Abstract
Background
Immunotherapy with PD-1 or PD-L1 blockade fails to induce a response in about 80% of patients with unselected non-small cell lung cancer (NSCLC), and many of those who do initially respond then develop resistance to treatment. Agonists that target the shared interleukin-2 (IL-2) and IL-15Rβγ pathway have induced complete and durable responses in some cancers, but no studies have been done to assess the safety or efficacy of these agonists in combination with anti-PD-1 immunotherapy. We aimed to define the safety, tolerability, and activity of this drug combination in patients with NSCLC.
Methods
In this non-randomised, open-label, phase 1b trial, we enrolled patients (aged ≥18 years) with previously treated histologically or cytologically confirmed stage IIIB or IV NSCLC from three academic hospitals in the USA. Key eligibility criteria included measurable disease, eligibility to receive anti-PD-1 immunotherapy, and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients received the anti-PD-1 monoclonal antibody nivolumab intravenously at 3 mg/kg (then 240 mg when US Food and Drug Administration [FDA]-approved dosing changed) every 14 days (either as new treatment or continued treatment at the time of disease progression) and the IL-15 superagonist ALT-803 subcutaneously once per week on weeks 1–5 of four 6-week cycles for 6 months. ALT-803 was administered at one of four escalating dose concentrations: 6, 10, 15, or 20 μg/kg. The primary endpoint was to define safety and tolerability and to establish a recommended phase 2 dose of ALT-803 in combination with nivolumab. Analyses were per-protocol and included any patients who received at least one dose of study treatment. This trial is registered with ClinicalTrials.gov, number NCT02523469; phase 2 enrolment of patients is ongoing.
Findings
Between Jan 18, 2016, and June 28, 2017, 23 patients were enrolled and 21 were treated at four dose levels of ALT-803 in combination with nivolumab. Two patients did not receive treatment because of the development of inter-current illness during enrolment, one patient due to leucopenia and one patient due to pulmonary dysfunction. No dose-limiting toxicities were recorded and the maximum tolerated dose was not reached. The most common adverse events were injection-site reactions (in 19 [90%] of 21 patients) and flu-like symptoms (15 [71%]). The most common grade 3 adverse events, occurring in two patients each, were lymphocytopenia and fatigue. A grade 3 myocardial infarction occurred in one patient. No grade 4 or 5 adverse events were recorded. The recommended phase 2 dose of ALT-803 is 20 μg/kg given once per week subcutaneously in combination with 240 mg intravenous nivolumab every 2 weeks.
Interpretation
ALT-803 in combination with nivolumab can be safely administered in an outpatient setting. The promising clinical activity observed with the addition of ALT-803 to the regimen of patients with PD-1 monoclonal antibody relapsed and refractory disease shows evidence of anti-tumour activity for a new class of agents in NSCLC.
Introduction
Immunotherapy with monoclonal antibodies targeting PD-1 and PD-L1 has transformed the treatment of non-small-cell lung cancer (NSCLC) since initial regulatory approval in 2015.1–4 These treatments exert their effect by disrupting the interaction between the inhibitory ligand PD-L1 and its receptor PD-1, leading to functional recovery of anti-tumour lymphocytes. Although second-line treatment with anti-PD-1 immunotherapy can induce durable clinical responses, roughly 80% of unselected NSCLC patients will not respond to treatment, and nearly all of those who do initially respond will eventually develop resistant disease.1,2 Recent studies also suggest that targeting the PD-1 pathway can benefit patients in the first line, either before or in combination with platinum-based chemotherapy.3,4 For patients with disease resistant to PD-1 blockade, the use of combination therapies offers the potential to target additional pathways to improve the proportion of patients achieving a response. In metastatic melanoma, the addition of the anti-CTLA-4 monoclonal antibody ipilimumab to anti-PD-1 monoclonal antibody treatment with nivolumab improves the efficacy of single-agent nivolumab therapy, although many patients report grade 3 and 4 adverse events.5 This combination of ipilimumab and nivolumab received the first ever US Food and Drug Administration (FDA) approval for the combination of two immunotherapy drugs in 2015, and is being investigated in NSCLC (NCT02477826). The assessment of other PD-1-targeted treatments in combination has also shown promise, including the use of use of anti-CD137 (4–1BB) monoclonal antibodies and pegylated IL-10.6,7
In contrast to previously used combinatorial agents, IL-2 and IL-15-based treatments target the shared IL-2Rβγ pathway in lymphocytes. Approved for use by the FDA nearly three decades ago, immunotherapy with single-agent IL-2 (aldesleukin) can induce complete and durable responses in patients with metastatic melanoma and renal cell carcinoma.8 However, effective recombinant human IL-2 treatment requires doses that invariably generate substantial toxicity, necessitating inpatient administration and intensive supportive care, restricting its widespread use. Another IL-2 and IL-15Rβγ agonist, IL-15, is closely related to IL-2 and signals through the shared receptor IL-2 and IL-15Rβγ. Both cytokines promote CD8-positive T-cell and natural killer (NK)-cell activation and proliferation. However, IL-2 preferentially leads to expansion of regulatory T cells that express the unique IL-2Rα subunit, facilitating high-affinity IL-2 binding. Treatment with single-agent, recombinant-human IL-15 (rhIL-15) was recently assessed in patients with metastatic melanoma and was shown to be safe, to act on IL-2Rβγ-positive lymphocytes such as CD8-positive T cells and NK cells, and to induce regression of lung metastases in two patients.9 Although rhIL-15 treatment is promising, pre-associating IL-15 with its soluble receptor (IL-15Rα) can lead to a roughly 50-fold increase in biological activity.10–13
ALT-803 is a pharmacological grade IL-15/IL-15Rα complex fused to an IgG1 Fc, in which IL-15 is additionally mutated (asn72asp) to further increase biological activity and agonism of the IL-2 and 15βγ receptor.14 Although IL-2 and IL-15Rβγ agonists and PD-1 monoclonal antibodies have shown clinical success as monotherapies in some patients, no published studies have assessed the administration of these two classes of agents concomitantly, although trials of similar concepts by other groups are ongoing (NCT02983045, NCT02964078, and NCT02452268). Preclinical studies have shown benefit of combining these two classes of drugs.15 In this trial, we assess the safety of the combination of ALT-803 and nivolumab immunotherapy, and the anti-tumour activity of cytokine treatment in the treatment of NSCLC.
