Abstract
Background
Low clearance transplant clinics (LCTCs) are recommended for the management of recipients with a failing kidney transplant (RFKT) but data to support their use is limited. We conducted a retrospective study to assess management of RFKT at 2 transplant centers, 1 with a LCTC (center A) and 1 without (center B).
Methods
Patients who transitioned to an alternative form of renal replacement therapy (RRT) between January 1, 2012, and November 30, 2016, were included. Patients with graft failure within a year of transplantation or due to an unpredictable acute event were excluded. Clinical data were collected after review of medical records.
Results
One hundred seventy-nine patients (age, 48.6 ± 13.4 years, 99 [55.3%] male, and mean transplant duration 10.3 ± 7.8 years) were included. RRT counseling occurred in 79 (91%) and 68 (74%) patients at centers A and B (P = 0.003), at median 135 (61-319) and 133 (69-260) days before dialysis after graft loss (P = 0.92). Sixty-one (34.1%) patients were waitlisted for retransplantation; 18 (32.7%) nonwaitlisted patients were still undergoing workup at center A compared with 37 (58.7%) at center B (P = 0.028). Preemptive retransplantation occurred in 4 (4.6%) and 5 (5.4%) patients at centers A and B (P = 0.35). At 1 year after initiation of dialysis after graft loss, 11 (15.3%) and 11 (17.2%) patients were retransplanted (P = 0.12), and mortality was 6.6% overall.
Conclusions
A dedicated LCTC improved RRT counseling and transplant work-up but did not lead to improved rates of retransplantation. Earlier consideration of retransplantation in LCTCs is required to improve RFKT outcomes.
Kidney transplant recipients (KTRs) returning to dialysis after graft loss (DAGL) make up around 4% of the incident dialysis population, and DAGL is now in the top 5 reasons for the initiation of dialysis overall.1-3 Although short-term outcomes in graft survival have improved significantly in recent years, there has been a more modest improvement in longer-term survival.4,5 Kidney transplant recipients with renal function that is approaching end stage are termed recipients with a failing kidney transplant (RFKT). Despite offering the best outcomes, only a minority of RFKT gets retransplanted and with transplant being the most prevalent form of renal replacement therapy (RRT), the number of patients requiring DAGL is likely to increase.6
There is evidence that RFKT get worse care than those with native disease.7,8 Moreover, patient outcomes during the period of worsening graft function is poor, and as estimated glomerular filtration rate (eGFR) declines, there is an associated increase in cardiovascular events and death.9 Patient outcomes are even worse when RFKT return to dialysis with mortality higher compared with similar patients awaiting transplant for the first time.10 Mortality on DAGL is 16% at 1 year and 33% at 3 years, most commonly due to cardiovascular disease, infection, and cancer.11,12
RFKT are therefore a high-risk cohort in whom there may be opportunities to improve care. Moreover, the switch from transplant to other forms of RRT can be a psychologically challenging time, highlighting the need for augmented support of this vulnerable group. The British Transplantation Society recommend managing RFKT in dedicated transplant clinics termed low-clearance transplant clinics (LCTC).1 However, evidence to support this practice is limited, and the benefit of such clinics is unknown. Accordingly, we undertook a retrospective study of the management of RFKT at 2 UK transplant centers, 1 with a dedicated LCTC, and another without.
MATERIALS AND METHODS
Study Design and Setting
We conducted a retrospective, cohort study at Bart’s Health (center A) and Royal Free Hospital (center B) NHS Trusts. Both centers provide tertiary nephrology and kidney transplant services to northeast and north central London and their surrounding regions respectively. The populations served by both centers are ethnically and socioeconomically diverse; 264 kidney transplants were performed across the sites between April 1, 2016, and March 31, 2017.13
Center A established a dedicated centralized LCTC in 2007. This provides multidisciplinary care to transplant patients with eGFR less than 30 mL/min per 1.73 m2. Patients are primarily reviewed by nephrologists, but also have access to transplant nurses specifically trained in the management of the complications of advanced chronic kidney disease (CKD), renal dieticians, and specialist renal pharmacists. There is a clinical focus on preparing patients for retransplantation and DAGL. Center B manages chronic transplant patients within the same clinic regardless of the degree of renal impairment. Patients are reviewed in both centralized and peripheral clinics. Patients in center B also have access to multidisciplinary services, equivalent to center A, but not within a dedicated LCTC.
