Abstract
An evolving reciprocal model posits that pain and tobacco smoking behavior interact in the manner of a positive feedback loop, resulting in greater pain and the maintenance of nicotine dependence. There is also reason to believe that abstaining from smoking may increase pain during the early stages of smoking cessation. The goal of this study was to test the effects of nicotine deprivation on experimental pain reactivity. Daily tobacco cigarette smokers (N = 165; 43% female; M CPD = 22) were randomized to either extended nicotine deprivation (12–24 hours smoking abstinence), minimal deprivation (2 hours smoking abstinence), or continued smoking conditions, prior to undergoing pain induction via topical capsaicin. As hypothesized, results indicated that extended deprivation (relative to continued smoking) increased capsaicin-induced pain intensity ratings, neurogenic inflammation, and mechanical hyperalgesia, thus implicating both central and peripheral mechanisms of action in the effects of smoking abstinence on pain reactivity. Pain intensity ratings were also positively correlated with nicotine withdrawal symptoms, and exploratory analyses suggest that pain sensitivity may increase with duration of smoking abstinence. Collectively, these findings indicate that smokers may experience a variety of negative pain-related sequelae during the early stages of a quit attempt. Future research should examine pain as a consequence or correlate of the nicotine withdrawal syndrome, and determine whether smokers may benefit from tailored cessation interventions that account for nicotine deprivation-induced amplification of pain.
Keywords: pain, nicotine, tobacco, smoking, deprivation, hyperalgesia
Pain and tobacco dependence are both highly prevalent and co-occurring conditions, with a combined annual economic burden in excess of $800 billion in the United States alone (Gaskin & Richard, 2012; US Department of Health & Human Services, 2014; Xu, Bishop, Kennedy, Simpson, & Pechacek, 2015). The prevalence of smoking among persons with pain (~42–68%; e.g., Hooten, Shi, Gazelka, & Warner, 2011; Jamison, Stetson, & Parris, 1991; Michna et al., 2004; Zvolensky, McMillan, Gonzalez, & Asmundson, 2009) appears to be substantially higher than rates observed in the general population (15%; Jamal et al., 2016), and an evolving reciprocal model of pain and smoking posits that these conditions interact in the manner of a positive feedback loop, resulting in greater pain and the maintenance of tobacco dependence (Ditre, Brandon, Zale, & Meagher, 2011; Zale, Maisto, & Ditre, 2015). Consistent with this perspective, cigarette smoking has been identified as a unique risk factor in the onset and progression of several painful conditions (e.g., Aho & Heliovaara, 2004; Shiri, Karppinen, Leino-Arjas, Solovieva, & Viikari-Juntura, 2010), situational pain has been shown to motivate smoking urge and behavior (e.g., Ditre & Brandon, 2008), and pain patients have reliably endorsed smoking to cope with pain (e.g., Jamison et al., 1991; Patterson et al., 2012).
Although accumulating research indicates that sustained smoking abstinence may decrease pain (e.g., Bastian et al., 2015; Behrend et al., 2012; Kaye, Prabhakar, Fitzmaurice, & Kaye, 2012), there is also reason to believe that pain reactivity may be exacerbated during the early stages of a quit attempt. For example, animal models have consistently demonstrated that nicotine deprivation increases reactivity to a variety of experimental pain assays, including hot plate, tail flick, and plantar stimulation (e.g., Grabus et al., 2005; Jackson, McIntosh, Brunzell, Sanjakdar, & Damaj, 2009). Among humans, nicotinic acetylcholine receptor availability during smoking abstinence has been positively correlated with experimental pain ratings (Cosgrove et al., 2010), and nicotine-deprived smokers have evinced greater sensitivity to laboratory pain induction than non-smokers (Baiamonte, Stickley, & Ford, 2016; Nakajima & Al’Absi, 2014; Perkins et al., 1994). However, no previous research has systematically-manipulated nicotine deprivation among current smokers to test the effects of early smoking abstinence on human experimental pain reactivity.
The primary goal of the current study was to test the effects of nicotine deprivation on experimental pain reactivity using an established capsaicin pain induction procedure. Specifically, we hypothesized that daily tobacco smokers randomized to extended nicotine deprivation (vs. continued ad lib smoking) would evince greater capsaicin-induced pain intensity, neurogenic inflammation, and mechanical hyperalgesia. A secondary goal was to examine associations between pain intensity ratings and nicotine withdrawal symptoms.
Method
Participant Recruitment
Participants were recruited from the local community via newspaper and internet advertisements for a two-session experimental study. Respondents were screened by phone for the following inclusion criteria: between 18–65 years of age; currently smoking ≥ 15 cigarettes per day; and ability to speak and read English. Respondents were excluded if they endorsed: a current attempt to reduce or quit smoking; self-reported chronic pain; current use of prescription medications for the treatment of pain; or a pepper allergy (contraindicated for capsaicin pain induction). Eligible respondents were scheduled for a baseline assessment session, and were instructed not to use any pain medication (e.g., acetaminophen or NSAIDs such as aspirin/ibuprofen) for 24 hours prior to the experimental session.
Procedure
At the baseline assessment session (Session 1), smoking status was verified via exhaled carbon monoxide (CO ≥ 8ppm) and self-report questionnaires were administered. Participants were then randomized to one of three conditions (extended deprivation [12–24hrs smoking abstinence], minimal deprivation [2-hours smoking abstinence], or continued [ad libitum] smoking), and scheduled to attend an experimental study session (Session 2). All participants were provided with appointment reminder cards and a reminder call (24 hours prior to the appointment) with detailed smoking instructions corresponding to the assigned condition. Although time of day for attending Session 2 was not standardized, mean start times were similar across conditions (extended deprivation [10:57 AM; SD = 117 min], minimal deprivation [11:46 AM; SD = 129 min], continued smoking [12:01 PM; SD = 135 min]). Upon arrival to Session 2, compliance with smoking instructions was determined via self-report and exhaled CO. Specifically, participants were required to self-report compliance with the smoking instructions, and provide an exhaled CO reading that fit within pre-determined parameters for their condition assignment (described below). Nicotine withdrawal and urge to smoke were assessed prior to pain induction. Pain intensity was assessed throughout the capsaicin application, and neurogenic inflammation and mechanical hyperalgesia were assessed upon removal of the stimulus. Participants were compensated $20 for attending Session 1, and $80 for attending Session 2.
Nicotine Deprivation Manipulation
The primary goal of this study was to compare smokers randomized to continued smoking (ad libitum smoking; biochemically verified via expired CO ≥ 8ppm; Benowitz et al., 2002) and those randomized to extended nicotine deprivation (12–24hrs smoking abstinence, to ensure sufficient differentiation from the continued smoking group; compliance was biochemically verified via CO < 8ppm or a 50% reduction from baseline; Benowitz et al., 2002; Evans, Sutton, Oliver, & Drobes, 2015; Piper & Curtin, 2006). Participants randomized to the extended nicotine deprivation group were instructed not to smoke or use any nicotine products in the 12–24 hours leading up to their experimental appointment. Exhaled CO requirements for this condition (e.g., at least a 50% reduction from baseline) were designed to reflect the half-life of exhaled CO, which can last up to 8 hours during sleep (Benowitz et al., 2002). Given that nicotine self-administration can produce acute analgesic effects (Ditre, Heckman, Zale, Kosiba, & Maisto, 2016), and that difficulty distinguishing withdrawal effects from effects of ongoing nicotine administration (i.e., via ad libitum smoking) is a known limitation of nicotine deprivation studies (Hughes, 1991), we also recruited a smaller group of participants who were randomized to minimal deprivation (2-hours smoking abstinence; biochemically verified via expired CO ≥ 8ppm; Benowitz et al., 2002; Kassel & Shiffman, 1997). The minimal deprivation duration was informed by the half-life of nicotine (i.e., 2-hours; Benowitz, Hukkanen, & Jacob, 2009; Benowitz et al., 2002; Benowitz & Jacob, 1994; Benowitz, Jacob, Denaro, & Jenkins, 1991), and the minimal deprivation and continued smoking groups were compared to assess whether continued smokers may be experiencing acute analgesic effects of nicotine.
