Abstract
To test the relative roles of perforin (pfp) vs. FasL in CTL control of autoreactive B cell expansion, we used the parent-into-F1 model of murine graft-vs.–host disease in which donor CD8 CTL prevent lupus like disease by eliminating activated autoreactive B cells. F1 mice receiving either pfp or FasL defective donor T cells exhibited an intermediate short-term phenotype. Pairing of purified normal CD4 T cells with either pfp or FasL defective CD8 T cell subsets resulted in impaired host B cell elimination and mild lupus like disease that was roughly equivalent in the two experimental groups. Thus, in addition to major roles in tumor and intracellular pathogen control, pfp mediated CD8 CTL killing plays a significant role in controlling autoreactive B cell expansion and lupus downregulation that is comparable to that mediated by FasL killing. Importantly, both pathways are required for optimal elimination of activated autoreactive B cells.
Keywords: Graft-vs.-host disease, T cells, cytotoxicity, lupus
1. Introduction
Systemic lupus erythematosus (lupus) is an immune mediated multi-system disease characterized by autoantibodies targeted to nuclear antigens. CD4+ T cells are central in driving B cell autoantibody production in both human and murine lupus [1-5] whereas CD8+ T cells are one of several potential down regulatory mechanisms in lupus and have been shown to limit experimental tolerance breaks in normal mice and to fail in lupus mice [6-9].
A useful model for studying the T cell-B cell mechanisms involved in lupus induction is the parent-into-F1 (P→F1) model of chronic graft-vs.-host disease (cGVHD) [10]. In this model, the transfer of parental strain CD4 T cells into semi-allogeneic F1 hosts induces a lupus-like disease as a consequence of donor T cell recognition of host allogeneic MHC II resulting in cognate help to host B cells. Donor CD8 T cells are critical for limiting host B cell expansion and lupus-like disease in this model. Specifically, the transfer of both donor CD4 and CD8 T cells prevents lupus expression because donor CD8 CTL specific for host MHC I eliminate host lymphocytes, to include B cells, resulting in an immunodeficient phenotype, acute GVHD (aGVHD). Ex vivo studies demonstrate that perforin (pfp) and Fas/FasL pathways together account for ~90% of the killing of host targets by donor CD8 CTL in this model [11]. In vivo studies also indicate a role for both CTL killing pathways in that the transfer of either pfp deficient (pfp KO) or FasL deficient (gld) donor T cells results in impaired elimination of host B cells at two weeks [12, 13]. Interestingly, long term pfp KO→F1 mice evolve into a lupus-like cGVHD phenotype [12] whereas the long-term phenotype of FasL defective donor transfers has not been reported in this model.
Taken together, these results suggest that pfp and Fas/FasL play important in vivo roles in limiting B cell expansion and preventing expression of lupus-like disease. Here we sought to determine whether both pathways contribute equally to suppression of lupus phenotype or whether one pathway is dominant. Given the significant roles of pfp in intracellular pathogen clearance and tumor immunity [14] and of Fas/FasL in homeostatic control of lymphocytes and retarding lupus [15, 16], it might be expected that the FasL pathway would have a greater role in limiting autoreactive B cell expansion and eventual lupus-like disease in the p→F1 model. Our results indicate that, both pathways are required for full control of autoreactive B cell expansion and contribute relatively equally.
2. Materials and Methods
2.1. Mice.
6-8 week old male and female normal C57BL/6 J (H-2b), FasL defective B6Smn.C3-Faslgld/J, pfp defective C57BL/6-Prf1tm1Sdz/J and normal DBA/2 (H-2d) were used as donor mice and B6D2F1J (BDF1) (H-2b/d) mice were used as recipient host mice. All strains were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal procedures were pre-approved by the Institutional Animal Care and Use Committee at the Uniformed Services University of Health Sciences.
2.2. Induction of GVHD.
Single cell suspensions of donor strain splenocytes were prepared as described [17] and transferred into age and sex matched BDF1 hosts by tail vein injection. The percentages of donor CD4+ and CD8+ T cells populations were analyzed by flow cytometry and donor inoculum adjusted prior to transfer such that all F1 mice received the number of donor CD4 and CD8 T cells designated in the Figure Legends. For Figs 1-4 this typically required 55-65 × 106 unfractionated donor splenocytes. In Fig. 5, GVHD was induced using purified donor T cell subsets achieved through negative selection using Dynal mouse CD4 or CD8 negative isolation kits (Invitrogen, Carlsbad, CA) which deplete B cells, NK cells, monocytes/macrophages, dendritic cells, granulocytes, platelets, erythrocytes and either CD8 or CD4 respectively. Purity of the donor inocula was confirmed by flow cytometry and was typically > 95%.
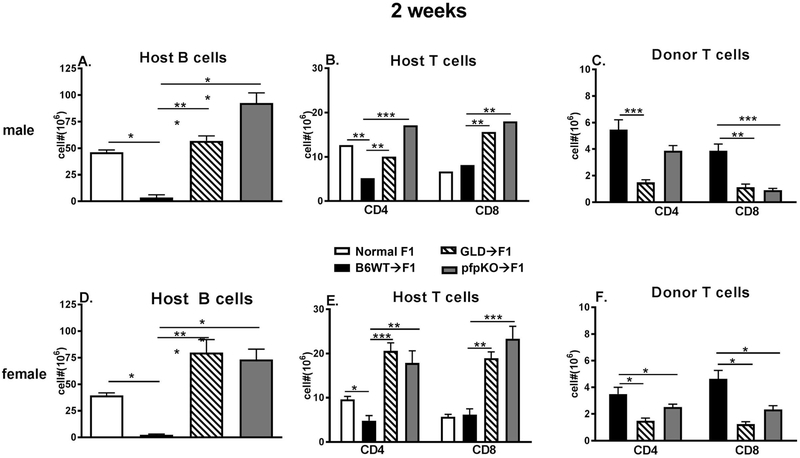
Fig. 1. Donor T cells require both perforin and FasL to mediate aGVHD phenotype at two weeks.
BDF1 mice received B6 WT, B6 GLD or B6 pfp KO donor splenocytes containing 7 ×106 CD4 T cells and 4-5 × 106 CD8 T cells as described in Methods. Donor and hosts were both either male (A-C) or female (D-F). Spleens were assessed at two weeks after donor transfer by flow cytometry for numbers of: (A, D) host B cells; (B, E) host CD4 and CD8 T cells and (C, F) donor CD4 and CD8 T cell engraftment. Values represent group mean ± SE (n= 5/grp). For all figures, *p<0.05, **p<0.01, ***p<0.001.