Participants
Transplant patients who transitioned to an alternative form of RRT (hemodialysis, peritoneal dialysis, retransplantation, or conservative management) between December 1, 2012, and November 30, 2016 were included. Patients who died with a functioning graft, who transitioned within the first year of transplantation, or in whom graft loss was due to an unpredictable acute event as determined by the study team were excluded.
Data Collection
Patients were identified and data collected from electronic medical records at both sites. The following were recorded for each patient:
(A) Demographic and baseline clinical data;
(B) Details of CKD parameters and their management.
Including hemoglobin, serum creatinine, urea, bicarbonate, calcium, phosphate, parathyroid hormone, blood pressure and their management (erythropoietin, phosphate binder, alphacalcidol, and sodium bicarbonate use). The last values measured before the initiation of DAGL were recorded.
(C) Details of the initiation of DAGL
Including documentation and timing of RRT counseling, modality restarted and location of restart (acute admission vs planned), retransplant wait listing and reason if not, and dialysis access at the time of restart.
(D) Patient outcomes at 1 year after RRT transition
Including survival and RRT modality at this time point.
For each site, mean values at the initiation of DAGL for each CKD parameter were determined, in addition to the number of patients managed with erythropoietin, bicarbonate supplementation, a phosphate binder, and activated vitamin D. The presence and timing of RRT counseling, the numbers of patients listed for retransplantation, preemptive retransplantation rates, and access for dialysis were calculated at each center. Unadjusted mortality and RRT modalities at 1 year were determined at both sites.
Outcome Measures
The primary outcome measure was the rate of preemptive retransplantation. Secondary outcomes were an assessment of CKD management in RFKT, the presence of RRT counseling, listing for retransplantation at the time of initiation of DAGL, and 1 year retransplantation and mortality rates.
Statistical Analysis
Variables are presented as either mean ± standard deviation (SD) or median ± interquartile range dependent on data distribution. Variables were compared between center A and center B using the Student t test and Mann-Whitney test if continuous, and using tests of proportionality (Fisher exact or χ2) if categorical. We compared 1-year unadjusted mortality between centers, using a log rank test to examine for differences in survival between these groups. Patients managed with conservative care (ie, not considered for retransplantation or dialysis) were excluded from the outcome analysis.
All analyses were conducted with Graphpad Prism version 7, with a P value less than 0.05 considered to represent statistical significance.
RESULTS
Participants
Graft failure or death with a functioning graft occurred in 426 patients during the study period. Predictable graft failure 1 year or more after transplantation occurred in 179 patients, and these patients were included in the study (Figure 1).
FIGURE 1.
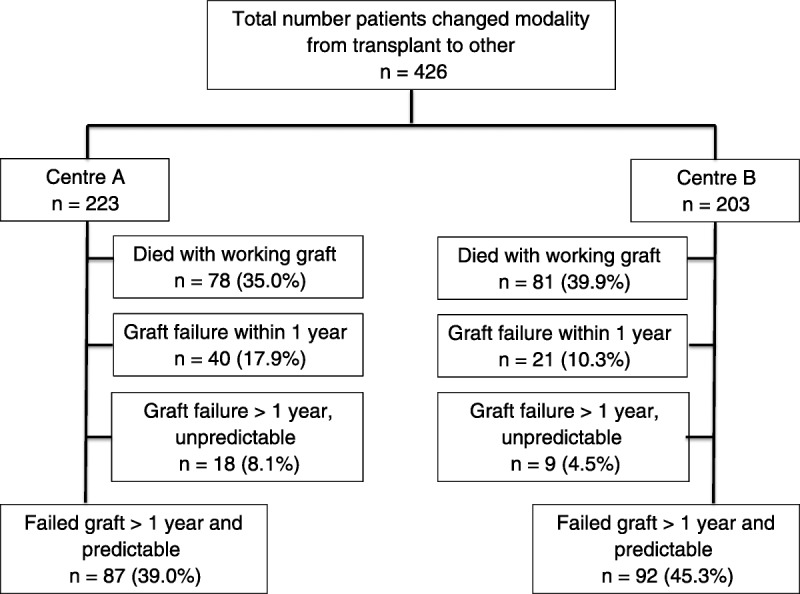
Cohort description.
