Abstract
Background:
Primary prevention of acute rheumatic fever is achieved by proper antibiotic treatment of group A β-hemolytic streptococcal (GAS) pharyngitis.
Methods:
To assess noninferiority of oral amoxicillin to intramuscular benzathine penicillin G (IM BPG). Children (2 to 12 years) meeting enrollment criteria were randomized 1:1 to receive antibiotic treatment in 2 urban outpatient clinics in Egypt and Croatia.
Results:
A total of 558 children (Croatia = 166, Egypt = 392) were randomized, with 368 evaluable in an intention-to-treat (ITT) analysis, and 272 evaluable in the per protocol (PP) analysis. In Croatia, ITT and PP treatment success rates were comparable for IM BPG and amoxicillin (2.5% difference vs 1.1% difference, respectively). In Egypt, amoxicillin was not comparable with IM BPG in ITT analysis (15.1% difference), but was comparable in PP analysis (−9.3% difference).
Conclusion:
If compliance is a major issue, a single dose of IM BPG may be preferable for treatment of GAS pharyngitis.
Keywords: streptococcal pharyngitis, randomized clinical trial, amoxicillin, intramuscular benzathine penicillin G
Introduction
Primary prevention of acute rheumatic fever (ARF) is accomplished by proper identification and antibiotic treatment of group A β-hemolytic streptococcal (GAS) pharyngitis.1–4 ARF results from an autoimmune response to infection with group A streptococcus and its sequelae, rheumatic heart disease (RHD), the most common cause of acquired cardiac disease worldwide.5 In affluent populations, ARF is now rare with an annual incidence estimated at 0.5 cases per 100 000 school-age children. In contrast, ARF remains an endemic disease in developing countries, with annual incidence rates ranging from 100 to 200 per 100 000 school-aged children and is a major cause of cardiovascular mortality.6 Antibiotic therapy can shorten the clinical course of GAS pharyngitis and prevent suppurative complications. However, the primary objective of antibiotic therapy is eradication of the GAS organism from the pharynx, which is necessary for prevention of ARF.1,7,8
In the 1950s, several studies demonstrated the efficacy of parenteral penicillin in the prevention of ARF in military recruits with tonsillitis.2,9–15 Since then, penicillin (either oral penicillin V or injectable benzathine penicillin) has remained the treatment of choice because it is cost-effective, has a narrow spectrum of activity, long-standing proven efficacy, and to date, penicillin-resistant GAS has not been documented. The World Health Organization, the American Heart Association, and the Infectious Diseases Society of America (IDSA) recommend oral penicillin V 2 to 4 times a day for 10 full days or a single intramuscular injection of benzathine benzyl-penicillin in patients without penicillin allergy.16–18 Oral antibiotics provide some advantages over parenteral treatment by reducing patient discomfort, needle-associated complications, providing shorter exposure to the medication, and less severe allergic reactions; however adherence to the prescribed oral treatment regimen is critical for treatment success. Thus, IM BPG is the preferred treatment for patients who are unlikely to complete a full 10-day course of oral therapy.17,19,20
Recent studies have suggested that a single daily dose of oral amoxicillin for 10 days may be as efficacious as oral penicillin V 2 to 3 times daily for 10 days.21–24 Amoxicillin is globally available, well-tolerated, and inexpensive, thus a single daily dose of amoxicillin may present a cost-effective and convenient alternative to oral or injectable penicillin in both high-and low-resource settings.
To date, there are no published reports in the literature directly comparing amoxicillin with injectable ben-zathine penicillin for treatment of GAS pharyngitis. Additionally, although the burden of ARF/RHD is largely in low-and middle-income countries, few clinical studies have been undertaken to assess treatment options for GAS pharyngitis in these regions. Thus, we compared the microbiological efficacy of a single intra-muscular injection of penicillin with a 10-day daily dose of oral amoxicillin for the treatment GAS pharyngitis in children in low-resource clinical settings.
