Abstract
Background
Posttransplant lymphoproliferative disorder (PTLD) is a neoplastic complication of transplantation, with early cases largely due to immunosuppression and primary Epstein-Barr virus infection. Etiology may differ for later-onset cases, but the contributions of immunosuppression, immune reactivity to the donor organ, and chronic B cell activation are uncertain.
Methods
We conducted a case-control study of late-onset PTLD (diagnosed >1 year posttransplant) in a cohort of liver recipients. We assessed serum samples (obtained >6 months before diagnosis in cases) from N = 60 cases and N = 166 matched controls for donor-specific antibodies (DSAs, evaluable for N = 221 subjects), immunoglobulin kappa and lambda free light chains (FLCs, N = 137), and B cell activating factor (BAFF, N = 226). Conditional or unconditional logistic regression was used to calculate adjusted odds ratios (aORs).
Results
Circulating DSAs were less common in PTLD cases than controls (18% vs 30%), although this difference was borderline significant (aOR, 0.51; 95% confidence interval [CI], 0.24-1.10; P = 0.09). Donor-specific antibodies against class II HLA antigens predominated and likewise showed a borderline inverse association with PTLD (aOR, 0.58; 95% CI, 0.27-1.24). The FLC levels were less frequently abnormal in cases than controls, but measurements were available for only a subset and confidence intervals were wide (elevated kappa: aOR, 0.57; 95% CI, 0.15-2.12; P = 0.40; elevated lambda: aOR, 0.68; 95% CI, 0.30-1.50; P = 0.34). B cell–activating factor levels were not associated with PTLD.
Conclusions
Our results suggest that circulating DSAs are associated with decreased risk of late-onset PTLD. Because DSAs may develop in the setting of underimmunosuppression, the inverse association with DSAs supports a role for immunosuppression in the etiology of late-onset PTLD.
Solid organ transplant recipients receive lifelong immunosuppressive therapy to prevent rejection of the donor organ. Posttransplant lymphoproliferative disorder (PTLD) is an important neoplastic complication of solid organ transplantation, comprising a spectrum of B cell disorders that includes reactive lymphoid hyperplasia, polyclonal proliferations, and non-Hodgkin lymphoma (NHL).1 Posttransplant lymphoproliferative disorder can be divided into early-onset cases (typically arising in the first 1 or 2 years posttransplant) and late-onset cases. Early-onset cases are closely associated with primary Epstein-Barr virus (EBV) infection in the setting of intensive induction immunosuppression.2,3,4 Epstein-Barr virus is also involved in late-onset PTLD, although a smaller proportion of cases is EBV-positive than for early-onset PTLD.2,4 HLA mismatch between donor and recipient is a risk factor for late-onset NHL.5
The level of immunosuppression is greatly reduced over time after liver transplantation, because of the physicians' perception that the risk of chronic rejection and loss of the donor liver is low. Much of the immune-related pathology in organ rejection is due to T cell responses against donor HLA and other antigens. However, a role for B cells and antibody-mediated rejection is increasingly recognized, including for liver transplants.6 Circulating donor-specific antibodies (DSAs) directed at HLA antigens can develop posttransplant (ie, de novo).7 Among liver recipients, de novo DSAs are associated with chronic rejection, decreased graft survival, and increased mortality.6,8
We previously showed that elevated serum levels of immunoglobulin free light chains (FLCs), which are antibody fragments released by activated B cells, are associated with elevated EBV viral load levels and development of PTLD in solid organ transplant recipients.9,10 Notably, some sera were obtained close to the time of PTLD diagnosis, so the FLCs may have been produced by B cells from the incipient tumors.9 Half of the PTLD cases in our prior study arose within the first year after transplantation, and the associations with FLC levels appeared largely restricted to this group.9 B cell activating factor (BAFF, also known as B lymphocyte stimulator) is a member of the tumor necrosis factor ligand family and plays a role in B cell homeostasis. Circulating BAFF levels are elevated among NHL patients from the general population.11
The contributions of immunosuppression, immune reactivity to the donor organ, and chronic B cell activation to late-onset PTLD remain uncertain. In the present study, we assessed a large population of liver recipients for the associations of serum DSAs, FLCs, and BAFF levels with subsequent risk of late-onset PTLD.
MATERIALS AND METHODS
We conducted a case-control study of late-onset PTLD nested in a cohort of more than 3400 adult liver-only recipients followed since 1985 at Baylor University Medical Center (Dallas, TX). Liver recipients were treated with cyclosporine-based or tacrolimus-based maintenance immunosuppression depending on era, using as a second agent azathioprine or mycophenolate mofetil. Alternatively, patients with hepatocellular carcinoma received sirolimus as a second agent (starting in 2001). All patients received a steroid taper. Induction was used in selective cases only. The study was approved by the Baylor institutional review board.
