Abstract
Eosinophilic solid and cystic (ESC) renal cell carcinoma (RCC) has recently been described as a potentially new subtype of RCC based upon morphologic and immunohistochemical features. These neoplasms typically demonstrate solid and cystic architecture, and the neoplastic cells contain voluminous eosinophilic cytoplasm with granular cytoplasmic stippling. There is frequently focal immunoreactivity for cytokeratin 20. While the initial cases all occurred in adult females and had benign outcome, we recently expanded the proposed spectrum of this neoplasm to include pediatric cases, multifocal neoplasms, and a case with hematogenous metastasis. ESC has been postulated to be analogous to a subtype of RCC consistently identified in tuberous sclerosis complex patients, and while previous work has demonstrated loss of heterozygosity at the TSC1 locus and copy number gains at TSC2 in ESC RCC, these genes have not been sequenced in ESC RCC. Using capture-based and amplicon-based next generation sequencing, we now demonstrate the consistent presence of either TSC1 or TSC2 gene mutations in pediatric ESC RCC (8 of 9 cases) and adult ESC RCC (6 of 6 cases). We also found these mutations in some neoplasms with variant morphology and thus potentially expand the spectrum of ESC RCC. These include 1 of our adult cases which demonstrated dominant “type 2” papillary RCC morphology and 2 of 3 previously unclassified pediatric RCC with features of ESC RCC minus granular cytoplasmic stippling. We also demonstrate TSC mutations in a case of so-called “oncocytoid RCC after neuroblastoma” with identical morphology and immunoprofile, providing a molecular link between the latter and ESC RCC. In summary, ESC RCC consistently harbors TSC1 or TSC2 mutations, which are infrequently seen in established subtypes of RCC. These findings support TSC1/2 mutation as a molecular marker of ESC RCC, and suggest expansion of the clinicopathologic spectrum to include neoplasms with papillary architecture, occasional cases lacking well-developed granular cytoplasmic stippling, and a subset of RCC with oncocytic features in patients who have survived neuroblastoma.
Keywords: pediatric, renal cell carcinoma, TSC, eosinophilic solid and cystic RCC
Introduction
Eosinophilic solid and cystic (ESC) RCC has recently been described as a potentially new subtype of RCC1,2. ESC RCC has been reported in adult female patients and has been postulated to be the sporadic counterpart to one subtype of tuberous sclerosis complex (TSC)-associated RCC3,4. Histologically, these neoplasms show solid and cystic architecture, and the neoplastic cells contain voluminous eosinophilic cytoplasm with granular cytoplasmic stippling. They frequently demonstrate at least focal expression of cytokeratin 20 (CK20). All cases reported in the initial two series occurred in females, and none of these patients had metastases at the time that they were reported.
Our group recently reported 10 eosinophilic solid and cystic (ESC) RCCs within a cohort of previously “unclassified” eosinophilic renal cell carcinomas in young patients, defined in that study as RCC in patients <35 years of age5. Our study expanded the proposed clinicopathologic spectrum of ESC RCC in several ways. First, our ESC RCC cases occurred in young patients (median age, 27 years), including 3 teenagers. Second, four of our cases occurred in males. Third, one case developed metastases to the lung and liver. Fourth, five of our cases (50%) were multifocal. Fifth, 5 of the 8 cases tested (62.5%) demonstrated focal labeling for cathepsin K, typically a marker of MiT family translocation RCC and PEComas. We also noted, however, that some additional cases in our cohort of eosinophilic RCC closely resembled ESC RCC, but were not perfect matches. Specifically, several of these cases had many of the morphologic features (solid architecture with cysts, abundant eosinophilic cytoplasm, and focal cytokeratin 20 immunoreactivity) of ESC RCC, but lacked well-developed granular cytoplasmic stippling.
Comprehensive DNA sequencing of ESC has not previously been reported. In the current study, we examined a subset of our cohort of ESC RCC in young patients for cancer-associated mutations, and found consistent inactivation of TSC genes (TSC1 or TSC2). We then screened a larger group of ESC RCC in children and adults, found similar TSC mutations, and expanded the study to analyze cases with variant morphology to potentially expand the spectrum of ESC.
Material and Methods
Institutional review board approval
This study was approved by the Institutional Review Board of the Johns Hopkins Hospital and other participating institutions.
Cases
We screened five ESC RCC from young patients previously included in our prior study5 (Prior ESC RCC #1, 2, 3, 5, 6) for cancer-associated mutations using capture-based next generation sequencing. Following the identification of TSC1 or TSC2 mutations in all cases in this cohort (see Results), we then studied a larger series of cases (n = 34) using amplicon-based NGS to specifically assess for TSC1 and TSC2 mutations. These included seven of the previously reported ESC RCCs from young patients [three of the five ESC RCCs initially evaluated with the hybrid capture technique (Prior ESC RCC #3, 5, and 6) along with Prior ESC RCC #4, 7, 9, and 10]5. We also studied: three previously described eosinophilic RCCs from young patients which had features of ESC but were considered unclassifiable because they lacked characteristic granular cytoplasmic stippling (Unclassified ESC-like #1-3)5; six new, previously unreported ESC RCCs from adults (New ESC RCC #1-6); one tuberous sclerosis complex-associated RCC; and one so-called “oncocytoid RCC after neuroblastoma.” As negative controls, we also included 4 oncocytomas, 4 chromophobe RCCs, 4 clear cell RCCs, 3 papillary RCCs, and 1 clear cell papillary RCC, all displaying prototypical histomorphology.
DNA Extraction
Tumor H&E slides were examined and marked by two pathologists (PA and DNP) for subsequent macro-dissection, DNA isolation, and molecular testing. Areas enriched with tumor were then scraped from adjacent 5-micron thick formalin-fixed paraffin-embedded (FFPE) sections (3 to 7 unstained slides per sample) and transferred to Sarstedt (Sarstedt AG & Co. KG, Nümbrecht, Germany) screw top tubes for DNA extraction. Adjacent normal tissue was separately isolated and processed for select cases when available (Prior ESC RCC #6 and the Tuberous Sclerosis Complex-associated RCC case).
Extraction and purification was performed with the automated Siemens Tissue Preparation System (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY, USA). Genomic DNA was quantified using the Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA).
Capture-based next generation sequencing
DNA libraries were prepared using Agilent SureSelectXT reagents (Agilent Technologies, Inc., Santa Clara, CA, USA) with genomic regions of interest captured by means of an Agilent custom-designed bait set (>600 genes, full coding regions; low coverage of exon 1 in a subset of genes). The libraries were sequenced to an average unique read depth of greater than 500X using Sequencing by Synthesis (SBS) 2 × 100 base pairs (bp) paired-end cluster generation on the Illumina HiSeq 2500 platform (Illumina, Inc., San Diego, CA, USA). FASTQ files were generated from Binary Cluster Files (.bcl) using the Illumina bcl2fastq v1.8.4 software with parameters set as per vendor’s specifications. FASTQ files were aligned to the human genome reference hg19 (GRCh37) using the Burrows-Wheeler Aligner v0.7.10 algorithm with default settings. BAM files (.bam) were generated using Picard Tools v1.119 and variant calling was performed using in-house variant caller algorithm (MDLVC v5.0) cross referenced with HaplotypeCaller (Genome Analysis Tool Kit 3.3) under discovery mode in the coding regions of target genes. Variants were classified according to the American College of Medical Genetics and Genomics (ACMG) 2015 Standards and Guidelines, and all variant calls were inspected using Integrated Genomics Viewer v2.3.4 (IGV; Broad Institute, MIT Harvard, Cambridge, MA, USA).
Amplicon-based next generation sequencing
DNA libraries were prepared using Illumina’s TruSeq Custom Amplicon oligonucleotide probes (175 bp amplicons) designed for dual-strand sequencing (separate primer sets for positive and negative DNA strands resulting in two separately generated amplicon pools per sample), which decreases the number of false positive calls that arise from deamination events during formalin fixation, and TruSeq Custom Amplicon Low Input Library Prep Kit as per vendor’s specifications. Genomic targets included the entire coding regions for the following 19 genes: TSC1, TSC2, PTEN, MTOR, FH, VHL, NF2, BAP1, PBRM1, CDKN2A, SETD2, SMARCA4, SMARCB1, TCEB1, KMT2A, KMT2C, KMT2D, KDM5C, and KDM6A.
The libraries were sequenced using SBS 2 × 100 bp paired-end cluster generation on the Illumina HiSeq 2500 platform. Sequencing data was analyzed using Illumina’s online BaseSpace® Sequence Hub Amplicon DS v1.2 App.
Only variants with quality scores of 100 detected in both amplicon pools were considered for further analysis. All variant calls were manually inspected in IGV for quality. Variants within homopolymeric regions were excluded from the study.