Materials and Methods
This randomized, open label multicenter clinical trial— known as the Treatment of Pharyngitis Study (TOPS)— was nested in a larger descriptive study designed to evaluate signs and symptoms of GAS pharyngitis in children and develop new clinical guidelines for diagnosis of GAS pharyngitis in low-resource settings without a laboratory. Data from this study describing differences in presentation of GAS pharyngitis have been published elsewhere.25,26
Patients
Between August 2001 and April 2003, children between the ages of 2 and 12 years presenting to outpatient clinics in Cairo, Egypt and Zagreb, Croatia with complaint of sore throat were enrolled in the parent descriptive study after informed consent.26 Children were excluded if the parent/guardian reported oral antibiotic use in the past 3 days or injectable penicillin in past 28 days prior to screening, had a history of rheumatic fever or rheumatic heart disease, required hospitalization for any reason at the time of enrollment, or had previously been enrolled in the study.
After enrollment, a physical examination was performed, demographic and clinical data were collected, and 2 throat swabs were simultaneously obtained for standard throat culture and immediate GAS screening using a rapid antigen detection kit. Patients with a positive rapid test were offered enrollment in the TOPS substudy. Children with a known hypersensitivity to penicillin or likely to require treatment with other antimicrobials during the study period were excluded at the time of enrollment. Children with a positive rapid antigen detection test and negative throat culture completed their prescribed treatment regimen, however, were excluded from the trial.
Study Design
Patients were randomized to receive oral amoxicillin suspension (750 mg once a day for all weight categories) given once daily for 10 days or a single dose of intra-muscularly administered benzathine penicillin G (IM BPG; 600 000 units if body weight <27 kg; 1.2 million units if body weight ≥27 kg). All drugs distributed to patients were obtained from local sources in Egypt and Croatia. Parents/guardians of children in the amoxicillin arm were instructed to administer the antibiotic at the same time every day for 10 days. All patients were scheduled for a follow-up visit (days 21–28), during which a physical examination and repeat throat culture were performed. Compliance, adverse events and drug tolerability were also assessed by parental report during this visit.
In each study site, participants were stratified by age (2 to 5 years and >5 years) and randomly assigned on a 1:1 basis (with permuted blocks of 6 to 10) to a treatment group by sequential selection from a random number table. Treatment allocation results generated by the study coordinator in Baltimore and shipped in presealed envelopes to study sites. Randomization results were kept in sealed envelopes and all study team members were blinded from the contents until treatment administration. Because of obvious differences in routes of treatment administration, masking of patients and physicians was not feasible. However, the primary endpoints were objective microbiologic measures that were analyzed by laboratory personnel blinded to the treatment.
This study was conducted in accordance with Good Clinical Research Practice guidelines and the Declaration of Helsinki. The study protocol, questionnaires, and informed consent forms were approved by both local and national ethics committees at participating clinical sites, the World Health Organization (WHO), and the Committee on Human Research at the Johns Hopkins Bloomberg School of Public Health. Informed consent was obtained from a parent or legal guardian and child assent was obtained from all children aged 5 years or older. All study sites used a common study protocol and data collection forms which were translated into local language.
Outcome Measures
The primary outcome of the study was bacteriologic treatment success, which was defined as eradication of GAS from the pharynx at the follow-up visit. Eradication was defined as no GAS present on the throat culture. Compliance with the amoxicillin treatment regimen was assessed at the follow-up visit. Parents/guardians were given a Whatman no. 3 filter paper strip and asked to dip it into the child’s urine once on the seventh day of treatment, allow it to air dry, place it in an envelope and bring it back to the clinic at the follow-up visit. Filter strips were analyzed for antimicrobial activity using a modification of the technique of Markowitz and Gordis.27 The primary measure of compliance was the presence of anti-microbial activity in the urine-impregnated filter paper strips. For those who did not return the filter paper strip, compliance was measured by parent/guardian report during the exit interview. Patients who did not fulfill at least one of these criteria were considered noncompliant.