Data were collected from patients at planned intervals and clinical events (eg, acute rejection, hospitalization, death). Serum samples (stored at −80°C) were obtained at 0, 0.25, 0.5, 1, 2, 5, 10, and 15 years posttransplant.
In the present study, cases (N = 60) were liver-only recipients in this cohort who developed late-onset PTLD (diagnosed after 1 year posttransplant) and who had an appropriate prediagnostic serum sample available for testing. Specifically, we selected the most recent serum sample before PTLD diagnosis, excluding the 6-month period immediately before PTLD diagnosis (to minimize reverse causation, ie, PTLD producing DSAs or FLCs). Controls were liver-only recipients who were individually matched to cases and were alive and PTLD-free at the time of PTLD in their matched case (measured from liver transplantation). Matching was based on transplant number (first vs 2+), age at transplant (±5 years), use of induction immunosuppression, maintenance immunosuppressant regimen at 3 months posttransplant (use of mammalian target of rapamycin [mTOR] inhibitor, number of maintenance medications), and availability of the same serum sample for testing as in the matched case. We selected up to 3 controls for case based on availability, which resulted in N = 166 controls.
For all assays, samples were arranged in sets keeping the cases and matched controls together, and randomly sorted within sets so that the testing laboratories were blinded to case-control status. For DSAs, sera were tested using a Luminex-based single antigen bead system (LABScreen, One Lambda, Canoga Park, CA) for IgG antibodies against multiple HLA antigens (N = 100 for class I, N = 98 for class II). The assay results were compared with the known donor HLA antigens to determine which antibodies represent DSAs. Because most preformed DSAs resolve by 1 year posttransplant,12 we assumed that detected DSAs had arisen de novo. Due to incomplete data for some donors or antigens, results were missing for 3 (5%) cases and 2 (1%) controls. We also captured antibodies directed against self-HLA or any HLA antigen regardless of whether it was present in the donor or recipient. The BAFF levels were measured in mean fluorescence intensity (MFI) units using a Luminex-based bead assay (Terasaki Research Institute, Los Angeles, CA).
Free light chains were measured using the Freelite assay (The Binding Site, Birmingham, UK) performed on a SPA PLUS (Special Protein Analyzer) platform. Due to the presence of lipemia, FLC results were not measurable for 24 cases (40%) and 65 controls (39%). The assay separately measures kappa and lambda FLCs. For interpretation, we describe FLC levels as normal versus elevated (upper limit of normal: 1.94 mg/dL for kappa, 2.63 mg/dL for lambda). Based on the kappa/lambda FLC ratio (normal range, 0.26-1.65), we also categorize FLC elevations in 2 patterns: monoclonal (abnormal ratio: N = 69, of which 68 had elevated kappa and/or lambda) and polyclonal (normal ratio with elevated kappa and/or lambda: N = 58).9 Because FLCs are elevated in people with renal insufficiency, we used the serum creatinine (available for 80% of subjects with FLC values) to assess the glomerular filtration rate (GFR) based on the Modification of Diet in Renal Disease formula.13
Among controls, we compared FLC and BAFF levels between recipients with and without DSAs using the Wilcoxon test, and assessed the correlation of kappa and lambda FLCs using the Spearman statistic. Conditional logistic regression, which accounts for the matching factors, was used to estimate adjusted odds ratios (aORs) comparing BAFF levels in cases and controls. Because of missing data for FLCs and DSAs, we compared levels in cases and controls after breaking the matching, using unconditional logistic regression to derive aORs adjusted for sex, age at transplant, and days from transplant to sample draw date. Fisher exact test was used when comparing the prevalence of antibodies against class I or self HLA, which were rare, in cases and controls. Finally, in a sensitivity analysis restricted to subjects transplanted in recent years (2000-2010), we used the Fisher exact test to compare the prevalence of DSAs and BAFF elevations in cases and controls.
RESULTS
The study included 60 PTLD cases and 166 controls (Table 1). Cases were described as having lymphoproliferative disorder not otherwise specified (33%), B cell NHL (58%), or T cell NHL (8%). Most cases (93%) occurred after a first liver transplant, the majority (63%) were male, and the median age at transplantation was 50 years. Only 13% received induction immunosuppression. At 3 months posttransplant, most cases received 2 maintenance immunosuppressant medications, and none was receiving immunosuppression with an mTOR inhibitor. Controls were matched well to cases on these characteristics (Table 1).
