graft Failure | GVHD (Grade III/IV acute, chronic) | Overall survival | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| CD34+ selection | Elhasid 2000 [62] | 1st Haplo: CD34+ selection (5 × 104 CD3+ cells/kg) ATG (d-6/4/2, +2/4/6/8/10/12) |
1 | Day +8 | Yes | None, NA | Alive (+2 yrs) |
| 2nd Haplo: CD34+ selection (3 × 104 CD3+ cells/kg) ATG (d-5 to -1) |
Day +10 | No | None, None | ||||
|
| |||||||
| Rossi 2003 [63] | CD34+ selection (1 × 104 CD3+ cells/kg) ATG (d-6 to -3) |
1 | Day +12 | No | None, None | Alive (+18 mon) | |
|
| |||||||
| Dufort 2012 [64] | CD34+ selection and OKT3 depletion (median 1.15 × 105 CD3+/kg) ATG (d-6 to -3) |
3 | 100% (median d+11) | 0% | 0%, 0% | 100% (+5,6,16 mon) | |
|
| |||||||
| Zecca 2014 [65] | CD34+ selection (median of 1.4 × 104 CD3+/kg) ATG (d -6 to -3) |
12 | 75% (median d+12) | 17% | 0%, 35% | 83% (+5 yr) | |
|
| |||||||
| TCR αβ+ depletion | Bertaina 2014 [66] | TCR αβ+ depletion (median 4 × 104 TCR αβ+ CD3+/kg ATG (d-5 to -3) |
4 | 100% (median d+13) | 0% | 0%, 0% | 100% (+18 mon) |
|
| |||||||
| PTCy | Thakar 2012 [67] | PTCy 25 mg/kg × 2 d | 3 | 100% (median d+15) | 0% | 33%, 33% | 67% (+2, 3 yrs) |
|
| |||||||
| CAMPATH | Rihani 2010 [68] | Alemtuzumab 0.2 mg/kg in donor cell bag (9.4 × 106 CD3+ cells/kg) ATG (d-6 to -2) |
1 | Day +9 | No | None, None | Alive (+15 mon) |
|
| |||||||
| Fetomaternal microchimerism | Yabe 2004 [69] | NIMA 1/10,000 chimeric level identified in 1 pt, not determined in 1 pt ATG x 4 days |
2 | 100% (median d+18) | No | 0%, 100% | Alive (+18, 30 mon) |
GVHD, graft versus host disease; Haplo, haploidentical stem cell transplant; NA, not applicable; ATG, anti-thymocyte globulin; TCR, T cell receptor; PTCy, post-transplant cyclophosphamide
In summary, nearly all patients have a suitable donor today. While an healthy HLA matched sibling donor certainly remains the donor of choice, and an HLA matched unrelated volunteer donor is the next best alternative, use of less well matched donors can still achieve reasonable levels of success in marked contrast to that observed a decade ago. The rationale for donor selection prioritization is summarized in Table 3.
Table 3.
Donor selection considerations and order of preference
| Ideal Donor | 8/8 HLA matched sibling donor | High (best) overall survival No need for TBI Excellent engraftment Minimal rates of GVHD with TCD Related donor less expensive to acquire |
|
|---|---|---|---|
|
| |||
| Alternative Donors | Excellent | 8/8 HLA matched non-genotypic related donor 7/8 HLA matched sibling/related donor 8/8 HLA matched unrelated donor |
Results similar between the 3 sources High overall survival Benefit from low dose TBI Excellent engraftment Low rates of GVHD with TCD Related donor less expensive to acquire |
| Good | 5–6/6 HLA matched unrelated UCB donor | Good overall survival Good engraftment Minimal GVHD |
|
| Less Suitable | 7/8 HLA matched unrelated donor 4/6 HLA matched unrelated UCB donor |
Adequate overall survival Less reliable engraftment Moderate rates of GVHD Equivalent expense to acquire HSCs Selection based on urgency and institutional preference |
|
|
| |||
| Investigational Donor | Haploidentical related donor | Use based on institutional preference Consider alternatives such as androgens to delay transplant |
|
HLA, human leukocyte antigen; TBI, total body irradiation; GVHD, graft-versus-host disease; TCD, T cell depletion; UCB, umbilical cord blood
3.0 AlloHCT and FA associated hematologic malignancies
3.1 Role of FA Group and BM Cytogenetics
The intrinsic DNA repair defect in FA cells contributes to baseline dyserythropoiesis [71], progressive BMF and in a proportion of patients, to MDS and/or acute leukemia. Early onset of MDS and acute leukemia is more frequent in patients with biallelic mutations in FANCD1/BRCA2 and FANCN/PALB2 [17, 19]. In most cases in all FA groups, MDS and acute leukemia are preceded by clonal chromosomal abnormalities with a preponderance of +1q, +3q, −7q, and RUNX1 abnormalities [71–73]. As reviewed recently by Peffault de Latour and Soulier [74], detection of these abnormalities can be challenging. While standard BM karyotyping may reveal +1q and −7p clones, +3q and RUNX1 abnormalities may require further investigation by FISH or next generation sequencing for their identification. Moreover, the presence of somatic mosaicism may lead to clonal hematopoietic populations with clonal fluctuations with gain and loss of abnormalities over time [75]. Alter et al. [75] examined the impact of morphologic MDS, cytogenetic changes, and clonal abnormalities in 41 patients with FA. Five year overall survival was very poor in those with morphologic MDS without a detectable clone (9%) and morphologic MDS with a clonal cytogenetic abnormality (0%). Complex cytogenetic abnormalities and isolated aberrations in chromosomes 3p, 7p, or RUNX1 correlate with clinically poorer outcomes and should prompt alloHCT.
3.2 Role of Pre-Transplant Induction Chemotherapy
The role for induction chemotherapy prior to alloHCT for MDS and AML remains unclear with inconsistent results in case reports or small case series. Generally, the inherent hypersensitivity to genotoxic therapies often leads to significant treatment-associated toxicities and prolonged marrow aplasia at least with standard regimens. Therefore, several groups have investigated the potential role of low to moderate dosed FLU, cytarabine and Neupogen (mini-FLAG) regimen. Mehta et al. [76] evaluated a low dosed FLAG induction regimen prior to alloHCT in 4 FA patients. It was well tolerated with one surviving the subsequent alloHCT. Talbot et al. [77] used a higher moderately dosed FLAG. It was also well tolerated in 6 FA patients with MDS/AML with 4 alive at the time of the report (death due to relapse in one and severe chronic GVHD in the other) [74]. Whether pre-transplant induction therapy offers any benefit over transplant alone in FA patients with MDS/acute leukemia still remains unclear. However, there appears to be one exception. Patients with FANCD1/BRCA2 with AML or ALL appear to tolerate conventional doses of chemotherapy with hematopoietic recovery in some cases. In this group, pre-transplant induction therapy may have a more important role.
