Supplemental digital content is available in the text.
Abstract
Background
Viral blips reflecting polymerase chain reaction (PCR) artefacts or transient low-level replication are well described in the human immunodeficiency virus setting. However, the epidemiology of such blips in transplant recipients screened for cytomegalovirus (CMV) with PCR remains uncertain and was investigated in a cohort of solid organ and hematopoietic stem cell recipients.
Methods
Eligible recipients had known donor/recipient CMV IgG serostatus, and 3 CMV PCRs ≥. The CMV PCR triplicates (3 consecutive CMV PCRs) were defined; the first CMV PCR was always negative, and the time between the second and third samples was 7 days ≤. A positive second but negative third sample represented a blip. Odds ratio (OR) for factors associated with a triplicate being a blip was estimated by binomial regression adjusted for repeated measurements. Whether blips affected the hazard ratio (HR) for subsequent CMV infection was determined with a Cox model.
Results
851 recipients generated 3883 CMV PCR triplicates. The OR of a triplicate representing a blip decreased with increasing viral load of the second sample (vs 273 IU/mL; >273-910 IU/mL: odds ratio [OR], 0.2; 95% confidence interval [CI], 0.1-0.5; >910 IU/mL: OR, 0.08; 95% CI, 0.02-0.2; P ≤ 0.0002) and increased with intermediary-/low-risk serostatus (vs high risk) (OR, 2.8; 95% CI, 1.2-5.5; P = 0.01). Cumulative exposure to DNAemia in the CMV blips greater than 910 IU/mL indicated increased HR of subsequent CMV infection (HR, 4.6; 95% CI, 1.2-17.2; P = 0.02).
Conclusions
Cytomegalovirus blips are frequent; particularly when the viral load of the first positive PCR is < 910 IU/mL, and serostatus risk is intermediary/low. Accumulating blips suggest intermittent low-level replication. If blips are suspected, confirmation of ongoing replication before initiation of treatment is prudent.
Posttransplant cytomegalovirus (CMV) infection remains a serious complication to both solid organ transplantation (SOT) and hematopoietic stem cell transplantation (HSCT). Currently, 2 main strategies, or a hybrid hereof, are applied with the aim to prevent CMV disease.1,2 Universal prophylaxis with valganciclovir for 3 to 12 months after transplantation is typically used among SOT recipients (1), whereas the preemptive strategy consisting of screening with CMV PCR and treatment in case of emerging CMV infection is generally used for HSCT recipients and in SOT recipients after valganciclovir prophylaxis is stopped.2
The indication for starting antiviral therapy in case of a positive CMV PCR result remains to be defined. There are 2 issues to consider. Despite the recent introduction of a World Health Organization (WHO) standard for CMV PCR,3 assay variability remains which may generate false positive values.4,5,6,7 Conversely, low-level positive reads may reflect early signs of ongoing viral replication.8,8,9,10 Antiviral therapy should only be initiated in the latter situation.
In the human immunodeficiency virus (HIV) setting, screening with PCR technology is known to identify isolated positive results, entitled “blips”.11,12,13 These blips may either constitute a false positive read due to assay variability or reflect transient low level viral replication. The implications and consequences of HIV blips have hitherto been extensively debated, even though many observations indicate they do not reflect ongoing replication.11,12,13,14,15
In the field of CMV infection, the presence of solitary CMV PCR reads or blips, as opposed to low-level CMV replication, has not previously been described. Here we report their prevalence, risk factors, and biological implications when screening a large and unselected cohort of SOT and HSCT recipients with extensive and complete follow-up.
MATERIAL AND METHODS
Definitions of the CMV PCR Triplicate, CMV Infection and CMV blip
To investigate the frequency and impact of CMV blips, we created a model named the CMV PCR triplicate. The CMV PCR triplicate consist of 3 consecutive samples, with 7 days or less between the second (indicator sample) and the third (response sample) CMV PCRs. All available CMV PCRs were considered for inclusion, and there are 3 different types of triplicates (Figure 1):
FIGURE 1.
