Abstract
Background:
Chronic fatigue syndrome (CFS) is characterized by prolonged fatigue and other physical and neurocognitive symptoms. Some studies suggest that CFS is accompanied by disruptions in the number and function of various lymphocytes. However, it is not clear which lymphocytes might influence CFS symptoms.
Purpose:
To determine if patient reported fatigue symptoms and physical functioning scores significantly changed across time with lymphocyte counts as evidence of a relation among chronic fatigue symptoms and the immune response.
Methods:
The current longitudinal, naturalistic study assessed the cellular expression of three lymphocyte subtypes -- natural killer (NK) cells (CD3-CD16+ and CD3-CD56+) and naïve T cells (CD4+CD45RA+) -- to determine whether changes in lymphocytes at 4 time points across 18 months were associated with clinical outcomes, including CFS symptoms, physical functioning, and vitality, among patients with chronic fatigue.. Latent growth curve models were used to examine the longitudinal relationship between lymphocytes and clinical outcomes.
Results:
Ninety-three patients with Fukuda-based CFS and seven with non-CFS fatigue provided study data. Results indicated that higher proportions of naïve T cells and lower proportions of NK cells were associated with worse physical functioning, whereas higher proportions of NK cells (CD3-CD16+) and lower proportions of naïve T cells were associated with fewer CFS symptoms.
Conclusion:
These findings suggest that lymphocytes are modestly related to clinical outcomes over time.
Keywords: chronic fatigue syndrome, functional status, longitudinal, lymphocyte subsets
Introduction
Background
Chronic fatigue syndrome (CFS), as defined by the 1994 criteria [1], is a debilitating condition involving six months or more of new onset fatigue accompanied by at least four other symptoms, such as muscle pain, concentration difficulties, post-exertional malaise, and unrefreshing sleep that result in functional limitations. The Institute of Medicine (IOM) recently proposed a new case definition that retains the emphasis on chronic, new onset fatigue and functional limitations, in addition to post-exertional malaise, unrefreshing sleep, and cognitive impairment or orthostatic intolerance, termed systemic exertion intolerance disease (SEID) (IOM, 2015) [2]. Comparisons of the 1994 and IOM criteria found comparable proportions of patients meeting both CFS and SEID criteria [3]. The community prevalence of CFS has been estimated at 0.42% [4], with most cases reported among women. Because CFS is a diagnosis of exclusion, without sensitive or specific biomarkers, patients with presumptive CFS should be evaluated carefully for other conditions that could cause a similar set of symptoms [1]. Despite the absence of a consistent biomarker, a good deal of interest centers on the potential role of physiological systems, such as the immune system, in the onset and course of CFS.
Differences in subsets of T and natural killer (NK) lymphocytes have been found between CFS patients and healthy controls, in that patients with CFS often have lower counts of NK cells and/or reduced cytotoxic function of their NK cells compared to healthy control patients, but these differences are inconsistent [5]. Clinical observations that a virus-like illness often precedes the onset of CFS have inspired hypotheses of immune system activation, which would be reflected in increasing numbers of T cell subsets in CFS patients, including memory T cells [6]. Contrary to this expectation, several studies of CFS patients have reported reductions in the number or percentage of naïve T cells (i.e., T cells bearing the CD45RA antigen), with no change in memory T cells (T cells bearing the CD45RO antigen) [7–9]. Other studies have found no differences in naïve T cells between CFS patients and controls [10].
It has also been suggested that immune system down-regulation characterizes CFS, most notably a “low NK cell syndrome” subset of CFS characterized by chronic fatigue, loss of interest in one’s usual activities, and low-grade fever [11]. Among other functions, NK cells are implicated in immunoregulation and response to infection. Compared to healthy controls, CFS patients have been reported to exhibit reductions in the absolute number [12–14] as well as the percentage [15–18] of NK cells (CD16+, CD56+). However, other studies have found no differences in NK cells between CFS patients and controls [10,19–23].
