Abstract
Oxytocin (OT) is a hypothalamic nonapeptide that mediates a host of physiological and behavioral processes including reproductive physiology and social attachments. While the OT sequence structure is highly conserved among mammals, New World monkeys (NWMs) represent an unusual ‘hot spot’ in OT structure variability among mammals. At least six distinct OT ligand variants among NWMs exist, yet it is currently unclear whether these evolved structural changes result in meaningful functional consequences. NWMs offer a new area to explore how these modifications to OT and its canonical G-protein coupled OT receptor (OTR) may mediate specific cellular, physiological, and behavioral outcomes. In this review, we highlight relationships between OT ligand and OTR structural variability specifically examining coevolution between OT ligands, OTRs, and physiological and behavioral phenotypes across NWMs. We consider whether these evolved modifications to the OT structure alter pharmacological profiles at human and marmoset OTRs including changes to receptor binding, intracellular signaling, and receptor internalization. Finally, we evaluate whether exogenous manipulation using OT variants in marmoset monkeys differentially enhance or impair behavioral processes involved in social relationships between pairmates, opposite-sex strangers, and parents and their offspring. Overall, it appears that changes to OT ligands in NWMs result in important changes ranging from cellular signaling to broad measures of social behavior.
Keywords: oxytocin, oxytocin receptor, marmoset, social behavior, receptor pharmacology, cell signaling
Introduction
Oxytocin (OT) can fairly be characterized as an essential element or the sine qua non of eutherian, placental mammals. This nine amino acid peptide hormone, synthesized by neurons in the supraoptic (SON) and paraventricular (PVN) nuclei of the neurohypophyseal tract, regulates the two physiological characteristics that are ubiquitous for female placental mammals. First, peripherally-secreted OT acts upon cells within the myometrium and endometrium of the uterus to stimulate uterine smooth muscle contractions associated with parturition and live birth (Arrowsmith & Wray 2014). Second, OT acting on the myoepithelial cells in the mammary gland stimulates milk ejection during lactation (Crowley 2015). Clearly, successful offspring birth and postnatal growth are critically dependent on these two OT-mediated facets of female reproductive physiology.
In addition to these important peripheral functions, OT release from oxytocinergic neurons, particularly from the PVN, also project to critical mesolimbic and forebrain regions, both through direct synaptic connections, and via volume transmission from neuronal dendrites and soma (Ludwig & Leng 2006). These projections are known to mediate a host of social processes. The canonical social relationship mediated by OT is the bond between mothers and their offspring (Rilling & Young 2014). These social bonds ensure continued spatial proximity of mother to offspring and offspring receipt of lactational sustenance, both of which are necessary for growth and survival. It is no surprise, then, that the same neural architecture that underlies maternal-offspring attachments also mediate other social processes. This neural architecture appears to be critical for close affiliative social relationships, and OT is an important element in the complex phenomenon associated with the rewarding properties of affiliative social interactions (Lieberwirth & Wang 2014; Numan & Young 2016). These social relationship traits include pair bonding among reproductive adults, “friendships” among nonreproductive partners and social group members, and more multifaceted sociocognitive traits such as trust, emotional intelligence, social memory, in-group social preferences, and altruism (Feldman 2017).
The hypothalamus also contains neurons that synthesize an additional nonapeptide, arginine vasopressin (AVP). AVP has considerable structural similarity to mammalian OT, differing only at positions 3 and 8 in the mature peptides. AVP exerts significant effects on peripheral function (particularly osmoregulation and vascular tone), but, like OT, AVP projections in the CNS are involved in the regulation of multiple behavioral systems including social attachments among adults, social communication and cognition, and aggression (Caldwell & Young 2006; Caldwell et al. 2008; Albers 2012; Goodson 2013; Kelly & Goodson 2014). In this review we focus primarily on OT, but given the structural similarity of AVP and its important role in the modulation of social behavior, we also explore details of AVP signaling at OT receptors (OTRs).