3.4 Transplant Outcomes in FA MDS/Acute Leukemia
Not surprisingly, alloHCT in FA patients with MDS or acute leukemia is less satisfactory relative to those with BMF. Conditioning regimens have most often consisted of CY 40 mg/kg, FLU, ATG and either TBI or Busulfan. Use of T cell depleted donor grafts has been variable. In various small series consisting of 6 to 21 patients [78–81], overall survival at 3 to 5 years has ranged from 33 to 80% with deaths most often due to relapse or opportunistic infection. Ayas et al. [81] reviewed outcomes for 113 patients (54 with isolated cytogenetic abnormalities, 45 with MDS, 12 with AML, 2 with ALL) reported to the CIBMTR between 1985 and 2007. Six of 14 patients with leukemia received cytoreductive chemotherapy prior to alloHCT. Sixty percent received radiation (TBI or limited field radiation). Stem cell source was BM (85 patients - 73 related and 12 unrelated), PBSC (13 patients - 9 related and 4 unrelated), and UCB (15 patients - 2 related and 13 unrelated). Overall survival was 64%, 58%, and 55% at 1, 3, and 5 years, respectively. Factors impacting 5 year survival was age at transplant (≤14 years 69% vs. >14 years 39%, p=0.001), or the development of grade II–IV acute GVHD (HR 1.94, p=0.02), and chronic GVHD (HR 2.79, p=0.01). Similarly, Peffault de Latour et al. [36] reported on 795 FA patients with 58 having MDS or AML reported to the EBMT between 1972 and 2010. About half were treated with MSD with the rest having a MUD. Interestingly, graft failure was higher in those with MDS/AML compared to BMF (HR 3.17, p-0.001). One year cumulative incidence of relapse for the 58 MDS/AML patients was 7% and 14% after MSD and MUD alloHCT, respectively. Multivariate analysis demonstrated that patients with MDS/AML had a 2-fold greater risk of death compared to BMF patients (HR 2.10, p=0.0002). Pre-existing clonal evolution was also an independent risk factor for development of secondary malignancy (HR 4.56, p=0.03).
In summary, the best outcomes are realized when FA patients proceed to alloHCT prior to development of clonal abnormalities, MDS, or leukemia transformation, highlighting the importance of close surveillance with regular blood counts and annual bone marrow evaluations including cytogenetic and FISH analyses (with even closer monitoring if clonal cytogenetic changes are demonstrated). For those with advanced MDS or acute leukemia, the utility of induction cytoreductive therapy prior to alloHCT remains unclear except in those with FANCD1/BRCA2 possibly. When used, prolonged cytopenias should be anticipated and a urgent donor search be initiated if transplant is an option. For those eligible for allo-HCT, the optimal conditioning regimen for MSD/leukemia has yet to be identified. While more intensive myeloablative conditioning may reduce the risk of relapse, higher dose therapy often leads to end-organ toxicities and infections. Importantly, MDS and leukemia in a patient with FA does not preclude successful alloHCT with about half alive at 5 years.
4.0 Alternatives to AlloHCT
4.1 Gene Therapy
Researchers in the field of gene therapy hypothesize that autologous infusion of gene corrected hematopoietic stem and progenitor cells (HSPCs) may delay or eliminate the risk of BMF, MDS and acute leukemia in patients with FA. Corrected cells should exhibit a survival and proliferative advantage over uncorrected FA cells which are prone to oxidative stress, pro-inflammatory cytokine sensitivity, and ultimately apoptosis. However, successful application of gene therapy for FA has yet to be effective due to several important obstacles (Table 4).
Table 4.
Gene therapy limitations
| Limitation | Description | Potential solution(s) |
|---|---|---|
|
| ||
| Insertional mutagenesis | Incorporation of viral vector DNA into a host gene driving malignant transformation or cell death | Identification of vectors less prone to high risk sites of DNA insertion (ex. Lentiviral vectors) Use of gene editing methods (ex. Meganucleases, CRISPR-Cas, Transcription activator-like effector-based nucleases (TALENs), Zinc finger nucleases) |
|
| ||
| Genetic heterogeneity | 21 known gene mutations leading to FA | Target highest frequency complementation group, FANCA (accounts for 60% of cases) [6] |
|
| ||
| Non-hematopoietic disease manifestations | Somatic cells continue to express FA mutations and contribute to risk of solid tumor development | Correction of FA HSCs may improve immune surveillance for transformation of somatic cells Avoid exacerbating exposures (chronic GVHD, mutagens, UV radiation) |
|
| ||
| FA HSPCs: | ||
| - Acquired mutations | FA HSPCs accumulate mutations over time resulting at times in MDS or leukemia | Derive healthy HSPCs from somatic induced pluripotent stem (iPS) cells (not yet demonstrated scientifically) [83–86] |
| - Sensitivity to apoptosis | DNA damage prompts apoptosis | |
| - Low HSPC numbers | FA patients with 2-fold reduction in overall cellularity and 6-fold fewer CD34+ HSPCs compared to healthy controls [82] | Utilize ex vivo HSPC expansion strategies such as aryl hydrocarbon receptor antagonist StemRegenin1 [87], Notch ligand [88, 89], nicotinamide analogs [90], copper chelators [91, 92] |
FA, Fanconi anemia; HSCs, hematopoietic stem cells; GVHD, graft-versus-host disease; HSPCs, hematopoietic stem and progenitor cells; MDS, myelodysplastic syndrome
Studies of gene therapy in murine models of FA [93] and with human FA cell lines [94] are ongoing with some promising results. Proceedings of the 1st International Fanconi Anemia Gene Therapy Working Group Meeting from November 2010 outlines expert opinions on optimal strategies for proceeding to clinical trials, including details on vector design, hematopoietic cell preparation, and methods of transduction [95]. European Union researchers have also developed a working group for development of gene therapy trials for FANCA (www.EuroFancolen.eu). To date, three trials of gene therapy for FA have been conducted or are underway in the US (NCT00001399 NHLBI trial using a retroviral vector for correction of FANCC, NCT00272857 Children’s Hospital Boston trial using a retroviral vector for gene correction of FANCA, and NCT01331018 Fred Hutchinson Cancer Research Center trial using a lentiviral vector for correction of FANCA). Thus far, long term persistence of gene corrected hematopoietic cells has yet to be reported.
4.2 Epigenetic targeting
Given the phenotypic heterogeneity of any given gene mutation leading to FA, Belo et al. [96] proposed epigenetic modification as a new therapeutic strategy. Epigenetic modifications such as aberrant DNA methylation and decreased histone acetylation have been previously shown to silence tumor suppressor genes and interrupt DNA stability pathways, implicating involvement of such post-translational modifications in development of MDS, AML and solid tumors. Comparing epigenetic gene expression and DNA methylation patterns of tumor suppressor genes of 12 FA patients to 14 normal controls, Belo found decreased expression in FA of DNA methyltransferase genes (DNMT1, DNM3Tβ) and histone modifying genes (CIITA, PAK1, RNF20, HDACs 2, 8, 9, 10 and 11, and SETD6). In addition there was a global pattern of hypomethylation in FA cells compared to healthy controls. In vitro experiments with the histone deacetylase (HDAC) inhibitor, Vorinostat, induced differential expression of aberrantly expressed genes and reduced DEB sensitivity in 6 FA patient samples.
4.3 Androgens
Androgen therapy to promote hematopoiesis was first evaluated in the 1950s with response rates exceeding 50%. In 70 FA patients treated with androgens between 1976 and 2014, 68% of the 37 patients with sufficient data demonstrated a partial or complete response with 88% of responses in 2 lineages [97]. Until recently, its mechanism of action has been unclear. In a preclinical FA model [98], androgens suppress osteopontin transcription in turn leading to HSC cycling and the production of blood cells. However, prolonged use leads to HSC exhaustion and BMF. Therefore it is not surprising perhaps that the response to androgens has often been temporary with most proceeding to alloHCT. In addition, androgens are associated with hepatic adenoma, virilization and premature fusion of growth plates, limiting its long-term use. Importantly, prior use of androgens is an independent risk factor for poor survival in alloHCT recipients regardless of stem cell source [31, 47, 79].
4.4 Investigational
Efforts to delay or halt FA-associated BMF with anti-oxidants or anti-inflammatory agents are in various stages of development (Table 5).
Table 5.