Experimental Pain Induction
Capsaicin is a vanilloid receptor agonist derived from chili peppers that induces prolonged activation of cutaneous nociceptors and mimics the spontaneous burning pain associated with neuropathic and inflammatory clinical pain states (Arendt-Nielsen & Andersen, 2005; Benarroch & Low, 1991; Hsieh & Lin, 1999; Parkhouse & Le Quesne, 1988). The capsaicin model permits tests of several pain-related outcomes (e.g., neurogenic inflammation, mechanical hyperalgesia; described in further detail below) that reflect different neural mechanisms of action (e.g., LaMotte, Lundberg, & Torebjork, 1992; Simone & Ochoa, 1991; Torebjork, Lundberg, & LaMotte, 1992). A 10% capsaicin solution was applied to the non-dominant volar forearm via a 1.5cm × 1.5cm gauze pad (Baron et al., 1999), which was covered to prevent movement or evaporation during the procedure. Capsaicin pain typically peaks in approximately 15–20 minutes (Geber et al., 2007; Petersen, Jones, Segredo, Dahl, & Rowbotham, 2001), and the stimulus was removed after 30 minutes.
Outcome Measures
Pain intensity
Capsaicin-induced pain intensity was assessed using a numerical rating scale (NRS) ranging from 0 (no pain) to 10 (pain as bad as you can imagine). Ratings were made at five-minute intervals. Total ratings were obtained by calculating the area under the response curves (AUC) for each participant using the trapezoidal method (Matthews, Altman, Campbell, & Royston, 1990).
Neurogenic inflammation
Neurogenic inflammation was quantified as the area of visible skin flare (i.e., redness that extends beyond the capsaicin application area; Helme & McKernan, 1985). The flare boundary was traced onto transparent acetate and scanned to generate an area value in pixels (Helme & McKernan, 1985) that was subsequently converted into squared centimeters.
Mechanical hyperalgesia
Mechanical hyperalgesia (i.e., increased sensitivity to mechanical stimulation) was assessed using a 6.65 von Frey hair (Bell-Krotoski, Fess, Figarola, & Hiltz, 1995). A standardized 300 grams of force was applied at points 1 centimeter apart along 8 linear paths radiating from the center of the application site, forming 8 concentric von Frey rings (Modir & Wallace, 2010). Participants rated pain intensity at each point using a numerical scale ranging from 0 (no pain) to 10 (pain as bad as you can imagine). Two measures of mechanical hyperalgesia were quantified, including sensitivity (by calculating AUC for each ring), and area of mechanical hyperalgesia (via several steps that have been described previously; Gottrup et al., 2004).
Other Measures
Biochemical verification of smoking status
Expired carbon monoxide (CO) was measured at both baseline and experimental sessions using a CoVita ToxCO™ CO monitor.
Tobacco dependence
The Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989) is a well-established six item scale that has been positively associated with biological markers of smoking dependence (e.g., salivary cotinine; Heatherton et al., 1989; Payne, Smith, McCracken, McSherry, & Antony, 1994).
Nicotine withdrawal
The Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986) was used to assess the severity of nine prototypical nicotine withdrawal symptoms at both sessions. Participants rated each item on a Likert scale from 0 (none) to 6 (severe) based on their experience over the past 24 hours. Responses were averaged to generate a total score. Internal consistency in the current sample was good (α = .88).
Urge to smoke tobacco
The Brief Questionnaire of Smoking Urges (QSU-Brief; Cox, Tiffany, & Christen, 2001) is a ten item measure that assesses current urge to smoke using a seven-point Likert scale ranging from 1 (strongly disagree) to 7 (strongly agree). The QSU-Brief demonstrated excellent internal consistency in the current sample (α = .95).
Past four-week pain severity
The Short Form Health Survey-12 (SFHS; Ware, Kosinski, & Keller, 1996) includes a single item that was used to assess the presence of past-four-week bodily pain (i.e., “How much bodily pain have you had during the past four weeks?”; Ware et al., 1996). Response options consisted of none, very mild, mild, moderate, severe, and very severe.
Anxiety symptoms
The Generalized Anxiety Disorder-7 (GAD-7; Spitzer, Kroenke, Williams, & Lowe, 2006) is a seven item measure that was selected for its utility as a broad-based index of anxiety and worry. Each item is rated on a four-point Likert scale ranging from 0 (not at all) to 3 (nearly every day), and a total score is generated by summing all items. Internal consistency in the current sample was excellent (α = .91).
Depression symptoms
The Center for Epidemiological Studies-Depression scale (CES-D; Radloff, 1977) is a twenty item measure that uses a four-point Likert scale ranging from 0 (rarely or none of the time) to 3 (most or all of the time) to assess the presence of psychological and physiological symptoms of depression over the past week. Total scores are calculated by summing all items, and internal consistency in the current sample was excellent (α = .90).
Data Analysis
Analyses were conducted using SPSS Statistics 21 (IBM SPSS Statistics, 2012). First, a manipulation check was performed by testing group differences in time since last cigarette, exhaled CO, MNWS total scores, and QSU-B total scores upon arrival to Session 2 using t-tests. We then tested for potential analgesic effects of ad libitum smoking by comparing the continued smoking and minimal deprivation conditions on AUC pain intensity scores, area of neurogenic inflammation, and area of mechanical hyperalgesia.
For the primary outcomes, we first tested the unadjusted effects of extended deprivation (vs. continued smoking) on capsaicin-induced pain intensity, neurogenic inflammation, and mechanical hyperalgesia using Analysis of Variance (ANOVA). We then adjusted for statistical covariates using Analysis of Covariance (ANCOVA). Past four-week pain intensity differed as a function of deprivation manipulation condition (p < .05; see Table 1), and was retained as a covariate in all analyses. Age, race, depression, and anxiety were also retained as covariates, due to observed associations with the primary outcome variables (i.e., AUC pain intensity, area of visible flare, or area of mechanical hyperalgesia). For the secondary outcome, we tested bivariate associations between AUC pain intensity ratings and individual MNWS nicotine withdrawal scores among the extended deprivation and continued smoking groups. Finally, we conducted exploratory analyses of unadjusted pain reactivity outcomes across all three experimental conditions.
Table 1.