Fig. 4. Increased splenic IL-21 gene expression is seen long term.
Experimental protocol and groups are as shown for Fig. 2. Host F1 spleens were assessed at 12 weeks for cytokine gene expression as determined by real time-PCR for IL-21 and the IFI genes Mx1 and OAS.
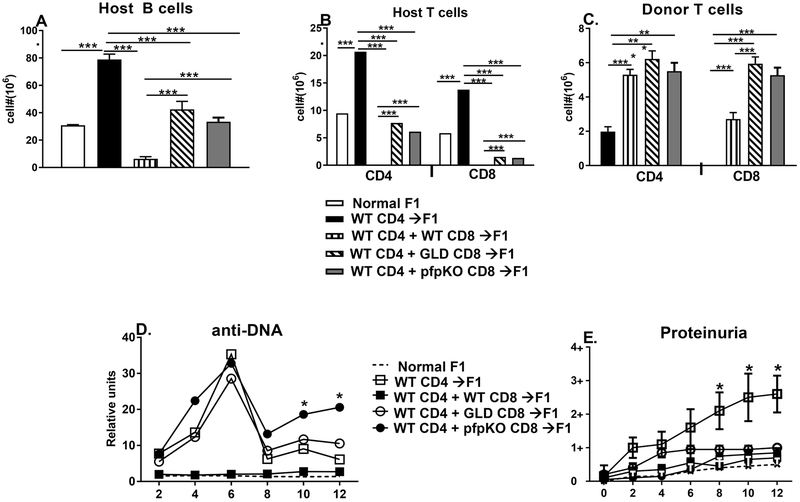
Fig. 5. Both perforin and FasL are important in long term prevention of cGVHD.
BDF1 mice were either uninjected or received 8 × 106 purified CD4 from B6 WT spleens alone or in conjunction with ~4 × 106 purified CD8 T cells from either B6 WT, B6 GLD or B6 pfp KO splenocytes. Mice were assessed at 12 weeks for (A) splenic host B cells; (B) splenic host CD4 and CD8 T cells; (C) splenic donor CD4 and CD8 T cells; (D) serum anti-DNA ab; and (E) proteinuria. Values represent group mean ± SE (n= 5/grp).
2.3. Flow cytometric analysis.
Spleen cells were first incubated with anti-murine Fcγ receptor II/III mAb, 2.4G2 for 10 min and then stained with saturating concentrations of Alexa Fluor 488-conjugated, APC-conjugated, biotin-conjugated, PE-conjugated, FITC-conjugated, PerCPCy5.5-conjugated, Alexa Fluor 647-conjugated and Pacific Blue-conjugated mAb against CD3, CD4, CD8, CD19, B220, H2-Kb, I-Ab, H-2Kd, and I-Ad, purchased from either BD Biosciences (San Jose, CA), BioLegend (San Diego, CA), eBioscience (San Diego, CA), or Invitrogen (Carlsbad, CA). Biotinylated primary mAb were detected using PE-Texas Red-streptavidin (BD Biosciences, San Jose, CA). Cells were fixed in 1% paraformaldehyde before reading.
Multi-color flow cytometric analyses were performed using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Gating strategies: lymphocytes were gated by forward and side scatter and fluorescence data were collected for a minimum of 10,000 gated cells. Studies of donor T cells were performed on a minimum of 5,000 cells collected using a lymphocyte gate that was positive for CD4 or CD8 and either positive (host origin) or negative (donor origin) for MHC class I of the uninjected parent e.g. for B6→F1 mice donor cells are H-2Kd negative. B cells were gated as positive for B220 and either positive (host origin) or negative (donor origin) for MHC Class II of the uninjected parent.
2.4. Cytokine Expression by Real Time PCR.
Real time PCR was performed as described [17]. Briefly, splenocytes (1 × 107) were homogenized in 1 ml of RNA-STAT-60 (Tel-Test, Friendswood, TX). cDNA was synthesized from mRNA using TaqMan® Reverse Transcription Reagents kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed using pre-made primers and probes from TaqMan® Gene Expression Assays and TaqMan® Universal PCR Master Mix (Applied Biosystems) for the following targets: IL-21, OAS, MX-1 with 18s rRNA as an internal control. The calculation of relative gene expression differences was done by comparative 2-ΔΔCT method. The result was expressed as fold change in the experimental groups compared to normal uninjected B6D2F1 control mice.
2.5. Serological studies.
Mice were bled at the times indicated and sera tested by ELISA for the presence of IgG antibodies to calf thymus DNA (Sigma-Aldrich, Atlanta, GA). Units were calculated as described [17].
2.6. Proteinuria.
Urine protein was quantitated by dipstick (Albustix, Bayer Pittsburgh, PA).
2.7. Statistical Analysis.
Statistical comparisons were performed using Prism 5.0 (Graphpad Software, San Diego, CA). Mice were tested individually and data are expressed as group mean ± SEM. Statistical significance between two groups was analyzed using Student’s t-test. For multiple comparisons, two-way ANOVA with an additional Sidak-Bonferoni post-test was used, p-values < 0.05 were considered statistically significant.
3. Results
3.1. Donor T cells require both perforin and FasL to mediate aGVHD phenotype at two weeks.
In the p→F1 model of aGVHD, the first week after donor cell transfer is characterized by donor CD4 T cell help for: 1) host B cell expansion and 2) donor CD8 CTL maturation [10]. Typically, donor CD8 CTL numbers peak at ~10 days after donor transfer and the donor CD4 T cell driven expansion of host B cells is checked by donor CD8 CTL specific for host MHC I which eliminate host B cells. By day 14, near complete donor CD8 T cell elimination of host splenic B cells is seen and homeostatic down regulation of engrafted donor CD8 T cells occurs [10, 18]. To determine the relative importance of the perforin and FasL pathways in donor CD8 CTL elimination of expanding host B cells, we compared the ability of B6 pfp KO or FasL defective (B6 gld) donor T cells to eliminate host B cells at two weeks after transfer. Both male and female combinations were tested using donor T cell numbers that were just above the threshold for aGVHD induction i.e. ~50-55 × 106 unfractionated donor splenocytes containing ~ 8 × 106 CD4 and 5 ×106 CD8 T cells [19]. Controls consisted of age and sex matched, uninjected F1 mice (negative control) and B6 WT→F1 mice (positive control). As shown in Fig. 1, male and female B6 WT→F1 mice exhibit a typical two-week aGVHD phenotype i.e. relative to control F1 mice, there is profound elimination of host B cells (Figs. 1A, 1D, bars 2 vs. 1) and significant elimination of host CD4 T cells (Figs. 1B, 1E, bars 2 vs. 1) in conjunction with substantial engraftment of donor CD4 and CD8 T cells (Figs. 1C, 1F, bars 1 and 4). By contrast, both GLD→F1 and pfp KO→F1 mice exhibit impaired host killing as shown by significantly greater numbers of surviving host B cells (Figs. 1A, 1D, bars 3 & 4 vs. 2) and CD4/CD8 T cells (Figs. 1B, 1E, bars 3 & 4 vs. 2 and bars 7 & 8 vs. 6). Defective engraftment of both donor CD4 and CD8 T cell subsets is seen for both GLD and pfp KO donor cells (Figs. 1C, 1F bars 2 vs. 1 and bars 4 & 5 vs. 3) with the exception of male pfp KO→F1 donor CD4 T cell numbers. Taken together, GLD and pfp KO donor cells do not induce a typical aGVHD phenotype but instead induce an intermediate phenotype suggestive of cGVHD i.e. impaired elimination of host B cells in conjunction with reduced donor CD8 T cell engraftment.
