Abstract
Purpose
Radiation injury to the bronchial tree is an important yet poorly understood potential side effect in lung stereotactic ablative radiotherapy (SAbR). We investigate the integration of virtual bronchoscopy in radiotherapy planning to quantify dose to individual airways. We develop a risk model of airway collapse and develop treatment plans that reduce the risk of radiation-induced airway injury.
Methods and Materials
Pre- and post-SAbR diagnostic-quality CT scans were retrospectively collected from 26 lung cancer patients. From each scan, the bronchial tree was segmented using a virtual bronchoscopy system and deformably registered to the planning CT. Univariate and stepwise multivariate Cox regression were performed, examining factors such as age, co-morbidities, smoking pack years, airway diameter and maximum point dose (Dmax). Logistic regression was utilized to formulate a risk function of segmental collapse based on Dmax and diameter. The risk function was incorporated into the objective function along with clinical dose volume constraints for planning target volume (PTV) and organs at risk (OARs).
Results
Univariate analysis showed that segmental diameter (p = 0.014) and Dmax (p=0.007) were significantly correlated with airway segment collapse. Multivariate stepwise Cox regression showed that diameter (p = 0.015), Dmax (p < 0.0001) and pack years of smoking (p = 0.02) were significant independent factors associated with collapse. Risk-management-based plans enabled significant dose reduction to individual airway segments while fulfilling clinical dosimetric objectives.
Conclusion
To our knowledge, this is the first systematic investigation of functional avoidance in lung SAbR based on mapping and minimizing dose to individual bronchial segments. Our early results show that it is possible to substantially lower airway dose. Such dose reduction may potentially reduce the risk of radiation-induced airway injury, while satisfying clinically prescribed dosimetric objectives.
Keywords: Non-small-cell lung cancer (NSCLC), stereotactic ablative, radiation therapy, SAbR, SBRT, functional avoidance, airway mapping, bronchial tree
Introduction
Lung stereotactic ablative radiotherapy (SAbR), which involves the precise administration of very high, biologically potent doses in 1–5 fractions, has been shown in multicenter studies to yield 5-year primary tumor control >90% in inoperable Stage I non-small-cell lung cancer (NSCLC) patients.[1] While very few recent lung cancer therapies have had such a demonstrably positive impact on public health, the use of such potent doses leads to moderate-to-significant Grade ≥ 3 toxicity in the treatment of even peripheral tumors,[2] and potentially severe toxicity in centrally located tumors[3] and larger (> 5cm) early-stage tumors.[4]
In current clinical practice, lung SAbR is utilized to treat a small minority of the NSCLC population - inoperable, early-stage patients with small (< 5 cm), relatively peripheral lesions.[5, 6] Concerns of excessive pulmonary toxicity limit a large population of early-stage NSCLC patients from receiving the most potent forms of SAbR (e.g., single-fraction or three-fraction). There are several clinical trials investigating the use of SAbR for central lung lesions using gentler fractionation schema (5–8 fractions).[7–9] Long-term (5+ years) findings from these trials are awaited. There is also increased interest in using SAbR to treat patients beyond the medically inoperable group, including high- and standard-risk operable patients. For example, in a recent study describing a pooled analysis of two randomized phase III trials (STARS and ROSEL) in T1-2a, N0M0 operable NSCLC patients, Chang et al. compared SAbR with lobectomy, and found that SAbR was better tolerated and showed superior 3-year overall survival (95%) than surgery (79%).[10] Improved dose-sparing of functional lung will become ever more important in such patients as they will likely live longer and may experience late (> 5 years) recurrences and/or toxicity.[11] Furthermore, there is promising clinical evidence from retrospective multi-institutional studies (334 patients, 20 institutions, 1–5 fractions) on the use of SAbR for lung oligometastases showing ~78% two-year local control and > 50% two-year overall survival.[12] This has greatly increased the oncology community’s interest in using SAbR to treat pulmonary oligometastases for aggressive local control.[13] These treatments are subject to the same physical constraints as SAbR for primary disease due to concerns of toxicity. Thus, reducing toxicity may enable the expansion of SAbR to include patients with larger and/or multiple oligometastatic lesions in the lung.
