Abstract
Background
Wiskott-Aldrich syndrome (WAS) is a rare primary immunodeficiency caused by mutations in Wiskott-Aldrich syndrome protein (WASp), a key regulator of cytoskeletal dynamics in hematopoietic cells. A high proportion of patients experience autoimmunity caused by a breakdown in T- and B-cell tolerance. Moreover, excessive production of type I interferon (IFN-I) by plasmacytoid dendritic cells (pDCs) contributes to autoimmune signs; however, the factors that trigger excessive innate activation have not been defined.
Objective
Neutrophil extracellular traps (NETs) emerged as major initiating factors in patients with diseases such as systemic lupus erythematosus and rheumatoid arthritis. In this study we explored the possible involvement of aberrant neutrophil functions in patients with WAS.
Methods
We evaluated the expression of a set of granulocyte genes associated with NETs in a cohort of patients with WAS and the presence of NET inducers in sera. Using a mouse model of WAS, we analyzed NET release by WASp-null neutrophils and evaluated the composition and homeostasis of neutrophils in vivo. By using depletion experiments, we assessed the effect of neutrophils in promoting inflammation and reactivity against autoantigens.
Results
Transcripts of genes encoding neutrophil enzymes and antimicrobial peptides were increased in granulocytes of patients with WAS, and serum-soluble factors triggered NET release. WASp-null neutrophils showed increased spontaneous NETosis, induced IFN-I production by pDCs, and activated B cells through B-cell activating factor. Consistently, their depletion abolished constitutive pDC activation, normalized circulating IFN-I levels, and, importantly, abolished production of autoantibodies directed against double-stranded DNA, nucleosomes, and myeloperoxidase.
Conclusions
These findings reveal that neutrophils are involved in the pathogenic loop that causes excessive activation of innate cells and autoreactive B cells, thus identifying novel mechanisms that contribute to the autoimmunity of WAS.
Keywords: Neutrophil extracellular traps, Wiskott-Aldrich syndrome, type I interferon, autoimmunity
Graphical Abstract

Wiskott-Aldrich syndrome (WAS) is a primary immunodeficiency characterized by abnormal lymphocyte function that leads to recurrent infections and increased risk of systemic autoimmunity.1 The Wiskott-Aldrich syndrome protein (WASp), a multidomain protein expressed exclusively in hematopoietic cells, controls Arp2/3 activation and cytoskeletal dynamics. Loss of WASp or expression of a truncated form compromise immune cell functions, resulting in inefficient T- and B-cell responses and inability to cope with infections.1,2 In addition, autoimmune manifestations, such as hemolytic anemia, vasculitis, inflammatory bowel disease, and arthritis, are common in patients with WAS.3
The cellular defects underlying predisposition to autoimmunity are only partially understood. Defective functions of regulatory T cells have been demonstrated in patients with WAS and Wasp-null mice.4–6 Analysis of the B-cell compartment in patients with WAS and mouse models of the disease showed a B-cell intrinsic propensity to produce autoreactive antibodies, likely through dysregulated Toll-like and myeloid differentiation primary response gene–88 signaling.7,8 Patients with WAS and WASp-null mice have autoantibodies against several autoantigens and show an expansion of potentially autoreactive B-cell populations.9,10 Furthermore, we have shown that chronic activation of plasmacytoid dendritic cells (pDCs) and increased levels of type I interferon (IFN-I) contribute to WAS.11 Excessive IFN-I is a hallmark of human systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). In patients with these diseases, immune complexes of autoantibodies against double-stranded DNA (dsDNA) and RNA are internalized by pDCs and trigger activation of Toll-like receptor (TLR) and IFN-I production.12–14 Moreover, release of neutrophil extracellular traps (NETs) and oxidized mitochondrial DNA by neutrophils has emerged recently as a potent trigger for endosomal TLR activation, and as an induced of interferon-stimulated genes (eg, STING) in pDCs and myeloid cells in IFN-I–mediated autoimmune diseases.15–19 Cytokines and autoantibodies generated in response to these triggers promote further NETosis, thus placing neutrophils at the center of the proinflammatory loop that exacerbates autoimmune response.
Here, based on our previous finding of increased IFN-I levels in patients with WAS, we have investigated a possible contribution of aberrant neutrophil function to disease pathogenesis in patient with this syndrome.
METHODS
Patients
Diagnoses were clinically defined and confirmed by means of genetic analysis. A brief description of all patients is reported in Table E1 in this article’s Online Repository at www.jacionline.org. Human samples and deidentified clinical and laboratory data were obtained after informed consent and according to the Helsinki Declaration and TIGET PERIBLOOD or TIGET08B, TIGET02, and TIGET06 clinical protocols approved by the OSR Ethical Committee, and protocol 16-I-N139 approved by the National Institutes of Health’s Institutional Review Board. All results obtained from samples of patients with WAS were compared with those from age-matched healthy donors (HDs).
Mice
WASp−/− (WKO) mice on a C57BL/6 (CD45.2) genetic background were a gift from S. Snapper (Massachusetts General Hospital, Boston, Mass).20 Animals were bred and maintained in sterile isolators. In vivo experiments were performed by using WASp−/− homozygous female or male mice as WKO and wild-type (WT) WASp+/+ littermate mice as control animals. Animal care and treatment were conducted in conformity with institutional guidelines in compliance with national and international laws and policies (European Economic Community Council Directive 86/609; OJL 358; December 12, 1987). Protocols were approved by the Italian Ministry of Health (authorization no. 1155/2016-PR).
Cell isolation
Bone marrow (BM)–derived pDCs were differentiated in vitro from the BM of WT mice by using recombinant FLT-3 ligand (BMD-FLT3 pDCs); after 7 days, they were collected and purified with B220+microbeads, as previously described.11 For isolation of pDCs, B cells, and neutrophils from the spleen, a cell suspension was obtained and subjected to purification after mechanical disruption and RBC lysis. Cells were enriched from total splenic cells by using the mouse Plasmacytoid Dendritic Cell Isolation kit II, mouse neutrophil isolation kit, or biotin anti-CD19+ or CD43 (Ly-48) microbreads, respectively (Miltenyi Biotech, Bergisch Gladbach, Germany). For pDCs isolated from spleens, after obtaining the CD11c+, plasmacytoid dendritic cell antigen 1 (PDCA-1)+, and B220+ fraction, the cells were further enriched with a FACSAria III cell sorter (BD, Franklin Lakes, NJ). Purity was greater than 95%.
pDC and B-cell activation assay in vitro
Neutrophil supernatants were obtained by means of overnight culture of 1 × 106 purified splenic neutrophils in 100 μL of complete medium without stimulus. Neutrophil supernatants were harvested and clarified by means of centrifugation at 4°C (10,000g for 5 minutes), and aliquots were stored at −80°C until further use. For pDC stimulation, 1:5 dilution of neutrophil supernatant was added to 3.5 × 105 total splenic pDCs, followed by culture for 4 hours or overnight for mRNA and ELISA analysis of IFN-α production, respectively. For B-cell stimulation, 1.5 × 105 CD43− splenic B cells where cultured alone or with a 1:5 volume of neutrophil supernatant in the presence or absence of 5 μg/mL anti–B-cell activating factor (BAFF) or isotype control (AF2106 and AB108C, respectively; R&D Systems, Minneapolis, Minn) for 24 hours. Levels of the early activation marker CD69 were analyzed by using flow cytometry gating on CD19+/B220+ B cells.