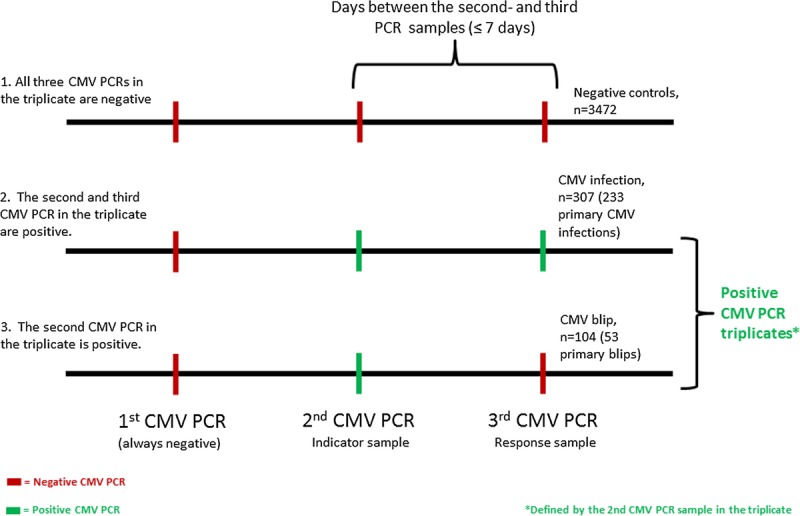
Definition of the CMV PCR triplicate, and the different outcomes. The CMV PCR triplicate consist of 3 consecutive samples, with ≤ 7 days between the second (index sample) and the third (response sample) CMV PCRs. There are 3 different outcomes of this model: (1) negative control triplicates, were all 3 CMV PCRs in the triplicate are negative; (2) CMV triplicates indicating CMV infection were both the indicator and response sample are positive; (3) CMV triplicates indicating CMV blips, where the indicator sample being the only positive sample.
The negative CMV triplicates (controls), in which all CMV PCR samples in the triplicate were negative.
The CMV infection triplicates, in which the first CMV PCR sample was negative, but the second (indicator) and third (response) CMV PCR samples were positive; this combination indicates CMV infection (ie, CMV infection is defined as ≥2 consecutive CMV PCRs ≥ 273 IU/mL taken within 7 days of each other).
The CMV blips triplicates, in which the first CMV PCR sample is negative, the second (indicator) CMV PCR sample is positive, but the third (response) CMV PCR sample is negative. This combination is classified as a CMV blip.
If the third value (response value) in the triplicate was negative (relevant for controls and blips), it could also be used as the first value in a subsequent triplicate, assuming all other inclusion criteria were met.
Patients
The SOT and HSCT recipients registered in the Management of Posttransplant Infections in Collaborating Hospitals (MATCH) program16 between January 1, 2010, and August 14, 2015 (n = 1,512) were considered for inclusion. All CMV PCR’s performed within the first 12 months following transplantation were investigated. The CMV IgG serostatus of donor (D)/recipient (R) (positive (+)/negative (−)) were determined before transplantation, and the eligible combinations for inclusion into the study were D+/R−, D+/R+ or D−/R+ (n = 1142). Furthermore, the recipients needed to have ≥3 CMV PCR analyses fulfilling the CMV triplicate definition previously described to be included in the study (n = 851).
Management of CMV
In general, the SOT recipients (heart, lung, liver, and kidney recipients) receive valganciclovir prophylaxis for 3 months after transplantation. During this time, the patients were monitored monthly with CMV PCR. Upon cessation of valganciclovir prophylaxis, the patients were monitored weekly with CMV PCR during month 4 to 6 posttransplant, and finally monthly until 1 year posttransplant. In case of a detected CMV infection, the patients were treated preemptively with valganciclovir and monitored weekly until 2 consecutive negative CMV PCR samples.
The HSCT recipients (myeloablative conditioning transplantation (MAC), nonmyeloablative conditioning (NMA) and umbilical cord blood [UCB] recipients) were monitored with CMV PCR weekly from week 4 to week 17 posttransplant, and then at weeks 19, 26, and 62. In case of graft-versus-host disease, weekly monitoring was continued. Emerging CMV infections were treated preemptively with valganciclovir.
In case of myelosuppression or resistance toward valganciclovir, both SOT and HSCT recipients were treated with foscovir.17
The CMV PCRs in the monitoring programs were performed on plasma. The COBAS Amplicor kit was used at our center throughout 2011 and was then discontinued. Since the beginning of 2011, the COBAS AmpliPrep/COBAS TaqMan was introduced as its substitute.18 Through simultaneous testing of the 2 different CMV PCR kits during 2011, they were found to be equivalent (conversion factor 1:1).
We have converted all CMV viral loads in the present study into IU/mL using the conversion factor for the COBAS AmpliPrep/COBAS TaqMan (1 copy/mL corresponding to 0.91 IU/mL). The lower limit of quantification of the CMV PCR kit used is 273 IU/mL.
The recipients were stratified according to risk of CMV infection as high, intermediary, or low risk, based on the Donor (D) and Recipient (R) CMV IgG serostatus.19,20 The high-risk group constituted of D+/R− SOT and D−/R+ HSCT recipients. D+/R+ constituted the intermediary risk group for both types of transplantations, and the low risk group D−/R+ for SOT and D+/R− for HSCT. Because of the low number of positive CMV PCR triplicates that originates from low risk patients (n = 25, 6% of the 411 positive CMV PCR triplicates), these are grouped together with the intermediary group.