Laboratory Methods
Two throat swabs were simultaneously obtained from each patient for both culture and rapid antigen assay using sterile cotton-tipped swabs. Throat culture specimens were plated on 5% sheep blood agar plates and incubated anaerobically at 37.0°C and examined at 24 and 48 hours for the presence of β-hemolytic streptococci and confirmed by bacitracin disc.28 The rapid test (Strep OIA MAX; Biostar Laboratories, Denver, CO) was performed according to the instructions in the manufacturer’s package insert by trained study personnel. This rapid test had internal positive and negative controls and has a reported sensitivity range of 79.5% to 98.1% and specificity of 96.9% to 99.0%, compared with throat culture.29–33 Both sites independently were provided with uniform training and regular site visits to ensure uniform laboratory procedures for both throat culture and rapid tests.
Statistical Methods
Assuming no difference in the treatment effect of the 2 study arms and a 90% eradication rate, we estimated that a sample size of 154 patients per treatment arm would have 80% power to demonstrate non-inferiority (defined as the upper 95% confidence limit of the treatment effect difference of ≤10%). Thus, if the treatment success for IM BPG was 90.0%, amoxicillin success would fall between 80.0% and 90.0% to be considered noninferior. For both the intent-to-treat (ITT) and per-protocol (PP) analysis, we were unable to reach the estimated sample size in each country, thus may not have had the power to detect a difference between treatment groups between countries.
Differences in discrete variables (GAS positivity rates, gender, and dichotomized age) were evaluated with 2-way contingency tables. Differences in continuous variables (age, weight, temperature) were tested using Student t tests. P < .05 (2-tailed) was considered significant. Logistic regression methods were used to compare crude risk difference between treatments, risk difference measures adjusted for age and gender in both the ITT and PP analysis, and compliance in the PP analysis for the outcome of treatment success. Data analyses were conducted using SAS v 9.1 and STATA 10.0 software.34,35
Analysis Groups
The ITT analysis included all patients who were enrolled in the study meeting the inclusion and exclusion criteria. The PP analysis included all patients in the ITT group who were compliant with treatment and returned for the follow-up visit.
Results
Patient Characteristics
A total of 558 children (Croatia n = 166, Egypt n = 392) were randomized to receive IM BPG or oral amoxicillin (Figure 1). Patients with discordant (negative) throat culture results from the initial visit were excluded from the trial, thus 121 patients in Croatia and 247 patients in Egypt were included in the ITT analysis. A total of 111 patients in Croatia and 161 in Egypt were classified as compliant and were eligible for the PP analysis (Table 1). Within countries, the demographic and clinical characteristics for patients were similar in both treatment arms; however, those in Croatia were more likely to be included in the analysis.
Figure 1.
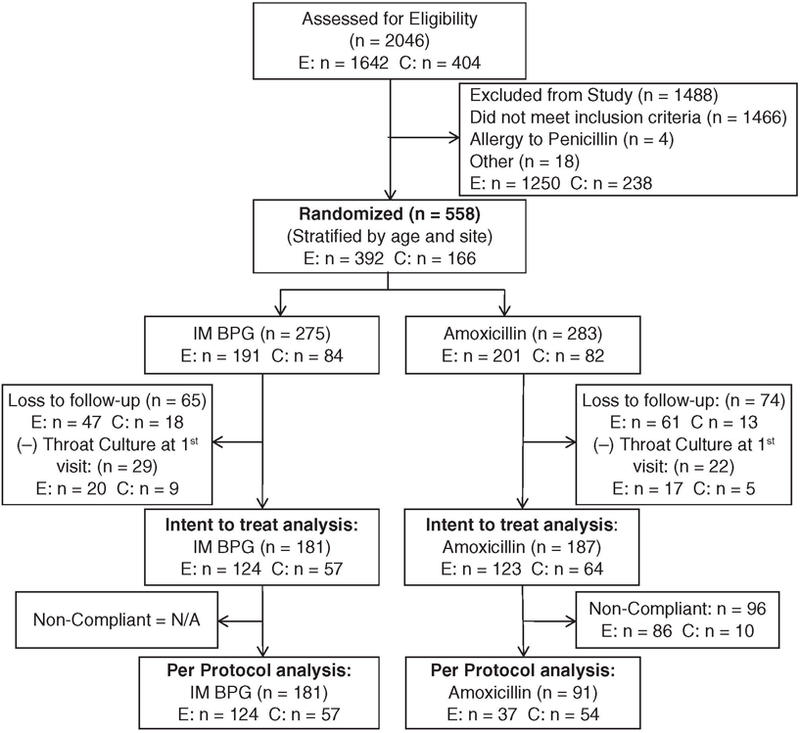
Flow diagram of enrollment and inclusion into the Treatment of Pharyngitis Study (TOPS) trial (E, Egypt; C, Croatia)
Table 1.