Baseline Sociodemographic, Smoking, and Pain Characteristics by Deprivation Condition
| Continued Smoking (n = 63) | Minimal Deprivation (n = 28) | Extended Deprivation (n = 74) | Total Sample (N = 165) | |
|---|---|---|---|---|
|
| ||||
| n (%) | n (%) | n (%) | n (%) | |
| Gender | ||||
| Male | 38 (60.3%) | 17 (60.7%) | 39 (52.7%) | 94 (57.0%) |
| Ethnicity | ||||
| Hispanic/Latino | 3 (4.8%) | 2 (7.1%) | 3 (4.1%) | 8 (4.8%) |
| Race | ||||
| White | 34 (54.0%) | 14 (50.0%) | 42 (56.8%) | 90 (54.5%) |
| Black/African American | 27 (42.9%) | 13 (46.4%) | 28 (37.8%) | 68 (41.2%) |
| American Indian/Alaska Native | 2 (3.2%) | 1 (3.6%) | 4 (5.4%) | 7 (4.2%) |
| Marital Status | ||||
| Single | 40 (63.5%) | 18 (64.3%) | 41 (55.4%) | 99 (60.0%) |
| Married | 9 (14.3%) | 3 (10.7%) | 14 (18.9%) | 26 (15.8%) |
| Divorced/Separated/Widowed | 14 (22.2%) | 7 (25.0%) | 19 (25.7%) | 40 (24.2%) |
| Education | ||||
| 0 – 11 Years | 17 (27%) | 6 (21.4%) | 15 (20.3%) | 38 (23.0%) |
| 12 Years | 23 (36.5%) | 11 (39.3%) | 28 (37.8%) | 62 (37.6%) |
| 12 – 15 Years | 17 (26.9%) | 10 (35.7%) | 16 (32.5%) | 51 (30.9%) |
| ≥ 16 Years | 6 (9.5%) | 1 (3.6%) | 7 (9.6%) | 14 (8.4%) |
| Household Income | ||||
| < 10,000 | 30 (47.6%) | 10 (35.7%) | 26 (35.1%) | 66 (40.0%) |
| 10,000 – 29,999 | 20 (31.7%) | 12 (42.9%) | 25 (33.8%) | 57 (34.6%) |
| 30,000 – 49,999 | 7 (11.1%) | 2 (7.2%) | 10 (13.5%) | 19 (11.5%) |
| ≥ 50,000 | 6 (9.5%) | 4 (14.4%) | 13 (17.7%) | 23 (13.9%) |
| Past Four-Week Pain* | ||||
| None | 10 (15.9%) | 2 (7.1%) | 13 (17.6%) | 25 (15.2%) |
| Very Mild | 20 (31.7%) | 6 (21.4%) | 13 (17.6%) | 39 (23.6%) |
| Mild | 14 (22.2%) | 9 (32.1%) | 13 (17.6%) | 36 (21.8%) |
| Moderate | 18 (28.6%) | 6 (21.4%) | 22 (29.7%) | 46 (27.9%) |
| Severe | 1 (1.6%) | 5 (17.9%) | 13 (17.6%) | 19 (11.5%) |
|
| ||||
| M (SD) | M (SD) | M (SD) | M (SD) | |
|
| ||||
| Age | 40.86 (12.75) | 45.39 (13.18) | 39.72 (12.19) | 41.12 (12.66) |
| Cigarettes per day | 20.24 (9.74) | 20.43 (11.43) | 24.14 (15.55) | 22.02 (12.99) |
| Years of daily smoking | 22.86 (11.71) | 28.50 (13.45) | 23.45 (12.64) | 24.08 (12.52) |
| Nicotine Dependence1 | 6.02 (1.86) | 5.64 (2.57) | 6.22 (2.57) | 6.04 (2.14) |
| Nicotine Withdrawal2 | 1.59 (0.85) | 1.65 (0.85) | 1.89 (0.96) | 1.73 (0.91) |
Note.
Fagerstrom Test of Nicotine Dependence;
Minnesota Nicotine Withdrawal Scale;
p < .05.
Given that outliers can influence results and lead to false positives (e.g., Rousseeuw & Hubert, 2011; Simmons, Nelson, & Simonsohn, 2011), we identified and excluded outliers using a conservative method (i.e., ± 2.5 median absolute deviations from each respective outcome) that is recommended by statisticians and researchers (e.g., Leys, Ley, Klein, Bernard, & Licata, 2013). There is also reason to suspect that such data points may be invalid in the context of the capsaicin pain model, which requires a high degree of experimenter precision and proper maintenance throughout the experimental period (e.g., accidental oversaturation or participant shifting can each expand the area of stimulation and influence pain reporting). Four outliers were excluded from the pain intensity analyses (extended deprivation: n = 1; continued smoking: n = 3), 12 were excluded from the inflammation analyses (extended deprivation: n = 7; continued smoking: n = 5), and eight were excluded from the mechanical hyperalgesia analyses (extended deprivation: n = 0; continued smoking: n = 8). Sensitivity analyses were conducted to determine whether results differed as a function of outlier exclusion.
Results
Participant Retention
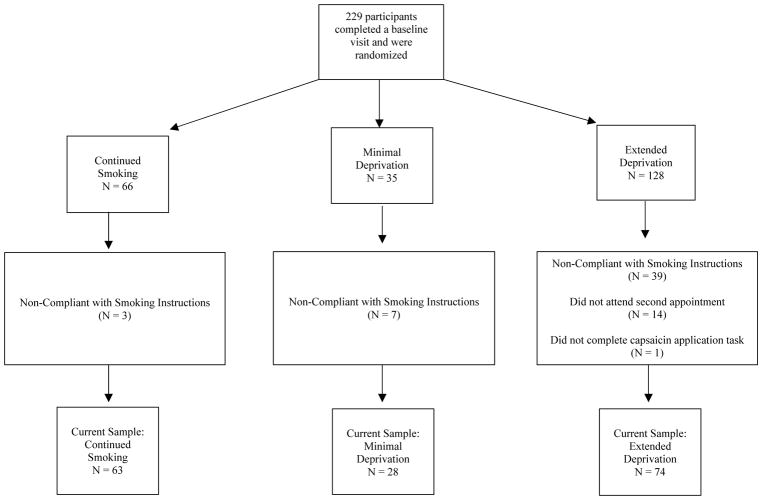
A total of 229 participants completed the baseline visit and were randomized to one of three experimental conditions (see Figure 1). Participants in the extended deprivation group were purposefully oversampled given known concerns regarding retention/compliance in studies that ask daily smokers to refrain from smoking for 12–24 hours (e.g., Perkins, Karelitz, Jao, Gur, & Lerman, 2013). As expected, participants randomized to extended deprivation were less likely to return for the experimental session or comply with smoking instructions than participants randomized to continued smoking or minimal deprivation (ps < .01). Specifically, 41% of participants randomized to extended deprivation either did not return for the experimental session (n = 14) or were determined to have not complied with the smoking instructions (n = 39). In the current study, those who did not return for Session 2 or comply with the smoking instructions reported lower urge to smoke at baseline (F [1, 227] = 4.575; p = .034) and greater nicotine withdrawal (F [1, 227] = 4.401; p = .037; see supplemental Table 1). No differences in any other sociodemographic, pain, and smoking characteristics were observed. The following analyses utilized the final sample (N = 165) who completed the experimental visit and were compliant with smoking instructions (n = 63 continued smoking; n = 74 extended deprivation; n = 28 minimal deprivation). All members of each group met the exhaled CO criteria required for their condition assignment.
Figure 1.
Flowchart depicting participant recruitment and retention.