For all positive CMV triplicates, administration of anti-CMV treatment in relation to the triplicate was registered as either starting before the indicator sample, at or after the indicator sample but before the response sample, or no treatment administered in relation to the CMV PCR triplicate (Figure 1).
Statistical Analyses
Baseline clinical characteristics and features of the included patients were described using standard descriptive statistics. Each included patient could be the cause of multiple CMV triplicates with different outcomes (negative control, CMV infection or CMV blip). The overall proportion of first-time blips in the cohort was calculated and stratified into 3 groups depending on the viral load in the indicator sample (Figures 1 and 2). This model was also stratified according to type of transplantation and risk of CMV infection according to D/R CMV IgG serostatus.
FIGURE 2.
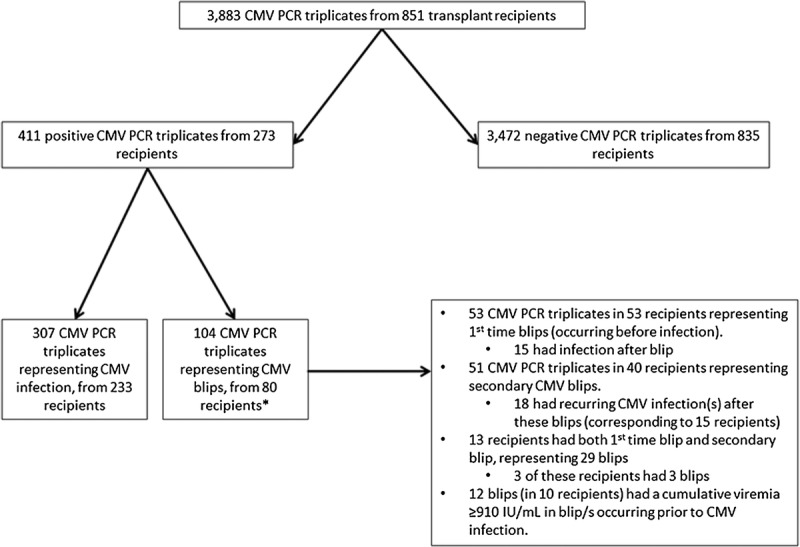
Flowchart of CMV triplicates and corresponding transplant recipients. *By definition, these 80 recipients have a blip before CMV infection or no CMV infection.
The odds ratio (OR) of the first positive CMV PCR being a CMV blip (ie, only CMV PCR triplicates with positive indicator samples, and only the first triplicate per patient) was modeled using logistic regression, investigating potential explanatory variables such as age, sex, calendar year, type of transplantation, risk of CMV infection, CMV viral load in the first positive sample, and administration of anti-CMV treatment between the first positive sample and the succeeding sample. Year of transplant and use of anti-CMV treatment were highly correlated and hence only use of anti-CMV treatment was included to avoid collinearity in the final model.
The analyses were repeated using all triplicates where the indicator sample was positive (ie, more than 1 triplicate per person), using binomial regression and adjusting for repeated measurements within patients.
Multivariate Cox regression was used to explore if CMV blips are a predictor of subsequent CMV infection. The model was adjusted for relevant covariates such as age, sex, calendar year, type of transplantation and CMV IgG serostatus. Patient follow-up was censored at the earliest of (1) 365 days posttransplant, (2) August 14 2015, for patients transplanted after the 14th of August 14, 2014; (3) 28 days after the last CMV PCR within the 1st year posttransplant, 4) death within the 1st year posttransplant or 5) CMV infection. Age at transplantation was divided into 2 categories: ≤ 16 years, >16 years. CMV blips were included as a time dependent variable. Death was included in the model as a competing risk.
First, we modelled the impact of the first CMV blip on CMV infection. The model was then repeated looking at the impact on CMV infection of repeated CMV blips as well as the impact of cumulative CMV viral load in blips (ie, the total viral load experienced in any blip/s occurring before CMV infection in each patient). Based on the observed viral load of the CMV blips, different viral load cut-offs were tested. Due to power, the final model focused at cumulative viremia of the blips ≤910 IU/mL and >910 IU/mL.
Two-sided P values were used for all analyses, and results were considered statistically significant at a level of 0.05 or less. The statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
A total of 851 patients had at least 1 CMV PCR triplicate that fitted 1 of the outcomes described in Figure 1. When comparing these 851 included patients with the 661 excluded patients, age and sex were comparable (P > 0.3). Among the excluded patients, the proportion of SOT recipients was significantly higher compared to the included patients (P < 0.001). Because these patients were excluded either due to missing serostatus, or being CMV IgG D−/R−, or because they did not fulfil the CMV PCR triplicate criteria, serostatus and screening with CMV PCR were not comparable with the included patients.