Since their inception, clinical radiation treatment planning techniques use a relatively simplistic model of the lung as a uniform, largely parallel organ, and rely on dose-volume metrics such as mean lung dose (MLD) and V20, V25 to predict toxicity. The results of multiple studies, including a 2010 quantitative analysis of normal tissue effects in the clinic (QUANTEC) meta-study of over 70 published articles, show that dose-volume metrics have limited predictive power in recommending tolerance dose thresholds for normal lung even in conventionally fractionated RT.[14–16] The predictive power of these quantities is further diminished in SAbR, where the irradiation fields and, consequently, the MLD, V20, V25 are substantially smaller than those encountered in conventionally fractionated lung RT (e.g., MLD ≈ 6 Gy; V20 ≈ 5% in SAbR, compared to ≈ 20 Gy and ≈ 30% respectively, in conventionally fractionated RT).[16, 17]
While this limited predictive power may be attributed to myriad factors including co-morbidities (e.g., emphysema, COPD), other patient-related factors (age, smoking habits), recent works have suggested that “dose-function” metrics, generated via image-based spatial mapping and avoidance of functional regions within the lung, may be more predictive of Grade ≥ 2 toxicity compared to conventional dose-volume metrics.[18, 19] To address these limitations of conventional RT planning, several groups have investigated strategies based on spatially mapping functional regions in the lung using ventilation/perfusion (V/Q) single photon emission computed tomography (SPECT) scans.[15, 20–23] By co-registering the V/Q images to planning CT images, these investigations have shown that one can create 3D conformal or intensity modulated radiotherapy (IMRT) treatment plans that avoid or minimize dose to regions exhibiting high ventilation/perfusion, while maintaining the original planning objectives. Other groups have investigated calculating localized volumetric changes from respiratory correlated four-dimensional CT (4DCT).[24–27] These volumetric changes serve as surrogates for lung ventilation and are used to create functional avoidance treatment plans, similar to those based on the V/Q SPECT scans.
All of these investigations suggest that more sophisticated imaging and treatment planning, that accounts for lung function, could potentially reduce radiation toxicity. However, a largely unexplored aspect of functional avoidance in lung SAbR is the dose-response of and radiation damage to the anatomical structures that are critical to the gas exchange process – namely, the elements of the airway tree and the pulmonary vasculature, collectively, branching serial structures (BSS). The lung is anatomically diverse and is composed of central and peripheral bronchi and major and minor pulmonary vessels, ultimately ending in a monotonous parallel parenchyma. Radiation damage to bronchi can cause airway stenosis, atelectasis (partial or complete lung collapse), ultimately leading to fibrosis.[28–30]. While gas exchange itself occurs in hundreds of millions of parallely-functioning alveoli,[31] airways (and vessels) are involved in the important steps immediately before (and after) gas exchange. While there have been investigations of the effect of radiation damage to relatively larger (main and lobar) bronchi, there have been no studies, to our knowledge, on understanding and quantifying radiation injury to peripheral, sub-lobar and, potentially, more radiosensitive airway elements.
In order to address this important gap in our current knowledge of lung toxicity, we propose a novel functional avoidance approach that uses virtual bronchoscopy to spatially map and incorporate central as well as peripheral airway segments in the treatment planning process. We describe a retrospective study using data from lung SAbR patients to calculate the dose to individual airway segments. We estimate the radiosensitivities of airway segments as a function of airway diameter and develop a risk-based approach to incorporate and avoid dose to specific airways in the treatment planning process. While we limit our current scope to mapping and dose-avoidance in airway elements, the overall approach may be extended (beyond our current scope) to dose-avoidance in pulmonary vessels.