Structural Changes in Nonapeptides
A necessary condition for both the peripheral and central effects of OT and AVP is the close coupling of the ligand with the cognate receptor(s). Nonapeptide-receptor binding results in a host of changes in cell properties that underlie the modulation of both physiological and behavioral states. Even subtle changes in ligand structure can alter binding properties with receptors and can differentially activate signaling cascades (Koehbach et al. 2013). Successful docking of nonapeptides with the extracellular and/or transmembrane elements of the receptors normally activate G protein-mediated intracellular processes (Busnelli et al. 2012; Stoop 2012; Stoop 2014; Grinevich et al. 2016), and, from a behavioral perspective, consequently alter neuronal function in cells that express receptors (Busnelli & Chini 2017). As with any ligand-receptor signaling system, a variety of alterations or modifications to the signaling molecule can enhance or inhibit cellular responses following OTR binding (Gimpl & Fahrenholz 2001; Zingg & Laporte 2003; Wyttenbach et al. 2008; Busnelli et al. 2016). An additional feature that makes the OT/AVP system intriguing is the ability of both nonapeptides to bind with some affinity with both OT and AVP receptors (Gruber et al. 2012; Manning et al. 2012). These ‘crosstalk’ interactions between OT and AVP can exert significant physiological changes (Chini et al. 1996; Meyer-Lindenberg et al. 2011) and alter behavioral outputs (Song et al. 2016; Caldwell 2017; Song & Albers 2017).
In the last few years, reports have emerged in the literature of nucleotide changes in the coding regions for the OT gene (OXT) that leads to altered OT peptide structure among some mammals, particularly in New World monkeys (NWMs) (Lee et al. 2011; Ren et al. 2015; Vargas-Pinilla et al. 2015). These findings serve as a springboard to reevaluate the assumption that the OT ligand is conserved across mammals, and to also explore the consequences of this variation for cellular and behavioral outcomes. This review will first highlight the broad phylogenetic and evolutionary lineage of nonapeptides, ultimately focusing on the recent evidence for changes in the OXT gene in NWMs. As of this writing, six structural OT variants (including the ‘consensus’ mammalian sequence: Cys–Tyr–Ile–Gln–Asn–Cys–Pro–Leu–Gly–NH2; Leu8-OT) have been identified in NWMs. We then characterize correlated changes in the OTR associated with ligand variation in NWMs. We also evaluate species differences among primates in AVP receptor structure, focusing on the AVP V1a receptor gene (AVPR1a). Second, we review details of nonapeptide ligand-receptor interactions in NWMs, focusing on receptor pharmacology and intracellular signaling pathways. This section highlights the ways in which ligand variation in OT can alter G-protein signaling cascades and points to the multiple ways in which ligand-receptor interactions may ultimately alter neural function serving as potent modulators for behavioral outputs. In our third section, we provide a description of the subtle and context-dependent nature of ligand-specific modulation of social behavior via exogenous manipulation of OT/AVP systems in marmoset monkeys (Callithrix spp.).
New World Monkey OT Evolution and Diversity
For those less familiar with primate taxonomy, we first provide a brief introduction to the evolution and phylogeny of the primate lineage, with the most detailed treatment of the NWMs. Diverging from other mammalian forms approximately 90 million years ago (Mya), the Order Primates are currently classified into five major groupings, as derived from extensive genomic phylogenies (Perelman et al. 2011). The most ancient primates include Lemuriformes (lemurs and lorises) and Tarsiformes (tarsiers). The monkey and ape-like primates (Simiiformes) include four major groupings including two groups of hominoid primates: Hylobatidae (gibbons and siamangs) and hominid primates (orangutans, gorillas, chimpanzees, bonobos, and humans). The African and Asian Old World primates (superfamily Cercopithecoidea; e.g., macaques, baboons, guenons) constitute the third grouping of Simiiformes, and the NWMs of South and Central America (parvorder Platyrrhini) fill out the primate order. NWMs are estimated to have diverged from Old World primates and hominoids ~43 Mya (Perelman, Johnson, Roos, et al. 2011). Within this taxonomic group there are 17 genera that are classified into three families (see Fig. 1). Atelidae are large-bodied (7 – 9 kg) and Pitheciidae are intermediate sized (1 – 3 kg), and generally have diets of fruits and leaves. Cebidae are small-bodied primates (0.12 – 2.5 kg) with more variable diets among species, including invertebrate and vertebrate prey (Hawes & Peres 2014).