Beyond case versus control comparisons, few studies have assessed the associations of immune parameters with measures of clinical status. Hassan and colleagues [20] found that the percentage of NK cells (CD16+) was positively associated with vitality scores on the Short Form-36 (SF-36) [24], but not with physical functioning scores. Furthermore, although the association of immune system dysregulation with CFS has been described as “persistent” [25], longitudinal studies are rare. Hardcastle and colleagues [26] examined 24 patients with CFS whose NK and T cells increased over a six-month period in patients classified as moderately and severely affected, respectively. Severely affected patients had poorer functional status than moderately affected patients, but the association of changes over time in immune and functional status variables was not examined. Levine and colleagues [22] reported the results of serial NK levels in eight family members with CFS; they were mostly in the low or low-normal range for two to five measurements per person over two years. Brenu and colleagues [15] assessed 67 patients with CFS and 21 controls on 3 occasions (baseline, 6 months, and 12 months), reporting reductions in the percentage of NK cells over time. The patients in the latter two studies were assumed to be symptomatic and to meet CFS case criteria at each assessment. Nevertheless, it would be informative to investigate the relationship between proportions of lymphocyte subsets and fluctuations in symptom severity and functional status in CFS patients.
An involvement of the immune system with CFS is intuitively and clinically appealing – an appeal that might help to explain the proliferation of studies on this topic. Several different explanations have been offered to account for the variability in immunologic findings noted above. Lyall and colleagues [5] concluded that findings of low levels of NK cells may be attributed to variability in studies’ methodology and quality. Alternatively, given the heterogeneous nature of CFS, lymphocyte variables might be relevant only to a subset of CFS patients, such as patients with the low NK cell syndrome proposed by Aoki and colleagues [11].
The present study examined the NK and naïve T cell lymphocyte subsets that have been found to discriminate between CFS patients and healthy controls in previous research. These NK and naïve T cell subsets were chosen for this study in order to characterize changes in lymphocyte levels over repeated measurements, and to determine the association between lymphocyte subsets and clinical status over time. Given the findings from the literature reviewed above, we offered the hypothesis that increases in naïve T cells and reductions in NK cells would be associated with more severe CFS symptoms and worse functional status over time.
Methods
Participants and Procedure
As described in detail previously [27], study participants were 100 patients aged 18–65 years who underwent extensive evaluations to determine whether they met the 1994 criteria [1] for CFS (N = 93) or non-chronic fatigue (N =7) The non-chronic fatigue group consisted of patients who reported having fatigue or fatigue symptoms, but who were not necessarily diagnosed with CFS. Evaluations included a physical examination, laboratory tests (including a complete blood count with differential and erythrocyte sedimentation rate), a review of all medical records, a self-report questionnaire on the presence or absence of all major and minor CFS case criteria, and a structured, computer-assisted psychiatric diagnostic interview, the Diagnostic Interview Schedule (DIS) Version III-A [28]. (All but one patient (99%), who did not experience unrefreshing sleep, appeared to meet SEID criteria [2] based on reviews of physical examination and self-report data).
This was a naturalistic, longitudinal study of the course of CFS in the patient sample. Participants completed 4 research appointments at 6-month intervals over 18 months. All procedures were reviewed and approved by the institutional review board at the authors’ institution. After hearing a description of the study, patients were asked to provide written informed consent to participate. At each research appointment, they provided a blood sample and completed a physical examination, a structured interview, and a questionnaire. Participants received honoraria for completing each of the four appointments.
Measures
Descriptive and clinical status measures were gathered by questionnaires and interviews. Descriptive variables included age, sex, length of illness, race (recoded as White versus non-White), employment status (recoded as working part- or full-time versus not working), and medications. Clinical status variables included a continuous measure of CFS-related symptoms and measures of functional status related to vitality and physical functioning, as described below.
CFS symptoms
Patients rated how often they experienced the nine CFS symptoms specified by Fukuda et al. [1] – fatigue, memory or concentration problems, sore throat, painful lymph nodes, muscle pain, joint pain, headaches, unrefreshing sleep, and post-exertion malaise – on a 5-point scale (0=not at all to 4=constantly), yielding possible scores ranging from 0 to 36. As previously reported, this measure had good discriminant validity and acceptable internal consistency, and did not decrease significantly over time in this sample [27].
Functional status
The SF-36 [24] assesses functional status on eight dimensions. Each score ranges from 0 to 100, with higher scores denoting better functioning and the normal range characterized as 80 and above. Scores for physical functioning and vitality were retained for analysis because of their demonstrated sensitivity to change over time in CFS samples [29], including demonstrable improvement over time in this sample [27].