Autoantibody array
Sera from WT and WKO animals were screened for the presence of autoantibodies by using an autoantigen proteomic microarray comprising 123 different antigens.
Autoantigen microarrays were manufactured, hybridized, and scanned by the Microarray Core Facility at the University of Texas Southwestern Medical Center (Dallas, Tex) in a blind manner. A heat map was generated based on the normalized fluorescence intensity of autoantibodies and on a color scale range between 12 and 22 SDs. To assess global differences in expression between groups, we computed permutation-based P values by using the global analysis of covariance method.21
Immunohistochemical analysis
Mouse bone samples were fixed in 4% paraformaldehyde (PFA) at 4°C for 48 hours, decalcified with an Ion Exchange Decal Unit (Biocare Medical, Pacheco, Calif), and paraffin embedded. Sections of 1.5 μm were used for hematoxylin and eosin staining to check for basic histopathologic changes. Moreover, sections were dewaxed and rehydrated, and endogenous peroxidase activity was blocked by 0.1% H2O2 for 15 minutes. Then sections were treated with pepsin enzyme (from porcine gastric mucosa, Sigma, St Louis, Mo; 1 mg/mL; antigen retrieval method), incubated with Rodent Block (Biocare Medical) to reduce background, and finally incubated overnight at 4°C with anti–myeloperoxidase (MPO) primary antibody (1:300; Abcam, Cambridge, Mass). Then the secondary antibody MACH 1 Universal HRP Polymer Kit (Biocare Medical) was added directly to the sections, reactions were developed in Biocare’s Betazoid DAB, and nuclei were counterstained with hematoxylin (Dako, Glostrup, Denmark).
Digital images were acquired with an Olympus XC50 camera mounted on a BX51 microscope (Olympus, Center Valley, Pa) using CellF Imaging software (Soft Imaging System GmbH, Muenster, Germany).
Visualization of NETs
Splenic or BM neutrophils were isolated as described above, and then 1.5 × 105 cells were seeded on poly-L-lysine–coated (0.01% in PBS) coverslips. Cells were maintained in RPMI 1640 supplemented with 1% FBS and 10 mmol/L HEPES. In experiments with stimulation, after 30 minutes of incubation at 37°C in a 5% CO2 atmosphere, coverslips were gently washed to remove unattached cells and then incubated in the presence of 100 nmol/L phorbol 12-myristate 13-acetate (PMA) or no stimulus (to measure spontaneously release of NETs) for 90 minutes at 37°C and 5% CO2. After stimulation, 1:15,000 SYTOX Green (Invitrogen, Carlsbad, Calif) was added for 15 minutes at room temperature. Cells were again washed and then fixed in 4% PFA in PBS, washed again, and blocked for 1 hour with 0.1% BSA. Cells were then incubated overnight in a humid chamber with rabbit anti-MPO or anti-elastase (ab65871 and ab21595, respectively; Abcam). Afterward, they were incubated for 1 hour at 37°C with Alexa Fluor 555–conjugated goat anti-rabbit IgG (Invitrogen). Samples were washed twice with PBS, and nuclei were counterstained with 4′-6-diamidino-2-phenylindole dihydrochloride. Coverslips were mounted on glass slides by using ProLong Gold antifade reagent (Invitrogen), and images were acquired on a Nikon A1+ confocal laser-scanning microscope with a ×63 oil objective at room temperature with NIS-Element Confocal software (Nikon, Tokyo, Japan). Images were quantified with ImageJ software (National Institutes of Health, Bethesda, Md).
Fluorimetric quantification of NET release from activated neutrophils
Extruded DNA was quantified with a fluorescence-based assay (SYTOX-based plate assay), as previously described,22,23 with minor modifications. Briefly, freshly isolated neutrophils (1 × 105 cells) were cultured in 96-well black plates (PerkinElmer, Waltham, Mass) in triplicates and stimulated either with 100 nmol/L PMA, 1:50 sera of patients with WAS, or 1:100 WT or WKO mice (depending on the assay) or left untreated. Cells were maintained at 37°C in a 5% CO2 atmosphere. SYTOX Green, a non–cell-permeant DNA binding dye, was added to the cells at 5 μmol/L concentration to detect extracellular DNA. After 90 minutes of incubation, fluorescence was quantified at 485/520-nm excitation/emission with a PerkinElmer EnVision Multilabel Reader. The relative fluorescence units from each treatment were normalized to the DNA fluorescence obtained by means of lysis (0.01% Triton X-100) and expressed as a percentage.
Cytokine, dsDNA, and αMPO ELISA
For detection of anti-dsDNA autoantibodies by means of ELISA, 96-well Immuno-Plates (Thermo Fisher Scientific, Waltham, Mass) were coated with 0.01% poly-L-lysine solution in PBS (Sigma-Aldrich, St Louis, Mo) for 1 hour at room temperature and then incubated overnight with 100 μg/mL salmon sperm dsDNA (Invitrogen) in borate buffer saline (pH 8.4). Plates were washed 3 times and blocked with 0.5% BSA/PBS for 3 hours at room temperature. Serially diluted serum samples in 0.05% Tween 20/0.5% BSA/PBS were added to the plates in duplicates and incubated for 2 hours at room temperature. Plates were then washed with 0.05% Tween 20/PBS, and goat anti–mouse IgG HRP (Cell Signaling Technology, Danvers, Mass) diluted at 1:2000 in 0.05% Tween 20/0.5% BSA/PBS was added to each well. Peroxidase reactions were developed by using TMB Peroxidase EIA Substrate (Sigma) and stopped with 2N H2SO4. OD450 was read by using the Bio-Rad Microplate Reader (Bio-Rad Laboratories, Hercules, Calif). For ELISA to MPO, wells were coated wells with 50 μg of recombinant mouse MPO (3667-MP-250; R&D Systems). Serum levels of BAFF and murine granulocyte colony-stimulating factor (G-CSF) were determined by using Quantikine ELISA kit mouse BAFF/BLys/TNFSF13B (R&D Systems) and murine G-CSF standard ABTS Elisa kit (PeproTech, Rocky Hill, NJ). Amounts of IFN-α were measured in supernatants of cultured cells or in a dilution of 1:10 mouse sera using anti-mouse IFN-α and comparing with the standard curve of mouse recombinant IFN-α (PBL InterferonSource, Piscataway, NJ).