Methods and Materials
Retrospective assessment of post-SAbR airway injury
Under IRB approval, pre- (≤ 3 months) and post-treatment (median follow-up: 8.5 months) diagnostic-quality CT scans (≤ 1 mm slice thickness) were retrospectively collected from 26 NSCLC patients treated with SAbR (50–60 Gy in 3–5 fractions). Patients were selected consecutively from 150 lung cancer patients who were treated with SAbR at our institution between 2012 and 2014. The selection criteria for this study were (i) lung cancer patients treated with SAbR, (ii) followup with CT scan, 8–14 months post-treatment, and (iii) image quality of the patient’s diagnostic and follow-up CT scan to be high enough so that their airway tree could be autosegmented. Patient demographics are shown in Appendix I. A commercial virtual bronchoscopy software, Lungpoint (Broncus Medical, San Jose, CA) was used to spatially map the airway tree in each CT dataset. The underlying algorithm, developed by Graham et al.,[32] performs airway autosegmentation from a diagnostic-quality CT scan using a 3-step process: (i) initial, conservative segmentation of major airways, (ii) exhaustive search for additional candidate locations, and (iii) a graph-based optimization to assess the cost-benefit of retaining candidate airways for final segmentation. The algorithm has been validated with patient data and has been found to delineate airways between the first and fourth generation (≥ ~2 mm diameter) with average accuracy of >95% compared to a gold-standard airway delineation by human experts. From each CT scan, the airway tree was segmented in LungPoint.
An in-house software application DICOManTX (UT Southwestern Medical Center, Dallas, TX) was further developed to enable the conversion of the segmented airway structures to DICOM-RT format. The pre-treatment CT with the airway tree (as an RT-Struct) was imported into the Eclipse treatment planning system and, using the deformable image registration tool available in Eclipse’s Velocity software (Varian Medical Systems, Palo Alto, CA), was registered to the planning CT. The planning CT was derived from the average of ten 4DCT phases in order to account for the effect of respiratory motion.
The regions of the airway tree in and near the radiation field were recontoured manually into individual segments. A total of 253 segments ranging from 2 to 14 mm in diameter were deemed evaluable. E.g., daughter segments that were downstream from a collapsed segment (assessed from the post-SAbR scan) were not considered evaluable in order to avoid erroneous counting of “open” airways. The dose to each evaluable segment from the clinically-delivered plan was calculated. All doses were converted to 5-fraction equivalent dose using the universal survival curve (USC) model, which combines the linear-quadratic and multi-target models in order to better describe survival data for high-potency ablative regimens such as SAbR.[33] For airways, in equation 10 of [33], we used α = 0.133, β = 0.037, and, D0 = 1.30, resulting in α/β = 3.59, Dq = 3.56 Gy, and Dt = 8.6 Gy.
On the post-treatment scan, the airway segments were evaluated for complete radiation-induced collapse with associated downstream atelectasis and/or fibrosis; i.e., the endpoint was the presence/absence of airway collapse. A radiation oncologist compared the pre- and post-treatment airway trees segmented by the virtual bronchoscopy software to determine the airways that had collapsed. For each collapsed airway substructure, collapse was differentiated from other morbidities such as radiation pneumonitis, fibrosis or atelectasis by visual inspection of the high-resolution pre- and post-treatment CT scans.
Statistical Analysis
Univariate and stepwise multivariate Cox regression were performed for clustered data. Clustered analysis was used in order to account for intra-patient correlation of airway elements. Factors examined were age, diagnosis of chronic obstructive pulmonary disease, segment diameter, emphysema, pack years of smoking and maximum point dose Dmax. In this study, Dmax was computed as D0.01cc, where, for a given structure, D0.01cc is the minimum dose to a voxel within the 0.01 cc volume that has received the highest dose. The stepwise multivariate Cox regression model is demonstrated in Appendix II.
Risk model of airway collapse
Based on the results from the analyses, logistic regression was utilized to formulate a risk probability function of segmental collapse based on Dmax and diameter. The risk function was modeled as
| (1) |
Risk model-based treatment planning
An intensity modulated radiotherapy (IMRT) treatment plan was created using a previously described particle swarm optimization-based treatment planning system implemented on a research graphics processing unit (GPU)-enabled Eclipse treatment planning system.[34, 35] The objective function consisted of dose volume constraints on OARs and PTV, and the weighted summation of probabilities of segmental collapse. The weights were calculated as diameter multiplied by a constant value, termed as airway protection factor (APF). The resulting objective function was formulated as
| (2) |
where
wi = APF * Diameteri
APF is a constant value ranging from 0 to ∞. The objective function was designed to progressively increase airway protection with increasing airway diameter by assigning a diameter-dependent weight. For instance, for APF=10, the weight for a 5 mm and a 10 mm airway was 50 and 100, respectively.