Lymphocyte subset analysis
The laboratory and procedures described in [30] were used for this sample. Whole blood specimens were collected in EDTA, stored at room temperature, delivered to the laboratory within 24 hours, and analyzed on delivery. Two-color flow cytometry analysis was used for lymphocyte phenotyping, with markers chosen on the basis of previous research indicating their ability to differentiate between CFS patients and controls, and to reduce the probability of Type I error. Dual marker analysis was used for CD3CD56 and CD3CD16 (NK cells) and CD4CD45RA (naïve cells). The percentages of CD3-CD16+, CD3–56+, and CD4+CD45RA+ were retained for analysis.
Data Analysis
A series of descriptive analyses characterized the sample. Latent growth curve models (LGCMs) were used to assess change over time in the immune parameters. In a LGCM, latent variables are used to describe a pattern of change and are represented by repeated measures of the same manifest variables. The latent variables are the initial (intercept) value and the slope value of the measure of interest; the slope value indicates the rate of change in the measure over time. Inter-individual variability can then be measured around the mean intercept and slope values. Change patterns and variability in a measure can be linear, quadratic, or cubic.
In LGCMs for the present study, the percentage of each lymphocyte was used as the measure of interest, and was assessed by using the intercept value and the slope value. The intercept represented the mean percentage of the lymphocyte from all subjects in the sample, and the slope represented the average rate of change in the percentage of the lymphocyte across four time points (i.e., visits at baseline, 6, 12, and 18 months). The intercept and slope values were represented and interpreted as correlation coefficients among the variables. In the LGCM analyses, the continuous measures of clinical status over time (CFS symptoms, vitality, and physical functioning) were modeled as time-variant covariates to allow for slowing or acceleration of improvement over time. All LGCMs were assessed with standard goodness of fit measures with the following parameter estimates indicative of acceptable fit: root mean square estimation of approximation (RMSEA) ≤0.050–0.080 (CI = 0.000–0.080), comparative fit index (CFI) ≥0.90, and standardized root mean square residual (SRMR) <0.08 [31]. All LGCM analyses were conducted by using Mplus software (base program), version 7.0.
Results
Participants
As reported previously [27], this sample had an average age of approximately 44 years (SD = 9.70); was predominantly female (82%), menopausal (54%), and White (92%); and had been ill for an average of slightly more than six years. Less than half (40%) were employed part- or full-time. Most (73%) were taking antidepressants, some (31%) were taking sedatives (narcotic analgesics, benzodiazepines), and nearly half (47%) denied alcohol use. None used tobacco products.
Association of Lymphocyte Subsets and Clinical Outcomes
Table 1 shows the average percentages of the lymphocyte and the clinical status variables at each study visit.
Table 1.
Mean and standard deviation for the clinical and lymphocyte variables over time
| Research Appointments |
||||
|---|---|---|---|---|
| Variable | Index visit | 6 months | 12 months | 18 months |
| CFS symptoms | 21.72 (4.38) |
21.03 (4.87) |
20.81 (5.69) |
20.41 (6.04) |
| SF-36 Vitality | 16.69 (13.76) |
24.40 (18.12) |
24.82 (18.37) |
26.14 (19.26) |
| SF-36 Physical Functioning | 44.75 (23.62) |
47.77 (24.00) |
50.16 (26.63) |
49.40 (26.00) |
| CD3-CD16+ (%) | 8.12 (9.34) |
7.66 (5.02) |
7.31 (4.99) |
7.79 (5.07) |
| CD3-CD56+ (%) | 4.32 (3.25) |
5.08 (3.37) |
5.08 (4.24) |
5.96 (4.33) |
| CD4+CD45 RA+ (%) | 19.79 (8.73) |
19.23 (8.02) |
19.07 (7.87) |
19.41 (7.78) |
CD3-CD16+
For this lymphocyte, a linear LGCM provided an acceptable fit with the data, with χ2 (41) = 62.40, p = 0.02, RMSEA = 0.079 (0.034–0.117), CFI = 0.90, and SRMR = 0.04. Significant variability was evident around the mean intercept value of this lymphocyte (est = 9.67, SE = 3.34, p = 0.004), but no significant change was noted in the slope value across time (est = −2.43, SE = 1.28, p = 0.06). None of the clinical status measures were significant predictors of immune status until Time 4 (18 months), when CFS symptoms (est = 0.16, SE = 0.07, p = 0.03) and physical functioning (est = 0.04, SE = 0.02, p = 0.05) both exhibited small effect sizes that were significantly and positively correlated with the percentage of CD3-CD16+. These results indicated that over time, worse CFS symptoms and better physical functioning were both associated with higher percentages of CD3-CD16+. Figure 1 depicts a LGCM of the longitudinal associations between CD3-CD16+ and physical functioning.