Single nucleotide polymorphism (SNP) array
Extracted genomic DNA was treated with the Infinium HD FFPE DNA Restore Kit (Illumina, San Diego, CA, USA) and genotyped using lllumina’s HumanCytoSNP-850K (v1.1) BeadChip platform (approximately 850,000 SNPs) and iScan microarray system. Data visualization was performed using KaryoStudio v2.0 and GenomeStudio Software v2.0 (Illumina). All determinations of chromosomal abnormalities, copy number variants, and copy-neutral loss of heterozygosity (LOH) were performed manually using B-allele frequency (BAF) and smoothed log-R ratio (LRR) data.
TSC1/TSC2 mutation calling
TSC1 and TSC2 variants were considered pathogenic if they had been reported in patients with familial and/or de novo tuberous sclerosis complex in the literature or in the tuberous sclerosis Leiden Open Variation Database (LOVD; www.LOVD.nl/TSC2; www.LOVD.nl/TSC1) (v2.0 Build 36)6. Variants that had not been previously reported were classified as either likely pathogenic (frameshift/nonsense variants considered likely impactful to gene function; pathogenic variants reported in the same genomic region) or variants of uncertain significance (VUS) (not present or present at frequencies below 0.1% in 1000 genomes database and/or located in a splice region). The following transcripts were used for annotation: TSC1 - NM_000368.4; TSC2 - NM_000548.3.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on the Benchmark XT autostainer (Ventana Medical Systems Inc., Tucson, AZ) for CK20 (ks20.8; Dako, Carpinteria, CA; pre-diluted).
Results
ESC RCC in Young Patients from Prior Study
In our prior study5, we classified ten cases as ESC RCCs. The median age was 27 years (range, 14 to 35 years). Six patients were female and 4 were male. All 10 neoplasms demonstrated the morphologic features described recently by Trpkov et al1,2: solid and cystic architecture, voluminous eosinophilic cytoplasm, granular cytoplasmic stippling, and focal CK20 expression. Five of 10 ESC RCC were multifocal (1 bilateral), while 3 of 10 ESC RCC patients had histories of prior malignancy. One patient developed metastatic disease involving the lung and liver. Clinical follow-up was available for 2 of 10 patients, and they showed no evidence of disease at 4.5 and 72 months. No patient had a reported personal or family history of tuberous sclerosis complex. Moreover, we confirmed specifically in 5 cases the absence of angiomyolipoma on renal imaging, the absence of suspicious skin lesions on physical examination, and no clinical history of unexplained seizures. Of 8 cases tested, 5 (62.5%) showed focal labeling for cathepsin K by IHC.
Capture-based next generation sequencing of a small cohort (n = 5) of the previously reported ESC RCCs from young patients (Prior ESC RCC #1, 2, 3, 5, 6)5 demonstrated TSC mutations in all cases (4 TSC2-mutated; 1 TSC1-mutated) (Figure 1). Matched normal tissue was available for one case (Prior ESC RCC #6) and was sequenced, confirming the somatic status of the variants identified. Of note, this patient had multifocal tumors (paraffin tissue from the other tumor in the same kidney was not available for testing). Four of the seven alterations detected (TSC2 p.Y130*; TSC2 splice acceptor variant c.976-1G>A between exon 10 and 11; TSC2 p.L361Sfs; TSC1 p.Y761*) have been previously reported in patients with tuberous sclerosis complex and are thought to be pathogenic7,8,9,10,11,12, while the other three (TSC2 p.L243Hfs; TSC2 p.D1677Afs; TSC1 p.L853Cfs) are considered to be likely pathogenic6,7.
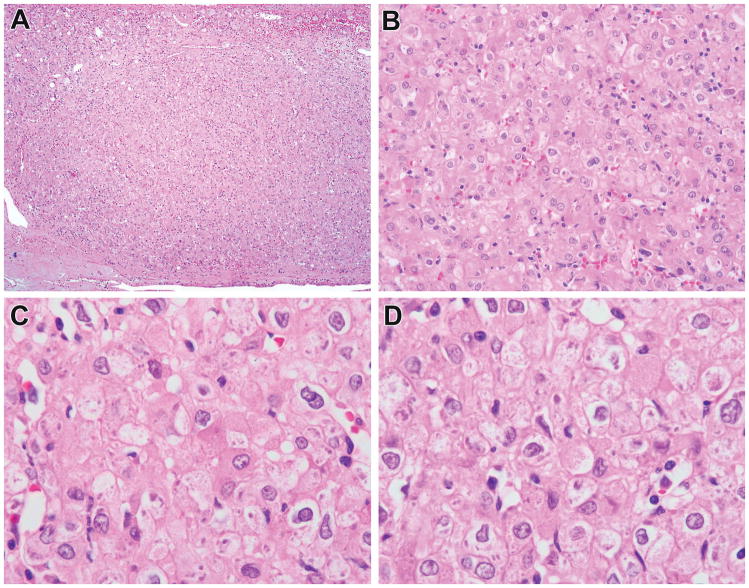
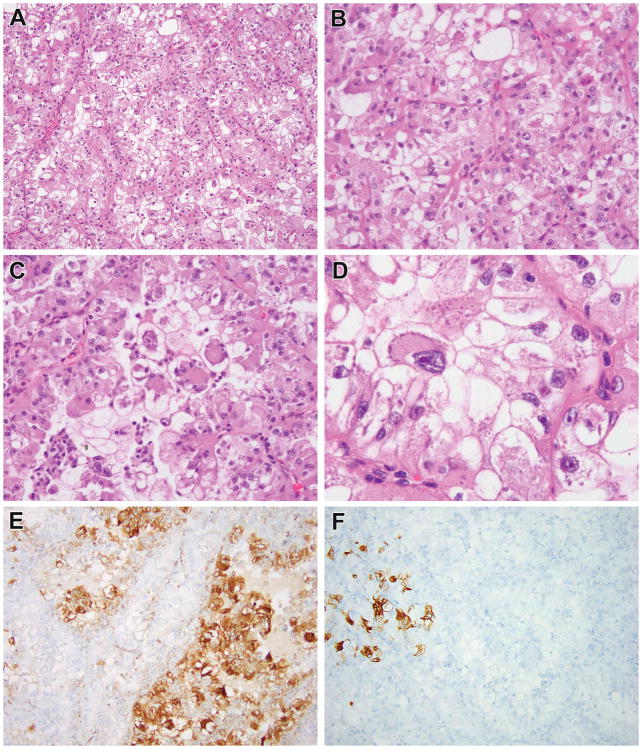
Figure 1.
Previously reported ESC RCC in young patient now showing to harbor TSC mutations. This tumor corresponds to ESC RCC #4 of reference 5, a 5.0 cm renal tumor in a 25-year-old male with a history of “bladder cancer” as a child. This tumor was solid (not cystic) throughout (A, B). The neoplastic cells had abundant eosinophilic cytoplasm, well defined cell borders, and prominent granular cytoplasmic stippling, characteristic of ESC RCC. This neoplasm demonstrated two TSC2 mutations.
TSC mutations were detected by amplicon-based next generation sequencing in 6 of 7 ESC RCCs from our previous study5, bringing the total to 8 of 9 ESC RCC in young patients associated with TSC mutations. Molecular results were orthogonally confirmed in the three cases (Prior ESC #3, 5, 6) tested by both methods with the exception of the TSC2 p.Y130* mutation detected by capture-based NGS in Prior ESC RCC #6. However, coverage of this region (chr16:2104297-2104441) by amplicon-based NGS was present in only one of two amplicon pools for that specimen. Among the prior ESC RCC cases tested using amplicon-based NGS only, two of the alterations (TSC2 p.F73Lfs; TSC2 c.774+1G>A), both detected in Prior ESC RCC #9, have been previously reported in cases of TSC6,8,13 and are thought to be pathogenic, two (TSC2 p.L853Afs; TSC1 p.P641Cfs) are considered likely pathogenic, and one (TSC2 c.1717-1G>C) a variant of uncertain significance (VUS).
We re-reviewed the single case of ESC RCC in a young patient (14-year-old female) in which we did not detect TSC mutations (prior ESC RCC #10). On review, this neoplasm demonstrated typical morphologic features of ESC RCC throughout (Figure 2). Review of the sequencing data showed adequate overall coverage for TSC1 and TSC2 (average depth of coverage, >1000 reads per amplicon pool), but scattered low covered areas (<100 reads) in either one or both amplicon pools.
Figure 2.
Previously reported ESC RCC that lacked detectable TSC mutations. This tumor corresponds to ESC RCC #10 of reference 5, multifocal cystic neoplasms in a 14-year-old female with a history of sickle cell trait. This neoplasm demonstrated solid and cystic areas and cells with voluminous cytoplasm lining the cysts, typical of ESC RCC (A, B). On high power examination, the neoplastic cells had granular pink cytoplasm with granular cytoplasmic stippling, characteristic of ESC RCC (C). The neoplasm demonstrated patchy immunoreactivity for cytokeratin 20 (D), again typical of ESC RCC. While this neoplasm demonstrated all of the hallmark features of ESC RCC, it lacked demonstrable mutations in TSC1 and TSC2.