Of the 851 included patients, 476 (56%) constituted SOT recipients and 375 (44%) constituted HSCT recipients. Most patients were recipients of kidney transplantation (28% of the transplantations), closely followed by MAC and NMA transplantations (23% and 19%, respectively) (Table 1). Heart and UCB transplantations only represented 4% and 2% of the patients, respectively.
TABLE 1.
Characteristics of 851 transplant recipients generating a total of 3,883 CMV PCR triplicates
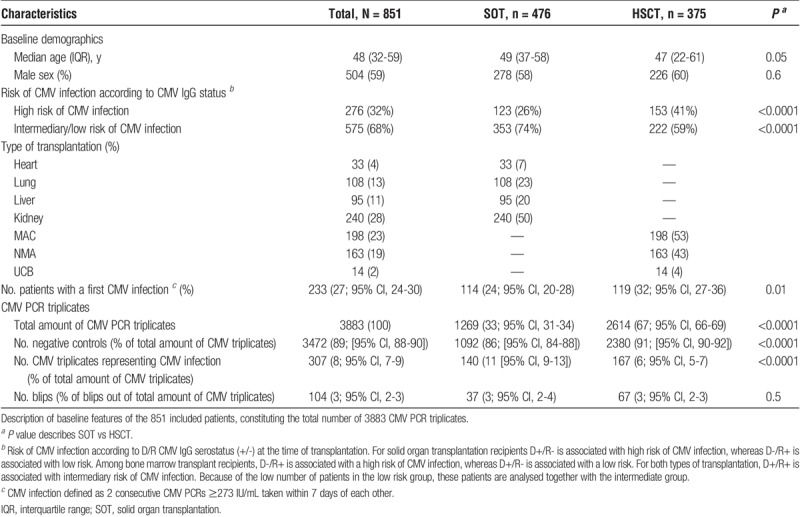
Age and sex were similarly distributed among the 2 groups of recipients (Table 1), although the HSCT recipients tended to be younger compared with the SOT recipients (P = 0.05).
Risk profiling (high and intermediate/low risk; see methods) based on D/R CMV IgG serostatus pretransplantation differed somewhat between SOT and HSCT recipients, with a higher proportion of high-risk serostatus recipients in the HSCT group (P < 0.0001) (Table 1).
CMV PCR Triplicates
In total, the recipients generated 3883 CMV PCR triplicates (Figure 2). 1269 of these came from the 476 (33%) SOT recipients and the remaining 2614 (67%) were generated by the 375 HSCT recipients. The majority (3472/3883 [89%; 95% confidence interval [CI], 88-90]) of the CMV PCR triplicates were negative controls, a distribution that also was found when stratifying for type of transplantation, even though the proportion was significantly higher among HSCT recipients (Table 1). Of the remaining 411 positive CMV PCR triplicates, 307 (8%; 95% CI, 7-9) represented CMV infections and 104 (3%; 95% CI 2-3) represented CMV blips (Figure 3). Of the 307 CMV infection triplicates, 233 were first time infections and the remaining 74 constituted recurrent infection/s. Thus, of the 851 included patients, 233 (27%; 95% CI, 24-30) developed a first episode of CMV infection (Table 1 and Figure 2).
FIGURE 3.
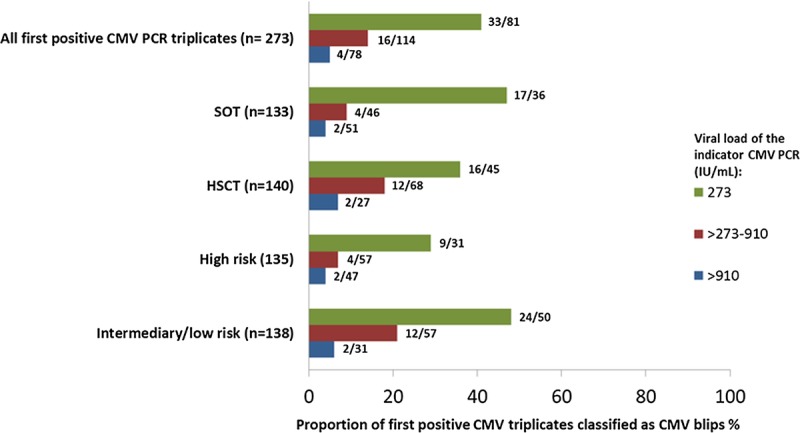
Distribution of CMV blips according to the viral load in the first positive (indicator) CMV PCR* and stratified for type of transplantation and donor/recipients CMV IgG serostatus. For solid organ transplantation recipients D+/R- is associated with high risk of CMV infection, while D-/R+ is associated with low risk. Among bone marrow transplant recipients, D-/R+ is associated with a high risk of CMV infection, whereas D+/R- is associated with a low risk. For both types of transplantation, D+/R+ is associated with intermediary risk of CMV infection. Due to the low number of patients in the low-risk group, these patients are analysed together with the intermediate group. Fractional numbers to the right of the bars indicate the number of triplicates representing a CMV blip within each viral load interval. *Negative control CMV PCR triplicates are not included in the figure.