Descriptive Data
Demographic and baseline clinical data are summarized in Table 1. Mean age at initiation of DAGL was 48.6 ± 13.4 years, 99 (55.3%) patients were male, and mean transplant duration was 10.3 ± 7.8 years. The most common causes of end-stage renal disease were unclear/small kidneys (n = 33, 18.4%), congenital kidney disease/reflux nephropathy (n = 27, 15.1%), hypertensive kidney disease (n = 23, 12.8%), IgA nephropathy (n = 19, 10.6%), and focal segmental glomerulosclerosis (n = 14, 7.8%).
TABLE 1.
Baseline demographic and clinical data of cohort
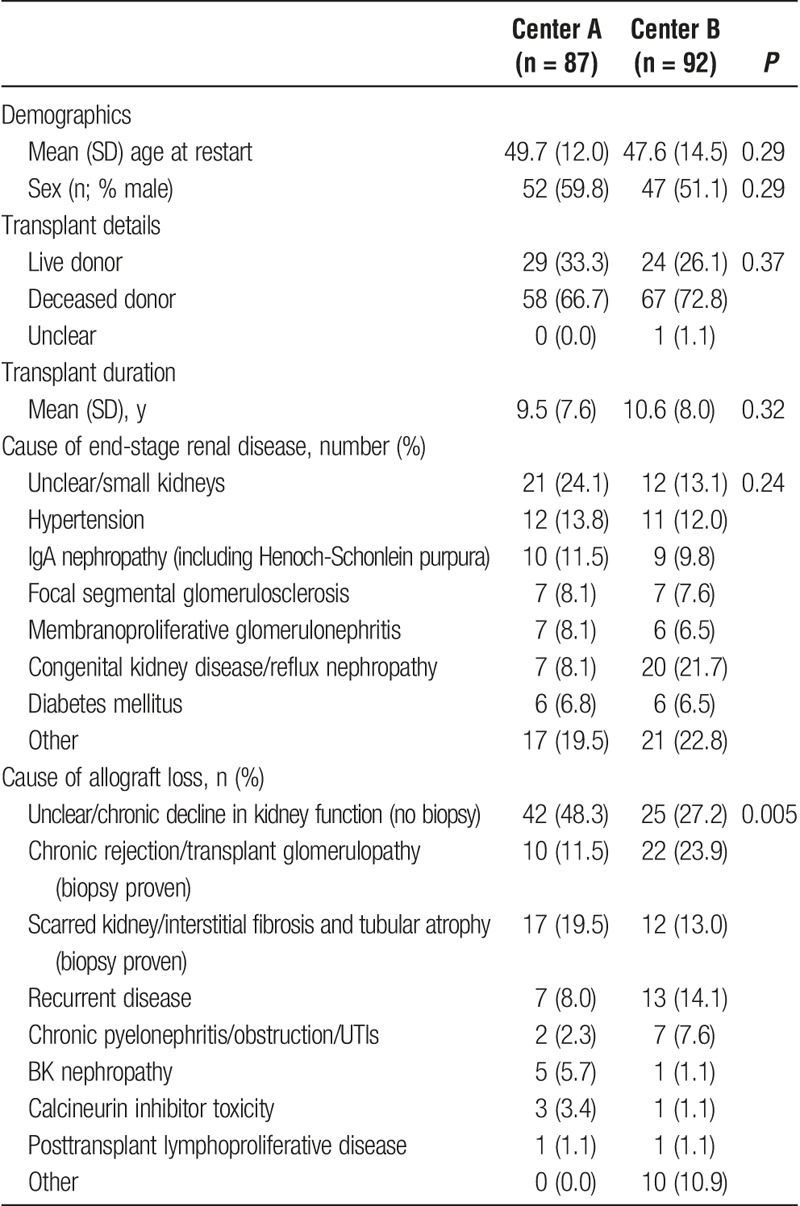
In 67 (34.0%) patients, the cause of allograft loss was undetermined. The cause of graft loss was made by biopsy diagnosis in 43 (49.4%) patients at center A and 52 (56.5%) patients at center B (P = 0.37). The most common causes of biopsy proven allograft loss were chronic rejection/transplant glomerulopathy (n = 32, 16.2%), interstitial fibrosis and tubular atrophy (n = 29, 14.7%), and recurrent disease (n = 20, 10.2%). There were significant differences in the causes of allograft loss between the sites, but not in other demographic or clinical parameters.