TABLE 1.
Characteristics of PTLD cases and controls
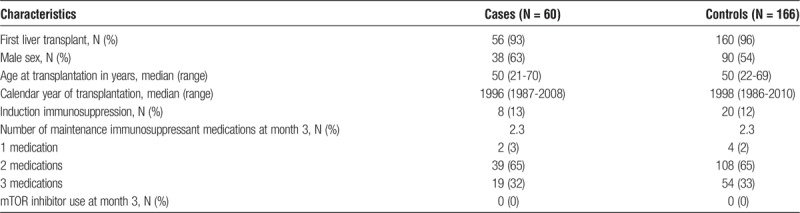
Posttransplant lymphoproliferative disorder was diagnosed a median of 6.4 years posttransplant (range, 1.0-19.5 years). Among cases, serum samples were obtained a median of 3.5 years posttransplant (range, 0.2-15.0 years) and 2.4 years before PTLD diagnosis (range, 0.5-9.6 years). Among controls, samples were obtained a median of 2.1 years posttransplant (range, 0.1-15.0 years).
Among controls, recipients with and without DSAs had similar levels of kappa FLC (median, 4.60 mg/dL vs 4.45 mg/dL; P = 0.70), lambda FLC (3.06 mg/dL vs 2.57 mg/dL, P = 0.14), and BAFF (1727 vs 1138 MFI units, P = 0.77). Kappa and lambda FLC levels were highly correlated with each other (Spearman R = 0.80, P < 0.001).
Donor-specific antibodies were present in 18% of cases and 30% of controls, which yielded an inverse association of borderline significance (aOR, 0.51; 95% CI, 0.24-1.10; P = 0.09; Figure 1). Most DSAs were directed against class II HLA, and class II DSAs were inversely associated with PTLD, again with borderline significance (aOR, 0.58; 95% CI, 0.27-1.24; P = 0.16; Figure 1). Class I DSAs were present in 4% of cases and 3% of controls (P = 1.00). Antibodies against self HLA were present in 5% of cases and 6% of controls (P = 1.00). Of note, none of the individuals with DSAs had been diagnosed or treated for antibody-mediated rejection during clinical follow-up. Results were similar when we restricted analyses to 47 cases and 139 controls with sera obtained after the first year posttransplant to capture only de novo DSA (results not shown).
FIGURE 1.
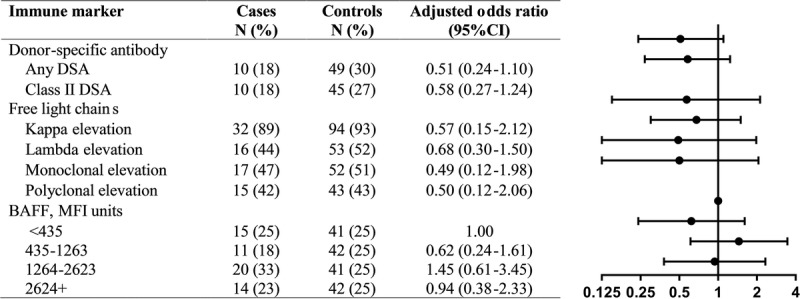
The figure shows the associations of donor-specific antibodies, FLCs, and BAFF levels with PTLD. The forest plot on the right graphically depicts the adjusted odds ratios and 95% confidence intervals. Measurements were available for N = 221 subjects for donor-specific antibody, N = 137 for FLCs, and N = 226 for BAFF. Conditional logistic regression was used for BAFF analyses, accounting for matched sets. Unconditional logistic regression was used for donor-specific antibody and FLC analyses, adjusted for sex, age at transplant, and days from transplant to sample draw date.
The FLC levels were less frequently abnormal in cases than controls, but measurements were available for only a subset of recipients and confidence intervals were very wide (elevated kappa: aOR, 0.57; 95% CI, 0.15-2.12; P = 0.40; elevated lambda: aOR, 0.68; 95% CI, 0.30-1.50; P = 0.34). Associations were similar for FLCs showing a monoclonal or polyclonal patterns (aORs, 0.49 [P = 0.31] and 0.50 [P = 0.34], respectively; Figure 1). Among subjects with measured FLC levels, renal function was similar in cases and controls (median GFR, 53 mL/min vs 52 mL/min). Associations between FLCs and case-control status were similar when we excluded individuals with GFRs below 30 mL/min (not shown). The BAFF levels were not associated with PTLD risk (Figure 1).