Figure 1.
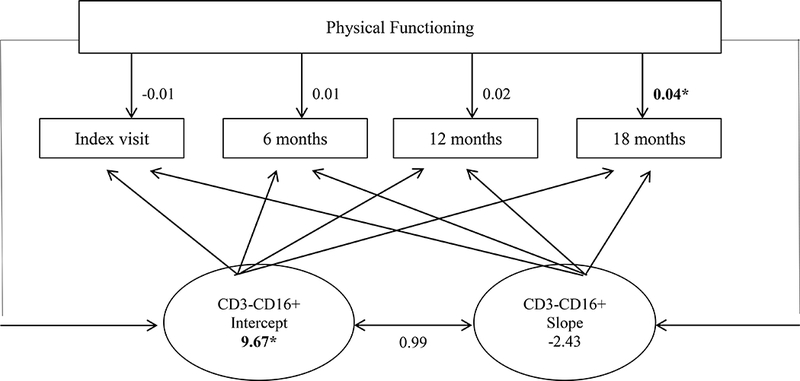
Diagram of the latent growth curve model of CD3-CD16+ and physical functioning over time (*p < .05)
CD3-CD56+
For this lymphocyte, a linear LGCM provided a good fit with the data, with χ2 (41) = 45.36, p = 0.30, RMSEA = 0.036 (0.000–0.086), CFI = 0.97, and SRMR = 0.04. Significant variability was evident around the mean intercept value for this lymphocyte (est = 5.58, SE = 1.78, p = 0.002), but no significant change was noted in the slope value (est = −1.21, SE = 0.98, p = 0.22). Neither CFS symptoms (est = −0.04–0.13, SE = 0.07–0.10, p = 0.16–0.71) nor vitality (est = −0.02–0.14, SE = 0.08–0.09, p = 0.13–0.83) were significant predictors of CD3-CD56+. Physical functioning was significantly and positively correlated with the percentage of CD3-CD56+, but only at Time 4 (est = 0.04, SE = 0.02, p = 0.04) and this effect size was small. This result indicates that patients with higher levels of physical functioning also had higher percentages of CD3-CD56+ at 18 months.
CD4+CD45RA+
For this lymphocyte, a quadratic LGCM provided a better fit for the change pattern than did a linear LGCM. The quadratic model was a good fit with the data, with χ2 (40) = 49.15, p = 0.15, RMSEA = 0.052 (0.000– 0.097), CFI = 0.97, and SRMR = 0.08. The lymphocyte percentage at the intercept value (est = 26.57, SE = 4.47, p < 0.001) revealed significant variability around the mean. Over time, the slope value was not significant for linear (est = −7.44, SE = 5.69, p = 0.19) or quadratic slope (est = 2.41, SE = 1.53, p = 0.12). The covariance between the quadratic slope and intercept was positive and significant (est = 2.64, SE = 1.30, p = 0.04), indicating that a higher initial percentage of CD4+CD45RA+ predicted a larger percentage increase over time. At Time 4 only, CFS symptoms and physical functioning were both negatively correlated with the percentage of CD4+CD45RA+, suggesting that more severe CFS symptoms (est = −0.20, SE = 0.08, p = 0.01) as well as better physical functioning (est = −0.05, SE = 0.02, p = 0.01) were both associated with lower percentages of this lymphocyte. Again, this effect was only found at Time 4, and the effect size was small.
Discussion
The goal of the current study was to discover correlations among lymphocyte cell surface markers and clinical outcomes associated with CFS (i.e., fatigue symptoms, physical functioning, and vitality) over an 18-month time period. This study of 100 people with chronic fatigue who we previously reported as showing improvements in vitality and physical functioning over time [27], showed no significant changes in the percentage of lymphocyte expression markers over the 18 months. This was reflected in the non-significant slope values from the LGCMs. Consistent with our hypothesis, we found that higher percentages of naïve T cells and lower percentages of NK cells were associated with worse physical functioning at 18 months. However, contrary to our hypothesis, we also found that higher percentages of NK cells (CD3-CD16+) and lower percentages of naïve T cells were associated with fewer CFS symptoms at 18 months, but not at any other time.