SNP array analysis showed whole chromosome gains of chromosomes 5, 11, and 14 in Prior ESC RCC #5 and segmental losses of chromosome 6 (6p22.1-p21.32; 6q21-q22.31; 6q25.2-q27) in Prior ESC RCC #2. No chromosomal aberrations were identified in Prior ESC RCC#6. SNP array was attempted in Prior ESC RCC #1 and #3 but could not be completed.
None of the seven Prior ESC RCC studied demonstrated known or likely pathogenic mutations in any of the 17 other RCC-associated genes (PTEN, MTOR, FH, VHL, NF2, BAP1, PBRM1, CDKN2A, SETD2, SMARCA4, SMARCB1, TCEB1, KMT2A, KMT2C, KMT2D, KDM5C, and KDM6A) in the amplicon-based next generation sequencing panel (not shown). A variant of unknown significance, however, was seen in Prior ESC RCC #9 in the VHL gene, p.N193K (NM_000551.3, VAF 40%).
The clinicopathologic features of these cases along with mutations are summarized in Table 1.
Table 1.
Clinicopathologic and Genetic Features of Cases
| Case # | Age | Sex | Clinicopathologic Features | Confirmed Absence of Renal AML, Seizures, Skin Lesions | TSC Alterations | ACMG Classification |
|---|---|---|---|---|---|---|
| Prior ESC1ǂ | 15 | M | Multifocal (3.8 cm, 1.2 cm), clinically bilateral, h/o brain tumor | TSC2 c.976-1G>A | Pathogenic | |
| Prior ESC2ǂ | 30 | F | 3.0 cm |
TSC1 c.2283delC, p.Tyr761* TSC1 c.2557_2558insGT, p.Leu853Cysfs |
Pathogenic Likely Pathogenic |
|
| Prior ESC3§ | 28 | F | Multifocal (2.4 cm. 0.35cm) | x | TSC2 c.727_734delCTCTGTCG, p.Leu243Hisfs | Likely Pathogenic |
| Prior ESC4 | 25 | M | 5.0 cm, h/o “bladder carcinoma” at 16 months | x |
TSC2 c.2554_2555insG, p.Leu853Alafs TSC2 c.1717-1G>C |
Likely Pathogenic VUS |
| Prior ESC5§ | 13 | F | Multifocal (9.0 cm, 4.0 cm), liver metastases | x | TSC2 c.5030delA, p.Asp1677Alafs | Likely Pathogenic |
| Prior ESC6§ | 26 | F | Multifocal (3.3 cm, 1.1 cm) |
TSC2 c.1080_1081insT, p.Leu361Serfs† TSC2 c.390C>G, p.Tyr130*†¥ |
Pathogenic Pathogenic |
|
| Prior ESC8 | 33 | M | 2.2 cm | TSC1 c.1920_1921insTGTG, p.Pro641Cysfs | Likely Pathogenic | |
| Prior ESC9 | 30 | F | 2.3 cm; h/o melanoma, thyroid carcinoma, splenic hemangioma | x** |
TSC2 c.217delT, p.Phe73Leufs TSC2 c.774+1G>A |
Pathogenic Pathogenic |
| Prior ESC10 | 14 | F | Multifocal (8.0 cm, 4.0 cm, 2.0 cm, 0.5 cm) | x | no pathogenic, likely pathogenic, or VUS alterations | |
| New ESC1 | 66 | F | 3.8 cm | x | TSC1 c.1835T>A, p.Leu612* | Likely Pathogenic |
| New ESC2 | 42 | F | 11.5 cm | x | TSC2 c.3415delG, p.Val1139Leufs | Likely Pathogenic |
| New ESC3 | 52 | F | Hematuria¶ |
TSC2 c.4730delG, p.Gly1577Alafs TSC2 c.188insA, p.Ile64Aspfs |
Pathogenic Likely Pathogenic |
|
| New ESC4 | 47 | F | 1.8 cm; papillary | x |
TSC1 c.444delT, p.Lys148Asnfs TSC1 c.1276G>T, p.Asp426Tyr |
Likely Pathogenic VUS |
| New ESC5 | 42 | F | 9.0 cm | x | TSC1 c.1978_1984delCACAGCA, p.His660Argfs | Likely Pathogenic |
| New ESC6 | 46 | F | 3.2 cm | x | TSC1 c.2514delA, p.Ser838Argfs | Likely Pathogenic |
| Unclassified ESC-like 1 | 15 | F | Focal Mart 1+¶ | no pathogenic, likely pathogenic, or VUS alterations | ||
| Unclassified ESC-like 2 | 27 | F | 3.0 cm; focal Melan A+ |
TSC2 c.4375C>T, p.Arg1459* TSC2 c.4947delC, p.Tyr1650Thrfs |
Pathogenic Pathogenic |
|
| Unclassified ESC-like 3 | 15 | F | Nausea¶, Vacuolated tumor | x | TSC1 c.231delC, p.Asn77Lysfs | Likely Pathogenic |
| TSC-Associated RCC | 9 | M | 0.9 cm; papillary |
TSC2 c.826_827delAT, p.Met276ValfsɅ TSC2 c.1119+1G>C† |
Pathogenic VUS |
|
| “Oncocytoid RCC s/p Neuroblastoma” | 40 | F | 3.0 cm; h/o neuroblastoma at 6 months of age, s/p adrenalectomy and radiation |
TSC2 c.731G>T, p.Cys244Phe TSC2 c.1189C>T, p.Gln397* |
VUS Likely Pathogenic |
|
Samples evaluated by capture-based NGS only;
Samples evaluated by both capture-based and amplicon-based NGS;
Variant detected by capture-based NGS, but not by amplicon-based NGS;
Confirmed somatic;
Confirmed germline;
Tumor size not recorded;
Premature stop codon. TSC1 transcript used: NM_000368.4. TSC2 transcript used: NM_000548.3.
seizures related to narcotic use noted; skin lesions noted and biopsied due to history of melanoma but all proved to be nevi. AML=angiomyolipoma
Previously Unreported Adult ESC RCC
We identified six new previously unreported adult cases of ESC RCC from our files. These patients ranged in age from 42 to 66 years, and all were females. The clinicopathologic features of these cases are summarized in Table 1. None of these cases had a reported clinical history or stigmata of tuberous sclerosis complex. Moreover, we confirmed in 5 cases specifically that no angiomyolipomas were present on renal imaging, no lesions were found on skin examination, and there was no history of seizures. Five of the six cases had been submitted in consultation to rule out MiT family translocation RCC. All five these cases demonstrated typical ESC RCC features, including solid and cystic architecture and voluminous eosinophilic cytoplasm with granular cytoplasmic stippling. All four cases tested demonstrated characteristic focal cytokeratin 20 immunoreactivity (Figure 3). All five cases demonstrated focal immunoreactivity for cathepsin k, but were negative for TFE3 and TFEB rearrangement by FISH. The sixth case of adult ESC RCC identified in the routine files of The Johns Hopkins Hospital was originally classified as a papillary RCC (Figure 4). On review by one author (PA), who had not originally seen this tumor but was subsequently made aware of it, this neoplasm had predominantly papillary architecture but displayed characteristic granular cytoplasmic stippling of ESC RCC and focal immunoreactivity for cytokeratin 20.
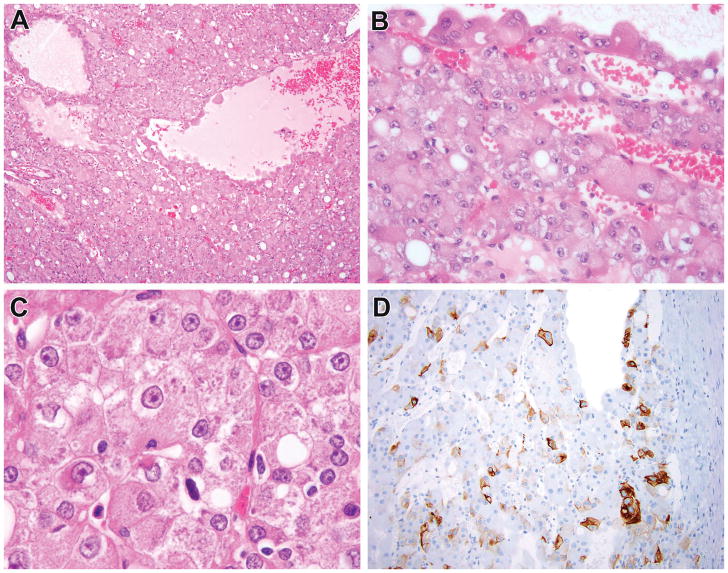
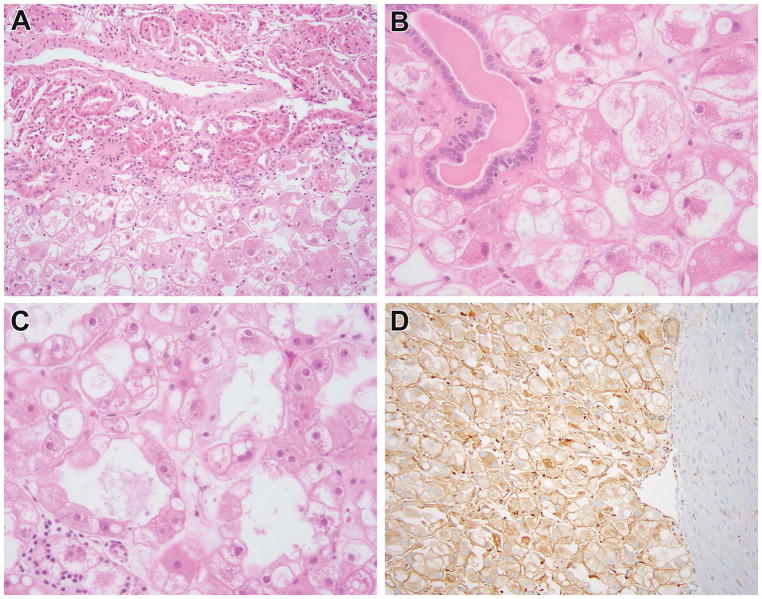
Figure 3.