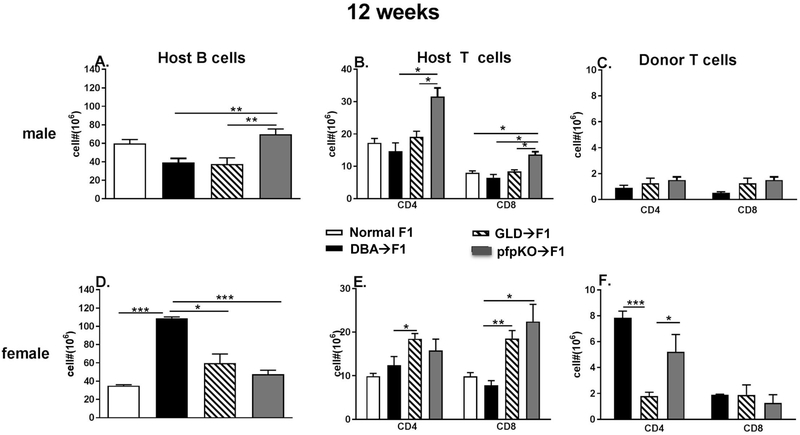
3.2. Donor cells defective in perforin or FasL induce an intermediate GVHD phenotype long term.
To determine whether the intermediate phenotype for pfp KO→F1 and FasL KO→F1 mice seen at two weeks was maintained long term or whether mice eventually evolve into either an aGVHD or cGVHD phenotype, GVHD was induced in pfp KO→F1 and gld→F1 mice and observed for 12 weeks. In these experiments, DBA→F1 mice serve as the positive control for cGVHD phenotype. Previous work has shown that DBA→F1 mice exhibit significant sex-based differences with female→Female transfers exhibiting stronger lupus-like parameters whereas male→male transfers exhibit milder disease that can resolve [20-22]. Such sex-based differences are reproduced in Fig. 2. For example, compared to uninjected control male F1 mice, male DBA→F1 mice exhibit no significant expansion of host B cells or host T cells (Figs. 2A, 2B, bars 2 vs. 1) and donor T cell engraftment is low level (Fig. 2C, bar 1). By contrast, compared to uninjected control female F1 mice, female DBA→F1 mice exhibit striking and significant increase in host B cells (Fig. 2D, bars 2 vs. 1), a modest (but not significant) increase in host CD4 T cells (Fig. 2E, bars 2 vs. 1) and high level engraftment of donor CD4 T cells that is ~ 4-fold greater than donor CD8 T cell engraftment (Fig. 2F, bars 1 vs 4). The effect of pfp or FasL defective donor cell transfers for male→male transfers was primarily limited to significant differences in host CD4 T cells. Specifically, pfp KO→F1 mice exhibited significant increase in host CD4 T cells vs. all other groups (Fig. 2B, bar 4). A lesser increase in host CD8 T cells for pfp→ F1 mice was also seen (Fig. 2B, bar 8).
Fig. 2. Defects in donor CD8 T cell perforin or FasL result in an intermediate cGVHD-like phenotype long term.
BDF1 mice received DBA, B6 GLD or B6 pfp KO donor splenocytes containing 7 ×106 CD4 T cells and 4-5 × 106 CD8 T cells as described in Methods. Donor and hosts were both either male (A-C) or female (D-F). Spleens were assessed 12 weeks after donor transfer by flow cytometry for numbers of: (A, D) host B cells; (B, E) host CD4 and CD8 T cells and (C, F) donor CD4 and CD8 T cell engraftment. Values represent group mean ± SE (n= 5/grp).
For female transfers, both pfp→F1 and gld→F1 mice exhibited a significant reduction in host B cell expansion vs. DBA→F1 mice (Fig. 2D, bars 3 & 4 vs. 2) however both pfp→F1 and gld→F1 mice exhibited increased host CD4 and CD8 T cell expansion vs. either untreated F1 or DBA→F1 mice (Fig. 2E, bars 3 & 4 vs 2; bars 7 & 8 vs. 6+). CD4 engraftment for female gld→F1 was significantly less than that of pfp→F1 mice or DBA→F1 mice (Fig. 2F, bars 1 & 3 vs. 2). All three female GVHD groups exhibited low level donor CD8 engraftment that did not differ significantly (Fig. 2F, bars 4-6).
DBA→F1 cGVHD mice also exhibit the reported sex-based differences in two lupus like parameters, proteinuria and serum anti-DNA ab. Male DBA→F1 mice exhibit low level (<2+) proteinuria (Fig. 3A) whereas female DB→F1 mice have significant proteinuria into the pathologic 3+ range by 10 weeks and sustained through week 14 (Fig. 3B). Both male and female DBA→F1 mice exhibited a striking increase in serum anti-DNA ab (Figs. 3C, 3D).
Fig. 3. Defects in donor CD8 T cell perforin or FasL result in attenuated lupus-like parameters long term.
Experimental protocol and groups are as shown in Fig. 2. Serial proteinuria is shown for males (A) and females (B). Serial serum anti-DNA levels are shown for males (C) and females (D).