Participant Characteristics
Participants were 165 current daily tobacco smokers (43% female; Mage = 41.12, SD = 12.66) who reported smoking an average of 22 cigarettes per day (M = 22.02, SD = 12.99) for 24 years (M = 24.08, SD = 12.52). As seen in Table 1, the mean FTND score was 6.04 (SD = 2.14), indicating a moderate level of nicotine dependence. Although participants denied having chronic pain, over one-third of the sample reported having experienced moderate-to-severe pain at some point over the past four-weeks. Approximately 40% of the sample identified as black or African American, more than half reported their highest level of education as high school graduate or less, and 40% reported an annual income of less than $10,000. Across all participants, significant positive correlations were observed between pain intensity ratings and areas of flare (r = .34, p < .001) and mechanical hyperalgesia (r = .47, p < .001). There was no significant correlation between the areas of flare and mechanical hyperalgesia (r = .09, p > .05).
Manipulation Checks
Continued smoking vs. Extended Deprivation
As expected, participants randomized to extended nicotine deprivation reported a longer duration of time since smoking their last cigarette (M = 17 hours, 31 minutes; SD = 6 hours, 7 minutes), relative to those randomized to continued smoking (M = 16 minutes; SD = 15 minutes; p < .001). Extended deprivation participants also evinced substantially lower exhaled CO readings (M = 5.93 [SE = .61] vs. M = 18.96 [SE = .66]; p < .001), and endorsed greater urge to smoke (QSU-B total M = 47.89 [SE = 1.72] vs. M = 34.77 [SD = 1.85]; p < .001), after controlling for baseline levels. We observed no differences with regard to past 24 hour MNWS nicotine withdrawal scores (p > .05).
Continued smoking vs. Minimal Deprivation
Participants randomized to minimal nicotine deprivation reported a longer duration of time since smoking their last cigarette (M = 2 hours, 5 minutes; SD = 21 minutes), relative to those randomized to continued smoking (M = 16 minutes; SD = 15 minutes; p < .001). After controlling for baseline levels, minimal deprivation participants also evinced lower exhaled CO (M = 15.41 [SE = 1.18] vs. M = 18.95 [SE = .79]; p = .014), and scored higher on a measure of urge to smoke (M = 46.81 [SE = 2.41] vs. M = 32.20 [SE = 1.59], p < .001). No differences were observed with regard to past 24 hour nicotine withdrawal (p > .05). We also observed no differences with regard to AUC pain intensity ratings, area of neurogenic inflammation, or area of mechanical hyperalgesia (all ps > .46), suggesting that continued smokers were not experiencing acute analgesic effects of nicotine.
Pain Intensity
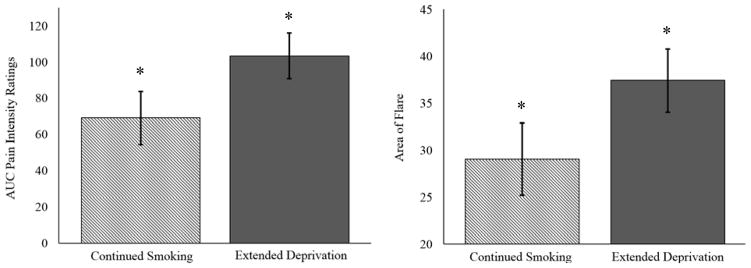
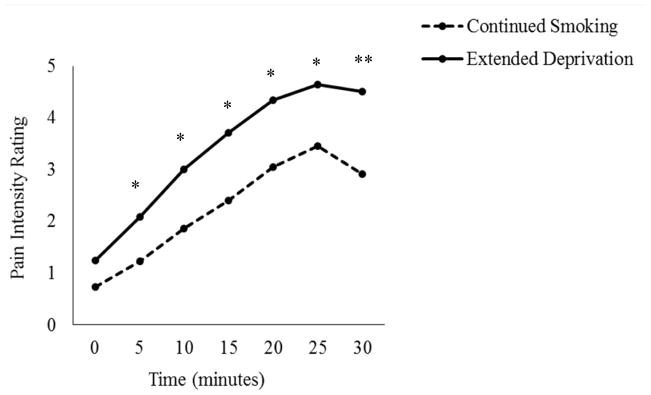
Smokers in the extended deprivation group reported greater pain over the course of the 30-minute capsaicin application period (M = 111.507, SD = 85.412), relative to those in the continued smoking group (M = 77.125, SD = 61.320) in both unadjusted (F [1, 131] = 6.826, p = .010, ηp2 = .050) and adjusted (F [1, 120] = 6.033, p = .015, ηp2 = .048; see Figure 2) analyses. Similarly, both unadjusted and adjusted analyses revealed that deprived smokers reported greater pain intensity at 5, 10, 15, 20, 25, and 30 minutes post-capsaicin application (all ps < .05; see Figure 3). Consistent with the typical ramp-up period for topical capsaicin (Geber et al., 2007; Petersen et al., 2001), no differences were observed at the 0 minute time-point (p > .05).
Figure 2.
AUC pain intensity ratings and area of neurogenic inflammation (flare) as a function of deprivation condition assignment. Note: Means statistically adjusted for age, race, past four-week pain severity, depression, and anxiety; * p < .05.
Figure 3.
Mean pain intensity ratings across the 30 minute capsaicin application period as a function of time and deprivation condition. Note: Means statistically adjusted for age, race, past four-week pain severity, depression, and anxiety; * p < .05, ** p < .01.
Neurogenic Inflammation
Extended nicotine deprivation also resulted in greater neurogenic inflammation. Specifically, smokers randomized to the extended deprivation condition exhibited a larger area of visible flare (M = 38.520 cm2, SD = 22.722 cm2) than participants randomized to continued smoking (M = 30.236 cm2, SD = 21.156 cm2) in both unadjusted (F[1, 123] = 4.404, p = .038, ηp2 = .035) and adjusted (F[1, 112] = 4.818, p = .030, ηp2 = .041; see Figure 2) analyses.
Mechanical Hyperalgesia
In both adjusted and unadjusted models, smokers in the extended deprivation condition (vs. continued smoking) evinced greater sensitivity to mechanical stimulation at each von Frey ring surrounding the application site (all ps < .05; see Table 2). Additionally, smokers randomized to extended deprivation demonstrated a larger area of mechanical hyperalgesia (M = 71.982, SD = 55.171), compared to participants in the continued smoking condition (M = 45.068, SD = 37.139) in both unadjusted (F[1, 127] = 9.783, p = .002, ηp2 = .072) and adjusted (F[1, 116] = 8.070, p = .005, ηp2 = .065) analyses. Thus, smokers randomized to extended deprivation evinced both greater mechanical pain sensitivity and hyperalgesia across a larger surface area.
Table 2.
Pain Ratings by von Frey Ring and Condition
| Continued Smoking M (SE) |
Extended Deprivation M (SE) |
|
|---|---|---|
| Ring 1 (outermost)* | 1.23 (1.15) | 3.82 (.96) |
| Ring 2** | 1.37 (1.25) | 4.41 (1.04) |
| Ring 3** | 2.19 (1.48) | 6.12 (1.24) |
| Ring 4 ** | 2.96 (1.75) | 8.21 (1.46) |
| Ring 5 ** | 4.13 (2.05) | 10.79 (1.71) |
| Ring 6 *** | 5.24 (2.42) | 13.70 (2.02) |
| Ring 7 *** | 7.13 (2.86) | 16.51 (2.38) |
| Ring 8 (innermost) ** | 8.50 (3.21) | 18.58 (2.67) |
Note. Means were statistically adjusted for age, race, past four-week pain severity, depression, and anxiety;
p < .05;
p < .01,
p < .001.