Methods
Study design and participants
In this non-randomised, open-label, phase 1b trial, patients aged 18 years and older with histologically or cytologically confirmed stage IIIB or IV NSCLC (or recurrent disease following previous radiotherapy or surgical resection) were enrolled from three academic hospitals in the USA. Eligible patients had measurable disease as assessed by Response Evaluation Criteria In Solid Tumors (RECIST, version 1.1), an Eastern Cooperative Oncology Group performance status of 0 or 1, adequate organ function, were eligible to recieve anti-PD-1 immunotherapy, had no known autoimmune disease, and had progressed after previous treatment. Patients who had progressed after at least 3 months of single-agent previous PD-1 immunotherapy with nivolumab, pembrolizumab, or atezolizumab were eligible. Those who previously responded or had stable disease as best response to anti-PD-1 immunotherapy before progression after a minimum duration of 3 months of treatment in the case of stable disease were defined as relapsed. Patients whose best response was progressive disease after a minimum duration of 3 months of single-agent PD-1 treatment were defined as refractory. Any previous treatment with other anti-PD-1, anti-PD-L1, anti-PD-L2, anti-CD137, or anti-CTLA-4 antibodies was an exclusion criterion for immunotherapy naive patients. Prior anti-PD-1 therapy was allowed for the relapsed and refractory population. Other key exclusion criteria included anti-cancer treatment within 14 days of registration; New York Heart Association Class III or IV heart failure and other cardiac morbidities; known autoimmune disease requiring active treatment; and CNS metastases, with the exception of patients who had asymptomatic CNS disease with metastases numbering five or fewer with no single lesion greater than 2 cm in diameter. Patients with symptomatic CNS disease were required to have lesions treated previous to enrolment. A full list of the inclusion and exclusion criteria is in the appendix (pp 54–56).
The study was designed in compliance with the International Conference on Harmonization Good Clinical Practice and the FDA regulations for the conduct and monitoring of clinical investigations and sound research practice. The study protocol, consent procedures, and amendments were approved by the local institutional review board of each treating institution. Written informed consent was obtained from all participating patients in accordance with the principles described by federal regulatory guidelines. Study data were collected and managed using REDCap electronic data capture tools hosted at The Medical University of South Carolina (MUSC; Charleston, SC, USA).16
Procedures
Patients received weekly subcutaneous ALT-803 injection at four escalating dose levels: 6, 10, 15, or 20 μg/kg on weeks 1–5 of four 6-week cycles in combination with intravenous nivolumab every 14 days. Treatment was administered by infusion nurses in the outpatient setting. Three patients were enrolled per ALT-803 dose level; an additional three patients received treatment in the 15 μg/kg cohort before proceeding to the highest dose level (the 20 μg/kg cohort). The initial protocol did not include the highest dose level of 20 μg/kg, but because of the absence of dose-limiting toxicity through the ALT-803 dose of 15 μg/kg, the trial was amended to add the dose level of 20 μg/kg on April 11, 2017 (as approved by the MUSC institutional review board). A low dose of 3 μg/kg of ALT-803 was also included in the protocol as a possible dose level to be explored in the event of toxicities at higher doses, but no patients were treated at that dose level. All study treatments were administered in the outpatient setting.
During the trial, the approved intravenous dose of nivolumab administered every 2 weeks was changed from 3 mg/kg to a flat 240 mg dose after FDA-approved dosing changed, and the protocol treatment schema was amended to reflect this change on Sept 20, 2016 (approved by the MUSC institutional review board). All patients given 20 μg/kg ALT-803 were given the nivolumab flat dosing regimen. Patients enrolled prior to this amendment were switched to nivolumab flat dosing at 240 mg every 14 days intravenously.
Efficacy was monitored at the beginning of each 6-week cycle and compared to baseline measurements using interval imaging ordered in accordance with RECIST 1.1 and interpreted using immune-related RECIST (irRECIST; version 1.1). Radio logical assessments in this phase 1b study were not confirmed. PD-L1 status was established in all enrolled patients using a commercially available, Clinical Laboratory Improvement Amendments (CLIA)-certified assays employing either the 22C3 (Dako, Santa Clara, CA, USA) or SP142 (Ventana, Tucson, AZ, USA) antibodies. Patients could discontinue study treatment for any of the following criteria for removal from study: all dose-limiting toxic effects, radiographic disease progression, clinical disease progression, inter-current illness or study treatment-related toxic effect that would in the judgment of the investigator affect assessments of clinical status to a significant degree or require discontinuation of study treatment, patient preference, treatment delay greater than 6 weeks for any adverse event, concomitant treatment with a prohibited drug, or patient non-compliance.
Safety was monitored in all patients with clinical and laboratory assessments during the 28-day dose-limiting toxicity observation period and throughout treatment. The National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) was used to grade adverse events. Patients continued to receive treatment until disease progression, unacceptable toxicity, or 24 months of treatment. Dose reductions were not permitted. Treatment delay for adverse events was allowed, with treatment allowed to restart if toxicity resolved to grade 0 or 1. Clinical and laboratory monitoring (haematological and chemistry) for safety was done with each dose administration.
For pharmacokinetic studies, analysis of serum ALT-803 was established before and after ALT-803 administration. At the time of the the initial dose of ALT-803, blood was collected at various timepoints (days 0, 4, 7, and 14), and serum samples were prepared. Serum concentrations of ALT-803 (pg/mL) were assessed by ELISA.
For immune correlate analysis, peripheral blood mononuclear cells and serum were obtained at the timepoints indicated (day 0, 6 h, 24 h, and days 4, 7, and 14). Briefly, peripheral blood mononuclear cells were stained for flow cytometric analysis and samples were acquired on a BD LSRFortessa (BD Biosciences, San Jose, CA, USA). Flow cytometric data were analysed using FlowJo software (Ashland, OR, USA). Serum was sent to Eve Technologies (Calgary, AB, Canada) for analysis using bead-based multiplexing technology. Genomic DNA was prepared from peripheral blood mononuclear cells and sent to Adaptive Biotechnologies (Seattle, WA, USA) for T-cell receptor (TCR) sequencing and analysis using the immunoSEQ Assay in view of the findings of previous studies showing an association between response to PD-1 blockade and changes in the TCR β-chain repertoire.17,18 Additional details of the procedure are included in the appendix (p 19).
Outcomes
The primary endpoint of the phase 1b study was the occurrence of dose-limiting toxicity (caused by both non-immune-related and immune-related adverse events) in order to define the safety and tolerability of escalating doses of ALT-803 used in combination with nivolumab and to establish a recommended phase 2 dose; the appendix provides a full definition of dose-limiting toxicity (appendix p 2). The secondary endpoints of the phase 1b study included duration of response, progression-free survival, overall survival, pharmacokinetics (ALT-803 Cmax and area under the curve), immunogenicity, plasma cytokine concentration, and lymphocyte subpopulation characterisation. In cases of pseudoprogression, this was defined as a more than 20% increase in tumour burden as measured by irRECIST 1.1 at timepoints up to 12 weeks previous to a partial or complete response. Progression-free survival and overall survival were measured from start of treatment to time of progression or death, respectively. Objective responses were defined as complete and partial responses as determined using RECIST (version 1.1). The proportion of patients achieving an objective response and disease control are post-hoc analyses for the phase 1b portion of the trial. All patients were included in analyses for primary and secondary endpoints per protocol. For the phase 1b part of the study, efficacy outcome stratified by PD-L1 status was not prespecified and is a post-hoc analysis.
Statistical analysis
We postulated that the addition of ALT-803 to nivolumab would be safe and that the combination would be more effective than single-agent nivolumab alone. Dose escalation was done according to a continual reassessment method with the following stopping rules: the sample size reached 21 patients, the recommended dose for the next cohort had already been given to 12 patients, or the estimated dose-limiting toxicity at dose –1 (ie, the lowest planned dose of 3 μg/kg) was 40% or higher.19 Skipping of dose levels was not permitted and so in the absence of dose-limiting toxicity, doses were considered sequentially with three patients per dose level.