Male pfp→F1 and gld→F1 mice exhibited mild proteinuria (Fig. 3A) and elevated serum anti-DNA ab levels (Fig. 3C) that did not differ significantly from male DBA→F1 mice. Similarly, female pfp→F1 and gld→F1 exhibited mild proteinuria (Fig. 3 B) and moderate elevations in serum anti-DNA ab (Fig. 3D) and both were significantly reduced vs. female DBA→F1 mice. Taken together, the results in Figs. 2 & 3 demonstrate that defects in donor T cell expression of pfp or FasL result in an intermediate phenotype at two weeks that does not eventually evolve into aGVHD but rather has features consistent with a mild lupus-like cGVHD. Importantly, the differences in donor and host T cell numbers seen for gld→F1 or pfp KO→F1 vs. DBA→F1 mice in Figs. 2B, 2E and 2F are not associated with significant changes in proteinuria or serum anti-DNA ab.
3.3. Long-term cytokine gene expression:
Sex based differences are seen in IL-21 whereas interferon alpha inducible (IFI) genes exhibit minimal expression. IL-21 is important cytokine in lupus pathogenesis (reviewed in [23]. Similarly, a significant upregulation of type I interferon inducible (IFI) genes is characteristic of many lupus patients [24]. The cohort shown in Fig. 3 was further examined at 14 weeks for splenic cytokine gene expression of IL-21 and the IFI genes M×1 and OAS. Significant differences were seen primarily for IL-21 expression. Both female and male DBA→F1 mice exhibited striking elevations of in IL-21 over uninjected control mice and female levels were roughly 2-fold greater than males (Figs. 4A vs. 4D, bar 1). For both sexes, IL-21 expression was significantly reduced in GLD→F1 and pfp KO→F1 vs. DBA→F1 (Figs. 4A, 4D, bars 2 & 3 vs. 1). Regarding IFI genes, male DBA→F1 mice showed an ~ 6-fold elevation of OAS expression over control (Fig. 4C, bar 1) whereas the OAS and MX-1 were minimally elevated if at all in the remaining male and female groups (Fig. 4B, 4C, 4E, 4F) over control. Of these cytokines, only the ~ 40-fold increase in IL-21 for female DBA→F1 is associated with greater disease severity.
3.4. Both pfp and FasL play important roles in controlling autoimmune B cell hyperactivity and cGVHD.
Previous work has shown that CD4 T cells from Fas deficient B6 lpr mice exhibit defective helper function for CD8 CTL relative to that of B6 WT [25]. To control for potential strain differences in CD4 Th cell activity, we paired normal B6 WT CD4 T cells with purified CD8 T cells from either WT, pfp KO or gld mice. Specifically, BDF1 mice received either: a) 8 × 106 B6 WT CD4 T cells alone (cGVHD control) or in conjunction with ~4 × 106 purified CD8 T from: b) WT (aGVHD control); c) pfp KO; or d) gld mice. Mice were monitored long term for cGVHD parameters. To be sure that we were off plateau, the dose of donor CD8 T cells used is at the lower limit for aGVHD induction. [19, 25]
The transfer of purified B6 CD4 T cells alone into F1 hosts results in typical features of cGVHD as previously described [10, 26] i.e., compared to uninjected control F1 mice at 14 weeks, there is significant expansion of host B cells, (Fig. 5A, bars 2 vs. 1), significant expansion of host CD4 and CD8 T cells (Fig. 5B, bars 2 vs. 1; bars 7 vs. 6), engraftment of donor CD4 T cells with no detectable donor CD8 T cell engraftment (Fig. 5C, bars 1, 5). B6 CD4→F1 mice also exhibit: 1) significant elevations in serum anti-DNA ab vs. uninjected control F1 mice with a peak at week 6 (Fig. 5D); and 2) a progressive and significant increase in proteinuria reaching levels between 2+ to 3+ (Fig. 5E). The transfer of both B6 WTCD4 and WT CD8 T cells converts cGVHD to aGVHD phenotype as previously described [10, 26] i.e. compared to uninjected control F1 mice, WT CD4 + WT CD8→F1 mice exhibit profound elimination of host B cells and T cells (Figs. 5A, bars 3 vs. 2; 5B, bars 3 vs. 2, bars 7 vs. 8), engraftment of both CD4 and CD8 B6 donor T cells (Fig. 5C, bars 2, 6), no significant elevation of serum anti-DNA ab levels or proteinuria vs. uninjected control F1 mice (Figs. 5D, 5E). Co-transfer of CD8 T cells defective in either pfp or FasL with B6 WT CD4 T cells results in a long term intermediate phenotype. Specifically, host B cell, CD4 T cell and CD8 T cell levels for either transfer are significantly reduced vs. the cGVHD control (B6 CD4→F1) but are significantly greater than the aGVHD control (B6 CD4+B6 CD8→F1) and values for host B cells and CD4 T cells are similar to untreated control F1 mice (Figs. 5A & 5B bars 4 and 5 vs. bars 2, 3 or 1; Fig 5B bars 9 and 10 vs. 6, 7, 8). Interestingly, donor CD8 T cell engraftment was significantly greater for both B6 pfp→F1 and B6 gld→F1 vs. B6 WT CD4+ B6 WT CD8→F1 (Fig. 5C, bars 7 & 8 vs. 6) despite both experimental groups exhibiting impaired killing of host B cells and killing of host CD4 T cells compared to that of B6 WT CD4+ B6 WT CD8→F1 (Figs 5A, 5B, bars 4 & 5 vs 3). Although both B6 CD4 + gld CD8→F1 and B6 CD4 + pfp KO CD8→F1 both exhibited elevations in serum anti-DNA ab comparable to WT CD4→F1 control cGVHD mice (Fig. 5D), they did not exhibit significantly elevated proteinuria (>2+) (Fig. 5E). Lastly, there were no significant differences between B6 CD4 + pfp KO CD8→F1 vs. B6 CD4 + gld CD8→F1 in: host B cells, host T cells, donor T cell engraftment, proteinuria or serum anti-DNA ab (except for weeks 10 and 12). (Fig. 5A-5E). Thus, both pfp and FasL pathways contribute relatively equally to aGVHD host lymphocyte elimination and the prevention of lupus like features.
4. Discussion
A central pathogenic mechanism in both human and murine lupus is CD4 T cell driven - B cell hyperactivity resulting in the production of autoantibodies targeting nuclear antigens [1-5]. Following a break in tolerance, autoantibodies are initially low titer and non-pathogenic [27] however over time, continued activation of autoreactive T and B cells results in clonal diversification, epitope spreading and eventually high affinity pathogenic autoantibodies that mediate clinical disease [28-32]. In human lupus, the initiation and expansion phases can precede clinical disease by years [28] making mechanistic studies of these early asymptomatic stages problematic.