Experimental therapies to prevent FA-associated BMF
| Reference | Intervention (mechanism) | Study design | Outcome | |
|---|---|---|---|---|
| Pre-clinical studies | Li 2012 [99] | Quercertin (flavonoid anti-oxidant) | Treatment of insulin resistant Fanca or Fancc deficient mice | Insulin sensitivity restored |
| Zhang 2016 [100] | Anti-TGF-β antibody (TGF-β contributes to HSPC growth, differentiation and apoptosis; hyperactive in FA) | Treatment of Fancd2−/− mice as well as a xenograph model of FA (NSG mice transplanted with human UCB CD34+ HSPCs transduced with a shFANCD2 lentivirus) | Improved hematopoiesis in both models | |
| Clinical study | Mehta 2012 [101] | Etanercept (TNF-α inhibition) | Once or twice weekly x 6 months in 6 FA patients with cytopenias | Well tolerated, but no improvement in hematopoiesis |
| Proposed studies | Fanconi Anemia Research Fund | N-acetylcysteine (anti-oxidant) | 2.7 gm/day orally x 6 months | Aim to evaluate impact on hematopoiesis |
| Clinical Trial NCT017220147 | Quercertin (flavonoid anti-oxidant) | Twice weekly x 16 weeks | Aim to evaluate impact on hematopoiesis, safety, pharmacokinetics, changes in insulin sensitivity |
HSPC, hematopoietic stem and progenitor cell; FA, Fanconi anemia; NSG, NOD/SCID gamma; UCB, umbilical cord blood
5.0 Impact of AlloHCT on FA-Associated Late Effects
FA patients are predisposed to a number of late effects, resulting from surgical complications following repair of severe cardiovascular and/or digestive tract malformations, the underlying DNA repair defect contributing to cancer predisposition, and various endocrinopathies.
Compared to the general population, FA patients have a markedly higher risk of malignancy, particularly squamous cell carcinomas of the aeroesophageal and/or anogenital tracts, in addition to MDS and AML [7, 8, 102–106]. In contrast to the general population, the peak hazard for solid tumors increases in a linear manner after the age of 10 years, increasing by >10%/year after the age of 40. However, the specific impact of alloHCT on the underlying risk of malignancy remains to be determined. In the large European cohort of FA patients undergoing alloHCT (n=795) from 1972–2009, Peffault de Latour et al. [36] demonstrated cancers had a major effect on long-term survival after alloHCT, with a history of chronic GVHD being a leading risk factor for malignancy. These data, as well as outcomes of 3 additional FA cohorts [30, 53, 107], suggest that both acute and chronic GVHD may contribute to development of epithelioid malignancies. Conversely, Risitano et al. [9], reporting on 180 FA patients, found no difference in solid tumor risk conferred by alloHCT compared to an untransplanted cohort. At this time, cancer risk is high in FA patients aged 30 years and older. While it is possible that alloHCT may increase the risk of cancer, only the occurrence of GVHD has been associated with risk of cancer after transplant. Donor source and conditioning regimen have not been found to be risk factors thus far.
Whether there are strategies to reduce the risk generally is of interest to the FA community. Much work has focused on understanding the role of HPV viral infection in head and neck squamous cell carcinoma (HNSCC) in FA. Katzenellenbogen et al. [107] reported on seroprevalence of skin and mucosal HPV types in 62 individuals with FA, with increases associated with age and HPV vaccination. Sauter et al. [108] found higher HPV seroprevalence among FA patients (n=126, 11.1% HPV positive) compared to their first-degree relatives (n=162, 2.5% HPV positive). Several groups have shown mechanisms by which HPV oncogenes E6 and E7 contribute to increased risk of mucosal carcinogenesis in FA (promoting genomic instability and DNA damage, p53 repression, as well as further impairment of the FA DNA repair signaling pathway) [109–113]. As FA patients respond appropriately to the HPV vaccine [114], vaccinations are recommended just as in the general population. Liberal use of sunscreens, avoidance of frequent dental radiographic evaluations, close surveillance of the oral cavity by the dentist and upper aeroesophageal tract by an ENT specialist are general recommendations for all FA patients regardless of alloHCT.
Similarly, endocrinopathies are common in FA patients with 80% demonstrating short stature, glucose intolerance, dyslipidemia, hypothyroidism, hypogonadism, pubertal delay and impaired fertility [115, 116]. With improved long-term survival after alloHCT, there are greater efforts to quantify and ameliorate the late effects of these endocrinopathies [117]. At the University of Minnesota [118], 44 patients with FA were followed after alloHCT with all receiving TBI 300 cGy. At least one endocrinopathy was reported in 86%, with 11% with ≥3 endocrine deficiencies. Specifically, Vitamin D deficiency was noted in 71%, hypothyroidism in 57%, hypogonadism in 27%, short stature in 50%, reduced total body and lumbar spine bone mineral density (BMD) in 57% and 21%, respectively. Compared to non-FA patients undergoing alloHCT for hematologic malignancies, patients with FA demonstrated greater rates of hypothyroidism, short stature, and reduced total body BMD. Similar post-alloHCT decreases in BMD were documented previously by this group [119].
In contrast to risk of malignancy, type of conditioning does play a role in potentially exacerbating some of the baseline endocrine risks in FA patients. While gonadal dysfunction is near universal in males with FA irrespective of transplant, use of TBI or busulfan leads to high risks of gonadal dysfunction and infertility. Particularly in patients with BMF where conditioning is not focused on eradicating malignant hematopoiesis, efforts are being made to preserve gonadal function by the reducing or eliminating TBI or Busulfan, or use of ovarian shielding in recipients of TBI. With regards to interventions, hormone replacement is the norm. Of note, growth hormone replacement is safe and often successful for treatment of short stature even after transplant [120].
6.0 Summary Recommendations
Close monitoring of complete blood counts with white blood cell differential every 3 months and annual bone marrow evaluations to assess the development and progression of clonal cytogenetic anomalies are indicated. Particularly in teenagers and older patients, increasing epithelioid tumor risk requires frequent evaluations of the aeroesophageal (biannual dental evaluations and annual direct endoscopic laryngoscopy) and anogenital tracts (annual PAP smears and evaluations). Patients should additionally be encouraged to reduce risk by avoiding environmental exposures such as alcohol, tobacco products, and ultraviolet radiation (particularly conscience of sunscreen and burn guard use). Patients and families affected by FA are encouraged to seek longitudinal comprehensive follow-up care at an established FA center, allowing for expert evidence-based multi-disciplinary care and for contribution to databases on FA natural history and responses to treatments/interventions. For example, such centers would include hematologists, oncologists, ENT, endocrinologists, dermatologists, gastroenterologists, nephrologists and orthopedic surgeons expert in FA and its varied manifestations (e.g. growth defects, hypothyroidism, insulin resistance; infertility; reconstructive surgeries of skeletal anomalies, cancer) as well as genetic counselors.
Any patient with childhood onset BMF should be screened for FA by chromosomal breakage analysis with subsequent fibroblast testing if signs/symptoms suggest somatic mosaicism may be responsible for false negative blood testing. Recent recommendations from Peffault de Latour prompt physicians to consider expanding chromosomal analysis of annual bone marrow evaluations to include FISH or next generation sequencing for identification of RUNX1 or +3q abnormalities to ensure malignant transformation is not overlooked [74]. Table 6 summarizes these recommendations including medical monitoring and risk reduction behaviors.
Table 6.