Patient Characteristics by Treatment Group
| Croatia (n = 166) |
Egypt (n = 392) |
|||||
|---|---|---|---|---|---|---|
| Patient Characteristics | IM BPG | Amoxicillin | IM BPG | Amoxicillin | ||
| Patients enrolled; n (%) | 84 (50.6) | 82 (49.4) | 191 (48.7) | 201 (51.3) | ||
| GAS + by throat culture; n (%) | 71 (82.6) | 76 (92.7) | 154 (80.6) | 162 (80.6) | ||
| Age in years; n (%) | ||||||
| <5 | 32 (38.1) | 34 (41.5) | 84 (44.0) | 88 (43.8) | ||
| ≥5 years | 52 (61.9) | 48 (58.5) | 107 (56.0) | 113 (56.2) | ||
| Gender; n (%) | ||||||
| Male | 55 (65.5) | 46 (56.1) | 115 (60.2) | 112 (55.7) | ||
| Female | 29 (34.5) | 36 (43.9) | 76 (39.8) | 89 (44.3) | ||
| Mean age in years ± SD | 5.6 ± 2.6 | 5.7 ± 2.4 | 5.3 ± 2.6 | 5.4 ± 2.5 | ||
| Mean weight in kg ± SD | 23.4 ± 9.1 | 24.0 ± 8.1 | 19.5 ± 7.4 | 18.9 ± 6.1 | ||
| Mean temperature in °C ± SD | 37.1 ± 0.8 | 37.6 ± 0.9 | 37.9 ± 0.9 | 37.7 ± 0.8 | ||
| Intent to treat analysis; n (%) | 57 (67.9) | 64 (78.0) | 124 (64.9) | 123 (61.2) | ||
| Per protocol analysis; n (%) | 57 (67.9) | 54 (65.9) | 124 (64.9) | 37 (18.4) | ||
Abbreviations: IM BPG, intramuscular benzathine penicillin G; GAS, group A β-hemolytic streptococcal; SD, standard deviation.
Treatment Success
In the ITT analysis, the difference in bacteriologic treatment success between the 2 antibiotic regimens varied by country. In Croatia, amoxicillin was not inferior to IM BPG, with no differences observed when further controlled by age and gender (2.5% difference, 95% confidence interval [CI]: −13.8, 18.9; Table 2). In Egypt, amoxicillin continued to be inferior to IM BPG after controlling for age and gender (−15.1% difference, 95% CI: −26.6, −3.5). Furthermore, in the PP analysis (excluding noncompliant patients), amoxicillin was not inferior to IM BPG in Croatia (1.1% difference, 95% CI: −16.2, 18.5), but remained inferior in Egypt, although no longer significant (−9.3% difference, 95% CI: −26.3, 7.8). When looking at specific stratum differences, the largest treatment difference (in favor of IM BPG) was seen in Egyptian girls younger than 5 years (38.2%, P < .001). However, this difference was no longer significant in the PP analysis, once compliance was controlled for (data not shown).
Table 2.
Bacteriological Treatment Success by Country
| Treatment Group |
Treatment Differencea |
Adjusted Treatment Differenceb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Amoxicillin Success Percentage (n/Total) |
IM BPG Success Percentage (n/Total) |
Percentage Difference |
95% CI | Percentage Difference |
95% CI | |||||
| Intention to treat analysis | |||||||||||
| Croatia (n = 121) | 70.3 (45/64) | 66.7 (38/57) | 3.6 | −12.9, 20.2 | 2.5 | −13.8, 18.9 | |||||
| Egypt (n = 247) | 61.8 (76/123) | 75.8 (94/124) | −14.0c | −25.5, −2.59 | −15.1c | −26.6, −3.4 | |||||
| Per protocol analysis | |||||||||||
| Croatia (n = 111) | 68.5 (37/54) | 66.7 (38/57) | 1.8 | −15.6, 19.3 | 1.1 | −16.2, 18.5 | |||||
| Egypt (n = 161) | 67.6 (25/37) | 75.8 (94/124) | −8.2 | −25.1, 8.6 | −9.3 | −26.3, 7.8 | |||||
Abbreviations: IM BPG, intramuscular benzathine penicillin G; 95% CI, 95% confidence interval.