RNA isolation, retrotranscription, and real-time PCR
Total RNA from isolated endogenous pDCs or CD19+ cells of pooled mice (n = 2–4 per experiment), isolated splenic neutrophils, total BM, total PBMCs, or splenic cells by using TRIzol reagent. For human samples, total RNA from isolated granulocytes was extracted with the Purelink RNA Mini Kit, according to the manufacturer’s instructions (Ambion, Foster City, Calif). A total of 1 μg of RNA was DNase treated with Amplification Grade I DNase I (Sigma-Aldrich) and retrotranscribed with SuperScript II (Invitrogen), according to the manufacturer’s instructions. Real-time PCR was performed (with 1 μL of 1:5 dilution of cDNA in duplicates for each gene) in a CFX96 Touch thermal cycler using SsoFast EvaGreen Supermix (Bio-Rad Laboratories). Murine primers were as follows: actin, 5′-GGCACCACACC TTCTACAATG-3′ and 5′-GTGGTGGTGAAGCTGTAGCC-3′; Il6, 5′-GAGGATACCACTCCCAACAGA-3′ and 5′-AAGTGCATCATCGTTGTT CAT-3′; Tnfa, 5′-CTGTAGCCCACGTCGTAGC-3′ and 5′-TTAAGATCCA TGCCGTTG-3′; Cramp, 5′-CTTCAACCAGCAGTCCCTAGACA-3′ and 5′-TCCAGGTCCAGGAGACGGTA-3′; Mpo, 5′-TGACGATTCAGCTTGG CAGG-3′ and 5′-TCCCCGTGCGTCATCGGTGGA-3′; Baff, 5′-GACGC GCTTTCCAGGGACCAG-3′ and 5′-GTCGGCGTGTCGCTGTCTGC-3′; and Ifna4, 5′-GCAGAAGTCTGGAGAGCCCTC-3′ and 5′-TGAGATGC AGTGTTCTGGTCC-3′. Human primers were as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TGCACCACCAACTGCTTA GC-3′ and 5′-GGCATGGACTGTGGTCATGAG-3′; LL37, 5′-GCAGTCAC CAGAGGATTGTGAC-3′ and 5′-CACCGCTTCACCAGCCC-3′; MPO, 5′-TTTGACAACCTGCACGATGAC-3′and 5′-CGGTTGTGCTCCCGAAG TAA-3′; defensin 1/3 (DEFA1/3), 5′-CTCCAAAGCATCCAGGCTCA-3′ and 5′-ATGCAGGTTCCATAGCGACG-3′; and neutrophil-expressed elastase (ELANE), 5′-CGCATCTTCGAAAACGGCTA-3′ and 5′-ACCCG TTGAGCTGGAGAATC-3′. Cycling conditions used were as follows: 95°C for 3 minutes, 40 cycles of 95°C for 5 seconds, and 60°C for 30 seconds, followed by an automatic melt curve. Expression levels of mouse genes were calculated with the comparative 2−ΔCt method by using actin as a reference. For human samples, values were calculated by using GAPDH as a reference.
Flow cytometry
Flow cytometry was performed with the FACSAria II flow cytometer, and analysis was performed with FACSDiva 6.1.1 software (BD Biosciences) or FlowJo 8.8.4 software (TreeStar, Ashland, Ore). For spleen and BM analysis, cells were harvested, minced, and then passed through a 70-μm nylon mesh filter (BD Falcon). Erythrocytes were lysed with ammonium-chloride-potassium buffer, and the resulting cell suspension was counted and maintained in ice. Cells were stained in PBS plus 1% BSA at a concentration of 2 × 106 cells per well and fixed in 1% PFA. Mouse antibodies included the following: CD11b–allophycocyanin (APC; M1/70), Ly6G–phycoerythrin (PE; 1A8), Ly6C–fluorescein isothiocyanate (HK1.4), CD19 (6D5)/CD3 (145-2C11)/NK1.1 (PK136)–Biotin, CD11c-PE (N418), PDCA-1–APC (927), Siglec H–PE (551), CD45R/B220–fluorescein isothiocyanate (RA3-6B2), CD23–PE-Cy7 (B3B4), CD21-APC (7E9), CD1d-PE (K253), and CD69-PE (H1.2-F3; BioLegend, San Diego, Calif). Dead cells were excluded by using the LIVE/DEAD Fixable Cell Stain Kit (Invitrogen). Gates were first set on live cells and singlets.
Adoptive transfer
Purified BM PMNs (>97%) were obtained from age- and sex-matched WT mice, as described above. After purification, PMNs were counted and stained with 3 μmol/L carboxyfluorescein succinimidyl ester (CFSE; BioLegend), according to the manufacturer’s instructions. Staining, viability, and purity were confirmed by means of FACS analysis. A total of 4 × 106 of CD11b+CFSE+Ly6G+ cells were injected through the tail vein in each recipient mouse. After 3 hours, mice were killed, and samples from spleens and BM were obtained to analyze the content of free and phagocytosed cells (CFSE+CD11b+Ly6G+ and CFSE+CD11b+Ly6G− cells, respectively).
Depletion studies
To deplete neutrophils, age- and sex-matched mice were injected intraperitoneally with an initial 400 μg, followed by 200 μg of anti-Ly6G (clone RB6-8C5; BioXCell, West Lebanon, NH) in 200 μL of PBS twice weekly. Control groups were injected with equal amount of rat isotype control IgG2b (Sigma) or PBS. After 4 weeks, mice were killed, and sample tissues were collected and analyzed for RNA and cytometry, as previously described.
Statistical analysis
Statistical analysis was performed by using the Wilcoxon-Mann-Whitney test or unpaired t test with Prism software (version 7.0; GraphPad Software, La Jolla, Calif). All values represent means ± SEMs.
RESULTS
Expression of NET-related genes and NETosis in patients with WAS
To address the role of neutrophils in the pathogenesis of human WAS, we assessed the expression of a set of genes associated with aberrant neutrophil function. In patients with SLE, chronic exposure to IFN-I induces expression of interferon-stimulated genes in neutrophils and primes cells to extrude NETs on encounter with immune complexes.15 Moreover, abundance of a subset of abnormal low-density granulocytes overexpressing mRNA for antimicrobial proteins (LL37 and defensins) and enzymes (MPO and elastase [ELANE]) contributes to enhanced tissue inflammation through release of highly immunogenic NETs.24–26 In the majority of WAS patients we observed increased expression of mRNA transcripts for LL37, MPO, DEFA1/3, and ELANE in the granulocyte-enriched fraction (Fig 1, A). The 3 patients with highest upregulation of NET-related genes (4N, 6N, and 1N) have a clinical history of severe autoinflammatory signs, such as vasculitis and arthritis, and patient 6N also has IgA nephropathy (see Table E1). We next examined whether sera from patients with WAS contain NET-inducing factors. Neutrophils from HDs were incubated with sera from 4 patients with WAS (indicated by asterisks in Table E1) or age-matched control subjects. NETosis was visualized by means of microscopy and measured with a SYTOX Green plate–based fluorimetric assay (Fig 1, B and C). We found that sera from patients with WAS, but not HD sera, induced extension of long DNA filaments positive for neutrophil elastase (NE) in a large fraction of cells. Quantification confirmed that sera from patients with WAS induced higher NET extrusion from control neutrophils. Together, these data suggest an involvement of aberrant neutrophil function in WAS pathogenesis.