Associations between Pain Intensity and Nicotine Withdrawal Symptoms
AUC pain intensity ratings were positively associated with MNWS nicotine withdrawal severity scores (r = .34, p < .001; see Table 3). Additionally, all individual withdrawal symptoms assessed by the MNWS were positively correlated with capsaicin-induced pain intensity ratings, including anger (r = .171, p = .049), anxiety (r = .260, p = .003), depressed mood (r = .207, p = .017), craving (r = .182, p = .036), difficulty concentrating (r = .212, p = .014), increased appetite (r = .274, p = .001), sleep problems (r = .277, p = .001), restlessness (r = .271; p = .002), and impatience (r = .296; p = .001).
Table 3.
Correlations between AUC Pain Intensity Ratings and Nicotine Withdrawal Symptoms
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 AUC- Pain Intensity | - | .34** | .17* | .26** | .21* | .18* | .21* | .27** | .28** | .27* | .30** |
| 2 MNWS – Total | - | .79** | .82** | .76** | .55** | .85** | .48** | .60** | .77** | .84** | |
| 3 MNWS – Anger | - | .77** | .64** | .40** | .64** | .29** | .25** | .52** | .67** | ||
| 4 MNWS – Anxiety | - | .64** | .46** | .69** | .31** | .28** | .50** | .70** | |||
| 5 MNWS – Depressed Mood | - | .28** | .66** | .34** | .34** | .40** | .59** | ||||
| 6 MNWS – Craving | - | .43** | .19* | .16 | .31** | .38** | |||||
| 7 MNWS – Difficulty Concentrating | - | .33** | .39** | .60** | .71** | ||||||
| 8 MNWS – Increased Appetite | - | .17 | .21* | .31** | |||||||
| 9 MNWS – Sleep Problems | - | .76** | .39** | ||||||||
| 10 MNWS – Restlessness | - | .63** | |||||||||
| 11 MNWS – Impatience | - |
Note.
p < .05;
p < .01.
Exploratory Analyses
Although primary analyses focused on comparisons between continued smoking and extended deprivation groups, exploratory examination of the unadjusted means across all three experimental conditions revealed that AUC pain ratings, area of flare, and area of mechanical hyperalgesia were each lowest for the continued smoking group, somewhat higher for the minimal deprivation group, and highest for the extended deprivation group (see Table 4). However, the only statistically-significant differences (in addition to the primary outcomes reported above) were observed between extended and minimally-deprived smokers for area of flare (p < .05), and between minimally-deprived and continued smokers for pain ratings on the three outermost von Frey rings (ps < .05).
Table 4.
Unadjusted Means and Standard Deviations for Pain Reactivity Outcomes as a Function of Condition Assignment
| Continued Smoking M (SD) |
Minimal Deprivation M (SD) |
Extended Deprivation M (SD) |
Total Sample M (SD) |
|
|---|---|---|---|---|
| Pain Intensity during Capsaicin Application | ||||
| 0 Minutes | 1.03 (1.62) | 1.71 (2.48) | 1.48 (2.40) | 1.35 (2.14) |
| 5 Minutesb | 1.55 (1.75) | 2.14 (2.32) | 2.48 (2.60) | 2.15 (2.32) |
| 10 Minutesb | 2.25 (2.18) | 2.82 (2.75) | 3.49 (3.24) | 3.01 (2.90) |
| 15 Minutesb | 2.70 (2.39) | 3.54 (3.48) | 4.03 (3.28) | 3.56 (3.14) |
| 20 Minutesb | 3.25 (2.67) | 4.00 (3.49) | 4.49 (3.37) | 4.05 (3.24) |
| 25 Minutesb | 3.47 (2.77) | 4.04 (3.79) | 4.66 (3.45) | 4.21 (3.36) |
| 30 Minutesb | 3.38 (2.82) | 3.89 (9.82) | 4.82 (3.52) | 4.20 (3.39) |
| AUC Total b | 77.13 (61.32) | 96.70 (83.64) | 111.51 (85.41) | 98.67 (79.59) |
|
| ||||
| Area of Flare b, c | 30.24 (21.16) | 31.95 (17.06) | 38.52 (22.72) | 39.04 (27.61) |
|
| ||||
| Area of Mechanical Hyperalgesia b | 45.07 (37.14) | 60.95 (56.78) | 71.98 (55.17) | 66.37 (55.95) |
|
| ||||
| Von Frey Pain Ratings | ||||
| Ring 1 (outermost)a, b | 1.12 (1.81) | 2.36 (3.49) | 3.96 (7.08) | 3.55 (6.82) |
| Ring 2a, b | 1.42 (2.35) | 3.02 (3.85) | 4.73 (7.63) | 4.14 (7.23) |
| Ring 3a, b | 2.05 (2.79) | 3.96 (5.06) | 6.44 (8.98) | 5.37 (8.25) |
| Ring 4b | 3.25 (3.54) | 5.27 (6.65) | 8.81 (10.43) | 7.10 (9.45) |
| Ring 5b | 4.79 (4.96) | 7.11 (8.75) | 11.51 (11.73) | 9.28 (10.70) |
| Ring 6b | 6.55 (5.88) | 9.91 (12.00) | 15.03 (13.79) | 12.09 (12.76) |
| Ring 7b | 9.10 (7.52) | 12.18 (14.68) | 18.40 (16.14) | 14.98 (14.79) |
| Ring 8 (innermost)b | 10.87 (9.14) | 15.52 (17.21) | 20.84 (18.12) | 17.35 (16.60) |
Note.
Significant (p < .05) difference between continued smoking and minimal deprivation conditions;
Significant (p < .05) difference between continued smoking and extended deprivation conditions.
Significant (p < .05) difference between minimal deprivation and extended deprivation conditions in primary outcome variable.
Sensitivity Analyses
As shown in Table 5, sensitivity analyses revealed that, when all outliers were included, extended deprivation vs. continued smoking comparisons were reduced to trend-level (p < .10) for AUC pain intensity outcomes, and were no longer statistically-significant for mean area of flare and mechanical hyperalgesia (ps > .10). Significant group differences were still observed for the three innermost von Frey pain intensity ratings (ps < .05).
Table 5.
Sensitivity Analyses of Primary Outcomes Comparing Continued Smoking and Extended Deprivation Groups with Outliers Included
| ContinuedSmoking M (SE) |
Extended Deprivation M (SE) |
|
|---|---|---|
| Pain Intensity during Capsaicin Application | ||
| 0 Minutes | .79 (.38) | 1.21 (.34) |
| 5 Minutes † | 1.41 (.43) | 2.10 (.38) |
| 10 Minutes | 2.14 (.55) | 2.95 (.49) |
| 15 Minutes † | 2.72 (.59) | 3.67 (.52) |
| 20 Minutes | 3.35 (.60) | 4.29 (.54) |
| 25 Minutes | 3.76 (.61) | 4.60 (.54) |
| 30 Minutes * | 3.14 (.60) | 4.48 (.53) |
| AUC Total † | 76.80 (14.82) | 102.16 (13.16) |
|
| ||
| Area of Flare | 32.33 (4.79) | 39.97 (4.26) |
|
| ||
| Area of Mechanical Hyperalgesia | 65.71 (10.36) | 73.84 (9.18) |
|
| ||
| Von Frey Pain Ratings | ||
| Ring 1 (outermost) | 4.61 (1.36) | 4.55 (1.20) |
| Ring 2 | 4.79 (1.43) | 5.17 (1.27) |
| Ring 3 | 5.76 (1.61) | 6.93 (1.43) |
| Ring 4 | 6.73 (1.84) | 9.19 (1.63) |
| Ring 5 † | 8.24 (2.06) | 11.89 (1.83) |
| Ring 6 * | 10.10 (2.42) | 15.23 (2.15) |
| Ring 7 * | 12.44 (2.79) | 18.36 (2.47) |
| Ring 8 (innermost) * | 14.03 (3.08) | 20.58 (2.73) |
Note. Analysis statistically adjusted for age, race, past four-week pain, anxiety, and depression.
p < .10,
p < .05.