Outcome Data
CKD Parameters at Restart
Table 2 outlines clinical and biochemical CKD parameters at the initiation of DAGL. Mean hemoglobin was lower in center A (89.4 ± 15.3 g/L) than center B (96.1 ± 17.1 g/L; P = 0.007). Diastolic blood pressure was better controlled in center A (81 ± 15 mm Hg) than center B (86 ± 13 mm Hg; P = 0.019). Alphacalcidol was used more frequently at center B (33 (37.9%) versus 59 (64.1%) patients; P = 0.0006), although this did not impact on phosphate or parathyroid hormone levels. There were no other statistically significant differences between the sites.
TABLE 2.
Clinical and biochemical parameters at restart, and CKD management before DAGL
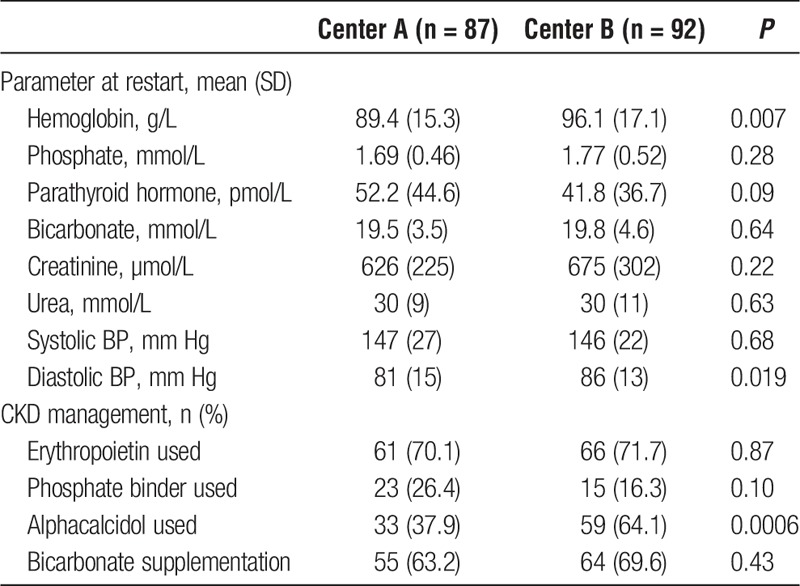
In centers A and B, respectively, hemoglobin was 100 g/L or greater in 25 (28.7%) and 34 (36.9%) patients (P = 0.27), phosphate was 1.5 mmol/L or less in 31 (35.6%) and 35 (38.0%) patients (P = 0.76), and bicarbonate was 22 mmol/L or greater in 21 (24.1%) and 32 (34.8%) patients at the initiation of DAGL.
Median eGFR at initiation of DAGL was 7.75 (6.0-10.0) mL/min per 1.73 m2 at center A and 7.43 (5.7-10.3) mL/min per 1.73 m2 at center B (P = 0.30).
Initiation of DAGL
Renal replacement therapy counseling was documented in 147 (82.1%) patients, but more so in center A than center B (79 [90.8%] vs 68 [73.9%] patients; P = 0.0034). If patients underwent RRT counseling, it occurred at a median 135 (61-319) days before restart at center A and 133 (69-260) days before restart at center B (P = 0.92).
Modalities of RRT restarted and vascular access in hemodialysis patients are outlined in Table 3. A total of 9 (5.0%) patients across both sites underwent preemptive retransplantation (center A, n = 4 [4.6%]; center B, n = 5 [5.4%], P = 0.35). Renal replacement therapy was restarted as an acute admission in 32 (38.6%) patients at center A, and 43 (49.4%) patients in center B (P = 0.33).
TABLE 3.