FIG 1.
Increased expression of neutrophil-related genes in patients with WAS. A, Expression of neutrophil-derived antimicrobial peptides (LL-37 and DEFA1/3]) and enzymes (MPO and ELANE]) was evaluated in granulocyte-enriched fractions from a cohort of 4 pediatric and 3 adult patients with WAS and the corresponding age-matched HD control subjects. Each patient is identified by a color code that refers to Table E1. For each sample, values are shown as relative abundance of the gene of interest relative to GAPDH as an internal control (2−ΔCt × 100). Significance was calculated by using the Mann-Whitney test. B, Sera from patients with WAS induce NET extrusion. Neutrophils isolated from 2 different HD donors were incubated with sera (1:50 dilution) from patients with WAS (marked with an asterisk in Table E1), age-matched HDs, or PMA as a positive control. Representative images of neutrophils labeled with elastase and 4′-6-diamidino-2-phenylindole dihydrochloride to visualize the nucleus. C, Quantification of NET extrusion by using a SYTOX Green assay. Data are expressed as the percentage of fluorescence obtained from each treatment compared with the maximum fluorescence signal obtained by using cell lysis. Values are pooled from 2 independent experiments with 4 sera per phenotype. *P < .05 and **P < .01, Mann-Whitney test.
Actin inhibitors favor NET release
To investigate the possibility that, in addition to cell-extrinsic factors, loss of actin organization in WAS-mutated neutrophils can facilitate NET release, we treated peripheral neutrophils isolated from HDs with an inhibitor of WASp (wiskostatin)27 or with Latrunculin A, a drug that blocks actin polymerization. Control and treated cells were allowed to adhere on slides and labeled with NE, nuclei were counterstained with 4′-6-diamidino-2-phenylindole dihydrochloride, and cells were then analyzed by using confocal microscopy to score the percentage of extruded NETs. Resting vehicle-treated neutrophils had a compact nucleus surrounded by cytoplasmic NE, whereas PMA-treated cells extruded long DNA structures that overlap with NE in up to 80% of cells (Fig 2, A). Treatment with both drugs induced a significant fraction (wiskostatin, 30.83 ± 3.33; Latrunculin A, 28 ± 4.74 [mean ± SEM]) of cells to undergo spontaneous NETosis (Fig 2, B), suggesting that an intact cytoskeletal network is essential to limit rupture of the nuclear membrane or translocation of granular contents.28 Thus cell-intrinsic factors related to loss of proper actin containment contribute to facilitate NETosis in WASp-mutated neutrophils.
FIG 2.
WAS inhibition and actin depolymerization promotes spontaneous NET release. A, Neutrophils from HDs were pretreated for 30 minutes with wiskostatin (Wstat; 100 nmol/L) or Latrunculin A (Lat-A; 500 nmol/L). After washing, cells were seeded and labeled with antielastase (red) and 4′-6-diamidino-2-phenylindole dihydrochloride (blue). PMA was used as a positive control of NET extrusion. Representative confocal images show NETs in treated neutrophils. Scale bar = 10 μmol/L. B, NETs were quantified manually from 10 randomized fields per sample for a total of 100 to 150 cells per condition and are expressed as percentage of total cells. Bars represent medians ± SEMs of 3 independent experiments using cells from different donors. **P < .01. DMSO, Dimethyl sulfoxide.
Aberrant neutrophil compartment in WKO mice
To better understand the role of neutrophils in the setting of WAS, we analyzed the neutrophil compartment in WASp-deficient animals (WKO).20 Frequencies of circulating neutrophils identified as Ly6G+CD11b+ cells were increased significantly in the blood of WKO animals compared with control mice. A similar increase was found in the spleen both in terms of frequencies and absolute numbers (Fig 3, A and B, and see Fig E1, A, in this article’s Online Repository at www.jacionline.org). In the BM, in contrast, similar frequencies of neutrophils were recorded in control and mutant mice. Surface expression of activation markers (CD11b median fluorescence intensity) was greater in circulating WKO neutrophils and equivalent in splenic neutrophils (Fig 1, C). Consistent with previous findings, the pDC (identified as PDCA-1+ and Siglec-H+) compartment was expanded (see Fig E1, B).11
FIG 3.
Accumulation and decreased clearance of neutrophils in WKO mice. Neutrophils frequencies were quantified by using flow cytometry in organs of 2- to 4-month-old WT or WKO mice. A, Neutrophils in peripheral blood, spleen, and BM were identified as Lin−CD11bhighLy6GhighLy6C− cells. B, Plots show frequencies of neutrophils on live cells in each organ. Statistical significance was determined with the Mann-Whitney test: *P < .05 and ***P < .001. C, CD11b expression (median fluorescence intensity [MFI]) on blood and spleen neutrophils (8 independent experiments, n = 2–4 mice each). ***P < .001. D, Immunohistochemical analysis of BM sections from WT and WKO BM stained with hematoxylin and eosin (H&E, left) and antibodies against MPO (right) to detect neutrophils. Images are one representative example of 4 samples analyzed. Scale bar = 200 μm for H&E and 100 μm for MPO, respectively. E, Neutrophil clearance in vivo. BM neutrophils from WT mice were labeled with 3 μmol/L CFSE and transferred adoptively into WT and WKO mice. Spleens were harvested after 3 hours, and neutrophil uptake was evaluated by using flow cytometry. Bars represent the ratio of free/engulfed neutrophils in the spleens. Data are means ± SEMs pooled from 3 independent experiments with 2 to 3 mice per group. ***P < .001, Mann-Whitney test.
To investigate the mechanisms leading to accumulation of activated neutrophils in WKO animals, we inspected BM specimens to evaluate granulocytic hyperplasia and MPO expression. As shown in Fig 3, D, we did not detect any substantial morphologic difference or a different frequency of MPO-positive cells, thus ruling out the presence of marked granulocytic hyperplasia in the BM. Levels of G-CSF were lower in WKO sera (P < .05), further excluding an increase in granulopoiesis and BM output (see Fig E1, C).
Because both defective phagocytosis and a proinflammatory environment coexist in the setting of WAS,29–31 we reasoned that neutrophil accumulation might be due to lack of clearance, causing persistence of inflammatory signals normally ceased by phagocytosis.32–34 To evaluate phagocytosis in vivo, we adoptively transferred CFSE-labeled WT neutrophils into WT or WKO recipients. The frequencies of free and engulfed neutrophils were measured in the spleen 3 hours after transfer (see Fig E1, D). We observed a higher number of free but not engulfed neutrophils in WKO spleens and a lower frequency of CD11b+ cells with engulfed fluorescent cells. The resulting free/phagocyte-associated neutrophils ratio was greater in WKO spleens, suggesting prolonged defective clearance (Fig 3, E).