Recommendations for FA management
| Congenital Anomalies: Baseline Assessments | ||
|---|---|---|
|
| ||
| System and examples | Subspecialty | Evaluation/Intervention |
|
Intracranial manifestations: Moya-moya, pituitary adenomas, medulloblastoma, hydrocephalus |
Neuroradiology | MRI brain (screening ± follow-up) |
| Microphthalmia, strabismus | Ophthalmology | Ophthalmology clinical exam |
| Sensorineural hearing loss | Audiology | Auditory brainstem response, audiogram, hearing aids |
| Congenital heart disease | Cardiology | Echocardiogram (screening ± follow-up) |
|
Gastrointestinal malformations: Tracheo-esophageal fistula, foregut atresias, malrotation |
Gastroenterology | Radiologic imaging, endoscopy as indicated |
|
Renal structural abnormalities: Hypoplastic, ectopic, horseshoe, or absent kidney |
Nephrology | Renal ultrasound (screening ± follow-up) |
|
Genitourinary malformations: Cryptorchidism, hypospadias, double ureter, uterine anomalies |
Urology and/or Gynecology | Radiologic imaging as indicated Consider surgical correction of malformations |
|
Musculoskeletal malformations: Radial ray defects, congenital hip dysplasia, vertebral anomalies |
Orthopedic surgery | Radiologic imaging as indicated Consider thumb pollicization |
|
| ||
| Fanconi Anemia Associated Disease Risks: Routine Assessments | ||
|
| ||
| Risk | Recommendation | Description |
| Bone marrow failure MDS | Hematology evaluation | Complete blood count with white blood cell differential every 3 months: Monitor for cytopenias, macrocytosis (increased erythrocyte mean corpuscular volume) |
| Acute leukemia | Bone marrow evaluation at least annually (more frequently if evidence of MDS): Aspirate for chromosomal analysis, FISH or NGS for cryptic anomalies, hematopathology for myelodysplastic changes, biopsy for cellularity |
|
| Epithelioid malignancies | Aeroesophageal evaluation | Biannual dental evaluations |
| Annual direct endoscopy laryngoscopy for HNSCC starting at 10 years of age | ||
| Anogenital tract evaluation | Annual anogenital exam and PAP smears for females starting at 15 years of age | |
| HPV vaccination | Begin series at age 9 years (both males and females) | |
| Environmental exposures | Avoid alcohol, tobacco products, UV radiation, liberal use of sunscreen/burn guards | |
| Osteopenia | Endocrinology evaluation | DEXA scan, Vitamin D, Calcium levels |
| Growth deficiency | Growth charting and growth hormone levels, consider growth hormone replacement | |
| Hypothyroidism | Thyroid function tests | |
| Impaired glucose metabolism | Oral glucose tolerance test | |
| Infertility | Assess HPG-axis, consider testosterone for males during puberty | |
MRI, magnetic resonance imaging; MDS, myelodysplastic syndrome; FISH, fluorescence in situ hybridization; NGS, next generation sequencing; HNSCC, head and neck squamous cell carcinoma; HPV, human papilloma virus; UV, ultraviolet; DEXA, dual-energy x-ray absorptiometry; HPG-axis, hypothalamic-pituitary-gonadal axis
The goal of alloHCT in FA is to eliminate cytopenias, MDS, and/or leukemia with consistent engraftment, no GVHD and minimal late effects. At this time, alloHCT indications include (1) severe BMF with risk of infection given low absolute neutrophil count (ANC <500 x 106/L), or anemia (hgb <8 g/dL) or thrombocytopenia (platelet count <20 x 106/L) approaching transfusion need (ideally blood product transfusions should be avoided prior to alloHCT to minimize risk of alloimmunization; however, when indicated, products should be leukoreduced and irradiated); (2) morphological MDS, or (3) leukemia. Generally, pre-emptive alloHCT should not be considered. However, in rare cases, patients with advancing (e.g increasing proportions over time) high-risk clonal cytogenetic abnormalities in the absence of morphological evidence of BMF, MDS, or acute leukemia should be considered for pre-emptive alloHCT due to high risk of disease transformation and subsequent poorer alloHCT outcomes. In isolated cases, patients with FANCD1/BRCA2 have also been considered for prophylactic alloHCT although this is controversial.
CONCLUSION
Sequential modifications to alloHCT regimens, specifically dose reductions of alkylating agents and irradiation, addition of FLU to conditioning, and T cell depletion of the donor graft, have dramatically improved outcomes for patients transplanted for FA associated BMF. Current rates of graft failure and acute GVHD are less than 10% with 5 year overall survival >90% for patients under the age of 10 years. Today, the expectation is that nearly every patient will have a suitable donor and that the outcome of transplant will likely be survival. Therefore, the goal now is to minimize the impact of alloHCT on the early and long-term risks of FA itself. Perhaps one day we can replace alloHCT altogether with FA targeted gene therapy or develop pharmacotherapeutic strategies to reduce hematopoietic stress and delay the risk of clonal disease that leads to BMF or malignant transformation.
EXPERT COMMENTARY
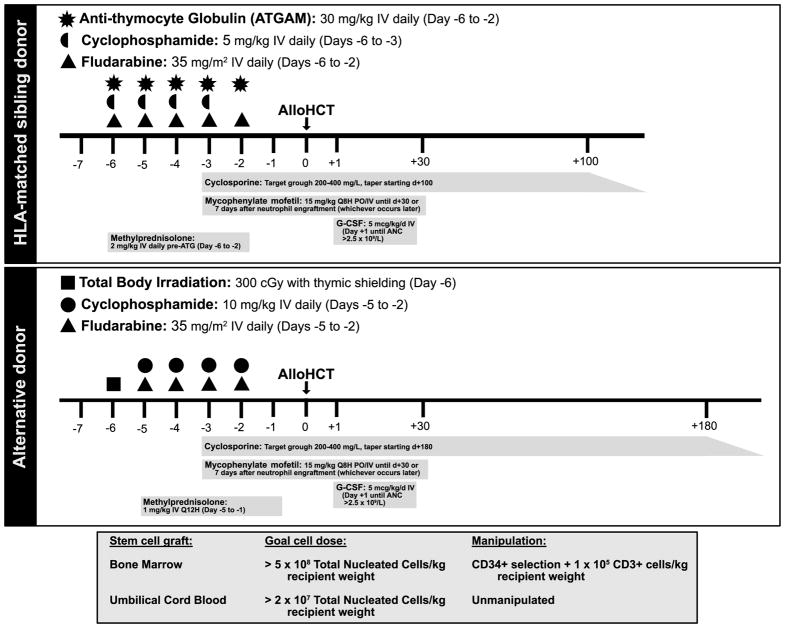
Improved understanding of the pathophysiology of FA has contributed to disease-specific modifications to alloHCT in this disease, making cure of FA-associated BMF, MDS, and leukemias more readily achievable over time. For BMF specifically, overall survival exceeds 90% with rates of graft rejection and GVHD <10% (using regimens, such as those outlined in Figure 1). Outcomes of alloHCT for MDS/leukemia lag behind, with 5 year overall survival for the latter merely 50–60%. Late effects and the interface between alloHCT complications such as GVHD, endocrinopathies, and subsequent epithelioid malignancies in FA require more evaluation to inform interventions. Development of effective, safe gene therapy or editing strategies to correct the specific mutations leading to the hematologic manifestations of FA provide hope for preemptive therapy obviating the need for alloHCT in the future.
Figure 1.
FIVE-YEAR VIEW
While subtle improvements in alloHCT outcomes for FA-associated BMF will likely be achieved with continued evolution of conditioning regimens and manipulation of donor grafts, identification of higher risk mutations for malignant transformation may prompt preemptive alloHCT for subpopulations of FA patients. Further, mechanisms underlying such transformation may inform less genotoxic, targeted therapies to replace or be used in combination with alloHCT to prevent or treat malignancies. With improved gene therapy and gene modification technology, we anticipate clinical trials will expand for correction of the hematologic manifestations of FA with gene-modified HSCs, greatly reducing the need for alloHCT in the future, and hopefully reducing late effects from chemotherapy, radiation and GVHD.
KEY ISSUES
-
Sequential improvements in conditioning regimens and donor cell manipulation for alloHCT in FA have resulted in better overall survival (5 yr OS: >90% in younger patients with BMF, 50–60% for those with hematologic malignancy)
Reduced transplant related mortality: Decreased doses of cyclophosphamide and irradiation
Reduced graft rejection (<10%): Administration of specified donor T cell dose; recipient lymphodepletion with fludarabine, serotherapy, and/or low dose irradiation
Reduced GVHD (<10%): TCD of donor graft, use of serotherapy for in vivo donor T cell depletion
Improved immune reconstitution: Administration of specific donor T cell dose
Gene therapy efforts, with less toxic targeted correction of FA mutations in hematopoietic cells, are well underway
Increasing knowledge of pathways downstream of FA gene mutations leading to BMF contribute to potential new therapeutic interventions to delay need for alloHCT
Identification of high risk FA mutations, or combinations of acquired mutations leading to leukemic transformation, may inform use of preemptive alloHCT
Further characterization of FA-associated late effects, including those exacerbated by alloHCT, such as epithelioid malignancies and endocrinopathies will contribute to future interventions or prevention strategies to limit such complications
Acknowledgments
Work was partially supported by the Fanconi Anemia Research Fund, Children’s Cancer Research Fund and Kidz1stFund.