The association of higher percentages of NK cells with better physical functioning and less severe CFS symptoms at the 18-month follow-up appears to be consistent with findings from other studies, in which healthy controls had higher levels of NK cells than did CFS patients [15–18, 32]. However, we also found some contradictory associations between immunological parameters and clinical outcomes. In our study, higher levels of the NK cell subtypes CD3-CD16+ and CD4+CD45 RA+ were associated with worse physical functioning and worse CFS symptoms, whereas several other studies have reported that lower levels of NK cells were associated with worse CFS symptoms and physical functioning [5–6].
Explaining Contradictory Findings
Several different explanations are possible for these contradictory results. First, our study only measured the percentages of the lymphocyte cell surface marker expression, as opposed to measuring functional activity among these markers. Therefore, it is possible that there are functional changes occurring within the immune cells that are not necessarily reflected by the expression of cell surface markers [10, 12, 15]. For example, NK cells are cytotoxic, and their ability to perform cell lyses does not necessarily correlate with the percentage or total count of cell surface makers, particularly in patients with CFS [10, 12, 15, 32]. In the current study if we had conducted molecular assays that more specifically measured functional activity and changes within the NK cells then perhaps we would have uncovered some informative relationships between cell function and clinical outcomes. Thus, our broader measure of cell surface expression may not have been intricate enough to detect a clear relationship among CFS symptoms and clinical outcomes.
Second, it is possible that strong relationships between immunological parameters and clinical outcomes simply do not exist in patients with CFS, despite findings from some older studies [32–33]. Although studies have supported the association of immunological parameters with CFS symptoms [19–20, 29], others show inconsistent associations with changes in immunological parameters that predict CFS symptoms [5, 34–36]. The estimates from our LGCMs were small in absolute value, suggesting weak correlations between immunological parameters and clinical outcome measures. However, given our small sample of 100 patients, these results should be interpreted with caution and ideally replicated with a larger sample size. We did not include a control group in the current study because the focus was to better understand how immune cell changes occur in patients with CFS over time, as well as how these changes may correlate with changes in clinical outcome status (e.g., non-CFS or CFS status). Regardless, including a control group of healthy individuals without CFS may have provided some clarity on how immune cells may change and compare across time.
Third, method variance might account for the contradictory results. Cellular and self-reported clinical and functional status measures vary in terms of the level of objectivity or subjectivity, and also focus on different levels of analysis. Cellular measures are objective, specific and at a fine-grained level, whereas self-report measures are subjective and broader in scope.
Relationships between immunological and clinical variables
Overall, the existing body of research might indicate that the relationship between immunological parameters and clinical outcomes is complex and is likely influenced by many factors. For example, the length of time that a person experiences CFS symptoms may partially account for differences in lymphocyte cell counts and functions. That is, lymphocyte counts could vary considerably across time depending on how long the patient has reported symptoms (or depending on how long the patient has been diagnosed with CFS) compared to when the lymphocytes are actually measured from a blood sample. For this reason, attempting to uncover a relationship among lymphocyte counts and/or activity and CFS symptoms could be difficult given the potential fluctuation in the immune system physiology. In the current study, the patients all differed in terms of the number of years (average was 6.16 years +/− 4.05) since they were first diagnosed with CFS, and thus, there could have been much variability in the immune cell counts, which may have made it difficult to detect a clear relationship to clinical outcomes.
There are also other physiological biomarkers such as cytokines, chemokines, and other lymphocyte cell surface markers that we did not measure in the current study, but have been found to be significant predictors of CFS in previous studies. For example, Brenu and colleagues [12] have conducted multiple biomarker studies with CFS patients and have reported increased anti-inflammatory cytokines (i.e., IL-10) and the pro-inflammatory cytokines (i.e., TNF-alpha and IFN-gamma), which may also influence the cytotoxic ability of the NK cells, as the surface markers Granzyme A and B and Perforin are responsible for cytotoxicity and can fluctuate in patients with CFS [12, 13, 15, 37]. Other researchers have reported increased expression of the CD69 marker and decreased expression of the CD25 surface marker in patients with CFS [10], which our study did not measure. Instead we examined a panel of markers including: CD2, CD8, CD4, CD20, CD16+/−, CD56+/−, CD45RO/RA, but found only slight changes in the CD3-CD16+, CD3-CD56+, and CD4+CD45 RA+ that are presented here. Although our panel of surface markers was broad, perhaps we omitted a few other markers such as the CD69, CD25, or CD11b that have been found to be significantly associated with CFS outcomes in other studies.