A granulocyte signature that includes inflammatory cytokines and DNA binding antimicrobial peptides (LL-37 in human subjects and cathelicidin-related antimicrobial peptide [CRAMP] in mouse) has been associated with aberrant neutrophil functions and increased NETosis in various autoimmune settings.35–37 In animal models of SLE, neutrophils were shown to be the main producers of IFN-I and BAFFs.38,39 To address this possibility in the setting of WAS, we isolated neutrophils from the spleens of WT and WKO animals and analyzed the expression of a set of relevant genes. WKO splenic neutrophils showed a significantly greater expression of inflammatory genes (TNFA, IL6, and IFNA), antimicrobial peptides, and neutrophil enzymes (CRAMP and MPO; Fig 4, A). Moreover, transcripts for BAFF were increased in WKO neutrophils compared with WT neutrophils, which was similar to what has been shown in an SLE mouse model.38 A similar increase in the expression of IFN-α, CRAMP, and BAFF mRNA transcripts was also found in total BM cells (see Fig E1, E). In addition, an array to detect antibodies against a panel of 123 autoantigens showed that WKO sera are highly enriched in IgG and IgM reactive to a broad panel of autoantigens, including antigens contained in NETs, such as histones H3 and H2b, anti-dsDNA, and MPO, some of which are also found to be increased in patients with SLE and RA (Fig 4, B).40–42
FIG 4.
NET-related transcripts and antibodies against NET components are increased in WKO mice. A, Relative gene expression of proinflammatory genes (IFNA, IL6, and TNF), antimicrobial peptide (CRAMP), MPO, and BAFF in splenic isolated neutrophils from WT or WKO adult mice. Data represent means ± SEMs of 4 to 10 mice per group. *P < .05, **P < .01, and ***P < .001, Mann-Whitney test. B, IgG and IgM against 123 antigens were tested in the sera of 4 WT and 6 WKO animals (2–4 months age). For each self-antigen, a colorimetric representation of relative autoantibody reactivity in each sample is shown according to the scale depicted. Red marks indicate autoantigens involved in neutrophil function. Statistical significance was evaluated by using the global analysis of covariance method.
Spontaneous NETosis in WKO neutrophils
We next used different approaches to directly evaluate NETosis in neutrophils derived from control or WASp-null mice. Initial steps of NETosis are characterized by nuclear decondensation and transition from a lobulated nuclear shape to a diffused nuclear morphology. In late stages, NETotic cells assume a spread-shaped morphology.43,44 We scored the fraction of neutrophils belonging to each of these categories by using confocal analysis on freshly isolated splenic neutrophils. In the absence of any added stimulus, 60% of WT nuclei showed the lobulated morphology typical of resting cells, 40% showed delobulated nuclei, and only a tiny fraction showed a spread morphology (<5%). In contrast, only 20% of WKO cells had lobulated nuclei at steady state, whereas most cells had a diffused (>60%) or spread (8%) nuclei (Fig 5, A). A similar increase in the frequency of delobulated and spread nuclei was observed in circulating WKO neutrophils (see Fig E2 in this article’s Online Repository at www.jacionline.org). Disintegration of nuclear membrane followed by translocation of granular material, such as elastase and MPO, in the nucleus is the first step in the process of extrusion of immunogenic NETs.45 To examine the extent of active NETosis in resting cells, we quantified the colocalization index of MPO and nuclear DNA at the single-cell level and found it to be significantly greater in WKO neutrophils (Fig 5, B). Finally, a SYTOX Green–based plate assay demonstrated an increase in NETs release by WKO cells with respect to values (Fig 5, C). Together, these data show that steady-state WKO neutrophils have a pronounced tendency to undergo spontaneous NETosis.
FIG 5.
Increased NETosis in WKO neutrophils. A, Neutrophil nuclear shapes were scored as lobulated (resting), delobulated (pre-NETotic), or spread (NETotic), as shown in the representative images. Bars represent means ± SEMs of 100 to 200 cells per genotype. Scale bar = 5 μm. B, Representative images show MPO associated to DNA as an index of NETosis. Plots show Manders coefficient (mean ± SEM) for MPO (red) in DNA (blue) calculated on 150 to 200 cells per genotype. C, Spontaneous NETosis in isolated neutrophils. Data are expressed as percentage of fluorescence on the maximum fluorescence signal obtained by cell lysis. Bars are means ± SEMs of 6 independent experiments. D, NETosis induced by WKO sera. BM neutrophils (1 × 105) from WT mice were incubated in medium (NS) or with a 1:100 dilution of sera from WT or WKO mice (2–4 months old). NETosis in Fig 5, C and D, was quantified by using a SYTOX Green plate assay. E, Schematic representation of experimental design. F, WT splenic pDCs were incubated with cell-free supernatants from resting WT or WKO splenic neutrophils. IFN-α transcripts (left) and protein (right) were evaluated by using RT-PCR and ELISA after 4 or 24 hours, respectively. Bars represent means ± SEMs of 6 and 4 independent experiments, respectively. *P < .05, **P < .01, and ***P < .001, Mann-Whitney test.
NETs are rich in immunogenic material, and their formation has been correlated to induction of IFN-α and of autoantibodies that in turn promote further NETosis. Therefore we evaluated the presence of soluble triggers of NETs by incubating sera from WT and WKO mice with WT neutrophils. WKO sera, but not WT sera, promoted NET release, which is in line with what we have observed using patient-derived sera (Figs 1, C, and 5, D).
NETs are linked directly to chronic IFN-I production and exacerbation of autoimmune disease in patients with SLE and type I diabetes through triggering of endosomal TLRs in pDCs.15,16,37 To understand whether a NET-pDC axis operates as driver of autoimmunity in the setting of WAS, we incubated pDCs with the cell-free supernatant of WT or WKO spleen-derived resting neutrophils (as shown in Fig 5, E). The supernatant of WKO neutrophils, but not of WT neutrophils, induced IFNA gene transcription and protein production by pDCs (Fig 5, F), demonstrating the presence of interferogenic soluble factors. Together, these results show that WKO neutrophils are prone to release NETs and that these spontaneously released NETs trigger IFN-I production by pDCs.