In a sensitivity analysis restricted to individuals transplanted during 2000 to 2010, we assessed 17 cases and 64 controls. Cases and controls were similar with respect to the prevalence of DSAs (19% vs 23%, P = 1.00), but cases appeared less likely than controls to have BAFF levels above the median value (6% vs 28%, P = 0.06).
DISCUSSION
In a case-control study of PTLD risk among liver recipients, we assessed associations with several circulating markers of donor-specific immune reactivity and B cell activation. The strongest association that we observed was approximately 40% to 50% reduction in the risk of late-onset PTLD associated with the detection of DSAs (specifically, class II DSAs), which was of borderline statistical significance. There was also a suggestion of an inverse association with FLC levels, but the wide confidence intervals preclude a firm conclusion. There was no association between BAFF levels and PTLD risk.
Donor-specific antibodies can be present before transplantation, but when identified after the first few months after transplant, most DSAs represent de novo B cell reactivity against the donor graft.7,8 As seen in our study, most DSAs are directed against class II HLA antigens, and in liver recipients, their presence is linked to an elevated risk of adverse outcomes, including chronic rejection of the donor organ.6,7,8 Underimmunosuppression, patient nonadherence with immunosuppressant medications, and HLA mismatch between donor and recipient are risk factors for the development of de novo DSAs.8,14,15
Antibody-mediated rejection is treated with intensification of immunosuppression, but DSAs were measured retrospectively for the recipients in this study, and none of the recipients with DSAs had been diagnosed with antibody-mediated rejection during their clinical follow-up. Instead, the inverse association that we observed between PTLD and DSAs could reflect that liver recipients with DSAs received less intensive immunosuppressive therapy than those without DSAs. Underimmunosuppression puts the graft at risk of rejection, but the lower levels of immunosuppression may allow for better control of EBV infection and thus translate into a decreased risk for PTLD. We did not see differences between cases and controls in the use of induction therapy or number of immunosuppressant medications at 3 months after transplantation, but it is possible that DSAs capture other qualitative differences in immunosuppression or differences between cases and controls at a later timepoint. Antibodies against class I or self HLA were rare and not associated with PTLD.
Circulating FLCs are also markers of B cell activation, but we did not find a clear relationship with risk of late-onset PTLD. Among individuals infected with human immunodeficiency virus, elevated FLC levels are associated with an increased risk of developing NHL.16,17 Our prior study found higher FLC levels in PTLD cases compared with controls, but many cases in that study had early-onset PTLD (ie, half of the cases were diagnosed <2 years posttransplant) and the FLC associations were strongest for the early-onset cases.9 In contrast, the present study had only 6 PTLD cases diagnosed less than 2 years posttransplant and, by design, none in the first year posttransplant. Also, compared with the intervals between FLC measurement and PTLD diagnosis in the present study (median, 2.4 years), the intervals in the prior study were much shorter (median, 0.3 year).9 Therefore, the FLC elevations in the prior study, detected close to the time of diagnosis, may have largely represented immunoglobulin fragments produced by the PTLD cells themselves, shortly before the tumor became clinically manifest and may not have reflected the underlying immune abnormalities early in the disease process leading to development of PTLD.
Elevated BAFF levels among kidney recipients have been linked with an increased risk of acute antibody-mediated rejection.18 In our main analysis, we did not find a clear relationship of BAFF levels with risk of late-onset PTLD, but in a sensitivity analysis restricted to the most recent transplants there was an inverse association, again suggesting that lower levels of immunosuppression may reduce PTLD risk by allowing better immune control of EBV. Increased BAFF levels have also been reported in patients suffering from autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, and Sjögren syndrome.19,20,21 Moreover, serum BAFF levels are predictive of NHL risk among human immunodeficiency virus-infected individuals.22
A strength of our study is its incorporation of multiple markers of immune reactivity to the donor organ and B cell activation, and it is the first study to assess DSAs and BAFF levels in association with PTLD. We also assessed these markers well in advance of PTLD onset in cases, so their measurements reflect the liver recipients’ immune status at a time when immunosuppression and B cell activation might be etiologically relevant. A limitation of this study is the modest number of PTLD cases overall. Furthermore, measurements on some recipients were missing due to assay difficulties (especially for FLCs, and to a lesser extent for DSAs), which reduced the available information and compelled us to break the case-control matching. FLC levels can be increased in people with poor renal function, but renal function did not differ between cases and controls. We lacked repeated measurements of these markers over time, which would have been informative regarding their evolution from the time of transplantation until PTLD diagnosis. Finally, because we studied late-onset PTLD, many of the transplants were performed in an earlier era. Although transplant practice, including immunosuppressive regimens and monitoring for rejection, has evolved over time, it is unclear whether those changes would have affected associations with the markers we measured. We conducted a sensitivity analysis restricted to transplants from 2000 onward, but the number of such transplants was limited.