Perhaps, the relationship among CFS and immune function might be mediated by psychological variables, such as depression, mood dysfunction, or by other health comorbidities such as a viral infection, chronic pain, or gut dysfunction, as several studies support a psychological or emotion component to CFS [39–43, 10, 33]. Depression in the current study was not common among our participants as only 4% of the sample had reported being diagnosed with depression [27]. Thus, we could not examine depression as a variable in our data analysis. However, future studies may benefit from examining how such variables may mediate the relationship between immune cells and clinical outcomes. Further, CFS is also a complex and multisystem condition where other biological systems such as the cardiovascular and gastrointestinal systems are likely affected. Therefore, future studies could examine how disruptions and functional changes within these systems might be contributing to the symptoms and health outcomes of CFS.
Despite the apparent inconsistency of some of our findings with those of other studies, the present work is in agreement with a few other studies supporting weak correlations among immune cell counts/function and clinical outcomes in patients with CFS [5, 34]. Further, our results add to the very limited number of longitudinal studies of immune variables in CFS [15, 26]. In addition, our use of the LGCM enabled us to study simultaneous change in variables over time while allowing for individual variability within the sample as well as for the influence of other variables on the outcome variable. With this approach, we could directly examine changes in lymphocytes over time while assessing how these changes were influenced by CFS symptoms and physical functioning. LGCMs are usually conducted with larger datasets with at least 300 or more participants, thus although appropriate for the type of data, our smaller sample of 100 may not have been enough to detect more significant changes with larger effects. Further, we do show that there are some changes among the lymphocyte cell markers, but these changes are small and they do not significantly reflect changes in clinical outcomes.
Conclusions
In conclusion, this study sought to characterize the association of immune function and clinical outcomes over time in a patient sample with fatigue of long duration. We found insignificant change over time in lymphocyte markers, and the changes that were observed were not strongly predictive of changes in clinical outcomes. More research is needed to understand the role of immunological variables in CFS symptoms. Ideally, future longitudinal studies might benefit from using functional lymphocyte molecular assays to examine how lymphocyte activity changes across time as symptoms first appear and then progress and/or improve across time. Such studies may offer meaningful and consistent patterns of immune cells changes that could be correlated with CFS symptoms and outcomes.
Acknowledgements
We thank Dr. Raymond Harris for his editing.
Sources of Support
This research was supported by NIH grant U19AI38429, Project 4.
Author Biographies
Melissa Mehalick, PhD
Dr. Mehalick is a research psychologist in the Department of Neurotrauma at the Naval Medical Research Center. She currently studies the physiological mechanisms and behavioral outcomes associated with blast-induced neurotrauma. Her research background also includes studies on the pharmacological management of chronic pain, as well as on enteric infections and the gut microbiome.
Karen B. Schmaling, PhD
Dr. Schmaling is a clinical psychologist and professor of psychology at Washington State University. Her research interests include psychosocial factors in chronic illnesses, and diversity in higher education.
Daniel E. Sabath, MD, PhD
Dr. Sabath is a hematopathologist who heads the hematology division in the Department of Laboratory Medicine at the University of Washington School of Medicine. His research interests include the development of new molecular diagnostic tests and tests for minimal residual disease in cancer.
Dedra Buchwald, MD
Dr. Buchwald is a Professor in the Elson S. Floyd College of Medicine and the director of the Initiative for Research and Education to Advance Community Health at Washington State University. Her research interests include health disparities, chronic unexplained clinical conditions, and twin studies.
Footnotes
Disclosure of Interest
The authors report no conflicts of interest.