Patient characteristics and clinical outcomes are presented using simple descriptive statistics. The product-limit (ie, Kaplan-Meier) estimator was used to estimate median progression-free survival and overall survival with corresponding 95% CIs. Progression-free survival data were censored for participants who were lost to follow-up but progression free at the time of study withdrawal, and for patients on study and progression free as of Nov 30, 2017. Overall survival data were censored for participants still alive as of Nov 30, 2017. Data analyses were done with R (version 3.2.3). The appendix provides the methods used for the description of correlative science statistics.
The study is registered with ClinicalTrials.gov, number NCT02523469; phase 2 enrolment of patients is ongoing.
Role of the funding source
Altor Bioscience supplied ALT-803 and performed pharmacokinetic and immunogenicity assays of the study drugs, and provided input into the study design and interpretation of data, and in the writing, review, and approval of the manuscript. The other funders had no role in the study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Jan 18, 2016, and June 28, 2017, 23 patients were enrolled and 21 were treated and assessed for safety (table 1, appendix p 3). Two patients did not receive any treatment because of the development of inter-current illness during enrolment, one patient due to leucopenia and one patient due to pulmonary dysfunction. Of the 21 patients, three patients were enrolled in the 6 μg/kg ALT-803 dose cohort, three in the 10 μg/kg cohort, six in the 15 μg/kg cohort, and nine in the 20 μg/kg cohort. No patients with untreated CNS metastases were enrolled in the study. Trial enrolment was stopped on July 27, 2017, due to the trial meeting its primary endpoint of defining a recommended phase 2 dose.
Table 1:
Baseline characteristics
| Patients (n=21) | |
|---|---|
| Age (years) | 55(46–67) |
| Sex | |
| Men | 15 (71%) |
| Women | 6 (29%) |
| Ethnic origin | |
| White | 18 (86%) |
| Black | 2 (10%) |
| Asian | 1 (5%) |
| Smoking status | |
| Never | 9 (42%) |
| Former | 12 (57%) |
| Histology | |
| Squamous | 2 (10%) |
| Non-squamous | 19 (90%) |
| ECOG performance status at baseline | |
| 0 | 10 (48%) |
| 1 | 11 (52%) |
| PD-L1 expression | |
| 0–<l% | 10 (48%) |
| ≥1–<50% | 2 (10%) |
| >50% | 4 (20%) |
| Unknown* | 5 (24%) |
| Number of previous treatments regimens | 1–9 (2) |
| 1 | 10 (48%) |
| 2–3 | 8 (38%) |
| >3 | 3 (14%) |
| Previous platinum-based chemotherapy | 21 (100%) |
| Previous definitive chemoradiation | 4 (20%) |
| Prior PD-1 monoclonal antibody immunotherapy | 11 (52%) |
| Mutations | |
| KRAS | 6 (29%) |
| EGFR | 2 (10%) |
| ALK rearranged | 0 (0%) |
| RET rearranged | 1 (5%) |
| ROS1 rearranged | 0 (0%) |
| BRAF | 2 (10%) |
Data are median (IQR), n (%), or range (average). ECOG=Eastern Cooperative Oncology Group.
Tumour not available for testing.
Median duration of follow-up in all patients was 6·9 months (IQR 5·5–12·0 months). Safety and tolerability were assessed in the 21 patients who received at least one dose of ALT-803. Frequency and severity of adverse events did not increase with higher doses of ALT-803 and a maximum tolerated dose was not reached (appendix p 5). No dose-limiting toxicities were recorded. The most frequent adverse events were grade 1 or 2 and consisted of injection-site reactions, fever, fatigue, chills, nausea, pain, and flu-like symptoms (table 2, appendix p 5). Hypotension, when present, was mild, with no events worse than grade 2, and did not require inpatient care. Grade 3 lymphocytopenia occurred in two participants; both events resolved spontaneously and did not recur with continued treatment. ALT-803 did not increase the risk of immune-mediated adverse events associated with nivolumab, with only one episode of grade 2 pneumonitis observed on trial that resolved without steroid treatment. No grade 4 or 5 adverse events were recorded. A patient who experienced myocardial infarction was discontinued from the 15 μg/kg cohort; (judged to be possibly treatment related), and no other patients were discontinued from treatment for adverse events. All treatment-related adverse events were managed on an outpatient basis, with the exception of an admission for pain control in one patient who had extreme pseudoprogression previous to response. Pseudoprogression in this patient resulted in a nearly initial 300% increase in tumour volume (according to irRECIST 1.1) and a subsequent 84% decrease in tumour volume from baseline and ongoing treatment for more than 17 months. The recommended phase 2 dose of ALT-803 was established as 20 μg/kg subcutaneously once per week in combination with 240 mg intravenous nivolumab every 2 weeks.
Table 2:
Adverse events
| All treated patients (n=21) | ||
|---|---|---|
| Grade 1–2 | Grade 3 | |
| Constitutional | ||
| Injection-site reaction | 19 (90%) | 0 |
| Flu-like symptoms | 15 (71%) | 0 |
| Fever | 11 (67%) | 1 (5%) |
| Fatigue | 10 (47%) | 2 (10%) |
| Anorexia | 4 (19%) | 0 |
| Chills | 6 (29%) | 0 |
| Dizziness | 5 (24%) | 1 (5%) |
| Generalised muscle weakness | 2 (10%) | 0 |
| Gastrointestinal | ||
| Nausea | 8 (38%) | 0 |
| Constipation | 4 (19%) | 0 |
| Vomiting | 3 (14%) | 0 |
| Diarrhoea | 2 (10%) | 0 |
| Dry mouth | 2 (10%) | 0 |
| Mouth sore | 2 (10%) | 0 |
| Respiratory | ||
| Cough | 4 (19%) | 0 |
| Dyspnoea | 4 (19%) | 0 |
| Cardiovascular | ||
| Hypotension | 4 (19%) | 0 |
| Laboratory | ||
| Lymphocytopenia | 0 | 2 (10%) |
| Increased alkaline phosphatase | 0 | 1 (5%) |
| Anaemia | 0 | 1 (5%) |
| Increased aspartate transaminase | 0 | 1 (5%) |
| Pain or other | ||
| Pain | 7 (33%) | 0 |
| Rash (not otherwise specified) | 2 (10%) | 0 |
| Headache | 2 (10%) | 0 |
| Back pain | 1 (5%) | 1 (5%) |
| Depression | 1 (5%) | 1 (5%) |
| Abdominal pain | 0 | 1 (5%) |
| Myocardial infarction | 0 | 1 (5%) |
Data are n (%).
ALT-803 concentrations were established to define the pharmacokinetics after subcutaneous dosing (figure 1). Serum concentrations of ALT-803 reached near-peak concentrations between 2 and 6 h post-injection for all dose levels studied. Patients who received doses of 10 μg/kg and higher had a modest upward trend in peak concentrations (Cmax) after 6 h, which continued until at least 24 h post-injection. Dose cohorts of 10 μg/kg and higher had similar Cmax concentrations 24 h after treatment (figure 1).