The proposed treatment planning strategy was tested retrospectively on a patient data set with a clinical plan that prescribed a dose of 50 Gy in 5 fractions to PTV, created using our institution’s protocol (Appendix III). We tested our risk-based model for airway collapse with three different values of APF: 10, 20 and 50.
Results
Figure 1 shows (a, b) pre- and (d, e) 11 months post-SAbR axial and coronal CT slices from an NSCLC patient with an ~5 cm diameter upper left lobe tumor. Also shown are the corresponding segmented airway trees (c and f). Radiation-induced bronchial collapse, is observed in several segmental bronchi arising from the left lobar bronchus (f). Figure 1d shows fibrosis distant from the site of the tumor (yellow arrow), a probable side effect of the segmental bronchial collapse seen in (f). A corresponding reduction in the left lung volume is also observed, wherein the post-SAbR image (e) shows the left fissure being pulled superiorly and centrally compared to the pre-SAbR image (b).
Figure 1.
CT slices and segmented bronchial tree of a patient with a 5 cm diameter left-upper-lobe tumor. Pre-treatment (a) axial and (b) coronal slices along with (c) segmented airway tree. Post-SAbR (d) axial and (e) coronal CT slices along with (f) corresponding segmented airway tree, acquired 11 months after treatment. Red and yellow arrows indicate the tumor target and the distant fibrosis, respectively. The dashed line in b and e indicates the location of the fissure between the lobes.
Figure 2 shows the airway segments that were considered in this study for one patient, and, Appendix I shows how many airway segments per patient were studied.
Figure 2.
A visual demonstration of the airway segments, in vicinity of PTV, that were considered in our study (PTV is shown in brown.)
Table 1 shows the univariate Cox regression analysis results. Our analysis showed that significant independent patient-specific parameters for time to airway collapse were maximum dose (hazard ratio [HR], 1.07; 95% CI: 1.05–1.09), and diameter (HR, 0.79; 95% CI: 0.65–0.95). The univariate Cox regression p values were 0.014 and 0.007 for segmental diameter and Dmax, respectively. Multivariate stepwise Cox regression showed that diameter (p = 0.015), Dmax (p < 0.0001) and pack years of smoking (p = 0.02) were significant independent factors associated with collapse.
Table 1.
Univariate Cox regression analysis of airway collapse versus patient-specific parameters
| Parameter | Hazard Ratio | 95% C.I. for HR | p |
|---|---|---|---|
| Age | 1.00 | (0.958, 1.047) | 0.9472 |
| COPD | 0.76 | (0.296, 1.96) | 0.5724 |
| Diameter (mm) | 0.79 | (0.653, 0.954) | 0.0143 |
| Emphysema | 0.64 | (0.26, 1.558) | 0.3232 |
| Max Dose (Gy) | 1.07 | (1.05, 1.091) | <.0001 |
| Pack-years of smoking | 0.98 | (0.965, 1.0) | 0.0630 |
| Smoke during SAbR | 1.37 | (0.427, 4.367) | 0.6000 |
For our logistic regression model of Eq. (1), we achieved the following coefficients (rounded to two significant digits):
| (3) |
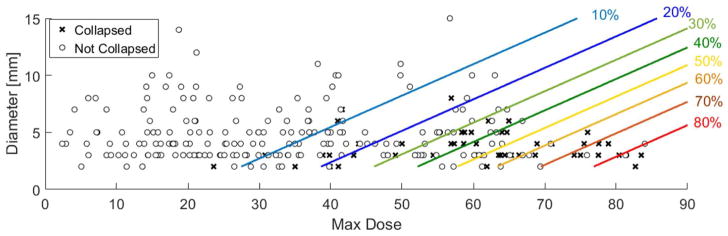
Appendix IV shows the corresponding probability curves versus airway diameter and maximum dose. Figure 3 shows the post-SAbR state of airway segments (collapsed/non-collapsed) as a function of Dmax and airway diameter along with the probabilities of collapse (Eq (1)). The parallel lines in this figure indicate equal probabilities of collapse across various diameters and values of Dmax.