Footnotes
Financial disclosure / Acknowledgements: The authors of this manuscript have no conflicts of interest or financial disclosures.
References
* Of interest
- 1.Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668(1–2):4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Dong H, Nebert DW, Bruford EA, et al. Update of the human and mouse Fanconi anemia genes. Hum Genomics. 2015;9(1):32. doi: 10.1186/s40246-015-0054-y. Summary of the first 19 human gene mutations associated with FA, their murine orthologs, and an evolutionary perspective on the role of these gene mutations. FA associated gene mutations continue to be discovered as demonstrated in references [3] and [4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JY, Virts EL, Jankowska A, et al. Complementation of hypersensitivity to DNA interstrand crosslinking agents demonstrates that XRCC2 is a Fanconi anaemia gene. J Med Genet. 2016;53(10):672–80. doi: 10.1136/jmedgenet-2016-103847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluteau D, Masliah-Planchon J, Clairmont C, et al. Biallelic inactivation of REV7 is associated with Fanconi anemia. J Clin Invest. 2016;126(9):3580–4. doi: 10.1172/JCI88010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butturini A, Gale RP, Verlander PC, et al. Hematologic abnormalities in Fanconi anemia: an International Fanconi Anemia Registry study. Blood. 1994;84(5):1650–5. [PubMed] [Google Scholar]
- 6.Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101(4):1249–56. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101(3):822–6. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93(4):511–7. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- 9.Risitano AM, Marotta S, Calzone R, et al. Twenty years of the Italian Fanconi Anemia Registry: where we stand and what remains to be learned. Haematologica. 2016;101(3):319–27. doi: 10.3324/haematol.2015.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagby GC, Alter BP. Fanconi anemia. Seminars in hematology. 2006;43(3):147–56. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11*.Walter D, Lier A, Geiselhart A, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520(7548):549–52. doi: 10.1038/nature14131. Describes the pathophysiology of the hematologic manifestations in FA, linking physiologic stress to DNA damage and ultimately bone marrow failure. [DOI] [PubMed] [Google Scholar]
- 12.Garaycoechea JI, Patel KJ. Why does the bone marrow fail in Fanconi anemia? Blood. 2014;123(1):26–34. doi: 10.1182/blood-2013-09-427740. [DOI] [PubMed] [Google Scholar]
- 13.Bagby GC., Jr Genetic basis of Fanconi anemia. Curr Opin Hematol. 2003;10(1):68–76. doi: 10.1097/00062752-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Garbati MR, Hays LE, Rathbun RK, et al. Cytokine overproduction and crosslinker hypersensitivity are unlinked in Fanconi anemia macrophages. J Leukoc Biol. 2016;99(3):455–65. doi: 10.1189/jlb.3A0515-201R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillio AP, Verlander PC, Batish SD, et al. Phenotypic consequences of mutations in the Fanconi anemia FAC gene: an International Fanconi Anemia Registry study. Blood. 1997;90(1):105–10. [PubMed] [Google Scholar]
- 16.Davies SM, Radloff GA, DeFor TE, et al. GST genotype may modify clinical phenotype in patients with Fanconi anaemia. British journal of haematology. 2005;131(1):118–22. doi: 10.1111/j.1365-2141.2005.05721.x. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JE, Tolar J, Levran O, et al. Germline mutations in BRCA2: shared genetic susceptibility to breast cancer, early onset leukemia, and Fanconi anemia. Blood. 2004;103(8):3226–9. doi: 10.1182/blood-2003-09-3138. [DOI] [PubMed] [Google Scholar]
- 18.Alter BP. The association between FANCD1/BRCA2 mutations and leukaemia. British journal of haematology. 2006;133(4):446–8. doi: 10.1111/j.1365-2141.2006.06049.x. author reply 8. [DOI] [PubMed] [Google Scholar]
- 19.Alter BP, Rosenberg PS, Brody LC. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. Journal of medical genetics. 2007;44(1):1–9. doi: 10.1136/jmg.2006.043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid S, Schindler D, Hanenberg H, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nature genetics. 2007;39(2):162–4. doi: 10.1038/ng1947. [DOI] [PubMed] [Google Scholar]
- 21.MacMillan ML, Wagner JE. Haematopoeitic cell transplantation for Fanconi anaemia - when and how? British journal of haematology. 2010;149(1):14–21. doi: 10.1111/j.1365-2141.2010.08078.x. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JE, Tolar J, Auerbach AD, et al. Thomas’ hematopoietic cell transplantation : stem cell transplantation. 5. Chichester, West Sussex, United Kingdom; Hoboken, NJ: John Wiley & Sons Inc; 2015. Hematopoietic cell transplantation for Fanconi anemia; pp. 884–907. [Google Scholar]
- 23.Gluckman E, Devergie A, Schaison G, et al. Bone marrow transplantation in Fanconi anaemia. British journal of haematology. 1980;45(4):557–64. doi: 10.1111/j.1365-2141.1980.tb07178.x. [DOI] [PubMed] [Google Scholar]
- 24.Berger R, Bernheim A, Gluckman E, et al. In vitro effect of cyclophosphamide metabolites on chromosomes of Fanconi anaemia patients. British journal of haematology. 1980;45(4):565–8. doi: 10.1111/j.1365-2141.1980.tb07179.x. [DOI] [PubMed] [Google Scholar]
- 25.Auerbach AD, Adler B, O’Reilly RJ, et al. Effect of procarbazine and cyclophosphamide on chromosome breakage in Fanconi anemia cells: relevance to bone marrow transplantation. Cancer Genet Cytogenet. 1983;9(1):25–36. doi: 10.1016/0165-4608(83)90021-3. [DOI] [PubMed] [Google Scholar]
- 26.Gluckman E, Devergie A, Dutreix J. Radiosensitivity in Fanconi anaemia: application to the conditioning regimen for bone marrow transplantation. British journal of haematology. 1983;54(3):431–40. doi: 10.1111/j.1365-2141.1983.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 27.Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86(7):2856–62. [PubMed] [Google Scholar]
- 28.Dufour C, Rondelli R, Locatelli F, et al. Stem cell transplantation from HLA-matched related donor for Fanconi’s anaemia: a retrospective review of the multicentric Italian experience on behalf of AIEOP-GITMO. British journal of haematology. 2001;112(3):796–805. doi: 10.1046/j.1365-2141.2001.02572.x. [DOI] [PubMed] [Google Scholar]
- 29.Farzin A, Davies SM, Smith FO, et al. Matched sibling donor haematopoietic stem cell transplantation in Fanconi anaemia: an update of the Cincinnati Children’s experience. British journal of haematology. 2007;136(4):633–40. doi: 10.1111/j.1365-2141.2006.06460.x. [DOI] [PubMed] [Google Scholar]
- 30.Deeg HJ, Socie G, Schoch G, et al. Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996;87(1):386–92. [PubMed] [Google Scholar]
- 31.Guardiola P, Pasquini R, Dokal I, et al. Outcome of 69 allogeneic stem cell transplantations for Fanconi anemia using HLA-matched unrelated donors: a study on behalf of the European Group for Blood and Marrow Transplantation. Blood. 2000;95(2):422–9. [PubMed] [Google Scholar]
- 32.Aker M, Varadi G, Slavin S, et al. Fludarabine-based protocol for human umbilical cord blood transplantation in children with Fanconi anemia. Journal of pediatric hematology/oncology. 1999;21(3):237–9. doi: 10.1097/00043426-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Tan PL, Wagner JE, Auerbach AD, et al. Successful engraftment without radiation after fludarabine-based regimen in Fanconi anemia patients undergoing genotypically identical donor hematopoietic cell transplantation. Pediatr Blood Cancer. 2006;46(5):630–6. doi: 10.1002/pbc.20538. [DOI] [PubMed] [Google Scholar]