Figure 1:
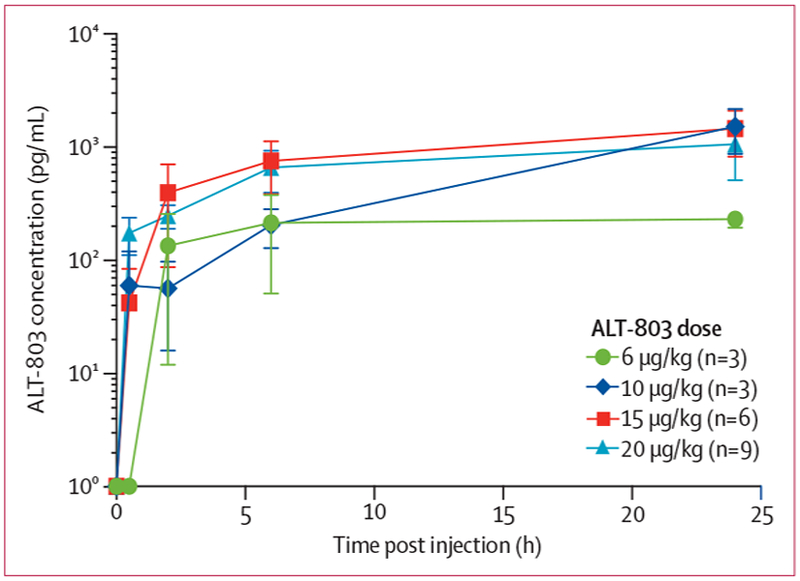
Pharmacokinetics of ALT-803 after subcutaneous administration
Seven (33%) of the 21 patients in the study developed detectable anti-ALT-803 antibody titres in sera after multidose treatment (two patients in the 10 μg/kg cohort, three patients in the 15 μg/kg cohort, and two patients in the 20 μg/kg cohort; appendix p 6). No anti-ALT-803 antibodies were detected in the 6 μg/kg cohort (n=3). In five of the seven patients with detectable anti-ALT-803 antibodies, these were initially recorded following administration of four doses of ALT-803. Titres in four of the seven positive patients were only detectable in one of the seven timepoints studied over the four 6-week treatment cycles. The highest titre recorded in the study was 315, which decreased to 153 or lower at subsequent timepoints. Three of seven patients had detectable titres at more than one timepoint. No anti-nivolumab antibodies were detected in any of the patients enrolled in this study. Of the seven patients who developed detectable anti-ALT-803 antibodies, three (42%) responded to treatment (all partial responses). No additional adverse events and no impairment in NK-cell counts based on the assessment of peripheral blood mononuclear cell samples were noted in these seven patients.
In the post-hoc analysis, six (29%) of 21 patients achieved an objective response (figure 2, table 3). All responses were partial responses, with no complete responses recorded. Nine (43%) of 21 patients experienced a decrease in the size of their target lesions, and 16 (76%) of 21 patients achieved disease control (stable disease, partial response, and complete response). Duration of response data will be reported separately. Progressive disease was recorded in ten patients and eight patients died. Median progression-free survival was 9·4 months (95% CI 3·0–not recorded), and median overall survival was 17·4 months (95% CI 9·0–not recorded), with six patients receiving ongoing treatment (figure 2B). Responses were recorded in patients with various different types of mutation status (appendix p 6). No dose-dependent response was noted (appendix p 7).
Figure 2: Post-hoc clinical activity of ALT-803 in combination with nivolumab.
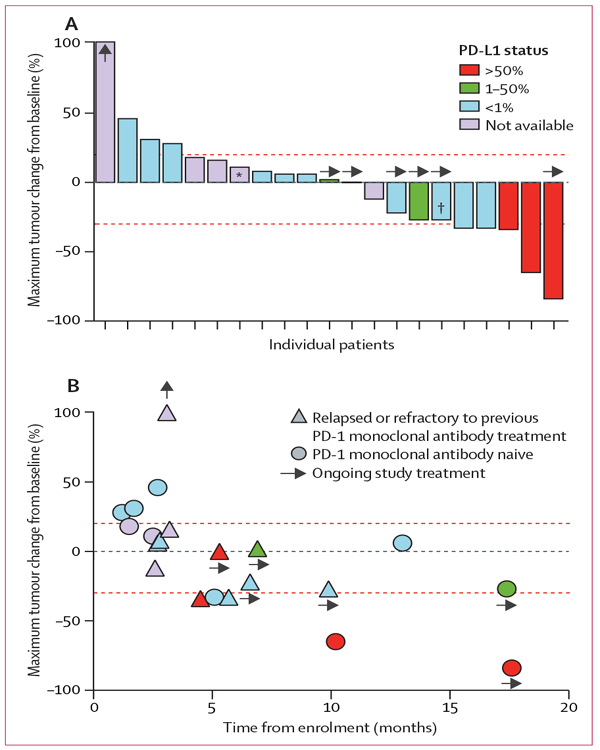
(A) Best observed response for 21 patients given ALT-803 in combination with the US Food and Drug Administration (FDA)-approved dose of nivolumab (3 mg/kg then 240 mg flat dose after change in FDA approval). Upward arrows indicate the patient had a 180% increase in size of tumour by RECIST and is out of scale for the figure. (B) Best response by patient versus duration of treatment on study. Upper red line is more than 20% over baseline tumour burden; lower red line is less than 30% from baseline tumour burden, both measured by RECIST criteria. RECIST=Response Evaluation Criteria In Solid Tumors. *Progression due to a new lesion. †Target lesion decrease of 27%, but met RECIST 1.1 criteria for partial response.
Table 3:
Post-hoc objective responses and disease control
| Objective responses (n, %, 95% CI) | Disease control (n, %, 95% CI) | |
|---|---|---|
| All patients | 6 (29%, 11–52) | 16 (76%, 53–92) |
| PD-1 relapsed and refractory | 3 (27%, 6–61) | 10 (91%, 59–99) |
| PD-LI negative (<1%) | 3 (30%, 7–65) | 7 (70%, 35–93) |
| PD-L1 positive (>50%) | 3 (75%, 19–99) | 4 (100%, 40–100) |
In a post-hoc exploratory analysis of response according to PD-L1 expression status, of four patients PD-L1 expression higher than 50% (classified as PD-L1-positive), three had objective responses and a fourth has stable disease with ongoing treatment at 6 months (figure 2). Both patients with PD-L1 expression between 1% and 50% have had stable disease at 6 and 17 months with ongoing treatment, the latter harbouring an EGFR exon 20 insertion and who has had a 27% reduction in the size of their target lesions (figure 2). In ten patients with PD-L1 expression lower than 1% (classified as PD-L1-negative), seven (70%) achieved stable disease or better, with three (30%) partial responses (table 3, appendix p 8).