ESC RCC in adult patient which harbored TSC2 mutation (New ESC #3). This was a renal tumor in a 52-year-old female who presented with hematuria. The neoplasm demonstrated characteristic solid and cystic architecture, with particularly large cells lining the cysts, typical of ESC RCC (A, B). The neoplastic cells demonstrated granular eosinophilic cytoplasm with prominent granular cytoplasmic stippling (C) and patchy labeling for cytokeratin 20 (D), typical of ESC RCC. This neoplasm demonstrated two TSC2 mutations.
Figure 4.
Adult ESC RCC with predominant papillary architecture shown to harbor TSC mutation (New ESC #4). This was a 1.8 cm renal tumor in a 47-year-old female. The neoplasm had dominant papillary architecture throughout (A) with only one focal (<1% of tumor) area of more typical solid growth (B, left of figure). On high power examination, the neoplasm demonstrated the prominent granular eosinophilic cytoplasm and granular cytoplasmic stippling (C), along with the patchy immunoreactivity for cytokeratin 20 (D) that are typical of ESC. This neoplasm demonstrated two TSC1 mutations.
All six of these neoplasms demonstrated TSC mutations (4 TSC1-mutated; 2 TSC2-mutated). Only one of the eight variants detected (TSC2 p.G1577Afs) in the new adult ESC cohort is thought to be pathogenic, having been previously reported in a familial case of TSC14. Six variants are considered to be likely pathogenic with analogous variants previously reported in familial and sporadic cases of TSC (TSC1 p.L612*15,16; TSC2 p.V1139Lfs6; TSC2 p.I64Dfs6; TSC1 p.K148Nfs6,13; TSC1 p.H660Rfs6; and TSC1 p.S838Rfs6), while one variant (TSC1 p.D426Y, New ESC RCC#4), is considered a VUS that appears to occur at a low frequency (rs765695557, ExAC 0.003%) in population databases and has not been reported in the literature or LOVD in individuals with tuberous sclerosis complex-related sequelae.
None of the six New ESC RCC studied demonstrated known or likely pathogenic mutations in any of the 17 other RCC-associated genes (PTEN, MTOR, FH, VHL, NF2, BAP1, PBRM1, CDKN2A, SETD2, SMARCA4, SMARCB1, TCEB1, KMT2A, KMT2C, KMT2D, KDM5C, and KDM6A) in the amplicon-based next generation sequencing panel (not shown). A variant of unknown significance, however, was seen in New ESC RCC #4 in the NF2 gene, p.A464V (NM_000268.3, VAF 51%).
Tuberous Sclerosis Complex-Associated RCC Case
This patient was a 9-year-old boy with a clinical history of tuberous sclerosis complex who was found to have a 9 mm renal mass. The neoplasm demonstrated nested papillary architecture and variably clear cytoplasm with ESC-like granular cytoplasmic stippling. The neoplasm demonstrated patchy immunoreactivity for cathepsin k and focal labeling for cytokeratin 20, similar to that previously reported in ESC RCC (Figure 5).
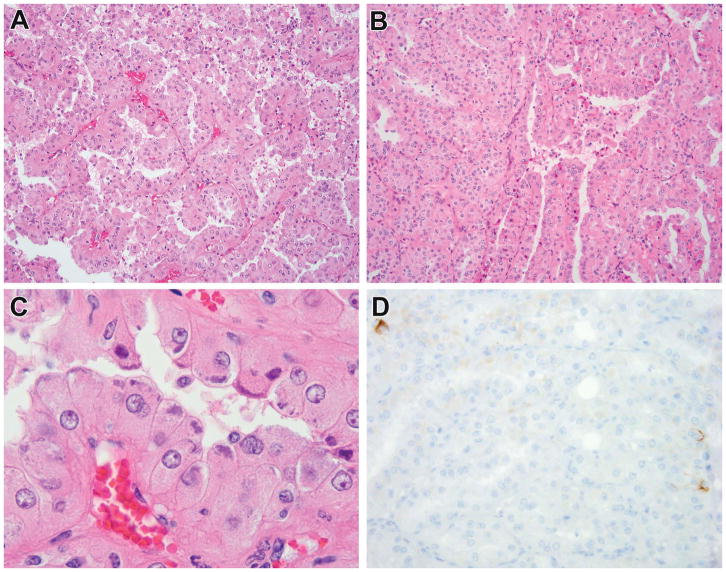
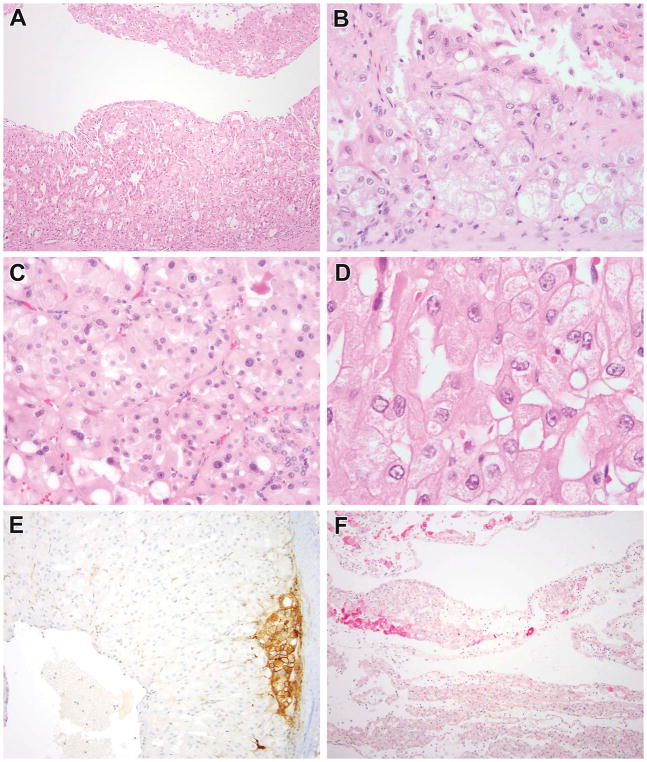
Figure 5.
Tuberous sclerosis complex-associated RCC demonstrated to have TSC gene mutations. This patient was a 9-year-old male with 9 mm renal tumor. The neoplasm demonstrated predominantly papillary architecture with solid areas, and cytoplasm that ranged from clear to granular eosinophilic (A, B). Focally, one could appreciate a biphasic appearance that suggested MiT family translocation carcinoma (C). This neoplasm demonstrated variably clear to eosinophilic cytoplasm and granular cytoplasmic stippling, similar to that seen in ESC RCC (D). The neoplasm demonstrated patchy immunoreactivity for cathepsin K (E) and for cytokeratin 20 (F). This neoplasm demonstrated both germline and somatic mutations in TSC2.
Molecular analyses of this case demonstrated both germline and somatic alterations in the TSC2 gene. Multiple TSC2 polymorphisms were detected in both the normal and tumor tissue (TSC2 c.482-3C>T, TSC2 p.L826M, TSC2 p.S1774T, and TSC2 p.G1439D) as well as one pathogenic variant (TSC2 p.M276Vfs) that has been reported in one familial case of TSC8 and multiple de novo cases17,18. A splice donor variant (TSC2 c.1119+1G>C) of unknown significance was also present in the tumor tissue. While this variant has one pathogenic submission in ClinVar (no assertion criteria provided, accessed Feb 18, 2018) and similar variants in the region reported in the LOVD database as pathogenic (TSC2 c.1119+1G>A; TSC2 c.1119+2T>G)6, the clinical significance of this specific splice variant is not certain.
Unclassified RCC with features of ESC
We noted in our prior study5 that four of 11 unclassified RCCs showed some features of ESC RCC, including focal CK20 expression, but lacked well-developed granular cytoplasmic stippling. We obtained material from 3 of these 4 cases, and found TSC mutations in 2 out of 3 (Figure 6, 7). Both of the TSC-mutated cases demonstrated prominent cell borders, giving a plant-like appearance to the tumor cells, along with at least focally-prominent nucleoli. One had prominent cytoplasmic vacuolization (Figure 6). One demonstrated focal immunoreactivity for Melan A. On re-review, no evidence of granular cytoplasmic stippling was found.