- 34.MacMillan ML, Auerbach AD, Davies SM, et al. Haematopoietic cell transplantation in patients with Fanconi anaemia using alternate donors: results of a total body irradiation dose escalation trial. British journal of haematology. 2000;109(1):121–9. doi: 10.1046/j.1365-2141.2000.01955.x. [DOI] [PubMed] [Google Scholar]
- 35.Wagner JE, Eapen M, MacMillan ML, et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109(5):2256–62. doi: 10.1182/blood-2006-07-036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peffault de Latour R, Porcher R, Dalle JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122(26):4279–86. doi: 10.1182/blood-2013-01-479733. [DOI] [PubMed] [Google Scholar]
- 37.Shimada A, Takahashi Y, Muramatsu H, et al. Excellent outcome of allogeneic bone marrow transplantation for Fanconi anemia using fludarabine-based reduced-intensity conditioning regimen. International journal of hematology. 2012;95(6):675–9. doi: 10.1007/s12185-012-1079-9. [DOI] [PubMed] [Google Scholar]
- 38.Benajiba L, Salvado C, Dalle JH, et al. HLA-matched related-donor HSCT in Fanconi anemia patients conditioned with cyclophosphamide and fludarabine. Blood. 2015;125(2):417–8. doi: 10.1182/blood-2014-10-605113. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhury S, Auerbach AD, Kernan NA, et al. Fludarabine-based cytoreductive regimen and T-cell-depleted grafts from alternative donors for the treatment of high-risk patients with Fanconi anaemia. British journal of haematology. 2008;140(6):644–55. doi: 10.1111/j.1365-2141.2007.06975.x. [DOI] [PubMed] [Google Scholar]
- 40.Bonfim C, Ribeiro GL, Bitencourt M, et al. Unrelated bone marrow transplantation (UBMT) for children and adolescents with Fanconi Anemia (FA) using cyclophosphamide, fludarabine and rabbit ATG: Analysis of 33 patients transplanted at a single institution. Biol Blood Marrow Tr. 2012;18(S2):S229–S. [Google Scholar]
- 41.Chao MM, Kuehl JS, Strauss G, et al. Outcomes of mismatched and unrelated donor hematopoietic stem cell transplantation in Fanconi anemia conditioned with chemotherapy only. Annals of hematology. 2015;94(8):1311–8. doi: 10.1007/s00277-015-2370-7. [DOI] [PubMed] [Google Scholar]
- 42.Locatelli F, Zecca M, Pession A, et al. The outcome of children with Fanconi anemia given hematopoietic stem cell transplantation and the influence of fludarabine in the conditioning regimen: a report from the Italian pediatric group. Haematologica. 2007;92(10):1381–8. doi: 10.3324/haematol.11436. [DOI] [PubMed] [Google Scholar]
- 43.Gluckman E, Rocha V, Ionescu I, et al. Results of unrelated cord blood transplant in fanconi anemia patients: risk factor analysis for engraftment and survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(9):1073–82. doi: 10.1016/j.bbmt.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Stepensky P, Shapira MY, Balashov D, et al. Bone marrow transplantation for Fanconi anemia using fludarabine-based conditioning. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(9):1282–8. doi: 10.1016/j.bbmt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 45*.MacMillan ML, DeFor TE, Young JA, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015;125(24):3798–804. doi: 10.1182/blood-2015-02-626002. Review of a single centers sequential advances in AD-HCT for the hematologic manifestations of FA, including addition of fludarabine to the conditioning regimen, optimization of TBI dosing, and addition of thymic shielding during radiation. Ultimately demonstrating achievement of outcomes rivaling that of MSD-HCT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonfim CM, de Medeiros CR, Bitencourt MA, et al. HLA-matched related donor hematopoietic cell transplantation in 43 patients with Fanconi anemia conditioned with 60 mg/kg of cyclophosphamide. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(12):1455–60. doi: 10.1016/j.bbmt.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasquini R, Carreras J, Pasquini MC, et al. HLA-matched sibling hematopoietic stem cell transplantation for fanconi anemia: comparison of irradiation and nonirradiation containing conditioning regimens. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(10):1141–7. doi: 10.1016/j.bbmt.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta PA, Davies SM, Myers KC, et al. Chemotherapy-only preprarative regimen for alternative donor hematopoietic cell transplantation for patients with Fanconi anmie (FA): Results of a multi-institutional study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(2 Supplement):S104–5. [Google Scholar]
- 49.MacMillan ML, Blazar BR, DeFor T, et al. Alternate Donor HCT for Fanconi Anemia (FA): Results of a Total Body Irradiation (TBI) Dose De-Escalation Study. Blood. 2008;112(11):2998. [Google Scholar]
- 50.MacMillan ML, Blazar BR, DeFor TE, et al. Thymic Shielding (TS) in Recipients of Total Body Irradiation (TBI) and Alternative Donor Hematopoietic Stem Cell Transplant (AD-HSCT): Reduced Risk of Opportunistic Infection in Patients with Fanconi Anemia (FA) Blood. 2006;108(11):3134. [Google Scholar]
- 51.Yabe H, Inoue H, Matsumoto M, et al. Allogeneic haematopoietic cell transplantation from alternative donors with a conditioning regimen of low-dose irradiation, fludarabine and cyclophosphamide in Fanconi anaemia. British journal of haematology. 2006;134(2):208–12. doi: 10.1111/j.1365-2141.2006.06128.x. [DOI] [PubMed] [Google Scholar]
- 52.Motwani J, Lawson SE, Darbyshire PJ. Successful HSCT using nonradiotherapy-based conditioning regimens and alternative donors in patients with Fanconi anaemia--experience in a single UK centre. Bone Marrow Transplant. 2005;36(5):405–10. doi: 10.1038/sj.bmt.1705071. [DOI] [PubMed] [Google Scholar]
- 53.Guardiola P, Socie G, Li X, et al. Acute graft-versus-host disease in patients with Fanconi anemia or acquired aplastic anemia undergoing bone marrow transplantation from HLA-identical sibling donors: risk factors and influence on outcome. Blood. 2004;103(1):73–7. doi: 10.1182/blood-2003-06-2146. [DOI] [PubMed] [Google Scholar]
- 54.Reisner Y, Kapoor N, Kirkpatrick D, et al. Transplantation for acute leukaemia with HLA-A and B nonidentical parental marrow cells fractionated with soybean agglutinin and sheep red blood cells. Lancet. 1981;2(8242):327–31. doi: 10.1016/s0140-6736(81)90647-4. [DOI] [PubMed] [Google Scholar]
- 55.Contreras TJ, Jemionek JF, Stevenson HC, et al. An improved technique for the negative selection of large numbers of human lymphocytes and monocytes by counterflow centrifugation--elutriation. Cell Immunol. 1980;54(1):215–29. doi: 10.1016/0008-8749(80)90203-8. [DOI] [PubMed] [Google Scholar]
- 56.Hertenstein B, Arseniev L, Novotny J, et al. A comparative review of methods for T cell depletion in the prophylaxis of graft-versus-host disease. BioDrugs. 1998;9(2):105–23. doi: 10.2165/00063030-199809020-00003. [DOI] [PubMed] [Google Scholar]
- 57.Chaleff S, Otto M, Barfield RC, et al. A large-scale method for the selective depletion of alphabeta T lymphocytes from PBSC for allogeneic transplantation. Cytotherapy. 2007;9(8):746–54. doi: 10.1080/14653240701644000. [DOI] [PubMed] [Google Scholar]
- 58.Auerbach AD. Umbilical cord blood transplants for genetic disease: Diagnostic and ethical issues in fetal studies. Blood Cells. 1994;20(2–3):303–9. [PubMed] [Google Scholar]