11 patients enrolled to the trial had previously received a single-agent PD-1 monoclonal antibody and progressed after at least 3 months of treatment (figure 2B). The proportion of PD-1 treatment-resistant patients who achieved disease control was 91% (ten of 11 patients), with three (27%) partial responses and seven (64%) patients with stable disease (figure 2B, table 3). Two objective responses occurred when ALT-803 was added to uninterrupted nivolumab at the time of relapse on single-agent anti-PD-1 immunotherapy, showing evidence of anti-tumour activity for ALT-803 in NSCLC. Furthermore, we noted a partial response to study treatment in a relapsed patient whose best response to 9·5 months of previous nivolumab therapy was stable disease. Stable disease was also achieved in another patient who was refractory to previous single-agent anti-PD-1 immunotherapy.
The clinical experience of one patient who had not received previous immunotherapy is noteworthy. This patient achieved an initial partial response (65% decrease in target lesion size) to study treatment in the 6 μg/kg ALT-803 dose cohort but then progressed after about 10 months on treatment. The patient then enrolled in a trial of docetaxel plus trametinib for patients whose tumours possess a KRAS mutation. Upon no response to this treatment, the patient was retreated with nivolumab and ALT-803 at 15 μg/kg using a single-patient investigational new drug application and the patient experienced a second, significant response (100% target lesion size decrease by RECIST 1.1, but this was only classed as a partial response due to the persistence of a non-target lesion, appendix p 9). This patient’s second experience with ALT-803 plus nivolumab was not included in any outcome measures of the trial.
Combination treatment-induced changes in the periphery were most evident in NK cells and to a lesser degree in CD8-positive T cells (as assessed in all 21 treated patients; figure 3A, appendix p 10). Within 7 days of initiation of study treatment, NK-cell frequency on average more than doubled at all dose levels of ALT-803 (figure 3A). The preferential expansion of NK cells over CD8-positive T cells has been previously reported with single agent recombinant IL-15,9 and could be a consequence of higher surface expression of the shared IL-2 and 15Rβγ (CD122 and CD132) on NK cells than CD8-positive T cells (appendix p 11). Although CD8-positive T-cell frequencies did not increase to the same extent as NK cells, some patients showed a notable increase in biological activity of CD8-positive T cells at cycle 1, day 4, based on Ki67 staining (figure 3B, C, appendix p 12). Importantly, we noted changes in NK cells and CD8-positive T cells after ALT-803 was added to continuous nivolumab treatment in two patients (Figure 3B, appendix p 10), showing that these biological effects were ALT-803 dependent (rather than being caused by nivolumab). Finally, as expected, we recorded minimal expansion of the regulatory T-cell population and an increase in the CD8-positive T cell to T regulatory cell ratio (appendix pp 10, 12, 13). In some patients, study treatment resulted in raised concentrations of inflammatory cytokines, notably interferon γ (IFNγ) and IL-6, which is indicative of the ability of IL-15-based treatments to induce systemic immune activation. Serum concentrations of these cytokines were highest 4–7 days after initiation of treatment (figure 3D). Induction of IFNγ as measured by fold change was notable in patients with PD-L1-negative tumours (those with <1% PD-L1 expression; appendix p 14). Consistent with an induction of systemic immune activation, study treatment was also associated with day 4 upregulation of HLA-DR expression on circulating CD11c-positive cells (figure 3E, appendix p 15). Finally, for comparison with other correlates, the appendix provides values from complete blood counts (appendix p 16).
Figure 3: Immune correlates of patients given ALT-803 in combination with nivolumab.
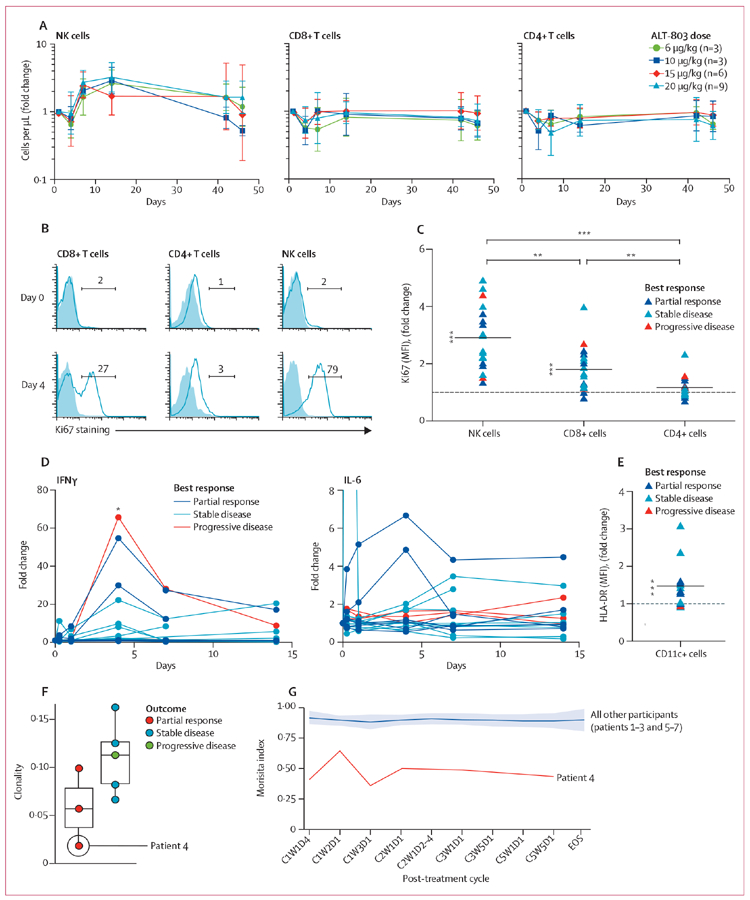
(A) The change in peripheral blood numbers of NK cells (CD56bright/dimCD16+CD3–), CD8-positive T cells (CD8+CD3+), and CD4-positive T cells (CD4+CD3+) in relation to pre-treatment assessment (appendix p 10 provides additional details). (B) Ki67 induction in immune cell subsets, measured in patient number 7 after the addition of ALT-803 treatment to ongoing nivolumab treatment. (C) Change in Ki67 concentrations (MFI) in lymphocyte subsets at day 4 versus pretreatment. Each symbol indicates one patient and colours indicate best response as depicted in the figure legend. Changes for NK cells and CD8-positive T cells differed significantly from 1, and all pairwise comparisons were statistically significant. (D) Serum interferon γ and interleukin-6 concentrations were measured longitudinally and the fold change was established in relation to pre-treatment assessment. Each line represents one patient. (E) Upregulation of HLA-DR on CD11c-positive cells was assessed by determining the fold change in percentage of HLA-DR-positive cells at day 4 versus pretreatment. Each symbol indicates one patient and the colours indicate best response. Fold changes differed significantly from 1. (F) T-cell receptor (TCR) immunosequencing of pretreatment peripheral blood mononuclear cells of the first seven trial patients. (G) Longitudinal analysis of TCR repertoire of first seven trial patients graphed as Morisita index. NK=natural killer. MFI=mean flouresence intensity. C=cycle. W=week. D=day. *p<0·01. **p<0·001. ***p<0·0001.