RRT modality restarted, location of initiation of DAGL (transplants excluded), hemodialysis access, and details of transplant relisting at the initiation of DAGL
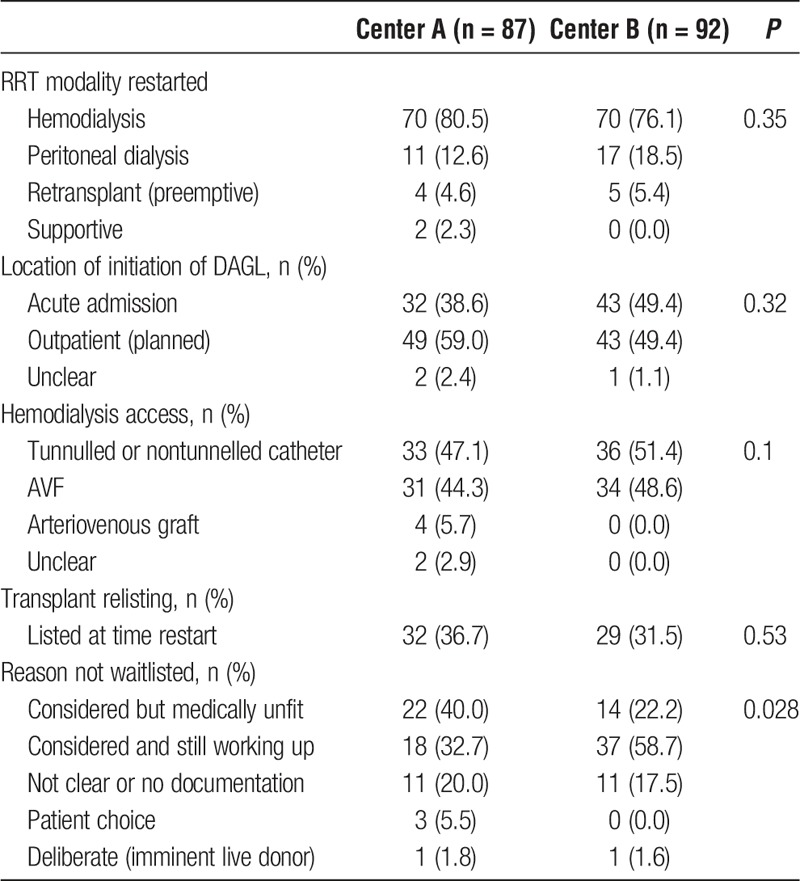
Sixty-one (34.1%) patients were waitlisted for transplant at the time of restart. There were significant differences in the reasons for not wait listing patients between centers, with more patients undergoing workup and deemed medically unfit at center A (n = 22 [40.0%] center A; n = 14 [22.2%] center B), and more patients still undergoing work-up at center B (n = 18 [32.7%] center A; n = 37 [58.7%] center B) (P = 0.028, Table 3).
1-Year Patient Outcomes
One hundred thirty-six patients completed 1 year of follow-up after changing modality and were included in the outcome analysis. The number of patients managed with hemodialysis, peritoneal dialysis, and transplanted at this point was 48 (66.7%), 5 (6.9%), and 11 (15.3%) at center A, and 44 (68.8%), 8 (12.5%), and 11 (17.2%), at center B, respectively (P = 0.12). The number of hemodialysis patients with an arteriovenous fistula (AVF) at 1 year was 27 (56.3%) at center A and 39 (88.6%) at center B (P = 0.0019).
The crude 1-year mortality rate was 6.6% (n = 9) across both sites (Figure 2). Death occurred at a mean time of 132 ± 112 days after initiation of DAGL. Causes of death were unclear (n = 5, 55.6%), malignancy, including posttransplant lymphoproliferative disease (n = 3, 33.3%), and stroke (n = 1, 11.1%).
FIGURE 2.
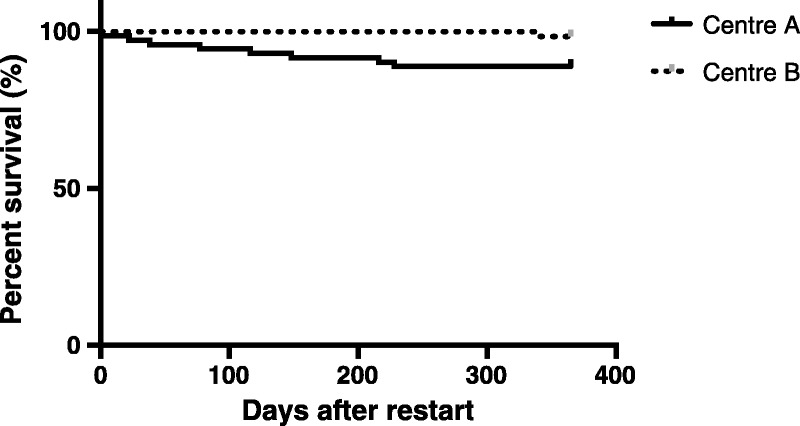
Kaplan-Meier survival analysis censored at 1 year comparing center A and center B. Probability of survival was greater at center B (log rank test P = 0.0248).