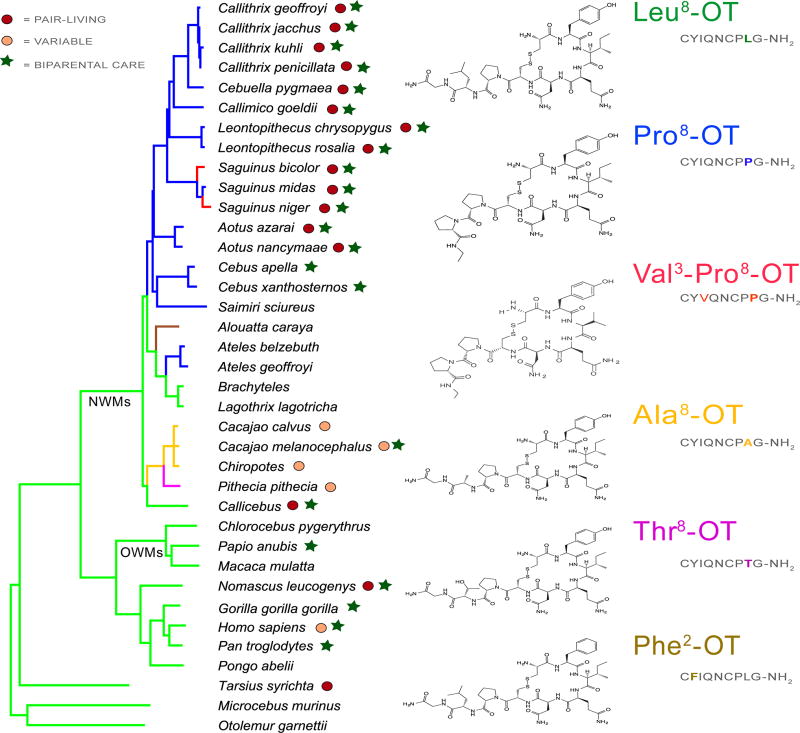
Figure 1.
Consensus primate phylogeny constructed from data from 10kTrees (Arnold et al. 2010) for selected genera/species for which OT ligand is known. Colored phylogenetic roots indicate separate OT ligands (2D structures shown for each OT ligand); red circles represent pair-living and beige variable pair-living/non-pair-living, and stars indicate the presence of biparental care via provisioning and/or protecting behavior for the species. Behavioral phenotypes derived from Lukas and Clutton-Brock 2013; French et al. 2017; and/or University of Michigan Museum of Zoology (animaldiversity.org).
Nonapeptide evolution
Hypothalamic nonapeptides have a long evolutionary history – these molecules are expressed in a number of invertebrate species, and appear to have emerged approximately 600 million years ago (Gruber et al. 2012; Beets et al. 2013). During the course of evolution, vasopressin-like molecules appear to be the ‘ancestral’ peptide in vertebrates (vasotocin), and the similarity of peptide structure and the close proximity of the coding regions for OXT and AVP genes on the same chromosome in primitive jawed fishes suggests a gene duplication event of very early evolutionary origin, first emerging in ancestral jawed fishes ca. 500 million years ago (Gruber et al. 2012; Beets et al. 2013; Koehbach et al. 2013). The vertebrate OT family (isotocin-mesotocin-oxytocin) is generally associated with reproductive function, while the AVP family (vasotocin-vasopressin) is presumed to have origins in fluid homeostasis (Acher et al. 1995). These two nonapeptide families have significant similarities beyond both possessing nine amino acids, including an N-terminal six-residue ring structure formed by a disulfide bridge between two Cys residues at positions 1 and 6, a flexible C-terminal tail structure with a Pro and Gly at positions 7 and 9, and Asn at position 5.
Given the importance of the peripheral actions of OT for reproduction in placental mammals, it is not surprising that an implicit assumption in the literature has been that the mature OT peptide is highly conserved among the nearly 4,000 species of eutherians (Acher et al. 1995; Caldwell et al. 2008; Donaldson & Young 2008; Insel 2010; Gruber 2014). Comparative genomics analyses have supported this proposition. Wallis (2012) analyzed variation in the 27 nucleotides that code for the mature OT peptide across placental mammals, and calculated dN/dS ratios (the number of nonsynonymous nucleotide substitutions relative to the number of synonymous substitutions), in which a ratio < 1.0 implies stabilizing selection for protein structure, while a ratio > 1.0 implies positive selection for diversity in nucleotide sequences and hence protein variability (Wallis 2012). The dN/dS ratio for eutherian OXT gene is remarkably low (0.009), reflecting a highly conserved protein structure that is consistent with the centrality of OT for critical reproductive and social processes in mammals. Wallis (2012) reported an even lower dN/dS ratio for nucleotides coding for AVP gene among mammals (0.005), again implying an extreme conservation in the AVP ligand structure.