In a comparison of measurements over the course of the first cycle performed on day 4, 7, and 14 after the start of treatment, the highest biological responses were on day 4 as reflected by both the increase in the staining intensity of Ki67 in NK cells (data not shown) and by increased concentration of circulating IFNγ (figure 3D), consistent with transient induction of immune responses between weekly ALT-803 administration. In the second cycle of treatment, peak day 4 responses were notably reduced in comparison to the first cycle (appendix p 17), suggesting that prolonged continuous treatment might lead to reduced biological responsiveness to subsequent treatment.
In an exploratory post-hoc analysis, we assessed differences in induced immune parameters related to patients’ best clinical response (figure 3F). In an initial subgroup analysis (n=7), participants responding to trial treatment showed reduced TCR clonality at baseline, as might be expected in patients treated with single-agent anti-PD-1 immuotherapy (figure 3F). Notably, one patient who initially showed pseudoprogression, which roughly tripled their tumour volume before they eventually achieved a notable response, showed the broadest baseline peripheral TCR repertoire and also the most dynamic change in the T-cell receptor repertoire following treatment (figure 3F, G). Multi-spectral imaging of pre-treatment tumour biopsy samples shows the relation between CD8-positive T cells and PD-L1-positive tumour and immune cells, and ongoing efforts will correlate multiple parameters of the tumour microenvironment with treatment response (appendix p 18).
Discussion
In this study we show that ALT-803 is safe, tolerable, and can be conveniently administered in combination with an anti-PD-1 checkpoint antibody for patients with NSCLC in an outpatient setting. The clinical experience of administering ALT-803 as part of this regimen is in stark contrast to that of IL-2, which requires supervision in the intensive-care setting due to its severe toxicity when given at its approved dose. More importantly, ALT-803 did not increase the severity of or induce additional adverse events associated with nivolumab immunotherapy, except for low-grade and transient constitutional adverse events such as fever and injection-site skin rash at a dose level as high as 20 μg/kg with weekly dosing. At this dose level, the activation of the immune system is evident based on NK and T-cell proliferation and induction of serum cytokine levels. Based on the combination of safety and potent biological activity, the 20 μg/kg weekly dosing was selected to move forward with the phase 2 portion of the trial. However, immune therapeutics can often be effective at a wide range of dose levels, and therefore future studies might be required to identify the optimal dosing of this agent with anti-PD-1 monoclonal antibodies treatment.
Notably, adverse events recorded in patients treated with ALT-803 and nivolumab in our study contrast markedly with published data about patients treated with ipilimumab (anti-CTLA-4 monoclonal antibodies) and nivolumab, the only FDA-approved dual checkpoint inhibitor treatment. Whereas ipilimumab and nivolumab treatment-related adverse events have led to treatment discontinuation in up to 39% of patients treated with the only approved regimen5 (although treatment discontinuations due to the toxicity of regimens modified for the NSCLC population seem to be less at 10–13%),20 toxicity due to ALT-803 plus nivolumab led to only one treatment discontinuation in our study. With regard to immune-mediated adverse events, we observed one case of grade 2 pneumonitis in our trial, which resolved without steroids, and no other immune-mediated adverse events associated with nivolumab exposure. Furthermore, there were no dose-limiting or grade 4 or 5 adverse events recorded with ALT-803 and nivolumab combination treatment. The fact that ALT-803 did not increase adverse events associated with nivolumab is consistent with patients who received combination treatment of ALT-803 with another immunotherapy (BCG) for non-muscle-invasive bladder cancer, such that the addition of ALT-803 did not exacerbate adverse events typically associated with BCG treatment.21
Furthermore, we report that ALT-803 can re-induce objective responses to anti-PD-1 immunotherapy after treatment relapse or failure. It has been reported that about 8% of patients with NSCLC treated past progression had partial responses with continued PD-1 monoclonal antibodies treatment.22 Although it is possible that this phenomenon might explain objective responses to ALT-803-nivolumab combination treatment after PD-1 treatment failure, the re-induction of response coincided directly with initiation of ALT-803 after progression in patients with relapsed disease treated with uninterrupted nivolumab. Moreover, disease control was asserted in all ten patients who had relapsed disease after previous anti-PD-1 immunotherapy. The ability of ALT-803 to safely re-induce objective responses to immunotherapy in patients whose disease has relapsed after previous single-agent PD-1 monoclonal antibodies treatment has important implications in solid tumour oncology beyond NSCLC. Taken together with compelling progression-free survival and overall survival data, the study results suggest that ALT-803 represents a novel immuno therapeutic agent that could augment the anti-tumour activity of checkpoint inhibitors in several treatment settings.
In second-line treatment for NSCLC, roughly 20% of patients achieve an objective response to anti-PD-1 treatment, but in patients with non-squamous NSCLC who have PD-L1 negative (<1%) tumours, only about 9% of patients achieve a clinical response.1 In our study with 19 (90%) of 21 patients with non-squamous NSCLC, the combination of ALT-803 and nivolumab induced clinical responses in 30% of PD-L1-negative patients and yielded disease control in 70%. In patients whose tumours lack PD-L1 expression, ALT-803 could therefore have substantial application in broadening the reach of benefit from checkpoint inhibitors.
Broadly, our results suggest that administration of an IL-2Rβγ agonist might be useful in overcoming resistance to anti-PD-1 monoclonal antibody treatment. Mechanistically, there are several possibilities that could facilitate improved response rates. Systemic induction of inflammatory cytokines such as IFNγ, could facilitate the conversion of an immunological cold tumour into a hot tumour. This theory could directly explain why patients with PD-L1-negative tumours might have an improved response rate with the addition of ALT-803 to their anti-PD-1 treatment. Another possibility is that ALT-803 drives expansion of neoantigen-reactive T cells, which is supported by our TCR sequencing data; however, this post-hoc subgroup analysis was small, and additional patient data will be needed for more conclusive assessment of these parameters. The low TCR clonality in the peripheral blood at baseline that we recorded is consistent with published reports with single-agent anti-PD-1 immunotherapy. This ability of ALT-803 to drive potent expansion of NK cells could have particular value because MHC class I is lost on a substantial proportion of NSLCL tumours,23 thereby making these tumours potentially susceptible to NK-cell-mediated killing. These questions are being addressed in our ongoing phase 2 study.
Although anti-ALT-803 antibodies were detected in seven study patients, there was no apparent association with toxic effects and no decrease in anti-tumour activity (as indicated by the fact that three of these seven patients had a partial response). Further study in additional patients is required to understand the biological effect of these antibodies. It is relevant that anti-drug antibodies have been reported in many trials using recombinant proteins and antibodies.24 In one study, anti-IL-2 antibodies were detected in roughly half of patients, and development of these antibodies was not associated with a decrease in the treatment effect.25
A crucial question for future studies is to determine the optimal timing and duration of adding an IL-2Rβγ agonist to anti-PD-1 treatment. Our immune correlative work provides some insights in this area. Consistent with previous reports with the administration of single-agent rhIL-15 in human patients,9 we detected induction of Ki67 in CD8-positive T and NK cells and serum cytokines such as IFNγ and IL-6 at early timepoints after ALT-803 and nivolumab administration. These changes observed in immune correlates correspond with fevers and flu-like symptoms, and were clearly related to ALT-803 because they were not recorded in patients receiving single-agent nivolumab. However, although inflammatory side-effects, including ALT-803 injection-site reactions, were recorded during the first cycle of treatment, by the second cycle of treatment these symptoms were greatly diminished. Notably, the reduction in inflammatory side-effects showed a strong correlation with reduced induction of Ki67 and IFNγ. Evidence of tolerance to sustained cytokine treatment has been previously described for IL-2 and IL-15.26–29 For comparison, it may be relevant that the FDA-approved dose of IL-2 is given in high-dose pulses that typically yield ongoing—even worsening—symptoms with subsequent treatment cycles. Thus, a crucial goal for future research could be to determine not only optimal dosing but also whether treatment breaks are important. Further studies are being planned to investigate the optimal dose and schedule of ALT-803 in combination with anti-PD-1 treatment, both preclinically and clinically (NCT03228667).