Figure 3.
Post-SAbR state of airway segments (collapsed/non-collapsed) as a function of Dmax and airway diameter. The lines indicate equal probabilities of collapse across various diameters and values of Dmax.
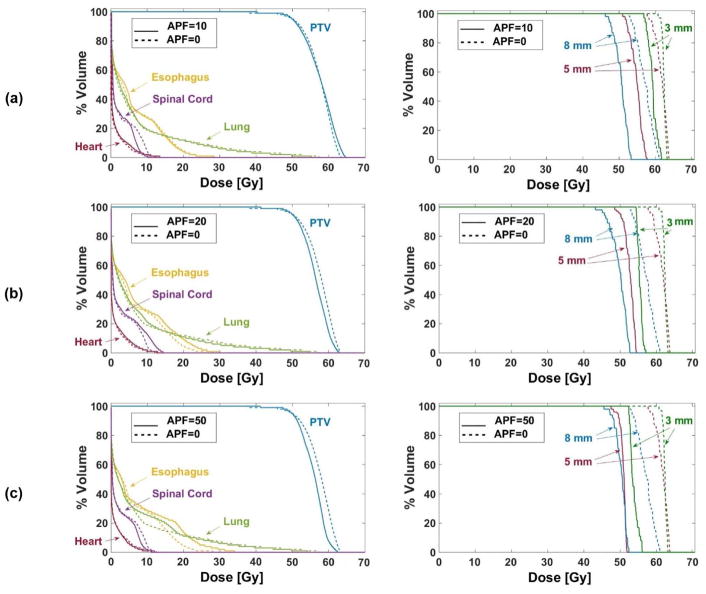
Figure 4 compares dose volume histogram (DVH) curves for PTV and OARs for one patient data set when the airways were considered and not considered in the treatment plans, with different values of weights on the airway dose avoidance. To illustrate the effect of incorporating the risk function, DVH curves of three individual airway segments with diameters 3, 5 and 8 mm are shown. Dose to individual airways for different values of APF is presented in Table 2. In addition, Fig. 5 demonstrates how increasing the importance of airway protection (by increasing APF) changed dose distribution and, consequently, the isodose contours.
Figure 4.
DVH curves for (left column) PTV, esophagus, spinal cord, heart and lung, and (right column) three airway segments ranging in diameter from 3–8 mm. Airway protection factors are (a) 10, (b) 20 and (c) 50. Solid lines correspond to plans that incorporated the risk model for airway segment collapse and dashed lines correspond to the original clinical plans that ignored dose to airway segments.
Table 2.
Dmax for OARs and three airway segments and Lung V13 with different levels of airway protection.
| Airway Protection Factor (APF) | Dmax [Gy] | Lung V13 (%) | |||||
|---|---|---|---|---|---|---|---|
| Airways | Heart | Spinal Cord | Esophagus | ||||
| 8 mm | 5 mm | 3 mm | |||||
| 0 | 61.32 | 60.04 | 62.03 | 12.43 | 11.97 | 28.10 | 18.81 |
| 10 | 53.23 | 50.76 | 52.79 | 13.34 | 9.98 | 28.33 | 18.49 |
| 20 | 52.72 | 54.52 | 57.11 | 12.82 | 14.29 | 29.79 | 17.52 |
| 50 | 52.4 | 51.89 | 56.07 | 11.00 | 11.19 | 34.01 | 18.82 |
Figure 5.
Dose color wash figures for one axial slide for the demonstrated plans in Figure 4. The airways are shown in brown and PTV is shown in red.