DISCUSSION
Key Findings
In this retrospective study, we assessed the management of 179 RFKT at 2 transplant centers in London, UK, 1 with a dedicated LCTC (center A), and 1 without (center B). Documented RRT counseling and retransplant workup was greater in center A, but this did not translate in to better retransplantation rates. Preemptive retransplantation occurred in 4 (4.6%) and 5 (5.4%) patients, and retransplantation at 1 year in 11 (15.3%) and 11 (17.2%) patients in centers A and B, respectively. At initiation of DAGL mean hemoglobin and blood pressure were 89.4 g/l and 147/81 mmHg at center A, and 96.1 g/l and 146/86 mmHg at center B, but there were no other differences in CKD parameters. Crude 1-year mortality was 6.6% overall, worse in center A than center B.
Interpretation
Advanced renal impairment is common in KTRs and 18% of transplant patients have an eGFR less than 30 mL/min per 1.73 m2 in the United Kingdom.8 RFKT are a high-risk cohort and the management of these patients in LCTCs is recommended.1 Such clinics cohort patients with advanced renal impairment and prioritize management of CKD complications, and preparation for retransplantation and DAGL. To the best of our knowledge, this is the first study to investigate the value of this approach.
RFKT are a unique and complex group of patients. Their management is challenging and must balance ongoing efforts to maximize current graft survival, managing the consequences of advanced renal impairment, and making decisions with regards to retransplantation and DAGL. It is a psychologically difficult time for patients with increased rates of depression and anxiety.14 Furthermore, the increased risk of cardiovascular disease, infection, and malignancy associated with prolonged immunosuppression must be addressed. Patient outcomes worsen as renal function declines in KTRs, and for each 5 mL/min per 1.73 m2 loss of GFR below an eGFR of 45 mL/min per 1.73 m2, there is a 15% increased risk of death.9 However, RFKT are relatively young; mean age was 48.6 years at initiation of DAGL in this study, younger than patients starting dialysis more generally, and therefore focusing efforts to improve care in this cohort may have potentially significant long-term consequences.
Management of the complications of advanced CKD is an important aspect of care in LCTCs. Guidelines recommend that blood pressure be controlled to less than 130/80 mm Hg (<125/75 mm Hg if proteinuria) given its beneficial effect on cardiovascular outcome, but there are no transplant-specific targets for management of anemia, CKD-mineral bone disorder, or acidosis, and clinical decisions are based on guidance for the native CKD population.1 Previous reports have highlighted deficiencies in the management of CKD complications in RFKT,8,15 and despite management in a dedicated LCTC, data from this study supported this finding. Mean hemoglobin, phosphate, and bicarbonate levels at initiation of DAGL at center A were 89.4 g/L, 1.69 mmol/L, and 19.5 mmol/L, respectively, as compared with values of 113 g/L, 1.65 mmol/L, and 20.3 mmol/L, in a cohort of CKD 5 T patients in Spain.15 Although some of the deficiency in management may be explained by our recording of the last CKD parameter before dialysis restart as opposed to values from a more stable CKD 5 T population, further efforts must be made both in clinic and at postclinic multidisciplinary transplant meetings to address these parameters and research undertaken to investigate whether improved control translates in to improved outcome specifically in the transplant setting.
There is conflicting evidence about when to restart DAGL,12,16,17 but, akin to the native setting, there is a suggestion of worse outcomes if dialysis is started early.12,17 The median eGFR in all patients at initiation of DAGL in this study was 7 mL/min/1.73 m2, reflecting current standard UK practice, and advice to start dialysis when eGFR falls to less than 6 mL/min/1.73 m2 even if asymptomatic.1 Initiation of DAGL should be planned, and 49.4% of patients in the center without a dedicated LCTC restarted dialysis via an acute admission. This has potentially adverse consequences for patients themselves, and also adds pressure to service provision within the health system more generally.