- 59.Verlinsky Y, Rechitsky S, Schoolcraft W, et al. Preimplantation diagnosis for Fanconi anemia combined with HLA matching. JAMA. 2001;285(24):3130–3. doi: 10.1001/jama.285.24.3130. [DOI] [PubMed] [Google Scholar]
- 60.Grewal SS, Kahn JP, MacMillan ML, et al. Successful hematopoietic stem cell transplantation for Fanconi anemia from an unaffected HLA-genotype-identical sibling selected using preimplantation genetic diagnosis. Blood. 2004;103(3):1147–51. doi: 10.1182/blood-2003-02-0587. [DOI] [PubMed] [Google Scholar]
- 61.Zierhut H, MacMillan ML, Wagner JE, et al. More than 10 years after the first ‘savior siblings’: parental experiences surrounding preimplantation genetic diagnosis. J Genet Couns. 2013;22(5):594–602. doi: 10.1007/s10897-013-9591-5. [DOI] [PubMed] [Google Scholar]
- 62.Elhasid R, Ben Arush MW, Katz T, et al. Successful haploidentical bone marrow transplantation in Fanconi anemia. Bone Marrow Transplant. 2000;26(11):1221–3. doi: 10.1038/sj.bmt.1702701. [DOI] [PubMed] [Google Scholar]
- 63.Rossi G, Giorgiani G, Comoli P, et al. Successful T-cell-depleted, related haploidentical peripheral blood stem cell transplantation in a patient with Fanconi anaemia using a fludarabine-based preparative regimen without radiation. Bone Marrow Transplant. 2003;31(6):437–40. doi: 10.1038/sj.bmt.1703903. [DOI] [PubMed] [Google Scholar]
- 64.Dufort G, Pisano S, Incoronato A, et al. Feasibility and outcome of haploidentical SCT in pediatric high-risk hematologic malignancies and Fanconi anemia in Uruguay. Bone Marrow Transplant. 2012;47(5):663–8. doi: 10.1038/bmt.2011.148. [DOI] [PubMed] [Google Scholar]
- 65.Zecca M, Strocchio L, Pagliara D, et al. HLA-haploidentical T cell-depleted allogeneic hematopoietic stem cell transplantation in children with Fanconi anemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(4):571–6. doi: 10.1016/j.bbmt.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 66.Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822–6. doi: 10.1182/blood-2014-03-563817. [DOI] [PubMed] [Google Scholar]
- 67.Thakar MS, Bonfim C, Sandmaier BM, et al. Cyclophosphamide-based in vivo T-cell depletion for HLA-haploidentical transplantation in Fanconi anemia. Pediatric hematology and oncology. 2012;29(6):568–78. doi: 10.3109/08880018.2012.708708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rihani R, Lataifeh I, Halalsheh H, et al. Haploidentical stem cell transplantation as a salvage therapy for cord blood engraftment failure in a patient with Fanconi anemia. Pediatr Blood Cancer. 2010;55(3):580–2. doi: 10.1002/pbc.22584. [DOI] [PubMed] [Google Scholar]
- 69.Yabe H, Inoue H, Matsumoto M, et al. Unmanipulated HLA-haploidentical bone marrow transplantation for the treatment of fatal, nonmalignant diseases in children and adolescents. International journal of hematology. 2004;80(1):78–82. doi: 10.1532/ijh97.04004. [DOI] [PubMed] [Google Scholar]
- 70.Airoldi I, Bertaina A, Prigione I, et al. gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood. 2015;125(15):2349–58. doi: 10.1182/blood-2014-09-599423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cioc AM, Wagner JE, MacMillan ML, et al. Diagnosis of myelodysplastic syndrome among a cohort of 119 patients with fanconi anemia: morphologic and cytogenetic characteristics. American journal of clinical pathology. 2010;133(1):92–100. doi: 10.1309/AJCP7W9VMJENZOVG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quentin S, Cuccuini W, Ceccaldi R, et al. Myelodysplasia and leukemia of Fanconi anemia are associated with a specific pattern of genomic abnormalities that includes cryptic RUNX1/AML1 lesions. Blood. 2011;117(15):e161–70. doi: 10.1182/blood-2010-09-308726. [DOI] [PubMed] [Google Scholar]
- 73.Tonnies H, Huber S, Kuhl JS, et al. Clonal chromosomal aberrations in bone marrow cells of Fanconi anemia patients: gains of the chromosomal segment 3q26q29 as an adverse risk factor. Blood. 2003;101(10):3872–4. doi: 10.1182/blood-2002-10-3243. [DOI] [PubMed] [Google Scholar]
- 74.Peffault de Latour R, Soulier J. How I treat MDS and AML in Fanconi anemia. Blood. 2016;127(24):2971–9. doi: 10.1182/blood-2016-01-583625. [DOI] [PubMed] [Google Scholar]
- 75.Alter BP, Caruso JP, Drachtman RA, et al. Fanconi anemia: myelodysplasia as a predictor of outcome. Cancer Genet Cytogenet. 2000;117(2):125–31. doi: 10.1016/s0165-4608(99)00159-4. [DOI] [PubMed] [Google Scholar]
- 76.Mehta PA, Ileri T, Harris RE, Williams DA, et al. Chemotherapy for myeloid malignancy in children with Fanconi anemia. Pediatr Blood Cancer. 2007;48(7):668–72. doi: 10.1002/pbc.20843. [DOI] [PubMed] [Google Scholar]
- 77.Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99(10):e199–200. doi: 10.3324/haematol.2013.098954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ayas M, Al-Jefri A, Al-Seraihi A, et al. Allogeneic stem cell transplantation in Fanconi anemia patients presenting with myelodysplasia and/or clonal abnormality: update on the Saudi experience. Bone Marrow Transplant. 2008;41(3):261–5. doi: 10.1038/sj.bmt.1705903. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell R, Wagner JE, Hirsch B, et al. Haematopoietic cell transplantation for acute leukaemia and advanced myelodysplastic syndrome in Fanconi anaemia. British journal of haematology. 2014;164(3):384–95. doi: 10.1111/bjh.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beier R, Maecker-Kolhoff B, Sykora KW, et al. Minimal antileukaemic treatment followed by reduced-intensity conditioning in three consecutive children with Fanconi anaemia and AML. Bone Marrow Transplant. 2015;50(3):463–4. doi: 10.1038/bmt.2014.283. [DOI] [PubMed] [Google Scholar]
- 81.Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(13):1669–76. doi: 10.1200/JCO.2012.45.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly PF, Radtke S, von Kalle C, et al. Stem cell collection and gene transfer in Fanconi anemia. Mol Ther. 2007;15(1):211–9. doi: 10.1038/sj.mt.6300033. [DOI] [PubMed] [Google Scholar]
- 83.Raya A, Rodriguez-Piza I, Guenechea G, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460(7251):53–9. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muller LU, Milsom MD, Harris CE, et al. Overcoming reprogramming resistance of Fanconi anemia cells. Blood. 2012;119(23):5449–57. doi: 10.1182/blood-2012-02-408674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muller LU, Schlaeger TM, DeVine AL, et al. Induced pluripotent stem cells as a tool for gaining new insights into Fanconi anemia. Cell cycle. 2012;11(16):2985–90. doi: 10.4161/cc.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chlon TM, Ruiz-Torres S, Maag L, et al. Overcoming Pluripotent Stem Cell Dependence on the Repair of Endogenous DNA Damage. Stem Cell Reports. 2016;6(1):44–54. doi: 10.1016/j.stemcr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–8. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varnum-Finney B, Brashem-Stein C, Bernstein ID. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101(5):1784–9. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 90.Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124(7):3121–8. doi: 10.1172/JCI74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peled T, Landau E, Prus E, et al. Cellular copper content modulates differentiation and self-renewal in cultures of cord blood-derived CD34+ cells. British journal of haematology. 2002;116(3):655–61. doi: 10.1046/j.0007-1048.2001.03316.x. [DOI] [PubMed] [Google Scholar]