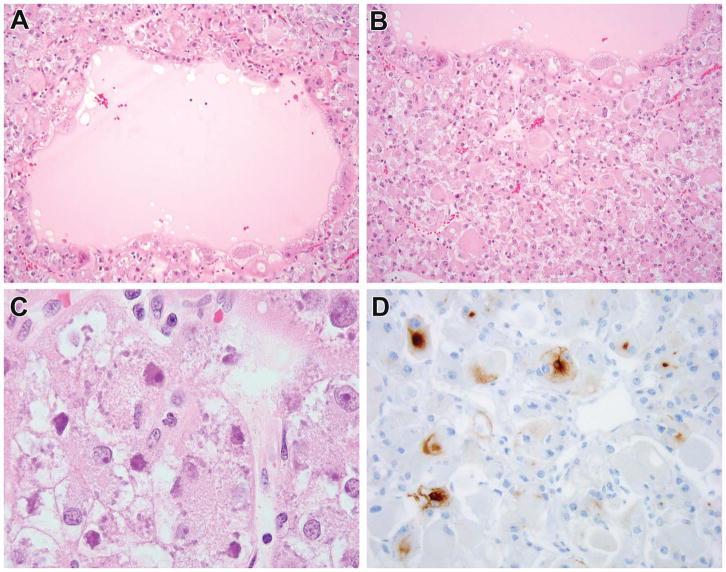
Figure 6.
Previously unclassified RCC with features of ESC now shown to harbor TSC mutation. This patient was a 15-year-old female who presented with nausea and was found to have a renal tumor. The neoplasm was unencapsulated (A) and entrapped negative renal tubules (B). The striking feature of the neoplastic cells was their prominent cell membrane giving them a plant-like appearance, along with prominent cytoplasmic vacuolization and prominent nucleoli (C). Granular cytoplasmic stippling was not seen. The neoplasm demonstrated patchy immunoreactivity for cathepsin k with strong staining in the area highlighted in this image (D). This neoplasm demonstrated a TSC1 gene mutation.
Figure 7.
Previously unclassified RCC with features of ESC now shown to harbor TSC mutation. This was a 15-year-old female who was found to have a renal tumor. The neoplasm demonstrated both solid and cystic architecture and cells with granular eosinophilic cytoplasm, typical of ESC (A, B). The cells had focally prominent nucleoli (C) and prominent plant-like cell borders (D), but lacked any evidence of granular cytoplasmic stippling that is typical of ESC. The neoplasm demonstrated patchy immunoreactivity for cathepsin K (E) and Melan A (F). This neoplasm was shown to harbor two TSC2 mutations.
Three TSC alterations were identified, one pathogenic (TSC2 p.R1459*, reported in over 40 cases of familial19,16 and sporadic cases6,8,13,17 TSC) and two likely pathogenic (TSC2 p.Y1650Tfs, TSC1 p.N77Lfs) with similar variants reported in a case of type 1 segmental postzygotic mosaicism of TSC20 and familial TSC21, respectively. None of these patients had a reported clinical history of tuberous sclerosis complex. Moreover, in Unclassified ESC-like RCC Case 3, we were able to specifically confirm the absence of renal angiomyolipomas on imaging, normal skin exam, and no clinical history of seizures.
None of the three Unclassified ESC-like RCC studied demonstrated known or likely pathogenic mutations in any of the 17 other RCC-associated genes (PTEN, MTOR, FH, VHL, NF2, BAP1, PBRM1, CDKN2A, SETD2, SMARCA4, SMARCB1, TCEB1, KMT2A, KMT2C, KMT2D, KDM5C, and KDM6A) in the amplicon-based next generation sequencing panel (not shown).
So called “Oncocytoid RCC after Neuroblastoma”
This patient was a 40-year-old female who had undergone adrenalectomy for neuroblastoma at the age of 6 months followed by adjuvant radiation therapy. Her course was complicated by chronic renal insufficiency associated with ureteral obstruction. At the age 40, she was found to have a renal mass on imaging, which proved to be a 3 cm, largely solid oncocytic neoplasm. The neoplasm had focal areas of papillary architecture, clusters of foamy macrophages, and lesional cells with abundant, voluminous eosinophilic cytoplasm associated with granular cytoplasmic stippling similar to that seen in ESC RCC. The neoplasm demonstrated focal immunoreactivity for cytokeratin 20 (rare positive cells), also similar to the pattern of ESC RCC. FISH showed no evidence of TFE3 or TFEB gene rearrangement, arguing against MiT family translocation carcinoma. SDHB immunoreactivity was intact. These features are typical of a subset of those reported by Medeiros et al as “oncocytoid renal cell carcinoma after neuroblastoma”22 (Figure 8).
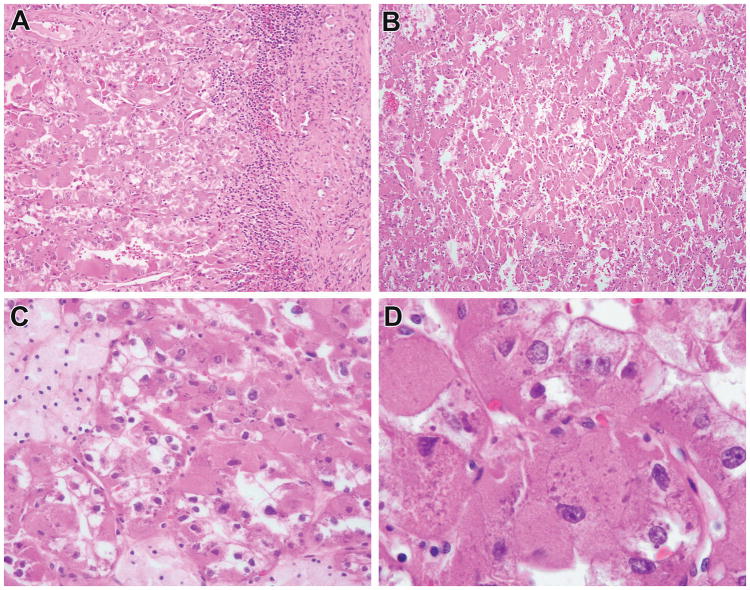
Figure 8.
So-called “oncocytoid RCC after neuroblastoma” now shown to harbor TSC mutations. This neoplasm occurred in a 40-year-old female with a distant history of neuroblastoma treated with surgery and radiation. The neoplasm was encapsulated in most areas but directly abutted the native renal parenchyma in other areas (A). The neoplasm had solid to papillary architecture (B) with some areas demonstrating prominent foam cells (C). The neoplastic cells had voluminous cytoplasm and demonstrated characteristic granular cytoplasmic stippling typically seen in ESC (D). The neoplasm demonstrated two TSC2 mutations.
This neoplasm demonstrated two TSC2 mutations: TSC2 p.Q397* and TSC2 p.C244F. A similar variant to the TSC2 p.Q397* found in this case has been reported in a patient with TSC9. Although the other variant detected, TSC2 p.C244F, has not been previously reported, missense variants involving this amino acid have been reported as “probably pathogenic” in the LOVD database (last accessed Feb 18, 2018)6.
This neoplasm did not demonstrate known or likely pathogenic mutations in any of the 17 other RCC-associated genes (PTEN, MTOR, FH, VHL, NF2, BAP1, PBRM1, CDKN2A, SETD2, SMARCA4, SMARCB1, TCEB1, KMT2A, KMT2C, KMT2D, KDM5C, and KDM6A) in the amplicon-based next generation sequencing panel (not shown). A variant of unknown significance, however, was seen in the NF2 gene, p.E463K (NM_000268.3, VAF 63%).
Negative Control Cases
None of the 16 negative control cases (4 oncocytomas, 4 chromophobe RCCs, 4 clear cell RCCs, 3 papillary RCCs, and 1 clear cell papillary RCC) demonstrated TSC1 or TSC2 mutations.
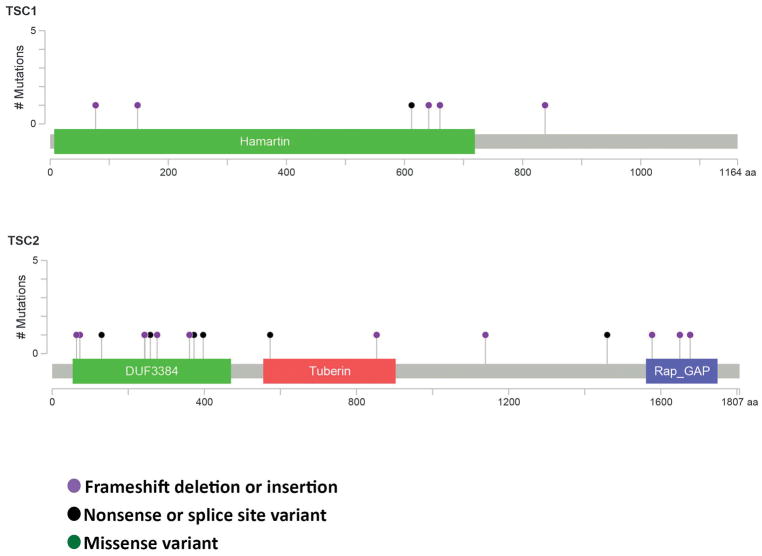
Figure 9 summarizes the mutations found in TSC1 and TSC2 in this study.