Difference in percentage success rates (amoxicillin – IM BPG).
Adjusted analysis: intention to treat, controlled for age and gender; per protocol, controlled for compliance, age, and gender (amoxicillin – IM BPG).
Statistically significant: treatment difference P = .016; adjusted treatment difference P = .011.
Treatment Compliance
Adherence to the amoxicillin treatment regimen varied significantly by country: In Croatia, 84.4% were classified as compliant by either detection of antimicrobial activity on the filter strip or patient report as compared to 30.1% in Egypt (P < .001; Table 3). In all strata (age and gender), children in Croatia were significantly more likely to be classified as compliant (P < .001). There were no observed differences in adherence by age or gender within countries.
Table 3.
Compliance With Amoxicillin Treatment Regimen by Patient Characteristic
| Patient Characteristics |
n (%) |
||||
|---|---|---|---|---|---|
| Croatia (n = 64) | Egypt (n = 123) | ||||
| Compliance measuresa |
54 (84.4) | 37 (30.1) | |||
| Positive urine filter strip only |
2 (3.7) | 0 (0.0) | |||
| Parent report only |
2 (3.7) | 0 (0.0) | |||
| Both measures of compliance |
50 (92.5) | 37 (100.0) | |||
| Gender | |||||
| Boysa | 33/39 (84.6) | 18/65 (27.7) | |||
| Girlsa | 21/25 (84.0) | 19/58 (32.8) | |||
| Age (years) | |||||
| <5a | 22/24 (91.7) | 17/57 (29.8) | |||
| ≥5a | 32/40 (80.0) | 20/66 (30.6) | |||
Statistical significance (P < .05) associated with the treatment compliance between sites.
Tolerability
Treatment-related adverse events occurred in 5.3% (10/187) of the patients in the amoxicillin arm, including skin rash 1.6% (3/187), diarrhea 1.6% (3/187), vomiting 0.5% (1/187), extreme sweating 0.5% (1/187), itching 0.5% (1/187), and nausea 0.5% (1/187). In the IM BPG group, 67.4% (122/181) reported discomfort at the site of injection. None of the adverse events required hospitalization, stopping the treatment regimen, medical attention, or withdrawal from the trial.
Discussion
Our study was designed to assess whether oral amoxicillin was not inferior to the long-standing recommended therapy of IM BPG in a low- and middle-income country setting. We found significant intersite variation in treatment success rates for both antibiotic regimens that were likely to be largely influenced by differences in patient compliance with the oral amoxicillin treatment regimen. In Croatia, the vast majority of patients were compliant with the oral treatment regimen; however, in Egypt, patient compliance was extremely low. The differences in the results of the ITT analysis reflect this discrepancy between sites. In Croatia, amoxicillin was not inferior to IM BPG in both the ITT and PP analyses. In Egypt, amoxicillin was inferior to IM BPG in the ITT but not the PP analyses; however, in all analyses, children who received IM BPG still had higher treatment success rates than those who were treated with amoxicillin.
Treatment success rates for the amoxicillin regimen were much lower than initially expected. Studies of once daily amoxicillin have reported treatment success rates ranging from 94.5% to 100.0%; in our study, treatment success ranged from 61.8% in Egypt to 70.3% in Croatia.21–24 Recent publications evaluating the effectiveness of IM BPG for GAS treatment have shown decreased rates reported in the range of 65% to 70%, which are comparable to our study, which ranged from 66.7% (Croatia) to 75.8% (Egypt).37 Additionally, our findings are consistent with recent reports of reduced microbiologic efficacy of both penicillin V and IM BPG in children with acute pharyngitis.38–40 To date, there have been no reports of penicillin-resistant GAS; how-ever, the continued decline in treatment success with penicillin should be monitored.