Discussion
The current study represents the first systematic laboratory investigation into the effects of nicotine deprivation on multiple indices of human pain reactivity. As hypothesized, participants randomized to extended 12–24 hour nicotine deprivation evinced greater capsaicin-induced pain intensity, neurogenic inflammation, and mechanical hyperalgesia than participants randomized to continued ad lib smoking. Pain intensity ratings were also positively correlated with individual nicotine withdrawal symptoms assessed using the MNWS. Collectively, these findings indicate that smokers may experience hyperalgesia or increased sensitivity to pain during the early stages of smoking abstinence.
These data also provide insight into potential mechanisms by which nicotine deprivation may exacerbate pain. For example, neurogenic inflammation reflects vasodilation caused by neuropeptide release from peripheral C-Fiber activation (Geber et al., 2007; Holzer, 1998), whereas mechanical hyperalgesia reflects an enhanced excitability of spinal dorsal horn neurons (Treede, Handwerker, Baumgärtner, Meyer, & Magerl, 2004) and the release of several pain-related neurotransmitters (i.e., glutamate, substance P, CGRP, somatostatin, and nitric oxide) at the central level (Sandkühler, 2009; Serra, Campero, & Ochoa, 1998; Ziegler, Magerl, Meyer, & Treede, 1999). Taken together, these results indicate that nicotine deprivation may influence both central and peripheral pain processes. This is consistent with previous findings that both central and peripheral nAChRs mediate the effects of nicotine deprivation on somatic symptoms of nicotine withdrawal (e.g., Cosgrove et al., 2009). Future research should clarify the role of nAChRs in pain-nicotine deprivation associations.
Although increased pain reactivity is considered to be characteristic of nicotine withdrawal across a well-established animal literature (e.g., Grabus et al., 2005; Jackson et al., 2009), researchers have just recently begun to examine potentially complex associations between the experience of pain and nicotine withdrawal among humans (e.g., Ditre, Kosiba, Zale, Zvolensky, & Maisto, 2016). We observed positive correlations between experimental pain ratings and individual nicotine withdrawal scores, and exploratory analyses including participants randomized to minimal deprivation further suggested a pattern of responses in which pain sensitivity appeared to increase with duration of smoking abstinence (see Table 4). Given that the minimal deprivation group was purposefully under-sampled, their performance should be interpreted with caution. However, these data provide initial evidence that two hours of nicotine deprivation may be sufficient to produce hyperalgesia, which would be consistent with previous work showing that many nicotine withdrawal symptoms emerge within the first few hours of smoking abstinence (Hendricks, Ditre, Drobes, & Brandon, 2006). Future research with larger samples is needed to adequately test the hypothesis that pain sensitivity may be incrementally related to duration of nicotine deprivation.
Several limitations of this study should be noted. First, it remains unclear whether the current results reflect a nicotine withdrawal effect (i.e., a biphasic transient change after abstinence) or a nicotine offset effect (i.e., a unidirectional change after abstinence; e.g., Hughes, 2007), and we did not account for factors that can influence plasma nicotine levels (e.g., trough levels, individual differences in clearance/tolerance effects; Benowitz et al., 2009). Future research should examine pain reactivity as a function of plasma nicotine levels, and employ a longer follow-up period to assess the time course of deprivation-induced hyperalgesia. Second, although participants were excluded if they endorsed taking prescription medications for pain, we did not assess all current medication use. Given that some medications (e.g., SSRIs) could influence pain reporting (e.g., Jung, Staiger, & Sullivan, 1997), future work would benefit from a more thorough assessment of current medication use. Third, although extended deprivation participants were asked to abstain from smoking for 12–24 hours prior to their appointment, the MNWS assessed withdrawal over the previous 24 hours. Thus, compliant participants could have smoked within the previous 24 hours, which may help to explain why we did not observe differences in MNWS scores as a function of deprivation condition assignment. Fourth, the attrition rate among extended deprivation participants (41%) may have introduced selection bias and is at the higher end of those observed in studies requiring similar durations of smoking abstinence (e.g., 12.3% – 44%; Brandon et al., 2011; Perkins et al., 2013; Robinson et al., 2007; Sweitzer et al., 2016), other sources of methodological variation notwithstanding (e.g., Perkins and colleagues enrolled smokers willing to engage in short-term practice quit attempts). Although baseline differences in past four-week pain severity could have further biased statistical tests, this variable was accounted for in adjusted analyses. It was also surprising that participants who did not return for the experimental session or comply with smoking instructions reported both lower urge to smoke and greater nicotine withdrawal at baseline, especially given that measures of urge and withdrawal are typically positively correlated (e.g., Tiffany, 1990; Toll, O’Malley, McKee, Salovey, & Krishnan-Sarin, 2007). Finally, sensitivity analyses revealed that statistically-significant findings were more consistently observed when outliers were excluded. Future research is needed to replicate these findings, perhaps using a combination of experimental pain modalities that assess both static and dynamic sensory functions (Arendt-Nielsen & Yarnitsky, 2009; Neziri et al., 2011).
Clinical implications of this work include the possibility that smokers may experience increased sensitivity to pain during the early stages of a quit attempt, and this may be especially important for the large population of smokers who live with comorbid chronic pain (e.g., Orhurhu, Pittelkow, & Hooten, 2015; Zvolensky et al., 2009). Given that previous work has shown situational pain to be a potent motivator of smoking behavior (Ditre & Brandon, 2008; Ditre, Heckman, Butts, & Brandon, 2010), and that that pain-related factors can account for unique variance in smoking lapse and relapse outcomes (LaRowe, Langdon, Zvolensky, Zale, & Ditre, 2017), future research should examine whether nicotine deprivation-induced hyperalgesia may precipitate relapse to smoking, and whether cessation interventions may benefit from tailoring to account for such effects. For example, given that nicotine can produce acute analgesia (Ditre, Heckman, et al., 2016), high dose nicotine replacement therapy may help to reduce deprivation-induced amplification of pain (e.g., Mills et al., 2012; Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006). Varenicline, a partial agonist of α4β2 nicotinic acetylcholine receptors that has demonstrated efficacy for smoking cessation (Jorenby et al., 2006), may also have utility in the mitigation of pain during the early stages of a quit attempt. Although this hypothesis has yet to be tested among humans, initial evidence derived from rodent models indicates that Varenicline can produce pain-inhibitory effects, as mediated by α3β4 nAChRs (AlSharari, Carroll, McIntosh, & Damaj, 2012). Finally, psychosocial cessation treatments may benefit from incorporating training in the use of more adaptive (i.e., non-smoking related) pain-coping strategies (e.g., relaxation, distraction, mindfulness; Davis, Zautra, Wolf, Tennen, & Yeung, 2015; Keefe, Rumble, Scipio, Giordano, & Perri, 2004) to enhance self-efficacy for quitting, reduce expectations that pain may impede smoking cessation (Ditre, Zale, Heckman, & Hendricks, 2017), and ultimately diminish pain-induced urge to smoke (Ditre et al., 2010).