Figure 9.
Map of mutations in TSC1 and TSC2 identified in this study. This figure was constructed using the cBioPortal MutationMapper (v1.0.1) (www.cbioportal.org)34,35.
Discussion
In this study, we demonstrate the consistent presence of TSC mutations in eosinophilic solid and cystic renal cell carcinoma (ESC RCC). Given the novel nature of these findings, we used two orthogonal sequencing techniques to assess for these mutations and showed concurrent results in cases studied by both methods. We did not find TSC mutations in our relatively small cohort of control cases studied, which is compatible with the relatively low frequency (approximately 5%) of TSC mutations found in the more common types of RCC reported in larger cohorts in the literature (Tables 2, 3). Our results are not entirely unexpected. ESC has been postulated to be analogous to a subtype of RCC consistently identified in tuberous sclerosis complex patients23, termed “eosinophilic macrocystic” RCC in one study3. Moreover, Trpkov et al. recently demonstrated loss of heterozygosity at the TSC1 locus and copy number gains at TSC2 in ESC RCC, though these genes were not sequenced. The presence of somatic TSC mutations in our cases supports the concept that ESC RCC is the sporadic counterpart of these tuberous sclerosis complex-associated RCC.
Table 2.
TSC mutations in Renal Cell Carcinoma detected in the AACR Project GENIE Public Cohort
| Renal Oncocytoma (n=23) |
Clear Cell RCC (n=463) |
Renal Angiomyolipoma (n=13) |
Unclassified RCC (n=72) |
Papillary RCC (n=96) |
RCC, Not Otherwise Specified (n=145) |
Chromophobe RCC (n=51) |
Renal Medullary Carcinoma (n=43) |
Renal Small Cell Carcinoma (n=1) |
|
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total TSC-Mutated Cases/Pts | 2 (8.7%) | 35 (7.6%) | 13 (100%) | 11 (15.3%) | 7 (7.3%) | 6 (4.1%) | 3 (5.9%) | 1 (2.3%) | 1 (100%) |
|
| |||||||||
| TSC1-Mutated | 2 (8.7%) | 24 (5.2%) | 1 (7.7%) | 6 (8.3%) | 2 (2.6%) | 2 (1.4%) | 0 | 1 (2.3%) | 0 |
| TSC2-Mutated | 0 | 11 (2.4%) | 12 (92.3%) | 5 (6.9%) | 5 (5.2%) | 4 (2.8%) | 3 (5.9%) | 0 | 1 (100%) |
|
| |||||||||
| TSC1 Mutations | n=3 | n=24 | n=1 | n=7 | n=2 | n=2 | n=0 | n=1 | n=0 |
|
| |||||||||
| Frameshift | 2 | 9 | 0 | 3 | 1 | 2 | 0 | 1 | 0 |
|
| |||||||||
| Nonsense | 0 | 7 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
|
| |||||||||
| Splice site | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
|
| |||||||||
| Missense | 1 | 7 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
|
| |||||||||
| TSC2 Mutations | n=0 | n=11 | n=13 | n=8 | n=5 | n=5 | n=3 | n=0 | n=1 |
|
| |||||||||
| Frameshift | 0 | 0 | 6 | 2 | 0 | 0 | 0 | 0 | 0 |
|
| |||||||||
| Nonsense | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 |
|
| |||||||||
| Splice site | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 0 |
|
| |||||||||
| Missense | 0 | 10 | 2 | 4 | 4 | 5 | 2 | 0 | 0 |
This query of the AACR Project GENIE Public Cohort (GENIE, 2018) v3.0.042 was performed using cBioPortal for Cancer Genomics (www.cbioportal.org)40,41 accessed on Feb 15, 2018. Metastatic lesions from primary-metastatic paired samples were excluded from the mutation counts if they contained identical mutations to the primary lesion.
Table 3.
Previously Reported TSC mutations in Renal Cell Carcinoma
| Chromophobe RCC (n=164) |
Clear Cell RCC (n=578) |
Clear Cell RCC w Sarcomaoid Features (n=106) | Renal Non-Clear Cell RCC (n=146)*34 | High Grade Unclassified RCC (n=62)39 |
|
|---|---|---|---|---|---|
|
| |||||
| Total TSC-Mutated Cases/Pts | 4 (2.4%) | 12 (2.1%) | 2 (1.9%) | 5 (3.4%) | 7 (11.3%) |
|
| |||||
| TSC1-Mutated | 3 (1.8%) | 3 (0.5%) | 1 (0.9%) | 3 (2.1%) | 4 (6.5%) |
| TSC2-Mutated | 1 (0.6%) | 9 (1.6%) | 1 (0.9%) | 2 (1.4%) | 3 (4.8%) |
|
| |||||
| Reported TSC1 Mutations | n=3 | n=3 | n=1 | n=3 | n=4 |
|
| |||||
| Frameshift | 1 | 1 | 0 | 0 | 2 |
|
| |||||
| Nonsense | 1 | 0 | 0 | 0 | 1 |
|
| |||||
| Splice site | 1 | 0 | 0 | 0 | 0 |
|
| |||||
| Missense | 0 | 2 | 1 | 3 | 1 |
|
| |||||
| Reported TSC2 Mutations | n=1 | n=10 | n=1 | n=3 | n=4 |
|
| |||||
| Frameshift | 0 | 0 | 1 | 1 | 0 |
|
| |||||
| Nonsense | 0 | 5 | 0 | 1 | 2 |
|
| |||||
| Splice site | 0 | 1 | 0 | 0 | 0 |
|
| |||||
| Missense | 1 | 4 | 0 | 1 | 2 |
Two cases from the renal non-clear cell RCC cohort34 were annotated as classic chromophobes: one case with a TSC1 missense mutation; and the other case with one TSC2 frameshift mutation and one TSC2 nonsense mutation. Three cases were annotated as “papillary”: two cases with one TSC1 missense mutation each; and the third case with one TSC2 missense mutation. This combined query contains samples from 7 studies: 1) Kidney Renal Clear Cell Carcinoma (BGI, Nat Genet 2012)35; 2) Clear Cell Renal Cell Carcinoma (U Tokyo, Nat Genet 2013)36; 3) Kidney Chromophobe (TCGA, Cancer Cell 2014)37; 4) Kidney Renal Clear Cell Carcinoma (TCGA, Nature 2013)33; 5) Renal Non-Clear Cell Carcinoma (Genentech, Nat Genet 2014)34; 6) Targeted gene sequencing in 62 high-grade primary Unclassified RCC (MSK, Nature 2016)39; and 7) Multiregion Sequencing of Clear Cell Renal Cell Carcinoma (IRC, Nat Genet 2014)38. The query was performed using cBioPortal for Cancer Genomics (www.cbioportal.org)40,41 accessed on Feb 15, 2018.
One consideration is that the TSC1/2 mutations we detected are germline, and that some/all of these patients have tuberous sclerosis complex which is a genetic disorder with known variable penetrane24. The minimal number of cases in the study with available non-neoplastic tissue from the kidney (one TSC-associated RCC, Prior ESC #6) is certainly a weakness of the study, though it reflects the consultative nature of these cases such that normal tissue was impossible for us to obtain. However, we strongly believe that the mutations we detected here are somatic for several reasons. First, no patient has clinical evidence of tuberous sclerosis complex, including 11 cases in which we directly confirm that renal angiomyolipomas, suspicious skin lesions, and history of unexplained seizures were not present. This is significant because 90% of patients with tuberous sclerosis complex have skin lesions (seen at all ages), 50–75% will have angiomyolipomas on renal imaging, and >70–80% will demonstrate a history of epilepsy24. Second, although detailed clinical history was not available for Prior ESC#6, normal tissue DNA was available and did not show evidence of TSC alterations.
Our results support all of the novel associations of ESC RCC reported previously by our group5, in that those cases consistently demonstrated the same TSC mutations seen in more typical adult ESC RCC. First, we document that TSC-mutated ESC can occur in young patients, as two of our TSC-mutated cases occurred in teenagers. Taken together with our prior study5, these findings indicate that the majority of previously unclassifiable pediatric RCC with eosinophilic cytoplasm can now be associated with one of three specific molecular alterations: fumarate hydratase (FH) deficiency, succinate dehydrogenase (SDH) deficiency, or TSC mutations. The former two can be readily identified by fumarate hydratase and SDHB immunohistochemistry, even though, as shown previously5, the morphology is often not that typically reported for these neoplasms in adults. Second, we confirm TSC-mutated ESC RCC in two males. Third, we confirm that TSC-mutated ESC RCC (Prior ESC RCC #5) may metastasize. Supporting this idea, an additional morphologically-defined ESC RCC with lymph node metastasis has recently been reported25. Fourth, we confirm that TSC-mutated ESC RCC can be multifocal in four of our previously reported cases, one of which was confirmed to harbor somatic mutations. Finally, we confirm that TSC-mutated ESC RCC typically demonstrates focal immunoreactivity for cathepsin k, which we found in all five new TSC-mutated adult ESC cases tested and over half of the previously reported ESC RCC in young patients26,27.