References
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A, et al. Chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med 1994;121(12):953–959. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Beyond myalgic ecncephalomyelitis/chronic fatigue syndrome: Redefining an illness. Washington DC: The National Academies; 2015. [PubMed] [Google Scholar]
- 3.Jason LA, Sunnquist M, Brown A, McManimen, Furst J. Reflections on the Institute of Medicine’s systemic exertion intolerance disease. Pol Arch Med Wewn 2015;125:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med 1999;159(18):2129–2139. [DOI] [PubMed] [Google Scholar]
- 5.Lyall M, Peakman M, Wessely S. A systematic review and critical evaluation of the immunology of chronic fatigue syndrome. J Psychosom Res 2003;55:79–90. [DOI] [PubMed] [Google Scholar]
- 6.Hanson SJ, Gause W, Natelson B. Detection of immunologically significant factors for chronic fatigue syndrome using neural-network classifiers. Clin Diagn Lab Immunol 2001;8: 658–662. doi: 10.1128/CDLI.8.3.658-662.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klimas NG, Salvato FR, Morgan R, Fletcher MA. Immunologic abnormalities in chronic fatigue syndrome. J Clin Microbiol 1990;28(6):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straus SE, Fritz S, Dale JK, Gould B, Strober W. Lymphocyte phenotype and function in the chronic fatigue syndrome. J Clin Immunol 1992;13(1):30–40. [DOI] [PubMed] [Google Scholar]
- 9.Tirelli U, Marotta G, Improta S, Pinto A. Immunological abnormalities in patients with chronic fatigue syndrome. Scand J Immunol 1994;40:601–608. [DOI] [PubMed] [Google Scholar]
- 10.Curriu M, Carrillo J, Massanella M, Rigau J, Alegre J, Puig J, et al. Screening NK-, B-, and T-cell phenotype and function in patients suffering from Chronic Fatigue Syndrome. J Transl Med 2013;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoki T, Miyakoshi H, Usuda Y, Herberman RB. Low NK syndrome and its relationship to chronic fatigue syndrome. Clin Immunol Immunopathol 1993;69(3):253–265. [DOI] [PubMed] [Google Scholar]
- 12.Brenu EW, van Driel ML, Staines DR, Ashton KJ, Ramos SB, Keane J, et al. Immunological abnormalities as potential biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med 2011;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maher KJ, Klimas NG, Fletcher MA. Chronic fatigue syndrome is associated with diminished intracellular perforin. Clin Exp Immunol 2005;142:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tirelli U, Pinto A, Marotta G, Crovato M, Quaia M, De Paoli P, et al. Clinical and immunological study of 205 patients with chronic fatigue syndrome: A case series from Italy. Arch Intern Med 1993;153:116–120. [PubMed] [Google Scholar]
- 15.Brenu EW, van Driel ML, Staines DR, Ashton KJ, Hardcastle SL, Keane J, et al. Longitudinal investigation of natural killer cells and cytokines in chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med 2012;10(88):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caligiuri M, Murray C, Buchwald D, Levine H, Cheney P, Peterson D, et al. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. J Immunol 1987;139:3306–3313. [PubMed] [Google Scholar]
- 17.Gupta S, Vayuvegula B. A comprehensive immunological analysis in chronic fatigue syndrome. Scand J Immunol 1991;33:319–327. [DOI] [PubMed] [Google Scholar]
- 18.Kibler R, Lucas DO, Hicks MJ, Poulos BT, Jones JF. Immune function in chronic active Epstein-Barr virus infection. J Clin Immunol 1985;5(1):46–54. [DOI] [PubMed] [Google Scholar]
- 19.Brenu EW, Staines DR, Baskurt OK, Ashton KJ, Ramos SB, Christy RM, et al. Immune and hemorheological changes in Chronic Fatigue Syndrome. J Transl Med 2010;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan IS, Bannister BA, Akbar A, Weir W, Bofill M. A study of the immunology of the chronic fatigue syndrome: Correlation of immunologic parameters to health dysfunction. Clinical Immunol Immunopath 1998;87(1):60–67. [DOI] [PubMed] [Google Scholar]
- 21.Landay AL, Jessop C, Lennette ET, Levy JA. Chronic fatigue syndrome: Clinical condition associated with immune activation. Lancet 1991;338:707–12. [DOI] [PubMed] [Google Scholar]
- 22.Levine PH, Whiteside TL, Friberg D, Bryant J, Colclough G, Hernerman RB. Dysfunction of natural killer activity in a family with chronic fatigue syndrome. Clinical Immunol Immunopath 1998;88(1):96–104. [DOI] [PubMed] [Google Scholar]