Discussion
In this work, we presented a significant shift in the conventional radiotherapy planning paradigm of regarding the lung as a parallelly functioning organ. Our initial findings suggest that it is important to consider the branching serial nature of airways in order to better understand and account for radiation injury within the lung. The example shown in Fig. 1 is illustrative of how radiation-induced damage to lobar and peripheral airways can lead to atelectasis, ultimately resulting in fibrosis distant from the site of intersection of the beams. Such information about radiation-induced airway injury, when combined with an appropriate statistical risk-based model allowed us to create treatment plans that fulfil clinical dose constraints and also avoid injury to central and peripheral airways. Avoiding radiation injury is likely to enable superior preservation of post-treatment lung function.
Our Cox regression analyses showed that there was 1.07 times higher chance of collapse per 1Gy increment in Dmax, after controlling for the effects of diameter and pack-years of smoking. There was 20% less chance of collapse per 1 mm increment in diameter, after controlling for the effects of Dmax and pack-years of smoking. Our airway-protected plans (Fig. 4) yielded substantially reduced dose to individual airway segments while satisfying the clinical dosimetric objectives for PTV and OARs (e.g., 95% of PTV was covered by 100% prescribed dose in all plans and maximum doses to heart, spinal cord and esophagus stayed below 38 Gy, 30 Gy and 35 Gy, respectively, as recommended in Appendix III). Increasing the APF reduced the dose to individual airways (Table 2). However, higher values of APF resulted in plans that begin to deviate from the clinical goals for PTV and OARs other than airways.
Our approach is distinct from and potentially complementary to other functional avoidance approaches based on SPECT V/Q mapping or 4DCT-based spatial mapping of ventilation. While lung functional avoidance approaches attempt to spatially map and avoid dose to regions of the lung that exhibit high level of function, as quantified by ventilation/perfusion, these approaches do not currently account for the “supply lines” upstream (airways) and downstream (pulmonary vessels).[18, 19, 25, 36–38] Our initial results indicate that, in addition to dose avoidance to functional lung regions, it is also critical to avoid excessive dose to these conduits, which make gas exchange possible.
We note the following limitations in this study. First, the number of patients is very limited at 26 and the model lacks external verification. Second, given the fact that this was a retrospective study, the pre- and the post-treatment CT scans were taken under free-breathing conditions and therefore were not necessarily acquired at the same respiratory phase or the same patient position (see Fig. 1b and e, as an example). These differences in acquisition and patient position can lead to variable uncertainties when we deformably register the high-resolution pre- and post-SAbR CT scans to the lower-resolution radiotherapy planning CT scan. We have currently opened a multi-institutional prospective protocol to acquire pre- and post-RT breath-hold CT scans with the patient in the same position as that used for CT simulation. Third, while the planning CT is generated from the average of ten phases of the 4DCT and therefore implicitly accounts for respiratory motion, the aforementioned uncertainties in registering the finer peripheral airways to the planning CT may result in under- or over-estimation of the extent of their motion. Fourth, we used apertures which had been created and sorted in Eclipse treatment planning system and then optimized aperture intensity weights using our in-house code, without changing the aperture shapes. Therefore, the final plans were deliverable because what we changed was the time (and monitor unit) at each aperture (see Appendix V) and not the aperture sequence or shape. However, since the optimization algorithm was allowed to remove some apertures by giving them 0 weights, the aperture sequence could be re-optimized after MU weight optimization for a more efficient delivery. Fifth, the only dosimetric parameter in our current probabilistic model was maximum dose for two reasons: (i) there were no data on the dose response of peripheral airways (to our knowledge), and, (ii) the airway was modeled as a branching serial structure and max dose is considered one of the most important dosimetric values for such structures. However, in future analyses, as we gather progressively more data, it may be worthwhile to consider other dose volume parameters. Finally, uncertainties due to deformable image registration (which can be up to ~5 mm [39]) were not explicitly accounted for in our treatment planning illustration, as our purpose was to show proof-of-concept. However, these uncertainties need to be carefully and quantitatively characterized, preferably on a patient-specific basis, before such planning strategies can be used in the clinic.
CONCLUSION
We presented a novel approach to map and quantify radiation injury to individual airways, thus addressing a major gap in our knowledge of lung toxicity. Furthermore, we developed a risk-management based treatment planning strategy to reduce post-SAbR radiation injury to airway segments. Our initial results suggest that the proposed plans may reduce airway injury and, thereby, post-SAbR toxicity through significant dose reduction to individual airway segments while satisfying volume dose constraints on PTV and other OARs.