Out of the 104 CMV PCR triplicates representing CMV blips, 53 were first-time blips that occurred before CMV infection. The remaining 51 CMV blips could either be a secondary blip situated any time between the first blip and any potential subsequent CMV infection/s, or they occurred after a CMV infection (Figure 2).
CMV Blips in First Positive Triplicate
The first positive CMV PCR triplicate (n = 273) of each patient were stratified into 3 different groups depending on the CMV viral load in the first positive indicator CMV PCR sample (Figure 3). The proportion of blips was lower the higher the viral load of the first positive indicator CMV PCR sample of the triplicate, and decreased with increasing viral load. This pattern also persisted after stratifying for type of transplantation and risk associated with CMV IgG serostatus (Figure 3).
The OR of the first positive indicator CMV PCR being a CMV blip rather than CMV infection was modelled for the 273 patients with first positive CMV PCR triplicates (Table 2). Given that the indicator CMV PCR in the triplicate was positive (and therefore that the outcome is either a blip or CMV infection), the odds of experiencing a CMV blip were almost 3 times higher in recipients with intermediary/low risk serostatus (OR, 2.8; 95% CI, 1.2-5.5; P = 0.01) in their first triplicate than recipients with high-risk serostatus. Furthermore, the odds of the triplicate being a CMV PCR blip decreased markedly the higher the viral load of the indicator sample measurement in the triplicate (Table 2). Use of anti-CMV medication within the duration of the triplicate did not predict whether the triplicate was a blip or an infection (P ≥ 0.1); additionally, sensitivity analyses using only triplicates were no treatment had been initiated between the indicator and response samples provided similar results (data not shown). However, when comparing the viral load of the indicator sample between triplicates were treatment was initiated versus no treatment administered in relation to the CMV PCR triplicate, the viral load of the indicator sample was significantly higher among triplicates were treatment was initiated (P < 0.0001).
TABLE 2.
The OR of the first positive indicator CMV PCR a being a CMV blip
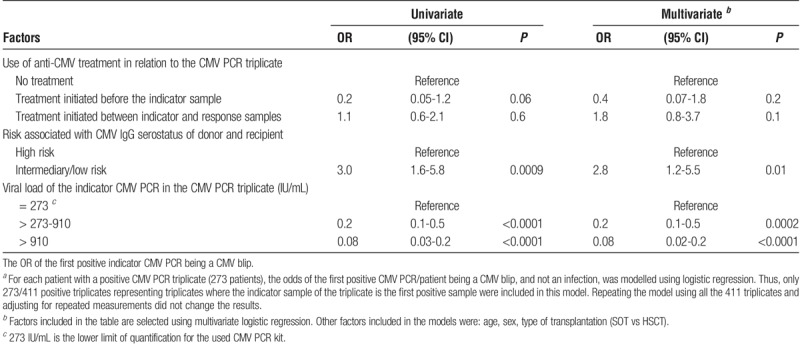
Using the data from all the 411 positive CMV PCR triplicates, the findings made for the first positive triplicate persisted (see Table S1, http://links.lww.com/TXD/A83).
Impact of CMV Blips on CMV Infection
The unadjusted hazard of subsequent CMV infection when comparing a single CMV blip before CMV infection with no blip was 1.8 (95% CI, 1.02-3.2; P = 0.04). However, this signal disappeared after adjustment (hazard ratio [HR], 1.46; 95% CI, 0.8-2.6), P = 0.2). When modelling the number of CMV blips before CMV infection, patients with ≥2 CMV blips had an increased hazard of CMV infection (HR, 2.8; 95% CI, 1.01-7.7; P = 0.05) when compared with no blips, whereas no increased hazard was detected for patients with only 1 blip (HR, 1.2; 95% CI, 0.6-2.4; P = 0.6). However, these estimates became nonsignificant when adding death as a competing risk to the model. This prompted us to investigate the cumulative exposure to viremia in the blips, as presented in the final model (Figure 4). Factors associated with significantly increased risk of CMV infection were: recipients of either lung transplantation (HR, 1.5; 95% CI, 1.03-2.3), P = 0.03) or UCB transplantation (HR, 4.3; 95% CI, 1.6-12.0), P = 0.004), high-risk serostatus (HR, 3.0; 95% CI, 2.3-4.0), P < 0.0001), and a cumulative viral load > 910 IU/mL in CMV blips preceding CMV infection (HR, 4.6; 95% CI, 1.2-17.2; P < 0.02).
FIGURE 4.