The presence of TSC mutations in ESC provides a plausible mechanism for focal cathepsin k immunoreactivity. In normal cells, the TSC gene products, TSC1 (hamartin) and TSC2 (tuberin), form a GTPase activating protein (GAP) complex and inhibit mTOR complex 1 (mTORC1). Loss or inhibition of the TSC complex leads to constitutive activation of mTORC1 and dysregulation of multiple cellular pathways including cell growth and proliferation. In osteoclasts, inhibition of mTORC signaling inhibits cathepsin K expression, so in ESC RCC one could hypothesize that increased mTORC signaling secondary to TSC mutations increases cathepsin K expression28,29. Interestingly, cathepsin k, a lysosomal cysteine protease normally regulated by MITF in activated macrophages and osteoclasts, is typically overexpressed in perivascular epithelioid cell tumors (PEComas)30 and pulmonary lymphangioleiomyomatosis31, which also demonstrate TSC1/2 inactivation and mTOR hyperactivation, as well as MiT family translocation RCCs26,27. Along these lines, we noted diffuse cathepsin k expression and focal CK20 labeling in one case of tuberous sclerosis complex-associated RCC studied in our cohort, though we have not seen consistent cathepsin k immunoreactivity in all tuberous sclerosis complex-associated RCC (P Argani, unpublished observations).
Our findings also potentially expand the morphologic spectrum of ESC RCC. Particularly noteworthy was New ESC RCC #4, which had extensively papillary architecture and was originally classified as papillary RCC. On careful review, this neoplasm demonstrated the characteristic granular cytoplasmic stippling of ESC RCC along with focal cytokeratin 20 immunoreactivity, as well as one small solid tubular area that was more typical of ESC RCC. It should be noted that focal papillary areas in ESC RCC were noted in a minority of cases in the studies by Trpkov et al1,2. These findings suggest that a subset of so called “type 2 papillary RCC,” now considered by the 2016 WHO classification to be a pattern that can be adopted by a wide range of RCC rather than a specific entity, may be related to ESC RCC32. Also, potentially expanding the spectrum of ESC RCC were two previously unclassified ESC-like cases notable for their prominent cell borders, reminiscent of the cytology of chromophobe RCC, and absence of well-defined, granular cytoplasmic stippling that now demonstrate TSC mutations. Of note, focal cytology reminiscent of chromophobe RCC was also noted in two cases reported by Trpkov et al.2; in our cases, the chromophobe RCC-like appearance was the dominant histologic finding. Also, one of these cases demonstrated focal immunoreactivity for Melan A, which has not been previously reported in ESC RCC, and both focally displayed more prominent nucleoli than those typically seen ESC RCC. One demonstrated prominent cytoplasmic vacuolization. Given that TSC mutations can rarely be detected in a small percentage of non-ESC RCC (see below), we believe that TSC mutation detection alone does not make the diagnosis of ESC. However, the shared morphologic features and TSC mutations, given the high frequency of TSC mutations in ESC (>90%) relative to other subtypes of RCC (<5%), strongly favor that these lesions are related to ESC.
While the detection of TSC mutations in ESC RCC suggests that molecular diagnostics can help clarify the spectrum of these lesions, several caveats should be noted. First, as noted above TSC mutations have been reported in small subset of other RCC types (Tables 2, 3)33–39,42. We reviewed online images of RCC associated with TSC mutations from the TCGA Kidney Renal Clear Cell Carcinoma cohort33 present on the cBioPortal for Cancer Genomics website (www.cbioportal.org)40,41 and found that at least some of the cases reported as clear cell RCC are morphologically compatible with that diagnosis (Supplemental Figure 1). While none of our cases demonstrated morphologic features of clear cell RCC or harbored known VHL gene alterations, we nonetheless believe that the mere detection of TSC mutations in an RCC does not in and of itself establish the diagnosis of ESC RCC. Second, it also remains possible that cases morphologically consistent with ESC RCC but lacking TSC mutations may harbor other alterations in the mTOR pathway that effects a similar phenotype. This concept is exemplified by translocation associated sarcomas, where variant fusions commonly exist such that the absence of a specific gene fusion does not exclude the diagnosis of most translocation-associated sarcomas. Along these lines, we re-reviewed the morphology of the one pediatric ESC RCC from our prior study which lacked detectable TSC mutations, and found it to be typical of ESC RCC. We suspect that this lesion may harbor a genetic alteration in a TSC gene that is not detectable using our techniques or a mutation that effects a similar phenotype to ESC RCC, perhaps targeting one of the other mTORC pathway genes.
The presence of TSC mutations in ESC RCC is intriguing given that a higher frequency (approximately 20%) of unclassified high grade RCC in one recently published cohort harbor mTOR pathway mutations, including 11% with TSC mutations39. Given that we have established the malignant potential of ESC RCC in the current study, one could speculate that some of the latter cases represent higher grade lesions in the ESC RCC spectrum. Regardless, the findings further support the need to consider therapy targeting the mTOR pathway in these RCC.
Our findings also support the link between the entities previously described as “oncocytoid renal cell carcinoma status post neuroblastoma” and ESC RCC. The former was proposed as a distinct neoplasm in the 2004 WHO classification of renal neoplasia, but was downgraded to a provisional entity in the current 2016 classification. It has been noted that such RCC occurring after neuroblastoma represent a heterogeneous group with many representing post therapy MiT family translocation carcinomas23,43. However, some of these cases have had a striking “oncocytoid” appearance described in the original paper by Medeiros et al., which has been noted to overlap with ESC RCC and tuberous sclerosis complex-associated RCC22,23. Our identification of a TSC mutation in one such case, along with the shared focal cytokeratin 20 immunoreactivity, further supports this association.
In summary, we have demonstrated the consistent presence of TSC mutations in an expanded spectrum of ESC RCCs. Our findings not only support the pathogenesis of ESC RCC as a sporadic counterpart of a subset of tuberous sclerosis complex-associated RCC with metastatic capacity, but also broaden the clinical presentation of ESC RCC to include males and multifocal lesions. Furthermore, we show that ESC RCC overlaps with a subset of RCCs that occur in patients who have survived neuroblastoma, so-called “oncocytoid RCC after neuroblastoma.”
Supplementary Material
Supplemental Figure 1: Clear cell RCCs associated with TSC mutations from the TCGA Kidney Renal Clear Cell Carcinoma cohort33. TSC2 p.L1216I (chr16:2131631; TCGA-B0-5709-01) (A). TSC1 p.G443Dfs*14 (chr 9:135782693; TCGA-B0-4842-01) (B). TSC2 p.R418S (chr16:2112006; TCGA-B0-4712-01) (C). Images were captured from scanned slides in the cBioPortal for Cancer Genomics website (www.cbioportal.org)34,35.
Acknowledgments
Supported in part by the National Institutes of Health Grant 5T32CA193145-02 (DNP), the Gary Hill Award (YL), Joey’s Wings (PA), and Dahan Translocation Carcinoma Fund (PA)
We thank the following physicians for providing clinical history and or unstained slides for this study: Julie Yin MD (Atlanta, GA), Marlyn Ciesla, MD (Elk Grove Village, IL), Maureen O’Sullivan, MD (Dublin, Ireland), Evita Sadimin, MD (New Brunswick, NJ), Semra Olgac, MD (Seattle, WA), Maria M. Picken, MD (Maywood, IL), Nancy Smith, MD (Ruston, LA), Laura Nadeau, MD (Troy, MI), Christopher Wixon, MD (San Diego, CA), and Mary J Reznicek (West Allis, WI).