Given these potent amplification mechanisms, it would seem that tolerance breaks would invariably lead to autoimmunity, however experimental immunization with nuclear autoantigens in normal mice results in transient autoantibodies without progression to lupus [6-9] supporting the idea that following a tolerance break, progression to lupus may represent a failure of normal down regulatory mechanisms [33]. CD8 T cells are major contributors to tolerance maintenance and defects in CD8 down regulation have been reported in NZB/W [7-9], MRL/lpr [34, 35], BxSB-Yaa [36, 37] and Yaa-related lupus mice [38].
In p→F1 mice, CD4 T cells drive host B cell hyperactivity and lupus-like features just as in human and spontaneous murine lupus [39, 40] however only in p→F1 lupus is the identity and specificity of the disease initiating CD4 T cells known. Specifically, parental strain donor CD4 T cells specific for host allogeneic MHC II provide cognate help (signal 2) to potentially all host B cells. Autoreactive B cells encountering their cognate ligand and binding to their B cell receptor (signal 1) then become mature autoantibody producing B cells/plasma cells. Similarly, donor CD8 CTL specific for host allogeneic MHC I use both perforin and FasL pathways to eliminate host splenocytes including autoantibody producing host B cells [10, 41]. In separate independent studies, it has been shown that the transfer of unfractionated B6 pfp KO or FasL defective C3H/gld donor parental strain splenocytes into normal BDF1 or B6C3F1 hosts respectively converts the expected aGVHD phenotype to an attenuated intermediate phenotype short term and in the case of pfp KO→F1 mice, a mild lupus-like cGVHD long term [12, 13]. Our study sought to determine the relative importance of these two major CTL killing pathways in lupus down regulation by performing a simultaneous direct comparison of pfp vs. FasL defective donor CD8 CTL using the same donor and host strains. Initially we compared unfractionated splenocytes containing equal numbers of donor T cells. Because Fas defective lpr CD4 T cells exhibit impaired help for CD8 CTL [25] in this model, we controlled for any potential differences in CD4 T cell help in pfp or FasL defective donors by using purified WT CD4 T cells paired with purified pfp or FasL defective CD8 T cells. Given the well established role of FasL in lymphocyte homeostasis and AICD [15, 42] and the major role of pfp killing in pathogen elimination [14], it might be expected that the FasL pathway would be the dominant mechanism by which B cell hyperactivity and lupus-like disease are controlled. Surprisingly, our results demonstrate that: 1) full control of B cell hyperactivity by CD8 CTL requires that both pathways be intact; and 2) the contributions by either pfp or FasL killing are relatively equal. With the loss of either donor CD8 CTL killing pathway, F1 mice exhibited incomplete elimination of host B cells compared to F1 mice receiving WT CD8 T cells. Importantly, it is the donor CD4 T cells that drive host B cell autoantibody production in this model and donor CD4 T cells are not targeted by donor CD8 T cells. Consequently, the engrafted donor CD4 T cells can continue to provide help to the surviving residual B cells as evidenced by the elevated serum anti-DNA ab levels seen in both pfp→F1 and GLD→F1, levels that did not differ significantly from cGVHD control mice receiving WT CD4 T cells alone. Nevertheless, despite the presence of elevated serum anti-DNA ab, neither pfp→F1 or GLD→F1 mice developed significantly elevated proteinuria whereas cGVHD control WT CD4→F1 mice had levels of >2+. We view this lack of proteinuria in the experimental groups as likely a threshold effect. The severity of cGVHD and elevation of serum anti-DNA levels in the p→F1 model is directly proportional to the number of donor CD4 T cells injected [43, 44] and in this study, we chose to use numbers of donor CD4 and CD8 T cells were just above the threshold for aGVHD and cGVHD induction i.e. 7-8 × 106 CD4 and ~4 × 106 CD8 T cells. Significant lupus-like renal disease in B6 CD4→F1 cGVHD is typically seen with transfers of 15-20 106CD4 T cells [25, 45]. Our study used donor cell doses just above the threshold for lupus induction but low enough to remain off plateau. In a previous study, we observed that the transfer of unfractioned pfp KO splenocytes into BDF1 mice also resulted in incomplete B cell elimination and autoantibody production however a mild ICGN was also seen. In that study, the number of donor CD4 and CD8 T cells transferred was not rigorously enumerated as in the present study. Thus, although neither pfp→F1 nor GLD→F1 mice exhibited significant proteinuria indicative of ICGN in this study, we do not conclude that the presence of a single CD8 CTL killing pathway is sufficient to protect against lupus like ICGN. With a stronger CD4 T cell helper signal (i.e. greater numbers of donor CD4 T cells transferred), we expect that the impaired CD8 CTL down regulation would be insufficient to prevent progression to ICGN.
A role for FasL in controlling autoimmunity is supported by the observation that crossing the lpr mutation onto lupus prone MRL+ mice, accelerates lupus-like renal disease [16]. The mechanism is thought to involve impaired Fas mediated deletion of both autoreactive CD4+ T cells and B cells [46-49] resulting in excessive CD4+ T cell help for intrinsically abnormal B cells [16]. Of note, crossing the lpr or gld mutations onto a non-autoimmune prone strain e.g., B6, results in lymphoproliferation similar to MRL/lpr mice but the lupus phenotype is attenuated with minimal/milder renal disease and a more restricted autoantibody profile that does not include the lupus-specific anti-Sm antibody [16, 50]. These results demonstrate that in the setting of a loss of tolerance (e.g., in MRL/+ mice), Fas/FasL mediated apoptosis is an important down regulatory mechanism which acts to retard autoimmunity. In the absence of a break in tolerance (e.g., in B6 mice), defective Fas function by itself may result in mild humoral autoimmunity but not florid lupus-like renal disease. Similarly, humans with defective Fas pathway function (Autoimmune Lymphoproliferation Syndrome) exhibit lymphoproliferation but lupus-like features are described in only a small subset [51]. In fact, most SLE patients do not have Fas/FasL mutations [52, 53] or defective in vitro Fas function but instead exhibit increased Fas expression on T cells [52, 54]. These results support the idea that Fas functions normally in SLE patients and acts in a down regulatory role aimed at normalizing the increased immune system activation characteristic of the disease.