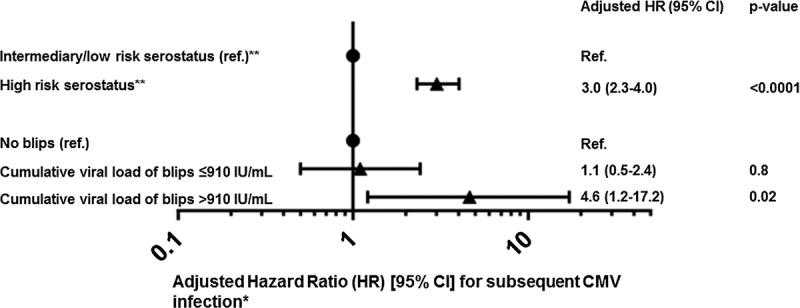
Forest plot of HR [95% CI] for a first CMV infection after transplantation. *Time dependent variables were updated accordingly, and death was included as a competing risk. The model also included age, sex, calendar year, and adjusted for type of transplantation (kidney, liver, heart, lung, myeloablative conditioning transplantation, nonmyeloablative conditioning and umbilical cord blood transplantations). Compared with kidney recipients, lung and umbilical cord blood transplantation recipients had an increased HR of subsequent CMV infection (lung (HR, 1.5; 95% CI, 1.03-2.3; P = 0.03) and umbilical blood cord (HR, 4.3; 95% CI, 1.6-12.0; P = 0.004) respectively. **For solid organ transplantation recipients CMV IgG D+/R- is associated with high risk of CMV infection, while D-/R+ is associated with low risk. Among bone marrow transplant recipients, D-/R+ is associated with a high risk of CMV infection, whereas D+/R- is associated with a low risk. For both types of transplantation, D+/R+ is associated with intermediary risk of CMV infection.
DISCUSSION
In this study, we demonstrate that CMV blips occur in approximately 19% (53/273) of the first positive CMV PCR samples obtained while screening SOT and HSCT recipients with CMV PCR. The CMV blips are particularly frequent if the viral load of the first positive PCR (the indicator sample in Figure 1) is at the detection limit or if the patient has intermediary/low risk serostatus. Furthermore, the cumulative viral load of CMV blips influence the risk of CMV infection, suggesting that these blips at least partly reflect low-level viremia rather than merely intermittent false positive results caused by the technology. Thus, the characteristics of CMV blips are important markers for subsequent infection. Upon detection of a first positive CMV PCR, these observations should be carefully considered by the clinician before initiation of anti-CMV treatment.
The term “viral blips” is well known within the HIV setting and entails detection of episodes of transient HIV viremia in patients who are considered suppressed on combined antiretroviral therapy.11,12,13 Through the years, the interpretation of HIV blips has been discussed extensively. A number of hypotheses have been suggested to explain this observation including random statistical and biological variation around mean viral loads,11 assay variations and artefacts of the PCR technology,21 poor adherence of the patient,12 and emerging drug resistance.22 However, a clear definition of HIV blips does not exist, and as a consequence the impact of HIV blips remains an issue for debate.11,12,13
International clinical practice guidelines based on CMV viral loads remains 1 of the most elusive issues to solve in the care of CMV in transplant recipients, even despite the introduction of an international WHO calibration standard for CMV PCR assays a couple of years ago.3 This is mainly due to the multifaceted challenges surrounding all involved steps in the in the CMV PCR assay procedures, that, if not standardized and optimized, may cause inter and intra assay variability.4,5,6 The present study is performed on plasma samples, using the same commercial assay over time, and the viral load levels have been converted according to the WHO standard for better interpretation of the results. As such, our results can be extrapolated to other sites using a similar approach, although it is recognized that interassay variability is a limitation.4,5,6,7,23,24 Furthermore, if whole blood and not plasma is used, it is likely that the epidemiology of CMV blips may be different and should be explored in settings using this approach.25
Our study does not address the most appropriate indication for when to commence antiviral therapy in patients with CMV infection. We defined CMV infection (as opposed to blips) conservatively. For example, if the first positive sample measured 273 IU/mL, and the next sample also measured 273 IU/mL, the CMV PCR triplicate was considered evidence of ongoing replication (Figure 1). Initiation of treatment in such a patient, provided that he or she is asymptomatic, may not be attractive to the clinician. Some studies would argue that the viral load threshold for initiating antiviral therapy is approximately 4000 IU/mL.26 However, of course, application of any threshold value assumes that the patient is suffering from CMV infection. Our data address the issue of how to interpret the first positive CMV PCR in the course of monitoring for emerging CMV infection. If the viral load is low, blips are relatively frequent.
The clinical dilemma that our results pose is that clinicians will only know that the first positive CMV PCR was a blip in hindsight (ie, until the next PCR is negative). However, we have previously demonstrated that the viral doubling time would allow confirming a positive CMV PCR of 910 IU/mL or less within approximately 4 days without risking the viral load of a potential infection reaching high levels.16 Therefore, our data would suggest a recommendation to confirm low-level CMV PCR results as soon as a positive result has been generated before commencing antiviral therapy.