Compliance with the amoxicillin treatment regimen is likely to be a major driver in the high rates of treatment failure in patients in Egypt. High rates of asymptomatic GAS carriage in Egyptian populations as compared to Croatia also have an impact on treatment success, since carriers are likely to continue to test positive for GAS despite treatment.37
Several limitations of the study may have affected our results and deserve mention. Because of financial constraints, we were unable to conduct M protein gene (emm) typing to identify GAS subtypes before and after therapy. Thus, it is possible that some patients who may have been reinfected after successful eradication of the initial infection were misclassified as treatment failures. Similarly, we were unable to conduct serologic analyses that would have allowed us to classify those patients who failed treatment as carriers. However, both carriers and those who reacquired GAS after successful treatment should have been equally distributed between the 2 treatment groups and therefore the observed treatment difference between regimens should not have been affected. Additionally, although we used a randomized, controlled design, both patients and clinicians were aware of the study assignments. Another limitation in our study was that both study drugs were obtained locally rather than from a central source, which could have resulted in differences in microbiologic efficacy of either treatment regimen between countries.
Finally, the power of this study to demonstrate statistically significant noninferiority of amoxicillin to IM BPG is limited by the sample size, which was inadequate because of high loss to follow up in both study sites. Although our study is underpowered, our findings add to the body of evidence that suggest that amoxicillin may provide an effective, well-tolerated treatment for GAS pharyngitis without the safety issues associated with a penicillin injection. Our study also highlights the importance of compliance and the extent to which it may be a problem, particularly in low-resource settings. Thus, in settings where compliance remains a major concern, a single dose of IM BPG may be preferable for the treatment of GAS pharyngitis.
In some countries, there is resistance to using injectable penicillin because of the perceived higher risk of anaphylaxis and the discomfort of intramuscular injections.8 However, more than 50 years of experience with penicillin has shown that although toxic reactions to intramuscular penicillin have been reported, severe reactions are exceedingly rare, especially in children.16,19 In our study, we did not observe any severe reactions to IM BPG.
When selecting an antibiotic regimen for the treatment of GAS pharyngitis, clinicians will consider various factors, including availability, bacteriologic and clinical efficacy, ease, and likelihood of patient adherence to the treatment regimen (based on frequency and duration of administration and palatability), cost, spectrum of antimicrobial activity, and potential side effects. There is no single antibiotic regimen that is 100% effective in eradicating GAS from the pharynx; thus there are multiple factors that will guide therapeutic choice. Recent studies have explored short course (3 to 6 days) treatments, which may be more convenient and improve compliance.41 These shorter course therapies should be considered and tested in low-resource settings, particularly in regions where ARF continues to be a major public health concern.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article:
This study was supported by USAID and the World Health Organization.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Catanzaro FJ, Rammelkamp CH Jr, Chamovitz R. Prevention of rheumatic fever by treatment of streptococcal infections. II. Factors responsible for failures. N Engl J Med 1958;259:53–57. [DOI] [PubMed] [Google Scholar]
- 2.Denny FW, Wannamaker LW, Brink WR, Rammelkamp CH Jr, Custer EA. Prevention of rheumatic fever: treatment of the preceding streptococcic infection. JAMA 1950;143:151–153. [DOI] [PubMed] [Google Scholar]