Perforin gene inactivation impairs resistance to tumors and viruses [14] but does not result in lupus in otherwise normal mice e.g. B6 pfp KO. However in Fas-intact MRL/+ mice, pfp gene inactivation accelerates humoral autoimmunity and lupus-like disease compared to Fas-intact, pfp-intact control MRL/+ mice [34] consistent with a major role for perforin, separate from Fas, in retarding autoimmunity in MRL/+ spontaneous lupus. Additionally, combined perforin-deficient, MRL/lpr mice exhibit greater accumulation of double negative T cells and accelerated mortality compared to perforin-intact MRL/lpr mice indicating that perforin also down regulates autoreactive T and B cells. These results in combination with the present study support the idea that, in addition to Fas mediated cytotoxicity, perforin mediated mechanisms, e.g., CD8 cytotoxicity play an important role in down regulating lupus.
Despite our results demonstrating that CD8 CTL use both perforin and FasL pathways to control B cell hyperactivity and previous reports that pfp and FasL are not defective in lupus patients, a large body of evidence demonstrates that in vitro and in vivo CTL function in both human and murine lupus is defective [35, 55-59]. For example, CD8 CTL are absolutely required for control of EBV and SLE patients exhibit defective EBV control [60]. Given the critical role of IL-2 in Th1/CTL responses, defective CD8 CTL in lupus is likely due not to defective Fas or pfp but rather to the well described IL-2 defect in human and murine lupus [61, 62]. A secondary, disease associated defect in IL-2/CTL is well recognized [62, 63] whereas a primary, pre-existing defect in IL-2 has long been suspected but difficult to address in either spontaneous murine lupus or human lupus since it is not clear when disease starts and secondary IL-2 defects begin. Moreover, the presence of a secondary defect hinders testing for a primary defect.
Recent work in the p→F1 model supports the concept that a primary defect in CD4 IL-2 production may predispose to lupus following a break in tolerance. Specifically, the transfer of DBA CD4 T cells into BDF1 hosts results in a more severe lupus-like disease than does the transfer of equal numbers of B6 CD4 T cells as shown by: a) greater numbers of DBA donor CD4 T follicular helper (Tfh) cells and greater help for B cell autoantibody production; b) more severe lupus-like renal disease; and c) fewer DBA donor CD4 Th1 cells resulting in reduced help for down regulatory CD8 T cells [64]. Importantly, DBA CD4 T cells exhibit defective in vitro IL-2 production prior to transfer and defective ex vivo IL-2 production following transfer whereas the B6 CD4 T cell IL-2 response is robust both in vitro and ex vivo.
Thus, following a loss of tolerance, the impaired IL-2 default response of DBA CD4 T cells skews towards lupus whereas the high IL-2 default B6 response skews towards milder lupus and eventual tolerance re-establishment. These results support a new paradigm in which a pre-existing, intrinsic defect in CD4 IL-2 contributes to lupus susceptibility both by promoting CD4 Tfh cell driven autoantibody formation and impairing the generation of down regulatory CD8 T CTL. Lastly, the view that defective IL-2 is central to lupus pathogenesis has recently been further supported by work in humans and mice demonstrating lupus improvement with low dose IL-2 supplementation [65, 66]. The presumed mechanism is due to improved Treg function however given the link between IL-2 and CD8 CTL induction, exogenous IL-2 could also be beneficial by boosting CD8 T cell down regulation to include CTL formation.
Highlights.
CD8 cytotoxic T cells are one of several down regulatory mechanisms in lupus that limit B cell expansion.
Both perforin and FasL pathways are required for optimal cytotoxic T cell elimination of activated autoreactive B cells.
Both killing pathways contribute relatively equally.
Acknowledgements
This work was supported by National Institutes of Health RO1AI047466 (CSV).
The opinions expressed herein are those of the authors and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD); or, the United States Army, Navy, or Air Force.
Abbreviations
- aGVHD
aGVHD
- BDF1
B6D2F1
- B6
C57BI/6
- cGVHD
cGVHD
- FasL
Fas ligand
- GVHD
graft -vs.-host disease
- KO
knock out
- p→F1
parent-into-F1
- pfp
perforin
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sobel ES, Cohen PL, Eisenberg RA, lpr T cells are necessary for autoantibody production in lpr mice, J Immunol, 150 (1993) 4160–4167. [PubMed] [Google Scholar]
- [2].Sobel ES, Kakkanaiah VN, Kakkanaiah M, Cheek RL, Cohen PL, Eisenberg RA, T-B collaboration for autoantibody production in lpr mice is cognate and MHC-restricted, J Immunol, 152 (1994) 6011–6016. [PubMed] [Google Scholar]
- [3].Koh DR, Ho A, Rahemtulla A, Fung-Leung WP, Griesser H, Mak TW, Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells, Eur J Immunol, 25 (1995) 2558–2562. [DOI] [PubMed] [Google Scholar]
- [4].Koshy M, Berger D, Crow MK, Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes, J Clin Invest, 98 (1996) 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peng SL, Madaio MP, Hughes DP, Crispe IN, Owen MJ, Wen L, Hayday AC, Craft J, Murine lupus in the absence of alpha beta T cells, J Immunol, 156 (1996) 4041–4049. [PubMed] [Google Scholar]
- [6].Mevorach D, Zhou JL, Song X, Elkon KB, Systemic exposure to irradiated apoptotic cells induces autoantibody production, J Exp Med, 188 (1998) 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singh RR, Ebling FM, Albuquerque DA, Saxena V, Kumar V, Giannini EH, Marion TN, Finkelman FD, Hahn BH, Induction of autoantibody production is limited in nonautoimmune mice, J Immunol, 169 (2002) 587–594. [DOI] [PubMed] [Google Scholar]
- [8].Hahn BH, Ebling F, Singh RR, Singh RP, Karpouzas G, L.A.C. A, Cellular and molecular mechanisms of regulation of autoantibody production in lupus, Ann N Y Acad Sci, 1051 (2005) 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karpouzas GA, La Cava A, Ebling FM, Singh RR, Hahn BH, Differences between CD8+ T cells in lupus-prone (NZB × NZW) F1 mice and healthy (BALB/c Ñ NZW) F1 mice may influence autoimmunity in the lupus model, Eur J Immunol, 34 (2004) 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Via CS, Advances in lupus stemming from the parent-into-F1 model, Trends Immunol, 31 (2010) 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shustov A, Nguyen P, Finkelman F, Elkon KB, Via CS, Differential expression of Fas and Fas ligand in acute and chronic graft-versus-host disease: up-regulation of Fas and Fas ligand requires CD8+ T cell activation and IFN-gamma production, J Immunol, 161 (1998) 2848–2855. [PubMed] [Google Scholar]