Some exceptions to this recommendation are well recognized and include isolated CMV infection in the lung (typically seen in lung transplant recipients). In such patients, CMV in plasma may be low (and undetectable in a significant proportion of the patients), whereas the virus replicates and cause disease in the lung compartment.27 Another exception is the CMV gastrointestinal disease, which may not always be associated with a positive CMV PCR signal in plasma/whole blood.28,29 Finally, our results indicate that high-risk recipients were less likely to experience blips compared to recipients of intermediary/low risk CMV IgG serostatus. Therefore, in these circumstances, the first low-level CMV PCR result may prompt initiation of antiviral therapy before the results of a confirmatory result becomes available.
We also found that CMV blips (1 or more) where the CMV PCR signal was above 910 IU/mL was associated with excess risk of subsequent genuine CMV infection, although we had few patients with high cumulative viral load blips, resulting in wider confidence intervals (Figure 4). The interpretation of these findings is that CMV blips may equally reflect transient low level CMV replication in some recipients perhaps due to some level—although varying—immune control of replication. In support of this, the odds of a first positive CMV PCR signal being a blip was higher in recipients with low to intermediate risk CMV IgG serostatus reflecting prior exposure to the infection. Conversely, lower viral load level blips were not associated with subsequent excess risk, and hence may reflect genuine false positive measurements generated by the PCR technology.
In this study, we combined SOT and HSCT recipients to improve power and assess the generalizability of the findings across transplant types. Results derived from univariate comparisons and after adjustment for potential confounders, including transplant type (Table 2), were broadly consistent. This indicates that the type of transplantation had little (if any) impact on our main findings, even though it is of importance to emphasize the associated heterogeneity that is introduced when combining SOT and HSCT in the analyses. Interestingly, use of anti-CMV therapy did also not impact our observations. These findings were consistent when restricting the analysis to triplicates observed without the recipient receiving any anti-CMV therapy, indicating that CMV DNA detected in plasma is reflecting release from cells likely outside the blood compartment, and dynamic changes in CMV PCR in plasma are protracted by the kinetic of release of CMV DNA from these compartments. However, it should also be kept in mind that the introduction of anti-CMV therapy was associated with higher viral load in the indicator sample, which can possibly also explain this lack of effect on the presence of CMV blips.
In conclusion, we have demonstrated that CMV blips are frequent while screening transplant recipients with plasma CMV PCR, and in particular, if the viral load of the first positive PCR is at the detection limit or the patient has intermediary-/low-risk serostatus. These observations need to be considered before initiation of anti-CMV treatment based on first positive CMV PCR samples. Furthermore, the cumulative viral load of CMV blips influence the risk of CMV infection, indicating that the characteristics of CMV blips may be important markers for subsequent infection and that that CMV blips at least partly reflect transient low-level CMV infection in transplant recipients. We encourage other clinics to apply the CMV PCR triplicate model to their cohort data, to further validate the reproducibility of this model.
Supplementary Material
Footnotes
This study is supported by grant [grant number DNRF126] from the Danish National Research Foundation. The funding source had no involvement in any part of the study design, data collection, data analysis, and interpretation of the data or in the writing of this article.
The authors declare no conflicts of interest.
I.P.L. was responsible for study design, collection of data, data analysis and interpretation, and, writing of the article. A.M. was involved in statistical analysis and writing of the article. J.D.L. and C.d.C.-B. were involved in study design, collection of data, data analysis and interpretation, and writing of the article. F.G., M.I., N.K., M.P., A.R., H.S., and S.S.S. provided scientific input and were involved with writing of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1. Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360. [DOI] [PubMed] [Google Scholar]
- 2. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Medicines & Healthcare Products Regulatory Agency. WHO International Standard 1st WHO International Standard for Human Cytomegalovirus for Nucleic Acid Amplification Techniques NIBSC code: 09/162. https://www.google.ca/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwi-Mn5lP7ZAhUmqFQKHWmGCPsQFggnMAA&url=http%3A%2F%2Fwww.nibsc.org%2Fdocuments%2Fifu%2F09-162.pdf&usg=AOvVaw3wRzXCbnueGNNVcT8iPBdt. Published September 2014.