Neutrophil depletion restores IFN-α levels and pDC activation
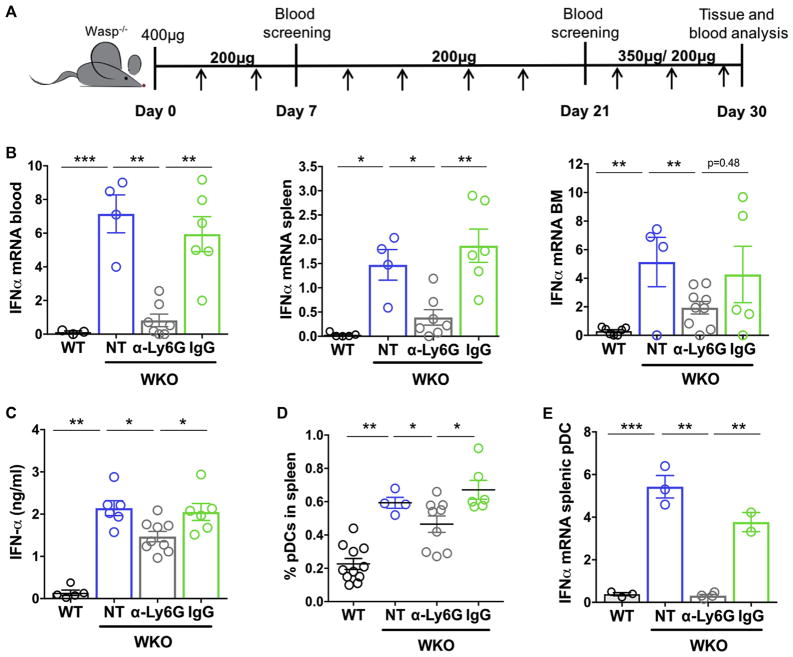
To assess more directly the role of neutrophils in the immunopathology of WAS, we performed depletion experiments in WKO mice. Animals were treated with repeated injection of anti-Ly6G or isotype control antibody (Fig 6, A). Depletion of neutrophils was efficient in all tissue analyzed (blood, BM, and spleen; see Fig E3, A and B, in this article’s Online Repository at www.jacionline.org). After 30 days of treatment, organs were harvested and analyzed. We first measured interferon transcripts in total blood cells, BM, and spleen. Compared with control untreated WKO mice or WKO mice treated with isotype control, neutrophil-depleted mice showed a significant reduction in IFN-I transcripts (Fig 6, B) in all compartments. Circulating IFN-α protein levels were also reduced significantly in depleted animals, although levels were still higher than in WT animals (Fig 6, C).
FIG 6.
Neutrophil depletion reduces IFN-I and pDC activation. A, Schematic representation of the timeline of neutrophil depletion. B, Two-month-old WKO mice were injected intraperitoneally with anti-Ly6G antibody or IgG2a isotype control (IgG), according to the scheme in Fig 6, A. After a 4-week treatment, sera, BM, blood, and spleen were collected for analysis. IFNA transcripts were evaluated in total tissues from the indicated organs of control and depleted groups. C, Circulating IFN-α protein levels in animal sera were measured by means of ELISA. Bars represent means ± SEMs of 3 independent experiments. D, pDC frequencies in spleens of the control and treated groups. Each dot represents a single mouse. E, IFNA transcripts were evaluated in isolated splenic pDCs from treated and untreated groups. Bars represent means ± SEMs of 3 to 4 animals per group from 3 independent experiments. *P < .05, **P < .01, and ***P < .001, Mann-Whitney test.
Because neutrophils induced pDC activation ex vivo (Fig 5, F), we next investigated whether depletion had an effect on pDC frequencies and activation in vivo. pDCs were significantly more abundant in WKO spleens and WKO mice treated with isotype control antibody than in control animals. In contrast, depleted animals showed a partial yet significant reduction in pDC frequencies (Fig 6, D). Importantly, steady-state IFN-I transcripts were abundant in splenic WKO pDCs but absent in pDCs from depleted animals (Fig 6, E). These results prove that neutrophils contribute to increased levels of circulating IFN-α and are a major trigger for IFN-I production by pDCs in vivo.
Neutrophils control the development of anti-dsDNA antibodies in WKO mice
IFN-α secreted by activated pDCs stimulates the maturation of dendritic cells and activation of T lymphocytes and provides activation signals for autoantibody-producing B cells, which in turn leads to a larger production of immune complexes.46,47 In addition, neutrophils sustain class-switching and antibody production by B cells and autoantibodies in SLE-prone mice, directly through secretion of BAFF or indirectly through invariant natural killer T cells.48
Therefore we sought to explore whether neutrophils influence development of autoreactive B cells in WKO animals. To this aim, we first performed coculture experiments using purified naive B cells and neutrophil-derived supernatants. Incubation of B cells with soluble factors derived from WKO neutrophils induced a significant upregulation of CD69 than that induced by WT neutrophil supernatants. The addition of blocking antibodies to soluble BAFF abolished neutrophil-induced B-cell activation (Fig 7, A).
FIG 7.
Neutrophils drive development of autoantibodies in WKO mice. A, B-cell activation by neutrophils. Naive CD43− splenic B cells were incubated with a 1:5 dilution of WT or WKO neutrophil-derived supernatant in the presence of isotype control or BAFF-neutralizing antibodies. CD69 expression on B cells was analyzed after 24 hours and expressed as the percentage of CD69+ cells on CD19+B220+ live cells. Data are representative of 3 independent experiments with similar results. B, Circulating levels of BAFF were quantified by means of ELISA in sera of control and treated groups (left), and BAFF transcripts were evaluated by using RT-PCR in peripheral blood cells (right). C, Antibodies to dsDNA, histones, and MPO were measured by means of ELISA in sera of the control and depleted groups after 1 month of depletion. OD values were calculated at 450 nm for serial dilution of sera. Data are presented as means ± SEMs of 1:400 dilution (anti-dsDNA and anti-histones) or 1:200 dilution (anti-MPO) for each group. Data are pooled from 3 independent experiments (n = 3–4 mice). *P < .05, **P < .01, and ***P < .001.
We next measured circulating BAFF protein and BAFF transcript levels in sera and peripheral blood of WT, WKO, and WKO neutrophil-depleted mice. Consistent with previous findings, BAFF levels were high in WKO mice,9,11 and depletion caused a slight but significant decrease in circulating BAFF protein and a complete rescue in BAFF transcripts in total PBMCs (Fig 7, B).
Finally, we evaluated the effect of depletion on the development of antibodies against autoantigens associated to NETs, such as dsDNA, nucleosomes, and MPO. Sera of WKO or isotype-treated WKO mice contained higher levels of antibodies against all antigens tested than WT sera. Remarkably, in neutrophil-depleted WKO animals the reactivity against these autoantigens was rescued to the level of WT animals (Fig 7, C).
Together, these data demonstrate that neutrophils, through multiple mechanisms that include BAFF secretion and release of immunogenic self-antigens, are directly involved in promoting the expansion, activation, or both of autoreactive B cells in WAS-null mice.
DISCUSSION
Despite enormous progress in understanding the cellular basis of WAS pathogenesis, the factors causing excessive chronic inflammation in patients with this syndrome remain poorly defined. By combining data in the mouse model of the disease and in patients, we propose that neutrophils are involved in the network of detrimental interactions that initiate and support excessive innate responses and development of autoantibodies.