- [12].Shustov A, Luzina I, Nguyen P, Papadimitriou JC, Handwerger B, Elkon KB, Via CS, Role of perforin in controlling B-cell hyperactivity and humoral autoimmunity, J Clin Invest, 106 (2000) R39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Via CS, Nguyen P, Shustov A, Drappa J, Elkon KB, A major role for the Fas pathway in acute graft-versus-host disease, J Immunol, 157 (1996) 5387–5393. [PubMed] [Google Scholar]
- [14].Walsh CM, Matloubian M, Liu CC, Ueda R, Kurahara CG, Christensen JL, Huang MT, Young JD, Ahmed R, Clark WR, Immune function in mice lacking the perforin gene, Proc Natl Acad Sci U S A, 91 (1994) 10854–10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S, Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis, Nature, 356 (1992) 314–317. [DOI] [PubMed] [Google Scholar]
- [16].Cohen PL, Eisenberg RA, Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease, Annu Rev Immunol, 9 (1991) 243–269. [DOI] [PubMed] [Google Scholar]
- [17].Soloviova K, Puliaiev M, Foster A, Via CS, The parent-into-F1 murine model in the study of lupus-like autoimmunity and CD8 cytotoxic T lymphocyte function, Methods Mol Biol, 900 (2012) 253–270. [DOI] [PubMed] [Google Scholar]
- [18].Puliaev R, Puliaeva I, Welniak LA, Ryan AE, Haas M, Murphy WJ, Via CS, CTL-promoting effects of CD40 stimulation outweigh B cell-stimulatory effects resulting in B cell elimination and disease improvement in a murine model of lupus, J Immunol, 181 (2008) 47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Puliaeva I, Soloviova K, Puliaiev M, Lang T, Puliaev R, Via CS, Enhancement of suboptimal CD8 cytotoxic T cell effector function in vivo using antigen-specific CD80 defective T cells, J Immunol, 186 (2011) 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Foster AD, Haas M, Puliaeva I, Soloviova K, Puliaev R, Via CS, Donor CD8 T cell activation is critical for greater renal disease severity in female chronic graft-vs.-host mice and is associated with increased splenic ICOS(hi) host CD4 T cells and IL-21 expression, Clinical immunology, 136 (2010) 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Foster AD, Soloviova K, Puliaeva I, Puliaiev M, Puliaev R, Finkelman F, Via CS, Donor CD8 T cells and IFN-gamma are critical for sex-based differences in donor CD4 T cell engraftment and lupus-like phenotype in short-term chronic graft-versus-host disease mice, J Immunol, 186 (2011) 6238–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grader-Beck T, Casciola-Rosen L, Lang TJ, Puliaev R, Rosen A, Via CS, Apoptotic splenocytes drive the autoimmune response to poly(ADP-ribose) polymerase 1 in a murine model of lupus, J Immunol, 178 (2007) 95–102. [DOI] [PubMed] [Google Scholar]
- [23].Comte D, Karampetsou MP, Tsokos GC, T cells as a therapeutic target in SLE, Lupus, 24 (2015) 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, Dong X, Chen W, Kim MH, Weng HH, Furst DE, Gorn A, McMahon M, Taylor M, Brahn E, Hahn BH, Tsao BP, Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus, Arthritis Rheum, 54 (2006) 2951–2962. [DOI] [PubMed] [Google Scholar]
- [25].Puliaeva I, Puliaev R, Shustov A, Haas M, Via CS, Fas expression on antigen-specific T cells has costimulatory, helper, and down-regulatory functions in vivo for cytotoxic T cell responses but not for T cell-dependent B cell responses, J Immunol, 181 (2008) 5912–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Puliaeva I, Puliaev R, Via CS, Therapeutic potential of CD8+ cytotoxic T lymphocytes in SLE, Autoimmunity Rev, 8 (2009) 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Craft J, Fatenejad S, Self antigens and epitope spreading in systemic autoimmunity, Arthritis Rheum, 40 (1997) 1374–1382. [DOI] [PubMed] [Google Scholar]
- [28].Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB, Development of autoantibodies before the clinical onset of systemic lupus erythematosus, N Engl J Med, 349 (2003) 1526–1533. [DOI] [PubMed] [Google Scholar]
- [29].Lehmann PV, Forsthuber T, Miller A, Sercarz EE, Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen, Nature, 358 (1992) 155–157. [DOI] [PubMed] [Google Scholar]
- [30].Fatenejad S, Brooks W, Schwartz A, Craft J, Pattern of anti-small nuclear ribonucleoprotein antibodies in MRL/Mp-lpr/lpr mice suggests that the intact U1 snRNP particle is their autoimmunogenic target, J Immunol, 152 (1994) 5523–5531. [PubMed] [Google Scholar]
- [31].Topfer F, Gordon T, McCluskey J, Intra- and intermolecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A), Proc Natl Acad Sci U S A, 92 (1995) 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shlomchik MJ, Craft JE, Mamula MJ, From T to B and back again: positive feedback in systemic autoimmune disease, Nature reviews. Immunology, 1 (2001) 147–153. [DOI] [PubMed] [Google Scholar]
- [33].Peterson LS, Winchester R, Systemic Lupus Erythematosus: Pathogenesis, in: Koopman WJ, Moreland LW (Eds.) Arthritis & Allied Conditions, Lippincott Williams & Wilkins; 2005, pp. 1523–1559. [Google Scholar]
- [34].Peng SL, Moslehi J, Robert ME, Craft J, Perforin protects against autoimmunity in lupus-prone mice, J Immunol, 160 (1998) 652–660. [PubMed] [Google Scholar]
- [35].Via CS, Shearer GM, Functional heterogeneity of L3T4+ T cells in MRL-lpr/lpr mice. L3T4+ T cells suppress major histocompatibility complex-self-restricted L3T4+ T helper cell function in association with autoimmunity, J Exp Med, 168 (1988) 2165–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McPhee CG, Sproule TJ, Shin DM, Bubier JA, Schott WH, Steinbuck MP, Avenesyan L, Morse HC 3rd, Roopenian DC, MHC class I family proteins retard systemic lupus erythematosus autoimmunity and B cell lymphomagenesis, J Immunol, 187 (2011) 4695–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H, CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice, Proc Natl Acad Sci U S A, 108 (2011) 2010–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H, Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance, Nature, 467 (2010) 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gleichmann E, Pals ST, Rolink AG, Radaszkiewicz T, Gleichmann H, Graft-versus-host reactions: clues to the etiopathology of a spectrum of immunological diseases., Immunol Today, 5 (1984) 324–332. [DOI] [PubMed] [Google Scholar]