Footnotes
Disclosures: None
References
- 1.Trpkov K, Hes O, Bonert M, et al. Eosinophilic, solid, and cystic renal cell carcinoma: clinicopathologic study of 16 unique, sporadic neoplasms occurring in women. Am J Surg Pathol. 2016;40:60–71. doi: 10.1097/PAS.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 2.Trpkov K, Abou-Ouf H, Hes O, et al. Eosinophilic solid and cystic renal cell carcinoma (ESC RCC): further morphologic and molecular characterization of ESC RCC as a distinct entity. Am J Surg Pathol. 41:1299–1308. doi: 10.1097/PAS.0000000000000838. [DOI] [PubMed] [Google Scholar]
- 3.Guo J, Tretiakova MS, Troxell ML, Osunkoya AO, et al. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. Am J Surg Pathol. 2014;38:1457–67. doi: 10.1097/PAS.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 4.Yang P, Cornejo KM, Sadow PM, Cheng L, et al. Renal cell carcinoma in tuberous sclerosis complex. Am J Surg Pathol. 2014;38:895–909. doi: 10.1097/PAS.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Reuter VE, Matoso A, Netto GJ, Epstein JI, Argani P. Re-evaluation of 33 ‘unclassified’ eosinophilic renal cell carcinomas in young patients. Histopathology. 2018;72:588–600. doi: 10.1111/his.13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fokkema IF, Taschner PE, Schaafsma GC, et al. LOVD v.2.0: the next generation in gene variant databases. Hum Mutat. 2011;32:557–563. doi: 10.1002/humu.21438. [DOI] [PubMed] [Google Scholar]
- 7.Beauchamp RL, Banwell A, McNamara P, et al. Exon scanning of the entire TSC2 gene for germline mutations in 40 unrelated patients with tuberous sclerosis. Hum Mutat. 1998;12:408–416. doi: 10.1002/(SICI)1098-1004(1998)12:6<408::AID-HUMU7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Sancak O, Nellist M, Goedbloed M, et al. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype--phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. EJHG. 2005;13:731–741. doi: 10.1038/sj.ejhg.5201402. [DOI] [PubMed] [Google Scholar]
- 9.Kwiatkowski DJ, Palmer MR, Jozwiak S, et al. Response to everolimus is seen in TSC-associated SEGAs and angiomyolipomas independent of mutation type and site in TSC1 and TSC2. EJHG. 2015;23:1665–1672. doi: 10.1038/ejhg.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung CC, Su YN, Chien SC, et al. Molecular and clinical analyses of 84 patients with tuberous sclerosis complex. BMC Med Genet. 2006;7:72. doi: 10.1186/1471-2350-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai TS, Huang MY, Chang YT, et al. High-resolution melting analysis is a more effective approach for screening TSC genes mutations. Genet Test Mol Biomarkers. 2011;15:415–421. doi: 10.1089/gtmb.2010.0133. [DOI] [PubMed] [Google Scholar]
- 12.Dabora SL, Sigalas I, Hall F, et al. Comprehensive mutation analysis of TSC1 using two-dimensional DNA electrophoresis with DGGE. Ann Hum Genet. 1998;62:491–504. doi: 10.1046/j.1469-1809.1998.6260491.x. [DOI] [PubMed] [Google Scholar]
- 13.Au KS, Williams AT, Roach ES, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med. 2007;9:88–100. doi: 10.1097/gim.0b013e31803068c7. [DOI] [PubMed] [Google Scholar]
- 14.Flader M, Kurzawa P, Maldyk J, et al. Papillary thyroid carcinoma in a boy with familial tuberous sclerosis complex attributable to a TSC2 deletion - a case report. Curr Oncol. 2017 Oct;24:e423–e428. doi: 10.3747/co.24.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 16.Young JM, Burley MW, Jeremiah SJ, et al. A mutation screen of the TSC1 gene reveals 26 protein truncating mutations and 1 splice site mutation in a panel of 79 tuberous sclerosis patients. Ann Hum Genet. 1998;62:203–213. doi: 10.1046/j.1469-1809.1998.6230203.x. [DOI] [PubMed] [Google Scholar]
- 17.Dabora SL, Sergiusz J, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail NF, Rani AQ, Nik Abdul Malik NM, et al. Combination of Multiple Ligation-Dependent Probe Amplification and Illumina MiSeq Amplicon Sequencing for TSC1/TSC2 Gene Analyses in Patients with Tuberous Sclerosis Complex. J Mol Diagn. 2017;19:265–276. doi: 10.1016/j.jmoldx.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Niida Y, Lawrence-Smith N, Banwell A, et al. Analysis of both TSC1 and TSC2 for germline mutations in 126 unrelated patients with tuberous sclerosis. Hum Mutat. 1999;14:412–422. doi: 10.1002/(SICI)1098-1004(199911)14:5<412::AID-HUMU7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Bessis D, Malinge MC, Girard C. Isolated and unilateral facial angiofibromas revealing a type 1 segmental postzygotic mosaicism of tuberous sclerosis complex with c.4949_4982del TSC2 mutation. Br J Dermatol. 2018;178:e53–e54. doi: 10.1111/bjd.15868. [DOI] [PubMed] [Google Scholar]
- 21.Peron A, Vignoli A, La Briola F, et al. Do patients with tuberous sclerosis complex have an increased risk for malignancies? Am J Med Genet A. 2016;170:1538–1544. doi: 10.1002/ajmg.a.37644. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros LJ, Palmedo G, Krigman HR, Kovacs G, Beckwith JB. Oncocytoid renal cell carcinoma after neuroblastoma: a report of four cases of a distinct clinicopathologic entity. Am J Surg Pathol. 1999;23:772–780. doi: 10.1097/00000478-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Falzarano SM, McKenney JK, Montironi R, et al. Renal cell carcinoma occurring in patients with prior neuroblastoma: a heterogenous group of neoplasms. Am J Surg Pathol. 2016;40:989–997. doi: 10.1097/PAS.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 24.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. NEJM. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 25.McKenney JK, Przybycin C, Trpkov K, Magi-Galluzzi C. Eosinophilic Solid and Cystic (ESC) Renal Cell Carcinomas Have Metastatic Potential. Histopathology. 2017 Dec 19; doi: 10.1111/his.13457. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Martignoni G, Pea M, Gobbo S, Brunelli M, Bonetti F, Segala D, Pan CC, Netto G, Doglioni C, Hes O, Argani P, Chilosi M. Cathepsin-K immunoreactivity distinguishes MiTF/TFE family renal translocation carcinomas from other renal carcinomas. Mod Pathol. 2009;22:1016–1022. doi: 10.1038/modpathol.2009.58. [DOI] [PubMed] [Google Scholar]
- 27.Martignoni G, Gobbo S, Camparo P, Brunelli M, Munari E, Segala D, Pea M, Bonetti F, Illei PB, Netto G, Ladanyi M, Chilosi M, Argani P. Differential expression of cathepsin-K in neoplasms harbouring TFE3 gene fusions. Mod Pathol. 2011;24:1313–1319. doi: 10.1038/modpathol.2011.93. [DOI] [PubMed] [Google Scholar]
- 28.Kneissel M, Luong-Nguyen NH, Baptist M, Cortesi R, Zumstein-Mecker S, Kossida S, O’Reilly T, Lane H, Susa M. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone. 2004;35:1144–56. doi: 10.1016/j.bone.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Medendrop K, van Groningen JJ, Schepens M, et al. Molecular mechanisms underlying the MiT translocation subgroup of renal cell carcinomas. Cytogenet Genome Res. 2007;118:157–165. doi: 10.1159/000108296. [DOI] [PubMed] [Google Scholar]
- 30.Martignoni G, Bonetti F, Chilosi M, et al. Cathepsin K expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Mod Pathol. 2012;25:100–111. doi: 10.1038/modpathol.2011.136. [DOI] [PubMed] [Google Scholar]
- 31.Martignoni G, Pea M, Reghellin D, et al. Molecular pathology of lymphangioleiomyomatosis and other perivascular epithelioid cell tumors. Arch Pathol Lab Med. 2010;134:33–40. doi: 10.5858/2008-0542-RAR1.1. [DOI] [PubMed] [Google Scholar]
- 32.Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO classification of tumours of the urinary system and male genital organs. 4. IARC; Lyon: 2016. [DOI] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durinck S, Stawiski EW, Pavía-Jiménez A, et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat Genet. 2015;47:13–21. doi: 10.1038/ng.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo G, Gui Y, Tang A, et al. Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nat Genet. 2011;44:17–19. doi: 10.1038/ng.1014. [DOI] [PubMed] [Google Scholar]
- 36.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 37.Davis CF, Ricketts CJ, Wang M, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26:319–330. doi: 10.1016/j.ccr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerlinger M, Horswell S, Larkin J, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–233. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YB, Xu J, Skanderup AJ, Dong Y, Brannon AR, Wang L, Won HH, Wang PI, Nanjangud GJ, Jungbluth AA, Li W, Ojeda V, Hakimi AA, Voss MH, Schultz N, Motzer RJ, Russo P, Cheng EH, Giancotti FG, Lee W, Berger MF, Tickoo SK, Reuter VE, Hsieh JJ. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat Commun. 2016 Oct 7;7:13131. doi: 10.1038/ncomms13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017;7:818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Argani P, Laé M, Ballard ET, Amin M, Manivel C, Hutchinson B, Reuter VE, Ladanyi M. Translocation carcinomas of the kidney after chemotherapy in childhood. J Clin Oncol. 2006;1(24):1529–34. doi: 10.1200/JCO.2005.04.4693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Clear cell RCCs associated with TSC mutations from the TCGA Kidney Renal Clear Cell Carcinoma cohort33. TSC2 p.L1216I (chr16:2131631; TCGA-B0-5709-01) (A). TSC1 p.G443Dfs*14 (chr 9:135782693; TCGA-B0-4842-01) (B). TSC2 p.R418S (chr16:2112006; TCGA-B0-4712-01) (C). Images were captured from scanned slides in the cBioPortal for Cancer Genomics website (www.cbioportal.org)34,35.