- 3.Rammelkamp CH, Wannamaker LW, Denny FW. The epidemiology and prevention of rheumatic fever. Bull N Y Acad Med 1952;28:321–334. [PMC free article] [PubMed] [Google Scholar]
- 4.Stollerman GH. Factors that predispose to rheumatic fever. Med Clin North Am 1960;44:17–28. [DOI] [PubMed] [Google Scholar]
- 5.Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet 2005;366:155–168. [DOI] [PubMed] [Google Scholar]
- 6.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005;5:685–694. [DOI] [PubMed] [Google Scholar]
- 7.Shulman ST. Complications of streptococcal pharyngitis. Pediatr Infect Dis J 1994;13(1 Suppl 1):S70–S74. [PubMed] [Google Scholar]
- 8.WHO/ISFC. Strategy for controlling rheumatic fever/rheumatic heart disease, with emphasis on primary prevention: memorandum from a joint WHO/ISFC meeting. Bull World Health Organ 1995;73:583–587. [PMC free article] [PubMed] [Google Scholar]
- 9.Houser HB, Wannamaker LW, Rammelkamp CH, et al. Prophylaxis of acute rheumatic fever by treatment of the preceding streptococcal infection with various amounts of penicillin. J Lab Clin Med 1950;36:839. [PubMed] [Google Scholar]
- 10.Bennike T, Brochner-Mortensen K, Kjaer E, Skadhauge K, Trolle E. Penicillin therapy in acute tonsillitis, phlegmonous tonsillitis and ulcerative tonsillitis. Acta Med Scand 1951;139:253–274. [DOI] [PubMed] [Google Scholar]
- 11.Bland EF, Duckett Jones T. Rheumatic fever and rheumatic heart disease: a twenty year report on 1000 patients followed since childhood. Circulation 1951;4:836–843. [DOI] [PubMed] [Google Scholar]
- 12.Wannamaker LW, Rammelkamp CH Jr, Denny FW, et al. Prophylaxis of acute rheumatic fever by treatment of the preceding streptococcal infection with various amounts of depot penicillin. Am J Med 1951;10:673–695. [DOI] [PubMed] [Google Scholar]
- 13.Denny FW, Wannamaker LW, Hahn EO. Comparative effects of penicillin, aureomycin and terramycin on streptococcal tonsillitis and pharyngitis. Pediatrics 1953;11: 7–13. [PubMed] [Google Scholar]
- 14.Chamovitz R, Catanzaro FJ, Stetson CA, Rammelkamp CH Jr. Prevention of rheumatic fever by treatment of previous streptococcal infections. I. Evaluation of benzathine penicillin G. N Engl J Med 1954;251:466–471. [DOI] [PubMed] [Google Scholar]
- 15.Brumfitt W, Slater JD. Treatment of acute sore throat with penicillin: a controlled trial in young soldiers. Lancet 1957;272:8–11. [DOI] [PubMed] [Google Scholar]
- 16.Bisno AL, Gerber MA, Gwaltney JM Jr, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Infectious Diseases Society of America. Clin Infect Dis 2002;35: 113–125. [DOI] [PubMed] [Google Scholar]
- 17.Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation 2009;119: 1541–1551. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO Expert Consultation on Rheumatic Fever and Rheumatic Heart Disease. In: Rheumatic Fever and Rheumatic Heart Disease: Report of a WHO Expert Consultation, Geneva October 29 to November 1, 2001 Geneva, Switzerland: World Health Organization. [Google Scholar]
- 19.Robertson KA, Volmink JA, Mayosi BM. Antibiotics for the primary prevention of acute rheumatic fever: a meta-analysis. BMC Cardiovasc Disord 2005;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baltimore RS. Re-evaluation of antibiotic treatment of streptococcal pharyngitis. Curr Opin Pediatr 2010;22:77–82. [DOI] [PubMed] [Google Scholar]
- 21.Clegg HW, Ryan AG, Dallas SD, et al. Treatment of streptococcal pharyngitis with once-daily compared with twice-daily amoxicillin: a noninferiority trial. Pediatr Infect Dis J 2006;25:761–767. [DOI] [PubMed] [Google Scholar]
- 22.Feder HM Jr, Gerber MA, Randolph MF, Stelmach PS, Kaplan EL. Once-daily therapy for streptococcal pharyngitis with amoxicillin. Pediatrics 1999;103:47–51. [DOI] [PubMed] [Google Scholar]
- 23.Lennon DR, Farrell E, Martin DR, Stewart JM. Once-daily amoxicillin versus twice-daily penicillin V in group A beta-haemolytic streptococcal pharyngitis. Arch Dis Child 2008;93:474–478. [DOI] [PubMed] [Google Scholar]