- 4. Preiksaitis JK, Hayden RT, Tong Y, et al. Are we there yet? Impact of the first international standard for cytomegalovirus DNA on the harmonization of results reported on plasma samples. Clin Infect Dis. 2016;63:583–589. [DOI] [PubMed] [Google Scholar]
- 5. Hayden RT, Sun Y, Tang L, et al. Progress in quantitative viral load testing: variability and impact of the WHO Quantitative International Standards. J Clin Microbiol. 2017;55:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayden RT, Gu Z, Sam SS, et al. Comparative performance of reagents and platforms for quantitation of cytomegalovirus DNA by digital PCR. J Clin Microbiol. 2016;54:2602–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayden RT, Preiksaitis J, Tong Y, et al. Commutability of the first World Health Organization International Standard for human cytomegalovirus. J Clin Microbiol. 2015;53:3325–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerna G, Lilleri D, Furione M, et al. Human cytomegalovirus end-organ disease is associated with high or low systemic viral load in preemptively treated solid-organ transplant recipients. New Microbiol. 2012;35:279–287. [PubMed] [Google Scholar]
- 9. Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42:1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraft CS, Armstrong WS, Caliendo AM. Interpreting quantitative cytomegalovirus DNA testing: understanding the laboratory perspective. Clin Infect Dis. 2012;54:1793–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829. [DOI] [PubMed] [Google Scholar]
- 12. Fung IC, Gambhir M, van Sighem A, et al. The clinical interpretation of viral blips in HIV patients receiving antiviral treatment: are we ready to infer poor adherence? J Acquir Immune Defic Syndr. 2012;60:5–11. [DOI] [PubMed] [Google Scholar]
- 13. Sörstedt E, Nilsson S, Blaxhult A, et al. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis. 2016;16:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masquelier B, Pereira E, Peytavin G, et al. ; APROCO/COPILOTE Study Group. Intermittent viremia during first-line, protease inhibitors-containing therapy: significance and relationship with drug resistance. J Clin Virol. 2005;33:75–78. [DOI] [PubMed] [Google Scholar]
- 15. Havlir DV, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA. 2001;286:171–179. [DOI] [PubMed] [Google Scholar]
- 16. Lodding IP, Sengelov H, da Cunha-Bang C, et al. Clinical application of variation in replication kinetics during episodes of post-transplant cytomegalovirus infections. EBioMedicine. 2015;2:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cunha-Bang C, Kirkby N, Sonderholm M, et al. The time course of development and impact from viral resistance against ganciclovir in cytomegalovirus infection. Am J Transplant. 2013;13:458–466. [DOI] [PubMed] [Google Scholar]
- 18. DiDomenico N, Link H, Knobel R, et al. COBAS AMPLICOR: fully automated RNA and DNA amplification and detection system for routine diagnostic PCR. Clin Chem. 1996;42:1915–1923. [PubMed] [Google Scholar]
- 19. da Cunha-Bang C, Sørensen SS, Iversen M, et al. Factors associated with the development of cytomegalovirus infection following solid organ transplantation. Scand J Infect Dis. 2011;43:360–365. [DOI] [PubMed] [Google Scholar]
- 20. Gor D, Sabin C, Prentice HG, et al. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant. 1998;21:597–605. [DOI] [PubMed] [Google Scholar]
- 21. Stosor V, Palella FJ, Jr, Berzins B, et al. Transient viremia in HIV-infected patients and use of plasma preparation tubes. Clin Infect Dis. 2005;41:1671–1674. [DOI] [PubMed] [Google Scholar]
- 22. Macias J, Palomares JC, Mira JA, et al. Transient rebounds of HIV plasma viremia are associated with the emergence of drug resistance mutations in patients on highly active antiretroviral therapy. J Infect. 2005;51:195–200. [DOI] [PubMed] [Google Scholar]
- 23. Hayden RT, Shahbazian MD, Valsamakis A, et al. Multicenter evaluation of a commercial cytomegalovirus quantitative standard: effects of commutability on interlaboratory concordance. J Clin Microbiol. 2013;51:3811–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayden RT, Yan X, Wick MT, et al. Factors contributing to variability of quantitative viral PCR results in proficiency testing samples: a multivariate analysis. J Clin Microbiol. 2012;50:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lisboa LF, Asberg A, Kumar D, et al. The clinical utility of whole blood versus plasma cytomegalovirus viral load assays for monitoring therapeutic response. Transplantation. 2011;91:231–236. [DOI] [PubMed] [Google Scholar]
- 26. Martín-Gandul C, Pérez-Romero P, Sánchez M, et al. Determination, validation and standardization of a CMV DNA cut-off value in plasma for preemptive treatment of CMV infection in solid organ transplant recipients at lower risk for CMV infection. J Clin Virol. 2013;56:13–18. [DOI] [PubMed] [Google Scholar]
- 27. Lodding IP, Schultz HH, Jensen JU, et al. Cytomegalovirus viral load in bronchoalveolar lavage to diagnose lung transplant associated CMV pneumonia. Transplantation. 2018;102:326–332. [DOI] [PubMed] [Google Scholar]
- 28. Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1687–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Razonable RR, Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev. 2013;26:703–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