Pseudoprogression in two patients on combination treatment with ALT-803 and nivolumab was recorded in this study. One patient experienced an extreme variant of pseudoprogression with tripling in the size of his tumours, causing severe pain, before he subsequently achieved a response to treatment. The potential for pseudoprogression must be considered in the clinical care of patients receiving this combination immunotherapy. The response assessment process might need adjustment to reflect this phenomenon and consideration must be given before treatment is stopped early in such patients. Our data indicate that ALT-803 and anti-PD-1 immunotherapy should be continued until confirmation of progression on subsequent imaging and careful assessment so as not to forgo an opportunity to experience benefit from combination treatment. The degree of pseudoprogression might, in fact, be a marker of quality of eventual response.30
Although our study is limited by the small number of treated patients, enrolment efforts are ongoing to accumulate experience with this treatment combination in a study population that more broadly mirrors the population of patients with NSCLC in the phase 2 portion of the clinical trial that stratifies patient based on PD-L1 status, prior exposure to anti-PD-1 immunotherapies, and EGFR mutation status. For example, there were fewer than expected current or former smokers and a high proportion of patients with PD-L1-negative tumours in our patient population. Additionally, this phase 1b study with a safety-related primary endpoint was not designed to directly assess the efficacy of ALT-803 and nivolumab compared with either single-agent nivolumab or second-line single-agent chemotherapy. Although we have determined a safe and tolerable dose for the ongoing phase 2 trial (NCT02523469), questions regarding the duration and timing of treatment, and dose-response association remain unanswered. Further accrual for the phase 2 trial will also allow us to achieve greater precision in the estimation of benefit for several subgroups based on genetic and tumour microenvironment characteristics. Moreover, since our correlate data show diminished effects at 6 weeks of treatment, de-intensification of administration of lymphocyte growth factor could potentially yield prolonged anti-tumour activity.
Overall, our findings from this phase 1b study show that ALT-803 can be safely administered with nivolumab entirely on an outpatient basis. To our knowledge, we provide the first findings for activity of an IL-15 treatment in patients with NSCLC. Perhaps of greatest importance, the ability of ALT-803, or an agonist targeting the shared IL-2 and IL-15Rβγ pathway, to re-induce responses in patients experiencing treatment failure with PD-1 treatments could have implications in a wide range of cancers.
Supplementary Material
Research in context
Evidence before this study
Although there is recent scientific literature documenting the efficacy of single-agent PD-1 or PD-L1-blocking agents in cancer in human beings, including with non-small-cell lung cancer (NSCLC), most patients with NSCLC do not respond to single-agent anti-PD-1 immunotherapy, and nearly all responders eventually progress. In contrast to agents that block immune checkpoints, interleukin-2 (IL-2) and IL-15-based cytokine treatments target the IL-2Rβγ pathway, thereby augmenting lymphocyte responses. For more than 20 years, IL-2 treatment has shown efficacy in metastatic melanoma and renal cell carcinoma, but there have been only anecdotal reports of efficacy in other cancers including NSCLC. Newer IL-2 and IL-15Rβγ targeting agents in development include NIZ985 (hetIL-15, IL-15 and IL-15Rα) and ALKS 4230 (IL-2 and IL-2Rα). We searched PubMed through Nov 17, 2017, for studies combining IL-2 or IL-15Rβγ agonists with PD-1-blocking or PD-L1-blocking agents using the following search terms: “IL-2 OR IL-15” and “nivolumab OR pembrolizumab OR atezolizumab OR avelumab OR durvalumab OR pidilizumab OR PD-1 OR PD-L1”. We also searched abstracts from the 2015, 2016, and 2017 American Society of Clinical Oncology (ASCO) annual meeting. Abstracts reported at ASCO 2017 included ongoing clinical studies combining a pegylated IL-2 molecule (NKTR-214) with PD-1 or PD-L1-blocking antibodies (NCT02983045). Although findings of preclinical studies show combining IL-2 and IL-15Rβγ agonists with anti-PD-1 monoclonal antibodies can improve anti-tumour responses, we found no published clinical studies assessing either the safety or efficacy of this combination in human patients.
Added value of this study
No dual immunotherapies or non-PD-1-directed targeted immunotherapeutics are approved for use in NSCLC; therefore, improved immunotherapeutic strategies to extend the therapeutic benefit to a larger proportion of patients with NSCLC and to resurrect clinical benefit when resistance to treatment occurs is a huge unmet need. This study is the first clinical report to assess safety or to report the efficacy of ALT-803 or any other IL-2 or IL-15Rβγ agonist cytokine administered in combination with anti-PD-1 immunotherapy. The current study shows a potential role for a new class of therapeutic agent for the treatment of NSCLC.
Implications of all the available evidence
Our findings show that cytokine treatment with an IL-2 and IL-15Rβγ superagonist at doses capable of inducing anti-tumour immune responses is safe and feasible in the outpatient setting and that the cytokine complex might safely be combined with anti-PD-1 immunotherapy. The ability of ALT-803 to re-induce immunotherapeutic response in PD-1-relapsed and refractory NSCLC should stimulate a surge of renewed interest in cytokine combination treatments for cancer.
Acknowledgments
Altor Bioscience provided financial support for this investigator-initiated study, and participated in the design of the study and the interpretation of data, as well as the writing, review, and approval of the manuscript.
TCR sequencing was funded by Adaptive Biotechnologies. This study was supported in part by the Biostatistics Shared Resource, the Clinical Trials Office, and the Cell Evaluation and Therapy core, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313). Also supported by the SCTR-Biomedical Informatics Center grant support (National Institutes of Health [NIH]/National Clinical Assessment and Treatment Service UL1 TR001450). JMW received support from the Hollings Cancer Center’s K12 Paul Calabresi Clinical and Translational Oncology Training Program (K12 CA157688). VV received support from an American Society of Clinical Oncology Career Development Award. We thank the Cancer Immunotherapy Trials Network and Low Country Hematology & Oncology. MPR received support from the NIH (P01CA154778) and the Cancer Research Institute. JMW would like to give special thanks to Mary Lou Wrangle. We also give special thanks to the patients who participated in the study and the friends and family who support them.
Funding
Altor BioScience (a NantWorks company), National Institutes of Health, and Medical University of South Carolina Hollings Cancer Center.