- 24.Shvartzman P, Tabenkin H, Rosentzwaig A, Dolginov F. Treatment of streptococcal pharyngitis with amoxycillin once a day. BMJ 1993;306:1170–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rimoin AW, Hamza HS, Vince A, et al. Evaluation of the WHO clinical decision rule for streptococcal pharyngitis. Arch Dis Child 2005;90:1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rimoin AW, Walker CL, Chitale RA, et al. Variation in clinical presentation of childhood group A streptococcal pharyngitis in four countries. J Trop Pediatr 2008;54: 308–312. [DOI] [PubMed] [Google Scholar]
- 27.Markowitz M, Gordis L. A mail-in technique for detecting penicillin in urine: application to the study of maintenance of prophylaxis in rheumatic fever patients. Pediatrics 1968;41:151–153. [PubMed] [Google Scholar]
- 28.Johnson DR, Kaplan EL, Sramek J, et al. Laboratory Diagnosis of Group A Streptococcal Infections Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 29.Daly JA, Korgenski EK, Munson AC, Llausas-Magana E. Optical immunoassay for streptococcal pharyngitis: evaluation of accuracy with routine and mucoid strains associated with acute rheumatic fever outbreak in the intermountain area of the United States. J Clin Microbiol 1994;32: 531–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harbeck RJ. Evaluation of two rapid antigen assays, Bio-Star Strep A OIA and Pacific Biotech CARDS O.S., and culture for detection of group A streptococci in throat swabs. J Clin Microbiol 1995;33:3365–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roe M, Kishiyama C, Davidson K, Schaefer L, Todd J. Comparison of BioStar Strep A OIA optical immune assay, Abbott TestPack Plus Strep A, and culture with selective media for diagnosis of group A streptococcal pharyngitis. J Clin Microbiol 1995;33:1551–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerber MA, Tanz RR, Kabat W, et al. Optical immunoassay test for group A beta-hemolytic streptococcal pharyngitis. An office-based, multicenter investigation. JAMA 1997;277:899–903. [PubMed] [Google Scholar]
- 33.Hart AP, Buck LL, Morgan S, Saverio S, McLaughlin JC. A comparison of the BioStar Strep A OIA rapid antigen assay, group A Selective Strep Agar (ssA), and Todd-Hewitt broth cultures for the detection of group A Streptococcus in an outpatient family practice setting. Diagn Microbiol Infect Dis 1997;29:139–145. [DOI] [PubMed] [Google Scholar]
- 34.SAS. SAS v9.1. Cary, NC: SAS Corporation; 2002. [Google Scholar]
- 35.STATA. STATA 7.0. College Station, TX: STATA Coporation; 2001. [Google Scholar]
- 36.Kaplan EL, Oakes JM, Johnson DR. Unexpected individual clinical site variation in eradication rates of group A streptococci by penicillin in multisite clinical trials. Pediatr Infect Dis J 2007;26:1110–1116. [DOI] [PubMed] [Google Scholar]
- 37.el-Kholy AM, Sorour AH, Rotta J, Guirguirs N. Group A beta hemolytic streptococci in skin lesions among an Egyptian school children population. J Hyg Epidemiol Microbiol Immunol 1973;17:316–322. [PubMed] [Google Scholar]
- 38.Kaplan EL, Johnson DR. Unexplained reduced microbiological efficacy of intramuscular benzathine penicillin G and of oral penicillin V in eradication of group a streptococci from children with acute pharyngitis. Pediatrics 2001;108:1180–1186. [DOI] [PubMed] [Google Scholar]
- 39.Pichichero ME. Explanations and therapies for penicillin failure in streptococcal pharyngitis. Clin Pediatr (Phila) 1992;31:642–649. [DOI] [PubMed] [Google Scholar]
- 40.Ovetchkine P, Levy C, de la Rocque F, Boucherat M, Bingen E, Cohen R. Variables influencing bacteriological outcome in patients with streptococcal tonsillopharyngitis treated with penicillin V. Eur J Pediatr 2002;161: 365–367. [DOI] [PubMed] [Google Scholar]
- 41.Altamimi S, Khalil A, Khalaiwi KA, Milner R, Pusic MV, Al Othman MA. Short versus standard duration antibiotic therapy for acute streptococcal pharyngitis in children. Cochrane Database Syst Rev 2009(1): 10.1002/14651858.CD004872.pub2. [DOI] [PubMed] [Google Scholar]