The main evidence supporting aberrant neutrophil function in patients with WAS is the transcriptional activation of genes for antimicrobial peptides and enzymes in granulocytes. In our cohort the abundance of transcripts for genes involved in aberrant neutrophil function was particularly pronounced in patients with severe autoinflammatory and autoimmune signs of the disease. A second observation is that sera from patients with WAS behave as strong NET inducers when incubated with normal neutrophils, indicating the presence of circulating inducers of NETs. Previous studies had shown in different cohorts that a fraction of patients with WAS display reactivity against nuclear antigens (SP100, Scl-70, and histones), dsRNA, mitochondrial antigens, and a slight reactivity to dsDNA and MPO.10,49 This pattern, although much attenuated, resembles the reactivity found in patients with SLE, further suggesting that antigens released by neutrophils during NETosis can serve as autoantigens and elicit an antibody response in patients with WAS. In patients with SLE, the increase in transcription of NET-related genes reflects the premature release of immature granulocytes characterized by abundant transcripts for enzymes and bactericidal proteins.25 In patients with RA, increased expression of defensive genes was associated with activation of mature neutrophils by circulating factors, such as IFN-I and immune complexes.42 The presence of immature granulocytes, granulocytic signature, circulating NET antigens, and pattern of autoantibodies in relation to clinical signs remains to be further investigated in larger WAS cohorts. Because direct analysis of NETs in patient-derived neutrophils was limited by the paucity of biological material, we used a WASp inhibitor and a broader inhibitor of actin polymerization in neutrophils from HDs. Spontaneous NET release in treated cells indicates that, in addition to cell-extrinsic factors, WASp-null neutrophils are inherently prone to release NETs.
Given that actin dynamics are important to maintain nuclear compartimentalization,50 stimulus-independent extrusion of nuclear material might depend on loss of mechanical constraints. Interestingly, translocation of NE into the nucleus requires F-actin degradation,45 suggesting that the major limit to nuclear invasion can be bypassed in WASp-null cells.
Using mouse disease models, we have confirmed and extended the observations made in patients and have addressed the mechanism underlying neutrophil-related defects. We observed an expanded neutrophil compartment in WKO mice and a diminished clearance of neutrophils in vivo, which is in line with the well-established phagocytic macrophage defects from patients with WAS.29 Inhibition of scavenger receptors by NETs along with defective C1q function has been proposed to explain reduced clearance of neutrophils from patients with SLE.51 A similar mechanism can cooperate with defective phagocytosis to determine neutrophil accumulation in the periphery of WASp-null animals.
Three complementary assays to measure NET extrusion provided direct evidence that WASp-null neutrophils release NETs spontaneously. Thus a cell-intrinsic predisposition to release NETs synergizes with defective disposal of dying neutrophils, leading to accumulation of neutrophil-derived immunogenic material. In support of this, cell-free neutrophil supernatants induced IFN-I gene transcription and protein production by pDCs in vitro. Based on a previous report in patients with SLE and diabetes, pDC activation is likely to be triggered by engagement of endosomal TLRs by nucleic acid in complex with cathelicidins, MPO, and autoantibodies.
Depletion studies helped to firmly establish the central role of neutrophils in immune deregulation. Neutrophil depletion was sufficient to rescue excessive IFN-I transcripts in total cells from the blood, spleen, and BM. This broad reduction suggests that in addition to contributing directly to the pool of IFN-I transcripts, neutrophils activate other cells to produce the cytokine. Indeed, splenic pDCs return to a resting state in depleted mice and stop to actively transcribe IFN-I genes, as happens in untreated WKO animals. This finding provides a strong mechanistic link between aberrantly activated neutrophils and chronic pDC activation in patients with WAS. Importantly, our results uncover the central role of neutrophils in promoting autoantibodies. Previous studies in conditional mice showed that WASp-null B cells are intrinsically prone to autoantibody development,8,52 suggesting that innate activation acts downstream of an initial break of tolerance at the B-cell level.
Our data add further evidence that neutrophil-derived signals feed autoreactive-prone B cells to sustain autoantibodies production. Direct activation of B cells through neutrophil-derived BAFF represents a first antigen-nonspecific mechanism to promote B-cell expansion, as indicated by BAFF production by WKO neutrophils (Fig 4, A), rescue of BAFF levels on depletion (Fig 7, B), and direct coculture experiments (Fig 7, A). This is in agreement with previous studies showing that neutrophils stimulate B cells through BAFF, a proliferation-inducing ligand in normal conditions53 and in the setting of SLE.38 Second, uncontrolled NETosis also supplies self-antigens against which antibodies are elicited, as shown by the reactivity against dsDNA, nucleosomes, and MPO, rescued on neutrophil depletion.
Altogether, this study unveils a novel pathogenic axis in the autoimmunity of WAS that, combined with previous findings of excessive IFN-I levels, might help to define the immune network underlying excessive inflammation and pathogenic B-cell responses.
Supplementary Material
Key messages.
Excessive activation and release of immunogenic extracellular traps by WASp-mutated neutrophils contribute to immune deregulation in patients with WAS.
Aberrant neutrophil activation stimulates production of IFN-I by pDCs and promotes activation of autoreactive B cells.
Acknowledgments
Supported by Italian Telethon grant GGP14281 to F.B. and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (to L.D.N.). K.E.C.-L. is supported by an ICGEB postdoctoral fellowship and CONACyT postdoctoral fellowship. N.C. is supported by an AIRC fellowship. The autoantibody arrays (UT Southwestern Medical Center and Microarray Core Facility) were performed in collaboration with Q.-Z. Li (Center for Immunology, University of Texas Southwestern Medical Center at Dallas, Dallas, Tex).