In conclusion, our results showed a borderline inverse association with class II DSAs, supporting a model in which immunosuppression is an important factor contributing to the development of late-onset PTLD. Additional methods for characterizing the level of immunosuppression, immunoreactivity to the donor, and B cell activation may provide further insight into the etiology of PTLD, allow for better tailoring of immunosuppressive regimens in transplant recipients, and reduce the incidence of PTLD.
Footnotes
Published online 15 May, 2018.
This study was supported by the Intramural Research Program of the National Cancer Institute.
The authors declare no conflicts of interest.
E.A.E., L.W.J., M.J.E., E.L.Y., R.M.P., G.B.K. participated in the research design. All authors participated in the writing of the article. E.A.E., L.W.J., M.J.E., O.L., K.M. participated in the performance of the research. E.A.E. and L.W.J. participated in data analysis.
REFERENCES
- 1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 2. Quinlan SC, Pfeiffer RM, Morton LM, et al. Risk factors for early-onset and late-onset post-transplant lymphoproliferative disorder in kidney recipients in the United States. Am J Hematol. 2011;86:206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibson TM, Engels EA, Clarke CA, et al. Risk of diffuse large B-cell lymphoma after solid organ transplantation in the United States. Am J Hematol. 2014;89:714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghobrial IM, Habermann TM, Macon WR, et al. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005;79:244–247. [DOI] [PubMed] [Google Scholar]
- 5. Hussain SK, Makgoeng SB, Everly MJ, et al. HLA and risk of diffuse large B-cell lymphoma after solid organ transplantation. Transplantation. 2016;100:2453–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Leary JG, Demetris AJ, Friedman LS, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jordan SC, Vo AA. Donor-specific antibodies in allograft recipients: etiology, impact and therapeutic approaches. Curr Opin Organ Transplant. 2014;19:591–597. [DOI] [PubMed] [Google Scholar]
- 8. Kaneku H, O'Leary JG, Banuelos N, et al. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013;13:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engels EA, Preiksaitis J, Zingone A, et al. Circulating antibody free light chains and risk of posttransplant lymphoproliferative disorder. Am J Transplant. 2012;12:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engels EA, Savoldo B, Pfeiffer RM, et al. Plasma markers of B-cell activation and clonality in pediatric liver and hematopoietic stem cell transplant recipients. Transplantation. 2013;95:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oki Y, Georgakis GV, Migone TS, et al. Elevated serum BLyS levels in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2007;48:1869–1871. [DOI] [PubMed] [Google Scholar]
- 12. O'Leary JG, Kaneku H, Jennings LW, et al. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. 2013;19:973–980. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Leary JG, Samaniego M, Barrio MC, et al. The influence of immunosuppressive agents on the risk of de novo donor-specific HLA antibody production in solid organ transplant recipients. Transplantation. 2016;100:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Everly MJ, Rebellato LM, Haisch CE, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95:410–417. [DOI] [PubMed] [Google Scholar]
- 16. Landgren O, Goedert JJ, Rabkin CS, et al. Circulating serum free light chains as predictive markers of AIDS-related lymphoma. J Clin Oncol. 2010;28:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vendrame E, Hussain SK, Breen EC, et al. Serum levels of cytokines and biomarkers for inflammation and immune activation, and HIV-associated non-Hodgkin B-cell lymphoma risk. Cancer Epidemiol Biomarkers Prev. 2014;23:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banham G, Prezzi D, Harford S, et al. Elevated pretransplantation soluble BAFF is associated with an increased risk of acute antibody-mediated rejection. Transplantation. 2013;96:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei F, Chang Y, Wei W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine. 2015;76:537–544. [DOI] [PubMed] [Google Scholar]
- 20. Vincent FB, Morand EF, Schneider P, et al. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. 2014;10:365–373. [DOI] [PubMed] [Google Scholar]
- 21. Nocturne G, Mariette X. Sjögren syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol. 2015;168:317–327. [DOI] [PubMed] [Google Scholar]
- 22. Epeldegui M, Magpantay L, Guo Y, et al. A prospective study of serum microbial translocation biomarkers and risk of AIDS-related non-Hodgkin lymphoma. AIDS. 2018;32:945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]