Although needing to be assessed on a case-by-case basis, of less debate is the optimal modality of RRT after allograft failure. Retransplantation, especially if preemptive, offers clear advantages in terms of quality of life and survival.3,18,19 Retransplantation was associated with a 45% and 23% reduction in 5-year mortality in diabetic (type 1) and nondiabetic kidney disease in the United States,3 and with an 88% reduction in overall mortality during 12 years follow-up in the United Kingdom.18 Preemptive retransplantation is recommended in RFKT when eGFR falls to 10 to 15 mL/min per 1.73 m2.1 In this study, retransplantation rates were low, with 5.0% of patients preemptively retransplanted and 16.2% of patients retransplanted at 1 year across both centers. This compares to previously reported preemptive transplant rates of 2.6% in Northern Ireland,18 and 15% to 29% retransplant rates at 5 years in the United States.3
Retransplantation requires early discussion of RRT options after allograft failure, in particular as rapid graft failure is commonly encountered as transplant patients approach end stage.18 For this reason, in addition to the other complexities surrounding retransplant workup outlined below, a relatively high eGFR cut off of 30 mL/min per 1.73 m2 was chosen for LCTC referral at center A. Admission that an allograft is failing may be a difficult process for both patients and healthcare professionals alike, and one of the key roles of a LCTC is to change expectations of the clinical interaction from one focused primarily on current graft survival to preparing for RRT after allograft loss. In the center with a dedicated LCTC, RRT counseling occurred more frequently; however, this occurred at a median time of between only 4 and 5 months before initiation of DAGL. The improved counseling, and indeed subsequent transplant workup in center A, did not translate in to improved preemptive retransplant rates and this delayed timing is likely to be the critical factor. Other challenges specific to retransplantation may have also impacted rates, including increased comorbidity (40% of patients were deemed medically unfit for retransplantation at center A), increased allosensitization, surgical complexities, and concerns of increased risk of recurrent disease if this was cause of initial allograft loss. This further emphasizes the need for early referral to LCTCs and subsequent retransplant work-up.
Aside from retransplantation, hemodialysis and peritoneal dialysis provide equivalent outcomes in RFKT,2 and decisions regarding modality are the same as in native kidney disease.20 Overall, 78.2% of patients in this study were treated with hemodialysis at restart compared with 15.6% with peritoneal dialysis; this compares to equivalent RRT rates of 73.1% and 19.2% of all dialysis starts in the United Kingdom in 2015.21 Approximately half of hemodialysis patients started dialysis with definitive access (ie, an AVF or graft) in this study, better than reported rates from North America.22,23 The presence of an access surgeon within the LCTC may improve this further, but must be balanced with the implications on resources available for other surgical work. Dialysis should start in a planned fashion, and the fact that 59% of patients in center B started dialysis via an acute admission highlights some deficiencies in planning and outpatient care. Lessons may be learned from the native low clearance setting, where more established protocols including early documented RRT and access plans exist.
1-year survival was better at both centers than published estimates.12 Death occurred in 9 patients overall, and in only 1 patient in center B. Given these low numbers, firm conclusions comparing the centers are difficult to make. This low mortality rate may reflect improved dialysis care, including the recognition in the centers studied that patients in the initial period on dialysis after allograft loss are high risk and require more intensive follow-up.
Strengths and Limitations
This study assessed the value of LCTCs in the management of RFKT. It highlights the value of collaborative research between transplant centers in the UK, and a relatively large cohort of patients was included with complete data in most. Although undertaken only in London, the findings are applicable to other settings within the region.
A center with a LCTC and 1 without were compared. Potential differences (eg, ethnic and socioeconomic) in the populations served by each center and other variables in care aside from the presence of a LCTC may explain center differences in practices and outcomes, and no attempt was made to adjust for these. The study was retrospective and observational, and some data was missing including details surrounding RRT counseling, the exact timing of this, and causes of death. Not all patients who transitioned to conservative care may have been captured, as modality may not have been changed on the electronic record. We assessed blood pressure control in RFKT, but did not collect data on other cardiovascular targets such as smoking and lipid control. Similarly, we did not record other aspects of care and outcomes after allograft loss, such as details on management of immunosuppression, graft nephrectomy, and sensitization rates.
CONCLUSIONS
A dedicated LCTC improved RRT counseling, reduced unplanned dialysis starts and increased retransplant workup but this did not translate in to improved rates of preemptive retransplantation, retransplant waitlisting, or 1-year survival. Altering patient and clinician expectations in LCTCs coupled with earlier consideration of retransplantation and DAGL are required to improve retransplantation rates and outcomes in this high-risk cohort.
Footnotes
Published online 2 May, 2018.
The authors declare no funding or conflicts of interest.
R.E., G.J., and R.T. designed the study. R.E., S.B., S.G.C., S.M.C., L.H., and A.T. collected and analyzed the data. R.E., G.J., and R.T. drafted the article. All authors have reviewed and critically appraised the content of the article and approved it for publication.