Supplementary Material
Summary.
Radiation injury to airways during lung stereotactic ablative radiotherapy can lead to reduced respiratory function post-radiotherapy. In this work, we use virtual bronchoscopy to map the airway tree and develop a risk-based model of the radiosensitivity of individual airway segments. Using this model, we develop a treatment planning strategy that mitigates the risk of airway collapse while meeting the prescribed clinical dosimetric goals for the tumor target and organs at risk.
Acknowledgments
We gratefully acknowledge research funding from the National Institutes for Health (R01 CA 202761).
Footnotes
Conflicts of Interest: Dr. Sawant has research support from the National Institutes of Health, Varian Medical Systems and VisionRT Ltd.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Timmerman RD, et al. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. International Journal of Radiation Oncology • Biology • Physics. 2014;90(1):S30. [Google Scholar]
- 2.Chi A, et al. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol. 2010;94(1):1–11. doi: 10.1016/j.radonc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo Y, et al. Dose--volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2012;83(4):e545–9. doi: 10.1016/j.ijrobp.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Wisnivesky JP, et al. Radiation therapy for the treatment of unresected stage I–II non-small cell lung cancer. Chest. 2005;128(3):1461–7. doi: 10.1378/chest.128.3.1461. [DOI] [PubMed] [Google Scholar]
- 6.Ward M, et al. IEEE Trans Neural Syst Rehabil Eng. 2014. A Flexible Platform for Biofeedback-driven Control and Personalization of Electrical Nerve Stimulation Therapy. [DOI] [PubMed] [Google Scholar]
- 7.Adebahr S, et al. LungTech, an EORTC Phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. Br J Radiol. 2015;88(1051):20150036. doi: 10.1259/bjr.20150036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura T, et al. Phase I study of stereotactic body radiation therapy for centrally located stage IA non-small cell lung cancer (JROSG10-1) Int J Clin Oncol. 2017;22(5):849–856. doi: 10.1007/s10147-017-1125-y. [DOI] [PubMed] [Google Scholar]
- 9.Bezjak A, et al. Efficacy and Toxicity Analysis of NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC) International Journal of Radiation Oncology • Biology • Physics. 96(2):S8. [Google Scholar]
- 10.Chang JY, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–7. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuo Y, et al. Preliminary report of late recurrences, at 5 years or more, after stereotactic body radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2012;7(2):453–6. doi: 10.1097/JTO.0b013e31823c5b29. [DOI] [PubMed] [Google Scholar]
- 12.Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol. 2010;5(7):1091–9. doi: 10.1097/JTO.0b013e3181de7143. [DOI] [PubMed] [Google Scholar]
- 13.Chmura SJ, Salama JK, Weichselbaum RR. Stereotactic radiotherapy for pulmonary metastases. Semin Thorac Cardiovasc Surg. 2013;25(4):292–9. doi: 10.1053/j.semtcvs.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Kong FM, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63(2):324–33. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Christian JA, et al. The incorporation of SPECT functional lung imaging into inverse radiotherapy planning for non-small cell lung cancer. Radiother Oncol. 2005;77(3):271–7. doi: 10.1016/j.radonc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Marks LB, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70–6. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker R, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys. 2013;85(1):190–5. doi: 10.1016/j.ijrobp.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 18.Farr KP, et al. Inclusion of functional information from perfusion SPECT improves predictive value of dose-volume parameters in lung toxicity outcome after radiotherapy for non-small cell lung cancer: A prospective study. Radiother Oncol. 2015;117(1):9–16. doi: 10.1016/j.radonc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Faught AM, et al. Evaluating the Toxicity Reduction With Computed Tomographic Ventilation Functional Avoidance Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;99(2):325–333. doi: 10.1016/j.ijrobp.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuire SM, et al. A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int J Radiat Oncol Biol Phys. 2006;66(5):1543–52. doi: 10.1016/j.ijrobp.2006.07.1377. [DOI] [PubMed] [Google Scholar]