Abbreviations
- APC
Allophycocyanin
- BAFF
B-cell activating factor
- BM
Bone marrow
- CFSE
Carboxyfluorescein succinimidyl ester
- CRAMP
Cathelicidin-related antimicrobial peptide
- DEFA1/3
Defensin 1/3
- dsDNA
Double-stranded DNA
- ELANE
Neutrophil-expressed elastase
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- G-CSF
Granulocyte colony-stimulating factor
- HD
Healthy donor
- IFN-I
Type I interferon
- MPO
Myeloperoxidase
- NE
Neutrophil elastase
- NET
Neutrophil extracellular trap
- pDC
Plasmacytoid dendritic cell
- PDCA-1
Plasmacytoid dendritic cell antigen 1
- PE
Phycoerythrin
- PFA
Paraformaldehyde
- PMA
Phorbol 12-myristate 13-acetate
- RA
Rheumatoid arthritis
- SLE
Systemic lupus erythematosus
- TLR
Toll-like receptor
- WAS
Wiskott-Aldrich syndrome
- WASp
Wiskott-Aldrich syndrome protein
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: A. Aiuti has received a grant from GlaxoSmithKline. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117:725–38. doi: 10.1016/j.jaci.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia Roncarolo M. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113:6288–95. doi: 10.1182/blood-2008-12-115253. [DOI] [PubMed] [Google Scholar]
- 3.Catucci M, Castiello MC, Pala F, Bosticardo M, Villa A. Autoimmunity in wiskott-Aldrich syndrome: an unsolved enigma. Front Immunol. 2012;3:209. doi: 10.3389/fimmu.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humblet-Baron S, Sather B, Anover S, Becker-Herman S, Kasprowicz DJ, Khim S, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest. 2007;117:407–18. doi: 10.1172/JCI29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maillard MH, Cotta-de-Almeida V, Takeshima F, Nguyen DD, Michetti P, Nagler C, et al. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med. 2007;204:381–91. doi: 10.1084/jem.20061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marangoni F, Trifari S, Scaramuzza S, Panaroni C, Martino S, Notarangelo LD, et al. WASP regulates suppressor activity of human and murine CD4(+)CD25(+) FOXP3(+) natural regulatory T cells. J Exp Med. 2007;204:369–80. doi: 10.1084/jem.20061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolhatkar NS, Brahmandam A, Thouvenel CD, Becker-Herman S, Jacobs HM, Schwartz MA, et al. Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome. J Exp Med. 2015;212:1663–77. doi: 10.1084/jem.20150585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, Jackson SW, Hudkins KL, Liu C, et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med. 2011;208:2033–42. doi: 10.1084/jem.20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castiello MC, Bosticardo M, Pala F, Catucci M, Chamberlain N, van Zelm MC, et al. Wiskott–Aldrich Syndrome protein deficiency perturbs the homeostasis of B-cell compartment in humans. J Autoimmun. 2014;50:42–50. doi: 10.1016/j.jaut.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castiello MC, Scaramuzza S, Pala F, Ferrua F, Uva P, Brigida I, et al. B-cell reconstitution after lentiviral vector-mediated gene therapy in patients with Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2015;136:692–702e2. doi: 10.1016/j.jaci.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prete F, Catucci M, Labrada M, Gobessi S, Castiello MC, Bonomi E, et al. Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J Exp Med. 2013;210:355–74. doi: 10.1084/jem.20120363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–17. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banchereau R, Cepika AM, Banchereau J, Pascual V. Understanding human autoimmunity and autoinflammation through transcriptomics. Annu Rev Immunol. 2017;35:337–70. doi: 10.1146/annurev-immunol-051116-052225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R, et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med. 2016;213:697–713. doi: 10.1084/jem.20151876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–53. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Biermann MH, Brauner JM, Liu Y, Zhao Y, Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front Immunol. 2016;7:302. doi: 10.3389/fimmu.2016.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 21.Hummel M, Meister R, Mansmann U. GlobalANCOVA: exploration and assessment of gene group effects. Bioinformatics. 2008;24:78–85. doi: 10.1093/bioinformatics/btm531. [DOI] [PubMed] [Google Scholar]
- 22.Carmona-Rivera C, Kaplan MJ. Induction and Quantification of NETosis. Hoboken (NJ): John Wiley & Sons; 2001. [DOI] [PubMed] [Google Scholar]
- 23.Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One. 2014;9:19–21. doi: 10.1371/journal.pone.0097088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann MH, Bruns H, Bäckdahl L, Neregàrd P, Niederreiter B, Herrmann M, et al. The cathelicidins LL-37 and rCRAMP are associated with pathogenic events of arthritis in humans and rats. Ann Rheum Dis. 2013;72:1239–48. doi: 10.1136/annrheumdis-2012-202218. [DOI] [PubMed] [Google Scholar]
- 25.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–52. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telles RW, Ferreira GA, Silva NPd, Sato EI. Increased plasma myeloperoxidase levels in systemic lupus erythematosus. Rheumatol Int. 2010;30:779–84. doi: 10.1007/s00296-009-1067-4. [DOI] [PubMed] [Google Scholar]
- 27.Peterson JR, Bickford LC, Morgan D, Kim AS, Ouerfelli O, Kirschner MW, et al. Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat Struct Mol Biol. 2004;11:747–55. doi: 10.1038/nsmb796. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Xu J, Zheng Y, Filippi M-D. Rho-GTPase Cdc42 regulates neutrophil polarity via crosstalk between Wiskott Aldrich syndrome protein (WASp), lipid raft reorganization, CD11b and microtubules. Blood. 2011;118:16. [Google Scholar]
- 29.Leverrier Y, Lorenzi R, Blundell MP, Brickell P, Kinnon C, Ridley AJ, et al. Cutting edge: the Wiskott-Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J Immunol. 2001;166:4831–4. doi: 10.4049/jimmunol.166.8.4831. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen DD, Wurbel M-A, Goettel JA, Eston MA, Ahmed OS, Marin R, et al. Wiskott-Aldrich syndrome protein deficiency in innate immune cells leads to mucosal immune dysregulation and colitis in mice. Gastroenterology. 2012;143:719–29e2. doi: 10.1053/j.gastro.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du H-Q, Zhang X, An Y-F, Ding Y, Zhao X-D. Effects of Wiskott-Aldrich syndrome protein deficiency on IL-10-producing regulatory B cells in humans and mice. Scand J Immunol. 2015;81:483–93. doi: 10.1111/sji.12282. [DOI] [PubMed] [Google Scholar]
- 32.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–9. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordy C, Pua H, Sempowski GD, He YW. Regulation of steady-state neutrophil homeostasis by macrophages. Blood. 2011;117:618–29. doi: 10.1182/blood-2010-01-265959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 38.Coquery CM, Wade NS, Loo WM, Kinchen JM, Cox KM, Jiang C, et al. Neutrophils contribute to excess serum BAFF levels and promote CD4 + T cell and B cell responses in lupus-prone mice. PLoS One. 2014 doi: 10.1371/journal.pone.0102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palanichamy A, Bauer JW, Yalavarthi S, Meednu N, Barnard J, Owen T, et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol. 2014;192:906–18. doi: 10.4049/jimmunol.1302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu Y, Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J Clin Cell Immunol. 2013 doi: 10.4172/2155-9899.1000139. 4pii: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu CL, Tangsombatvisit S, Rosenberg JM, Mandelbaum G, Gillespie EC, Gozani OP, et al. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther. 2012;14:R25. doi: 10.1186/ar3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sur Chowdhury C, Giaglis S, Walker Ua, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16:R122. doi: 10.1186/ar4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386–93. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8:883–96. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding C, Cai Y, Marroquin J, Ildstad ST, Yan J. Plasmacytoid dendritic cells regulate autoreactive B cell activation via soluble factors and in a cell-to-cell contact manner. J Immunol. 2009;183:7140–9. doi: 10.4049/jimmunol.0901175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol. 2012;90:498–504. doi: 10.1038/icb.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagglof T, Sedimbi SK, Yates JL, Parsa R, Salas BH, Harris RA, et al. Neutrophils license iNKT cells to regulate self-reactive mouse B cell responses. Nat Immunol. 2016;17:1407–14. doi: 10.1038/ni.3583. [DOI] [PubMed] [Google Scholar]
- 49.Crestani E, Volpi S, Candotti F, Giliani S, Notarangelo LD, Chu J, et al. Broad spectrum of autoantibodies in patients with Wiskott-Aldrich syndrome and X-linked thrombocytopenia. J Allergy Clin Immunol. 2015;136:1401–4. e1–3. doi: 10.1016/j.jaci.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatch EM, Hetzer MW. Nuclear envelope rupture is induced by actin-based nucleus confinement. J Cell Biol. 2016;215:27–36. doi: 10.1083/jcb.201603053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191:2647–56. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
- 52.Recher M, Burns SO, De La Fuente Ma, Volpi S, Dahlberg C, Walter JE, et al. B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood. 2012;119:2819–28. doi: 10.1182/blood-2011-09-379412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. B cell–helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2011;13:170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.