- 23.Natelson BH, LaManca JJ, Denny TN, Vladutiu A, Oleske J, Hill N, et al. Immunological parameters in chronic fatigue syndrome, major depression, and multiple sclerosis. Am J Med 1998;105(3A):43S–49S. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 25.Whiteside TL, Friberg D. Natural killer cells and natural killer cell activity in chronic fatigue syndrome. Am J Med 1998;105(3A): 27S–34S. [DOI] [PubMed] [Google Scholar]
- 26.Hardcastle SL, Brenu EW, Johnston S, Nguyen T, Huth T, Ramos S, Staines D, Marshall-Gradisnik S. Longitudinal analysis of immune abnormalities in varying severities of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients. J Trans Med 2015:13:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmaling KB, Fiedelak JI, Katon WJ, Bader JO, Buchwald DS. Prospective study of the prognosis of unexplained chronic fatigue in a clinic-based cohort. Psychosom Med 2003;65:1047–54. [DOI] [PubMed] [Google Scholar]
- 28.Robins L, Helzer J. Diagnostic interview schedule (DIS): version III-A. St. Louis (MO): Department of Psychiatry, Washington University School of Medicine; 1985. [Google Scholar]
- 29.Komaroff AL, Fagioli LR, Doolittle TH, Gandek B, Gleit MA, Guerriero RT, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med 1996;101:281–290. [DOI] [PubMed] [Google Scholar]
- 30.Sabath DE, Barcy S, Koelle DM, Zeh J, Ashton S, Buchwald D. Cellular immunity in monozygotic twins discordant for chronic fatigue syndrome. J Infect Dis 2002;185:828–832. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Wang X. Structural equation modeling In Balding J, Cressie NA, Fitzmaurice GH, Goldstein H, Johnstone IM, Molenberghs G, Scott DW, Smith AF, Tsay RS, Weisberg S (Eds), Wiley Series in Probability and Statistics: Vol. 1. Introduction: Model Evaluation (pp. 17–23). West Sussex, United Kingdom: Higher Education Press, 2012. [Google Scholar]
- 32.Barker E, Fujimura SF, Fadem MB, Landay AL, Levy JA. Immunologic abnormalities associated with chronic fatigue syndrome. Clin Infectious Dis 1994;18:36–41. [DOI] [PubMed] [Google Scholar]
- 33.Masuda A, Nozoe S, Matsuyama T, Tanaka H. Psychobehavioral and immunological characteristics of adult people with chronic fatigue and patients with chronic fatigue syndrome. Psychosom Med 1994;56:512–518. [DOI] [PubMed] [Google Scholar]
- 34.Peakman M, Deale A, Field R, Mahalingam M, Wessely S. Clinical improvement in chronic fatigue syndrome is not associated with lymphocyte subsets of function or activation. Clin Immunol Immunopath 1997;82:83–91. [DOI] [PubMed] [Google Scholar]
- 35.Swanink CMA, Vercoulen JHMM, Galama JMD, Roos MTL, Meyaard L, van der Ven-Jongekrijg J, et al. Lymphocyte subsets, apoptosis, and cytokines in patients with chronic fatigue syndrome. J Infect Dis 1996;173:460–463. [DOI] [PubMed] [Google Scholar]
- 36.Wilson A, Hickie I, Lloyd A, Hadzi-Pavlovic D, Boughton C, Dwyer J, et al. Longitudinal study of outcome of chronic fatigue syndrome. BMJ 1994;308:756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saiki T, Kawai T, Morita K, Ohta M, Saito T, Rokutan K, Ban N. Identification of marker genes for differential diagnosis of chronic fatigue syndrome. Mol Med 2008; 14:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robertson MJ, Schacterle RS, Mackin GA, Wilson SN, Bloomingdale KL, Ritz J, Komaroff AL. Lymphocyte subset differences in patients with chronic fatigue syndrome, multiple sclerosis and major depression. Clin Exper Immunol 2005;141:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadlandsmyth K, Vowles KE. Does depression mediate the relation between disability in chronic fatigue syndrome sufferers? J Psychosom Res 2009;66:31–35. [DOI] [PubMed] [Google Scholar]
- 40.Taillefer SS, Kirmayer LJ, Robbins JM, Lasry JC. Psychological correlates of functional status in chronic fatigue syndrome. J Psychosom Res 2002;53:1097–1106. [DOI] [PubMed] [Google Scholar]
- 41.Valero S, Saez-Francas N, Calvo N, Alegre J, Casas M. The role of neuroticism, perfectionism, and depression in chronic fatigue syndrome. A structural equation modeling approach. Compr Psychiatry 2013;54:1061–1067. [DOI] [PubMed] [Google Scholar]
- 42.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12; 1365–1371. [DOI] [PubMed] [Google Scholar]
- 43.Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: Effects of age, duration, of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett 2008; 29; 902–910. [PubMed] [Google Scholar]