- 21.Shioyama Y, et al. Preserving functional lung using perfusion imaging and intensity-modulated radiation therapy for advanced-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;68(5):1349–58. doi: 10.1016/j.ijrobp.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Lavrenkov K, et al. A potential to reduce pulmonary toxicity: the use of perfusion SPECT with IMRT for functional lung avoidance in radiotherapy of non-small cell lung cancer. Radiother Oncol. 2007;83(2):156–62. doi: 10.1016/j.radonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Meng X, et al. Changes in functional lung regions during the course of radiation therapy and their potential impact on lung dosimetry for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):145–51. doi: 10.1016/j.ijrobp.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaremko BP, et al. Reduction of normal lung irradiation in locally advanced non-small-cell lung cancer patients, using ventilation images for functional avoidance. Int J Radiat Oncol Biol Phys. 2007;68(2):562–71. doi: 10.1016/j.ijrobp.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto T, et al. Impact of four-dimensional computed tomography pulmonary ventilation imaging-based functional avoidance for lung cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(1):279–88. doi: 10.1016/j.ijrobp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, et al. Investigation of four-dimensional computed tomography-based pulmonary ventilation imaging in patients with emphysematous lung regions. Phys Med Biol. 2011;56(7):2279–98. doi: 10.1088/0031-9155/56/7/023. [DOI] [PubMed] [Google Scholar]
- 27.Vinogradskiy Y, et al. Use of 4-dimensional computed tomography-based ventilation imaging to correlate lung dose and function with clinical outcomes. Int J Radiat Oncol Biol Phys. 2013;86(2):366–71. doi: 10.1016/j.ijrobp.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller KL, et al. Bronchial stenosis: an underreported complication of high-dose external beam radiotherapy for lung cancer? Int J Radiat Oncol Biol Phys. 2005;61(1):64–9. doi: 10.1016/j.ijrobp.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 29.Kelsey CR, et al. Radiation-induced narrowing of the tracheobronchial tree: an in-depth analysis. Lung Cancer. 2006;52(1):111–6. doi: 10.1016/j.lungcan.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson K, et al. Retrospective cohort study of bronchial doses and radiation-induced atelectasis after stereotactic body radiation therapy of lung tumors located close to the bronchial tree. Int J Radiat Oncol Biol Phys. 2013;87(3):590–5. doi: 10.1016/j.ijrobp.2013.06.2055. [DOI] [PubMed] [Google Scholar]
- 31.Ochs M, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169(1):120–4. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 32.Graham MW, et al. Robust 3-D Airway Tree Segmentation for Image-Guided Peripheral Bronchoscopy. Medical Imaging, IEEE Transactions on. 2010;29(4):982–997. doi: 10.1109/TMI.2009.2035813. [DOI] [PubMed] [Google Scholar]
- 33.Park C, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(3):847–52. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 34.Modiri A, et al. Inverse 4D conformal planning for lung SBRT using particle swarm optimization. Phys Med Biol. 2016;61(16):6181–202. doi: 10.1088/0031-9155/61/16/6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aaron H, et al. Multi-GPU configuration of 4D intensity modulated radiation therapy inverse planning using global optimization. Physics in Medicine & Biology. 2018;63(2):025028. doi: 10.1088/1361-6560/aa9c96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theuws JC, et al. Dose-effect relations for early local pulmonary injury after irradiation for malignant lymphoma and breast cancer. Radiother Oncol. 1998;48(1):33–43. doi: 10.1016/s0167-8140(98)00019-x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, et al. Radiation-induced reductions in regional lung perfusion: 0.1–12 year data from a prospective clinical study. Int J Radiat Oncol Biol Phys. 2010;76(2):425–32. doi: 10.1016/j.ijrobp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Faught AM, et al. Evaluating Which Dose-Function Metrics Are Most Critical for Functional-Guided Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):202–209. doi: 10.1016/j.ijrobp.2017.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadoya N, et al. Multi-institutional Validation Study of Commercially Available Deformable Image Registration Software for Thoracic Images. International Journal of Radiation Oncology*Biology*Physics. 2016;96(2):422–431. doi: 10.1016/j.ijrobp.2016.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.