Supplementary Material
General Scientific Summary.
Relations between the experience of pain and nicotine withdrawal are of increasing scientific interest, and there is reason to suspect that abstaining from smoking may increase pain during the early stages of a quit attempt. This study indicates that nicotine deprivation can increase pain intensity ratings, neurogenic inflammation, and mechanical hyperalgesia among daily tobacco smokers. Results also provide initial evidence that pain sensitivity may be incrementally related to duration of smoking abstinence.
Acknowledgments
This research was supported by NIH Grant No. R21DA034285 awarded to Joseph W. Ditre. The study was approved by the Institutional Review Board at Syracuse University (Smoking and Mood Study; Protocol #12-228).
This research was supported by NIH Grant No. R21DA034285 awarded to Joseph W. Ditre.
Footnotes
There has not been prior dissemination of the data and ideas appearing in this manuscript.
Conflicts of interest
None.
References
- Aho K, Heliovaara M. Risk factors for rheumatoid arthritis. Annals of Medicine. 2004;36(4):242–251. doi: 10.1080/07853890410026025. [DOI] [PubMed] [Google Scholar]
- AlSharari SD, Carroll FI, McIntosh JM, Damaj MI. The antinociceptive effects of nicotinic partial agonists varenicline and sazetidine-A in murine acute and tonic pain models. Journal of Pharmacology and Experimental Therapeutics. 2012;342(3):742–749. doi: 10.1124/jpet.112.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Andersen OK. Capsaicin in human experimental pain models of skin, muscle and visceral sensitization. In: Malmberg AB, Bley KR, editors. Turning up the Heat on Pain: TRPV1 Receptors in Pain and Inflammation. Birkhäuser; Basel: 2005. pp. 117–144. [Google Scholar]
- Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. The Journal of Pain. 2009;10(6):556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Baiamonte BA, Stickley SC, Ford SJ. Nicotine Deprivation Produces Deficits in Pain Perception that are Moderately Attenuated by Caffeine Consumption. Journal of Psychoactive Drugs. 2016:1–7. doi: 10.1080/02791072.2016.1172745. [DOI] [PubMed] [Google Scholar]
- Baron R, Wasner G, Borgstedt R, Hastedt E, Schulte H, Binder A, … Fields HL. Effect of sympathetic activity on capsaicin-evoked pain, hyperalgesia, and vasodilatation. Neurology. 1999;52(5):923–932. doi: 10.1212/wnl.52.5.923. [DOI] [PubMed] [Google Scholar]
- Bastian LA, Fish LJ, Gierisch JM, Stechuchak KM, Grambow SC, Keefe FJ. Impact of smoking cessation on subsequent pain intensity among chronically Ill veterans enrolled in a smoking cessation trial. Journal of Pain and Symptom Management. 2015;50(6):822–829. doi: 10.1016/j.jpainsymman.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Behrend C, Prasarn M, Coyne E, Horodyski M, Wright J, Rechtine GR. Smoking cessation related to improved patient-reported pain scores following spinal care. Journal of Bone and Joint Surgery. 2012;94(23):2161–2166. doi: 10.2106/JBJS.K.01598. [DOI] [PubMed] [Google Scholar]
- Bell-Krotoski JA, Fess EE, Figarola JH, Hiltz D. Threshold detection and Semmes-Weinstein monofilaments. Journal of Hand Therapy. 1995;8(2):155–162. doi: 10.1016/s0894-1130(12)80314-0. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, Low PA. The acetylcholine-induced flare response in evaluation of small fiber dysfunction. Annals of Neurology. 1991;29(6):590–595. doi: 10.1002/ana.410290604. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of Experimental Pharmacology. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, III, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, … Tsoh J. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4(2) [Google Scholar]
- Benowitz NL, Jacob P. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clinical Pharmacology & Therapeutics. 1994;56(5):483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Denaro C, Jenkins R. Stable isotope studies of nicotine kinetics and bioavailability. Clinical Pharmacology & Therapeutics. 1991;49(3):270–277. doi: 10.1038/clpt.1991.28. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, … Small BJ. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 2011;218(2):391–403. doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, … Perry E. β2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Archives of General Psychiatry. 2009;66(6):666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, McKee S, Bois F, Alagille D, Tamagnan GD, … Staley JK. Beta2* nicotinic acetylcholine receptors modulate pain sensitivity in acutely abstinent tobacco smokers. Nicotine & Tobacco Research. 2010;12(5):535–539. doi: 10.1093/ntr/ntq040. ntq040 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Wolf LD, Tennen H, Yeung EW. Mindfulness and cognitive–behavioral interventions for chronic pain: Differential effects on daily pain reactivity and stress reactivity. Journal of Consulting and Clinical Psychology. 2015;83(1):24. doi: 10.1037/a0038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH. Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology. 2008;117(2):467–472. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychological Bulletin. 2011;137(6):1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. Journal of Abnormal Psychology. 2010;119(3):524–533. doi: 10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Zale EL, Kosiba JD, Maisto SA. Acute analgesic effects of nicotine and tobacco in humans: a meta-analysis. Pain. 2016 doi: 10.1097/j.pain.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Kosiba JD, Zale EL, Zvolensky MJ, Maisto SA. Chronic pain status, nicotine withdrawal, and expectancies for smoking cessation among lighter smokers. Annals of Behavioral Medicine. 2016;50(3):427–435. doi: 10.1007/s12160-016-9769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Langdon KJ, Kosiba JD, Zale EL, Zvolensky MJ. Relations between pain-related anxiety, tobacco dependence, and barriers to quitting among a community-based sample of daily smokers. Addictive Behaviors. 2015;42:130–135. doi: 10.1016/j.addbeh.2014.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, Heckman BW, Hendricks PS. A measure of perceived pain and tobacco smoking interrelations: pilot validation of the pain and smoking inventory. Cognitive Behaviour Therapy. 2017;46(4):339–351. doi: 10.1080/16506073.2016.1256347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Sutton SK, Oliver JA, Drobes DJ. Cortical activity differs during nicotine deprivation versus satiation in heavy smokers. Psychopharmacology. 2015;232(11):1879–1885. doi: 10.1007/s00213-014-3821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P. The economic costs of pain in the United States. The Journal of Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Geber C, Fondel R, Krämer HH, Rolke R, Treede RD, Sommer C, Birklein F. Psychophysics, flare, and neurosecretory function in human pain models: capsaicin versus electrically evoked pain. The Journal of Pain. 2007;8(6):503–514. doi: 10.1016/j.jpain.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gottrup H, Juhl G, Kristensen AD, Lai R, Chizh BA, Brown J, … Jensen TS. Chronic oral gabapentin reduces elements of central sensitization in human experimental hyperalgesia. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2004;101(6):1400–1408. doi: 10.1097/00000542-200412000-00021. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Batman AM, Tyndale RF, Sellers E, Damaj MI. Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology. 2005;178(2–3):183–192. doi: 10.1007/s00213-004-2007-3. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction. 1989;84(7):791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Helme R, McKernan S. Neurogenic flare responses following topical application of capsaicin in humans. Annals of Neurology. 1985;18(4):505–509. doi: 10.1002/ana.410180414. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. General Pharmacology: The Vascular System. 1998;30(1):5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Hooten WM, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152(1):223–229. doi: 10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh ST, Lin WM. Modulation of keratinocyte proliferation by skin innervation. Journal of Investigative Dermatology. 1999;113(4):579–586. doi: 10.1046/j.1523-1747.1999.00737.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Distinguishing withdrawal relief and direct effects of smoking. Psychopharmacology. 1991;104(3):409–410. doi: 10.1007/BF02246044. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine & Tobacco Research. 2007;9(3):315–327. doi: 10.1080/14622200701188919. 772841444 [pii] [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of α6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. Journal of Pharmacology and Experimental Therapeutics. 2009;331(2):547–554. doi: 10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, DBS, Graffunder CM. Current cigarette smoking among adults—United States, 2005–2015. MMWR. Morbidity and Mortality Weekly Report. 2016;65 doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Stetson BA, Parris WC. The relationship between cigarette smoking and chronic low back pain. Addictive Behaviors. 1991;16(3–4):103–110. doi: 10.1016/0306-4603(91)90002-Y. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, … Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. 296/1/56 [pii] [DOI] [PubMed] [Google Scholar]
- Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. Journal of General Internal Medicine. 1997;12(6):384–389. doi: 10.1046/j.1525-1497.1997.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Shiffman S. Attentional mediation of cigarette smoking’s effect on anxiety. Health Psychology. 1997;16(4):359–368. doi: 10.1037//0278-6133.16.4.359. [DOI] [PubMed] [Google Scholar]
- Kaye AD, Prabhakar AP, Fitzmaurice ME, Kaye RJ. Smoking cessation in pain patients. The Ochsner Journal. 2012;12(1):17–20. [PMC free article] [PubMed] [Google Scholar]
- Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LM. Psychological aspects of persistent pain: current state of the science. The Journal of Pain. 2004;5(4):195–211. doi: 10.1016/j.jpain.2004.02.576. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. Journal of Physiology. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe LR, Langdon KJ, Zvolensky MJ, Zale EL, Ditre JW. Pain-Related Anxiety as a Predictor of Early Lapse and Relapse to Cigarette Smoking. Experimental and Clinical Psychopharmacology. 2017 doi: 10.1037/pha0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology. 2013;49(4):764–766. [Google Scholar]
- Matthews J, Altman DG, Campbell M, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, … Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. Journal of Pain and Symptom Management. 2004;28(3):250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Annals of Medicine. 2012;44(6):588–597. doi: 10.3109/07853890.2012.705016. [DOI] [PubMed] [Google Scholar]
- Modir JG, Wallace MS. Human experimental pain models 3: heat/capsaicin sensitization and intradermal capsaicin models. Methods in Molecular Biology. 2010;617:169–174. doi: 10.1007/978-1-60327-323-7_14. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Al’Absi M. Nicotine withdrawal and stress-induced changes in pain sensitivity: A cross-sectional investigation between abstinent smokers and nonsmokers. Psychophysiology. 2014;51(10):1015–1022. doi: 10.1111/psyp.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neziri AY, Curatolo M, Nüesch E, Scaramozzino P, Andersen OK, Arendt-Nielsen L, Jüni P. Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain. 2011;152(5):1146–1155. doi: 10.1016/j.pain.2011.01.047. [DOI] [PubMed] [Google Scholar]
- Orhurhu VJ, Pittelkow TP, Hooten WM. Prevalence of smoking in adults with chronic pain. Tobacco Induced Diseases. 2015;13(1):17. doi: 10.1186/s12971-015-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse N, Le Quesne PM. Quantitative objective assessment of peripheral nociceptive C fibre function. Journal of Neurology, Neurosurgery & Psychiatry. 1988;51(1):28–34. doi: 10.1136/jnnp.51.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, Morasco BJ. Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. The Journal of Pain. 2012;13(3):285–292. doi: 10.1016/j.jpain.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM. Assessing nicotine dependence: A comparison of the Fagerström Tolerance Questionnaire (FTQ) with the Fagerström Test for Nicotine Dependence (FTND) in a clinical sample. Addictive Behaviors. 1994;19(3):307–317. doi: 10.1016/0306-4603(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Stiller RL, Scierka A, Goettler J, Reynolds W, Jennings JR. Effects of Nicotine on Thermal Pain Detection in Humans. Experimental and Clinical Psychopharmacology. 1994;2(1):95–106. [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC, Gur RC, Lerman C. Effects of bupropion on cognitive performance during initial tobacco abstinence. Drug and Alcohol Dependence. 2013;133(1):283–286. doi: 10.1016/j.drugalcdep.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KL, Jones B, Segredo V, Dahl JB, Rowbotham MC. Effect of remifentanil on pain and secondary hyperalgesia associated with the heat–capsaicin sensitization model in healthy volunteers. The Journal of the American Society of Anesthesiologists. 2001;94(1):15–20. doi: 10.1097/00000542-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Piper ME, Curtin JJ. Tobacco withdrawal and negative affect: an analysis of initial emotional response intensity and voluntary emotion regulation. Journal of Abnormal Psychology. 2006;115(1):96–102. doi: 10.1037/0021-843X.115.1.96. 2006-02317-011 [pii] [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Robinson JD, Cinciripini PM, Tiffany ST, Carter BL, Lam CY, Wetter DW. Gender differences in affective response to acute nicotine administration and deprivation. Addictive Behaviors. 2007;32(3):543–561. doi: 10.1016/j.addbeh.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw PJ, Hubert M. Robust statistics for outlier detection. Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discovery. 2011;1(1):73–79. [Google Scholar]
- Sandkühler J. Models and Mechanisms of Hyperalgesia and Allodynia. Physiological Reviews. 2009;89(2):707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J. Flare and hyperalgesia after intradermal capsaicin injection in human skin. Journal of Neurophysiology. 1998;80(6):2801–2810. doi: 10.1152/jn.1998.80.6.2801. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184(3–4):637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. American Journal of Medicine. 2010;123(1):87e87–35. doi: 10.1016/j.amjmed.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science. 2011;22(11):1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47(3):285–294. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Denlinger R, Forbes EE, Raiff BR, Dallery J, … Donny EC. Blunted striatal response to monetary reward anticipation during smoking abstinence predicts lapse during a contingency-managed quit attempt. Psychopharmacology. 2016;233(5):751–760. doi: 10.1007/s00213-015-4152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97(2):147. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota nicotine withdrawal scale. Psychology of Addictive Behaviors. 2007;21(2):216. doi: 10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. Journal of Physiology. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede R, Handwerker H, Baumgärtner U, Meyer R, Magerl W. Hyperalgesia and allodynia: taxonomy, assessment, and mechanisms. Hyperalgesia: Molecular Mechanisms and Clinical Implications. 2004;30:1–15. [Google Scholar]
- US Department of Health & Human Services. A Report of the Surgeon General. 2014. The health consequences of smoking—50 years of progress. [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual healthcare spending attributable to cigarette smoking: an update. American Journal of Preventive Medicine. 2015;48(3):326–333. doi: 10.1016/j.amepre.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, Ditre JW. Anxiety and Depression in Bidirectional Relations Between Pain and Smoking Implications for Smoking Cessation. Behavior Modification. 2015 doi: 10.1177/0145445515610744. 0145445515610744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E, Magerl W, Meyer R, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Brain. 1999;122(12):2245–2257. doi: 10.1093/brain/122.12.2245. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine & Tobacco Research. 2009;11(12):1407–1414. doi: 10.1093/ntr/ntp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.