- 92.Peled T, Mandel J, Goudsmid RN, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6(4):344–55. doi: 10.1080/14653240410004916. [DOI] [PubMed] [Google Scholar]
- 93.Molina-Estevez FJ, Nowrouzi A, Lozano ML, et al. Lentiviral-Mediated Gene Therapy in Fanconi Anemia-A Mice Reveals Long-Term Engraftment and Continuous Turnover of Corrected HSCs. Curr Gene Ther. 2015;15(6):550–62. doi: 10.2174/1566523215666150929110903. [DOI] [PubMed] [Google Scholar]
- 94*.Osborn MJ, Gabriel R, Webber BR, et al. Fanconi Anemia Gene Editing by the CRISPR/Cas9 System. Hum Gene Ther. 2015;26(2):114–26. doi: 10.1089/hum.2014.111. Example of gene editing with the CRISPR/Cas9 system to precisely target and correct FANCC mutations in a human patient’s fibroblasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tolar J, Adair JE, Antoniou M, et al. Stem cell gene therapy for fanconi anemia: report from the 1st international Fanconi anemia gene therapy working group meeting. Mol Ther. 2011;19(7):1193–8. doi: 10.1038/mt.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96*.Belo H, Silva G, Cardoso BA, et al. Epigenetic Alterations in Fanconi Anaemia: Role in Pathophysiology and Therapeutic Potential. PLoS One. 2015;10(10):e0139740. doi: 10.1371/journal.pone.0139740. Investigation of DNA methylation profiles of tumor suppressor genes between FA and control samples followed by in vitro treatment of FA cells with a histone deacetylase inhibitor as a method to investigate new therapeutic options targeting epigenetic changes in FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paustian L, Chao MM, Hanenberg H, et al. Androgen therapy in Fanconi anemia: A retrospective analysis of 30 years in Germany. Pediatric hematology and oncology. 2016;33(1):5–12. doi: 10.3109/08880018.2015.1129567. [DOI] [PubMed] [Google Scholar]
- 98.Zhang QS, Benedetti E, Deater M, et al. Oxymetholone therapy of fanconi anemia suppresses osteopontin transcription and induces hematopoietic stem cell cycling. Stem Cell Reports. 2015;4(1):90–102. doi: 10.1016/j.stemcr.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J, Sipple J, Maynard S, et al. Fanconi anemia links reactive oxygen species to insulin resistance and obesity. Antioxid Redox Signal. 2012;17(8):1083–98. doi: 10.1089/ars.2011.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H, Kozono DE, O’Connor KW, et al. TGF-beta Inhibition Rescues Hematopoietic Stem Cell Defects and Bone Marrow Failure in Fanconi Anemia. Cell stem cell. 2016;18(5):668–81. doi: 10.1016/j.stem.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehta PA, Svahn J, Davies SM, et al. Etanercept treatment in Fanconi anaemia; combined US and Italian experience. British journal of haematology. 2012;158(6):809–11. doi: 10.1111/j.1365-2141.2012.09250.x. [DOI] [PubMed] [Google Scholar]
- 102.Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. British journal of haematology. 2010;150(2):179–88. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tamary H, Nishri D, Yacobovich J, et al. Frequency and natural history of inherited bone marrow failure syndromes: the Israeli Inherited Bone Marrow Failure Registry. Haematologica. 2010;95(8):1300–7. doi: 10.3324/haematol.2009.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alter BP. Fanconi anemia and the development of leukemia. Best Pract Res Clin Haematol. 2014;27(3–4):214–21. doi: 10.1016/j.beha.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kutler DI, Patel KR, Auerbach AD, et al. Natural history and management of Fanconi anemia patients with head and neck cancer: A 10-year follow-up. Laryngoscope. 2016;126(4):870–9. doi: 10.1002/lary.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106*.Bonfim C, Ribeiro L, Nichele S, et al. Long-term Survival, Organ Function, and Malignancy after Hematopoietic Stem Cell Transplantation for Fanconi Anemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016 doi: 10.1016/j.bbmt.2016.03.007. A comprehensive evaluation of post-alloHCT late effects in a large population of FA patients and associations with patient, disease, and transplantation characteristics. [DOI] [PubMed] [Google Scholar]
- 107.Katzenellenbogen RA, Carter JJ, Stern JE, et al. Skin and mucosal human papillomavirus seroprevalence in persons with Fanconi Anemia. Clin Vaccine Immunol. 2015;22(4):413–20. doi: 10.1128/CVI.00665-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sauter SL, Wells SI, Zhang X, et al. Oral human papillomavirus is common in individuals with Fanconi anemia. Cancer Epidemiol Biomarkers Prev. 2015;24(5):864–72. doi: 10.1158/1055-9965.EPI-15-0097-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu GB, Chen J, Wu ZH, et al. Association of human papillomavirus with Fanconi anemia promotes carcinogenesis in Fanconi anemia patients. Rev Med Virol. 2015;25(6):345–53. doi: 10.1002/rmv.1834. [DOI] [PubMed] [Google Scholar]
- 110.Chlon TM, Hoskins EE, Mayhew CN, et al. High-risk human papillomavirus E6 protein promotes reprogramming of Fanconi anemia patient cells through repression of p53 but does not allow for sustained growth of induced pluripotent stem cells. Journal of virology. 2014;88(19):11315–26. doi: 10.1128/JVI.01533-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoskins EE, Morris TA, Higginbotham JM, et al. Fanconi anemia deficiency stimulates HPV-associated hyperplastic growth in organotypic epithelial raft culture. Oncogene. 2009;28(5):674–85. doi: 10.1038/onc.2008.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoskins EE, Morreale RJ, Werner SP, et al. The fanconi anemia pathway limits human papillomavirus replication. Journal of virology. 2012;86(15):8131–8. doi: 10.1128/JVI.00408-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park JW, Pitot HC, Strati K, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70(23):9959–68. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alter BP, Giri N, Pan Y, et al. Antibody response to human papillomavirus vaccine in subjects with inherited bone marrow failure syndromes. Vaccine. 2014;32(10):1169–73. doi: 10.1016/j.vaccine.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rose SR, Myers KC, Rutter MM, et al. Endocrine phenotype of children and adults with Fanconi anemia. Pediatr Blood Cancer. 2012;59(4):690–6. doi: 10.1002/pbc.24095. [DOI] [PubMed] [Google Scholar]
- 116.Petryk A, Kanakatti Shankar R, Giri N, et al. Endocrine disorders in Fanconi anemia: recommendations for screening and treatment. J Clin Endocrinol Metab. 2015;100(3):803–11. doi: 10.1210/jc.2014-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anur P, Friedman DN, Sklar C, et al. Late effects in patients with Fanconi anemia following allogeneic hematopoietic stem cell transplantation from alternative donors. Bone Marrow Transplant. 2016 doi: 10.1038/bmt.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barnum JL, Petryk A, Zhang L, et al. Endocrinopathies, Bone Health, and Insulin Resistance in Patients with Fanconi Anemia after Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016 doi: 10.1016/j.bbmt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petryk A, Polgreen LE, Barnum JL, et al. Bone Mineral Density in Children with Fanconi Anemia after Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 doi: 10.1016/j.bbmt.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Forlenza GP, Polgreen LE, Miller BS, et al. Growth hormone treatment of patients with Fanconi anemia after hematopoietic cell transplantation. Pediatr Blood Cancer. 2014;61(6):1142–3. doi: 10.1002/pbc.24910. [DOI] [PMC free article] [PubMed] [Google Scholar]