Footnotes
Declaration of interests
JMW reports grants from Altor Bioscience, during the conduct of the study. VV reports personal fees from Genentech, Merck, Bristol-Myers Squibb, Takeda, and Celgene, and grants and personal fees from AstraZeneca, outside the submitted work. JGR reports other from Imbio LLC, outside the submitted work. JSM reports grants from Altor Biosciences, outside the submitted work. JAR reports personal fees from Adaptive Biotechnologies, outside the submitted work. CMS reports personal fees from Adaptive Biotechnologies, outside the submitted work. ECY reports personal fees from Adaptive Biotechnologies, outside the submitted work. WLR reports grants from Bristol-Myers Squibb, Tesaro, MedImmune, Nektar Therapeutics, Aeglea Biotherapeutics, IRX Therapeutics, Merck, and Galectin Therapeutics, outside the submitted work. JOE is an employee and equity holder of Altor Bioscience. PRR is an employee and equity holder of Altor Bioscience and has a patent ALT-803 issued, and a patent ALT-803 plus checkpoint inhibitors pending. EKJ is an employee and equity holder of Altor Bioscience. ADR reports personal fees from Altor Bioscience, during the conduct of the study; and personal fees from Altor BioScience, outside the submitted work. HCW is an employee and equity holder of Altor Bioscience and has a patent ALT-803 issued, and a patent ALT-803 plus checkpoint inhibitors pending. MPR reports grants from Altor Bioscience, XOMA, and Neumedicines, outside the submitted work. In addition, MPR has a patent related to IL-2/anti-IL-2 monoclonal antibody complexes pending. All other authors declare no competing interests.
Contributor Information
John M Wrangle, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Vamsidhar Velcheti, Cleveland Clinic, Cleveland, OH, USA.
Manish R Patel, Department of Medicine, Division of Hematology, Oncology, and Transplantation, University of Minnesota, Minneapolis, MN, USA.
Elizabeth Garrett-Mayer, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Elizabeth G Hill, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
James G Ravenel, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Jeffrey S Miller, Department of Medicine, Division of Hematology, Oncology, and Transplantation, University of Minnesota, Minneapolis, MN, USA.
Mohammad Farhad, Earle A Chiles Research Institute, Portland, OR, USA.
Kate Anderton, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Kathryn Lindsey, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Michele Taffaro-Neskey, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Carol Sherman, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Samantha Suriano, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Marzena Swiderska-Syn, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Amy Sion, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Joni Harris, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Andie R Edwards, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
Julie A Rytlewski, Adaptive Biotechnologies, Seattle, WA, USA.
Catherine M Sanders, Adaptive Biotechnologies, Seattle, WA, USA.
Erik C Yusko, Adaptive Biotechnologies, Seattle, WA, USA.
Mark D Robinson, Institute of Molecular Life Sciences, University of Zurich, Zurich, Switzerland.
Carsten Krieg, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
William L Redmond, Earle A Chiles Research Institute, Portland, OR, USA.
Jack O Egan, Altor BioScience, Miramar, FL, USA.
Peter R Rhode, Altor BioScience, Miramar, FL, USA.
Emily K Jeng, Altor BioScience, Miramar, FL, USA.
Amy D Rock, Altor BioScience, Miramar, FL, USA.
Hing C Wong, Altor BioScience, Miramar, FL, USA.
Mark P Rubinstein, Department of Medicine, Division of Hematology and Oncology, and Department of Surgery Medical University of South Carolina, Charleston, SC, USA.
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017; 35: 3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. , and the KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 4.Langer CJ, Gadgeel SM, Borghaei H, et al. , and the KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17: 1497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377: 1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolcher AW, Sznol M, Hu-Lieskovan S, et al. Phase Ib study of utomilumab (PF-05082566), a 4–1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res 2017; 23: 5349–57. [DOI] [PubMed] [Google Scholar]
- 7.Naing A, Papadopoulos KP, Infante JR, et al. Clinical activity and safety of pegylated human IL-10 (AM0010) in combination with anti-PD1. Proc Am Soc Clin Oncol 2016; 34 (15 suppl): 3018–18. [Google Scholar]
- 8.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014; 192: 5451–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015; 33: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubinstein MP, Kovar M, Purton JF, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Ralpha. Proc Natl Acad Sci USA 2006; 103: 9166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol 2006; 177: 6072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois S, Patel HJ, Zhang M, Waldmann TA, Müller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumour action. J Immunol 2008; 180: 2099–106. [DOI] [PubMed] [Google Scholar]
- 13.Bergamaschi C, Rosati M, Jalah R, et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem 2008; 283: 4189–99. [DOI] [PubMed] [Google Scholar]
- 14.Rhode PR, Egan JO, Xu W, et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res 2016; 4: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desbois M, Le Vu P, Coutzac C, et al. IL-15 trans-signaling with the superagonist RLI promotes effector/memory CD8+ T cell responses and enhances antitumour activity of PD-1 antagonists. J Immunol 2016; 197: 168–78. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder A, Nathanson T, Funt SA, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: An exploratory multi-omic analysis. PLoS Med 2017; 14: e1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung YK. Dose finding by the continual reassessment method. Boca Raton: Taylor & Francis, 2011: 189. [Google Scholar]
- 20.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017; 18: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosser CJ, Nix J, Ferguson L, Wong H. Phase IB trial of ALT-803, an IL-15 superagonist, plus Bacillus Calmette Guerin (BCG) for the treatment of BCG-naive patients with non-muscle-invasive bladder cancer (NMIBC). J Urol 2017; 197: e175. [Google Scholar]
- 22.Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol 2017; 44: 3–7. [DOI] [PubMed] [Google Scholar]
- 23.Garrido F, Algarra I. MHC antigens and tumour escape from immune surveillance. Adv Cancer Res 2001; 83: 117–58. [DOI] [PubMed] [Google Scholar]
- 24.van Brummelen EM, Ros W, Wolbink G, Beijnen JH, Schellens JH. Antidrug antibody formation in oncology: clinical relevance and challenges. Oncologist 2016; 21: 1260–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scharenberg JG, Stam AG, von Blomberg BM, et al. The development of anti-interleukin-2 (IL-2) antibodies in cancer patients treated with recombinant IL-2. Eur J Cancer 1994; 30A: 1804–09. [DOI] [PubMed] [Google Scholar]
- 26.Hirakawa M, Matos TR, Liu H, et al. Low-dose IL-2 selectively activates subsets of CD4+Tregs and NK cells. JCI Insight 2016; 1: e89278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci USA 2010; 107: 21647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs JA, Vogel S, Metcalf JA, et al. Interleukin-2 induced immune effects in human immunodeficiency virus-infected patients receiving intermittent interleukin-2 immunotherapy. Eur J Immunol 2001; 31: 1351–60. [DOI] [PubMed] [Google Scholar]
- 29.Larsen CS, Ostergård L, Møller BK, Buhl MR. Subcutaneous interleukin-2 in combination with anti-retroviral treatment for treatment of HIV-1-infected subjects. Scand J Infect Dis 2000; 32: 153–60. [DOI] [PubMed] [Google Scholar]
- 30.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015; 33: 3541–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