- [40].Via CS, Shearer GM, T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease, Immunol Today, 9 (1988) 207–213. [DOI] [PubMed] [Google Scholar]
- [41].Via CS, Implications of the parent-into-F1 model for human lupus pathogenesis: roles for cytotoxic T lymphocytes and viral pathogens, Curr Opin Rheumatol, 22 (2010) 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S, Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand, Cell, 76 (1994) 969–976. [DOI] [PubMed] [Google Scholar]
- [43].Van FM Rappard-Van Der Veen, T. Radaszkiewicz, L. Terraneo, E. Gleichmann, Attempts at standardization of lupus-like graft-vs-host disease: inadvertent repopulation by DBA/2 spleen cells of H-2-different nonirradiated F1 mice, J Immunol, 130 (1983) 2693–2701. [PubMed] [Google Scholar]
- [44].Via CS, Shearer GM, Murine graft-versus-host disease as a model for the development of autoimmunity. Relevance of cytotoxic T lymphocytes, Ann.N Y.Acad.Sci, 532 (1988) 44–50. [DOI] [PubMed] [Google Scholar]
- [45].Rus V, Nguyen V, Puliaev R, Puliaeva I, Zernetkina V, Luzina I, Papadimitriou JC, Via CS, T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity, J Immunol, 178 (2007) 3962–3972. [DOI] [PubMed] [Google Scholar]
- [46].Russell JH, Rush B, Weaver C, Wang R, Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide, Proc Natl Acad Sci U S A, 90 (1993) 4409–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ettinger R, Panka DJ, Wang JK, Stanger BZ, Ju ST, Marshak-Rothstein A, Fas ligand-mediated cytotoxicity is directly responsible for apoptosis of normal CD4+ T cells responding to a bacterial superantigen, J Immunol, 154 (1995) 4302–4308. [PubMed] [Google Scholar]
- [48].Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC, CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells, Nature, 376 (1995) 181–184. [DOI] [PubMed] [Google Scholar]
- [49].Fukuyama H, Adachi M, Suematsu S, Miwa K, Suda T, Yoshida N, Nagata S, Transgenic expression of Fas in T cells blocks lymphoproliferation but not autoimmune disease in MRL-lpr mice, J Immunol, 160 (1998) 3805–3811. [PubMed] [Google Scholar]
- [50].Warren RW, Caster SA, Roths JB, Murphy ED, Pisetsky DS, The influence of the lpr gene on B cell activation: differential antibody expression in lpr congenic mouse strains, Clin Immunol Immunopathol, 31 (1984) 65–77. [DOI] [PubMed] [Google Scholar]
- [51].Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB, Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity, N Engl J Med, 335 (1996) 1643–1649. [DOI] [PubMed] [Google Scholar]
- [52].Mysler E, Bini P, Drappa J, Ramos P, Friedman SM, Krammer PH, Elkon KB, The apoptosis-1/Fas protein in human systemic lupus erythematosus, J Clin Invest, 93 (1994) 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ohsako S, Hara M, Harigai M, Fukasawa C, Kashiwazaki S, Expression and function of Fas antigen and bcl-2 in human systemic lupus erythematosus lymphocytes, Clin Immunol Immunopathol, 73 (1994) 109–114. [DOI] [PubMed] [Google Scholar]
- [54].Amasaki Y, Kobayashi S, Takeda T, Ogura N, Jodo S, Nakabayashi T, Tsutsumi A, Fujisaku A, Koike T, Up-regulated expression of Fas antigen (CD95) by peripheral naive and memory T cell subsets in patients with systemic lupus erythematosus (SLE): a possible mechanism for lymphopenia, Clin Exp Immunol, 99 (1995) 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tsokos GC, Balow JE, Cellular immune responses in systemic lupus erythematosus, Prog Allergy, 35 (1984) 93–161. [PubMed] [Google Scholar]
- [56].Tsokos GC, Smith PL, Christian CB, Lipnick RN, Balow JE, Djeu JY, Interleukin-2 restores the depressed allogeneic cell-mediated lympholysis and natural killer cell activity in patients with systemic lupus erythematosus, Clin Immunol Immunopathol, 34 (1985) 379–386. [DOI] [PubMed] [Google Scholar]
- [57].Charpentier B, Carnaud C, Bach JF, Selective depression of the xenogeneic cell-mediated lympholysis in systemic lupus erythematosus, J Clin Invest, 64 (1979) 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Via CS, Sharrow SO, Shearer GM, Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease, J Immunol, 139 (1987) 1840–1849. [PubMed] [Google Scholar]
- [59].Stohl W, Impaired polyclonal T cell cytolytic activity. A possible risk factor for systemic lupus erythematosus, Arthritis Rheum, 38 (1995) 506–516. [DOI] [PubMed] [Google Scholar]
- [60].Kang I, Quan T, Nolasco H, Park SH, Hong MS, Crouch J, Pamer EG, Howe JG, Craft J, Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus, J Immunol, 172 (2004) 1287–1294. [DOI] [PubMed] [Google Scholar]
- [61].Lieberman LA, Tsokos GC, The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity, J Biomed Biotechnol, 2010 (2010) 740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Via CS, Shearer GM, Defective in vitro IL-2 production in lupus is an early but secondary event paralleling disease activity: evidence from the murine parent-into-F1 model supports staging of IL-2 defects in human lupus, Autoimmunity, 43 (2010) 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Horwitz DA, The clinical significance of decreased T cell interleukin-2 production in systemic lupus erythematosus: connecting historical dots, Arthritis Rheum, 62 (2010) 2185–2187. [DOI] [PubMed] [Google Scholar]
- [64].Soloviova K, Puliaiev M, Haas M, Dalgard CL, Schaefer BC, Via CS, Intrinsic Differences in Donor CD4 T Cell IL-2 Production Influence Severity of Parent-into-F1 Murine Lupus by Skewing the Immune Response Either toward Help for B Cells and a Sustained Autoantibody Response or toward Help for CD8 T Cells and a Downregulatory Th1 Response, J Immunol, 195 (2015) 2985–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X, Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Zhang X, Su Y, Guo J, Shen N, Morand EF, Yu D, Li Z, Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus, Nat Med, 22 (2016) 991–993. [DOI] [PubMed] [Google Scholar]
- [66].Klatzmann D, Abbas AK, The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases, Nature reviews. Immunology, 15 (2015) 283–294. [DOI] [PubMed] [Google Scholar]