REFERENCES
- 1.Andrews PA; Standards Committee of the British Transplantation Society. Summary of the British Transplantation Society Guidelines for Management of the Failing Kidney Transplant. Transplantation. 2014;98:1130–1133. [DOI] [PubMed] [Google Scholar]
- 2.Perl J, Bargman JM, Davies SJ, et al. Clinical outcomes after failed renal transplantation—does dialysis modality matter? Semin Dial. 2008;21:239–244. [DOI] [PubMed] [Google Scholar]
- 3.Ojo A, Wolfe RA, Agodoa LY, et al. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation. 1998;66:1651–1659. [DOI] [PubMed] [Google Scholar]
- 4.Serur D, Saal S, Wang J, et al. Deceased-donor kidney transplantation: improvement in long-term survival. Nephrol Dial Transplant. 2011;26:317–324. [DOI] [PubMed] [Google Scholar]
- 5.Lodhi SA, Meier-Kriesche HU. Kidney allograft survival: the long and short of it. Nephrol Dial Transplant. 2011;26:15–17. [DOI] [PubMed] [Google Scholar]
- 6.Fuquay R, Teitelbaum I. Care of the patient after renal allograft failure: managing the present and planning for the future. Am J Nephrol. 2012;36:348–354. [DOI] [PubMed] [Google Scholar]
- 7.Gill JS, Abichandani R, Khan S, et al. Opportunities to improve the care of patients with kidney transplant failure. Kidney Int. 2002;61:2193–2200. [DOI] [PubMed] [Google Scholar]
- 8.Ansell D, Udayaraj UP, Steenkamp R, et al. Chronic renal failure in kidney transplant recipients. do they receive optimum care?: data from the UK Renal Registry. Am J Transplant. 2007;7:1167–1176. [DOI] [PubMed] [Google Scholar]
- 9.Weiner DE, Carpenter MA, Levey AS, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012;12:2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao PS, Schaubel DE, Jia X, et al. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49:294–300. [DOI] [PubMed] [Google Scholar]
- 11.Lea-Henry T, Chacko B. Management considerations in the failing renal allograft. Nephrology (Carlton). 2018;23:12–19. [DOI] [PubMed] [Google Scholar]
- 12.Gill JS, Abichandani R, Kausz AT, et al. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int. 2002;62:1875–1883. [DOI] [PubMed] [Google Scholar]
- 13.ODT Clinical—NHS Blood and Transplant. Organ specific reports. https://www.odt.nhs.uk/statistics-and-reports/organ-specific-reports/. Accessed December 31, 2017.
- 14.Szeifert L, Molnar MZ, Ambrus C, et al. Symptoms of depression in kidney transplant recipients: a cross-sectional study. Am J Kidney Dis. 2010;55:132–140. [DOI] [PubMed] [Google Scholar]
- 15.Marcén R, del Castillo D, Capdevila L, et al. Achieving chronic kidney disease treatment targets in renal transplant recipients: results from a cross-sectional study in Spain. Transplantation. 2009;87:1340–1346. [DOI] [PubMed] [Google Scholar]
- 16.Park JT, Yoo TH, Chang TI, et al. Predictors of mortality in patients returning to dialysis after allograft loss. Blood Purif. 2010;30:56–63. [DOI] [PubMed] [Google Scholar]
- 17.Molnar MZ, Streja E, Kovesdy CP, et al. Estimated glomerular filtration rate at reinitiation of dialysis and mortality in failed kidney transplant recipients. Nephrol Dial Transplant. 2012;27:2913–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCaughan JA, Patterson CC, Maxwell AP, et al. Factors influencing survival after kidney transplant failure. Transplant Res. 2014;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoll G, Muirhead N, Trpeski L, et al. Patient survival following renal transplant failure in Canada. Am J Transplant. 2005;5:1719–1724. [DOI] [PubMed] [Google Scholar]
- 20.Farrington K, Warwick G. Renal Association Clinical Practice Guideline on planning, initiating and withdrawal of renal replacement therapy. Nephron Clin Pract. 2011;118(Suppl 1):c189–c208. [DOI] [PubMed] [Google Scholar]
- 21.Gilg J, Methven S, Casula A, et al. UK Renal Registry 19th Annual Report: Chapter 1 UK RRT Adult Incidence in 2015: National and Centre-specific Analyses. Nephron. 2017;137(Suppl 1):11–44. [DOI] [PubMed] [Google Scholar]
- 22.Zhang JC, Al-Jaishi A, Perl J, et al. Hemodialysis arteriovenous vascular access creation after kidney transplant failure. Am J Kidney. 2015;66:646–654. [DOI] [PubMed] [Google Scholar]
- 23.Chan MR, Oza-Gajera B, Chapla K, et al. Initial vascular access type in patients with a failed renal transplant. Clin J Am Soc Nephrol. 2014;9:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]