In light of the evidence for conserved OT ligand structure in mammals, it comes as some surprise that there is, in fact, considerable variation in OT structures among the NWMs. The first data suggesting multiple ligand forms were published in 2011 (Lee et al. 2011), and indicated that four species of NWMs sequenced for OXT (Callithrix, Aotus, Saimiri, and Cebus) showed an in-frame nonsynonymous substitution (NS) in the OXT codon for the 8th amino acid (CCG→CTG). Wallis (2012) also reported a similar NS substitution in the tree shrew (genus Tupaia). This NS leads to an alteration in the amino acid at the 8th position from the consensus mammalian leucine (Leu) to a proline (Pro), yielding a unique nonapeptide variant, Pro8-OT. Lee et al. (2011) further showed that this substitution was expressed in mRNA and that neurohypophysial extracts contained Pro8-OT, indicating that the altered OXT codon at position 8 in these species is translated into a mature Pro8-OT structured peptide.
In 2015, two laboratories independently conducted a broader comparative analysis of NWMs (Ren et al. 2015; Vargas-Pinilla et al. 2015), both confirming the presence of Pro8-OT in the species sampled in Lee et al. (2011), and revealing additional variability in OXT in this taxonomic group, ultimately characterizing six OT-like variants (Leu8-OT, Pro8-OT, Val3-Pro8-OT, Phe2-OT, Ala8-OT, and Thr8-OT). Accession of available sequence data from NCBI for primates revealed that all species outside NWMs possess the consensus mammalian Leu8-OT. Fig 1 characterizes the mapping of these OT variants on the consensus molecular phylogeny of NWM, and full details on the amino acid substitutions in NWMs can be found in French et al. (2016). All three Families of NWMs have at least two genera that have divergent OT ligands from Leu8-OT, and there appears to have been multiple NS nucleotide changes in Pitheciidae and Atelidae, since exemplar genera contain at least three variants of OT. All genera of Cebidae, a Family that separated from the other NWM ~ 23 Mya, have OXT coding regions for Pro8-OT, suggesting that the mutation that was associated with the evolutionary divergence of this Family. Within the Cebidae, at least one species in the genera Saguinus expresses an additional NS in the codon at position 3 (Valine for Isoleucine; Vargas-Pinilla et al. 2015) as well as Proline at position eight. This finding, along with the emergence of relatively recent (7.5 – 9 Mya) variants in the other Families, indicates that mutations in the coding regions of OXT in NWM have continued after the separation of these families, and that some mutations have occurred in relatively recent evolutionary time. There has been a report of a seventh OT variant in NWMs, the howler monkey, Alouatta seniculus. Campbell and Cortés-Ortiz (personal communication, Nov 2017) have sequenced a Val3-Leu8-OT variant in this taxon. The potential existence of other, additional OT variants awaits a more complete and systematic sampling of species within the NWMs.
The OT variants in NWMs represent both structural and physicochemical divergence from consensus Leu8-OT. The Pro8 substitution, found in all Cebids and one genera of Atelidae, represents the most dramatic alteration from other variants, since the Pro-Pro sequence in the tail portion of the OT peptide at positions 7 and 8 places constraints on the rotational structure of the peptide, and can significantly alter ligand-cell membrane interaction profiles (Geisler & Chmielewski 2009). In addition, Pro8-OT differs in its hydrophilic profile from Leu8-OT, with greater hydrophilicity in Pro8 (Ren et al. 2015; Parreiras-e-Silva et al. 2017). For all OT variants in NWMs, each change in amino acid residues represents at least one physicochemical change (polarity, charge, or hydrophobicity), and hence each can be characterized as a ‘radical’ residue substitution (Zhang 2000). Indeed, Stoop (2012) argued that the structural changes associated with the Pro8 substitution represents a more dramatic change in the molecular architecture of OT than the corresponding changes from mesotocin to consensus Leu8-OT (Isoleucine to Leucine). In the OTR, both the N-terminus, which shows considerable structural variability across NWMs (Ren et al. 2015), and the first extracellular loop of the OTR are thought to be important for ligand selectivity and binding (Zingg et al. 2003), with the latter thought to be primarily interacting with the ligand C-terminus tail (where the majority of substitutions are found at the 8th position) of the OT peptide (Chini et al. 1996; Wesley et al. 2002). Thus, changes in peptide structure of this magnitude have the potential to dramatically alter either receptor binding properties or post-binding intracellular signaling.
In light of the widespread and potentially consequential variation in OT ligands among NWMs, it is notable that NWMs have identical predicted protein structure for AVP. Ren et al. (2014) characterized AVP coding sequences for 33 species of primates, representing all four major clades. In all cases, the amino acid sequences for AVP were identical to the majority of eutherian mammals (Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2; interestingly there are other variants (e.g., Lys8) present in a few nonprimate species). Among the available hominoid, prosimian, and Old World monkey species, all had identical nucleotides in the coding region for AVP (Ren et al. 2014).
Collectively, the findings on OT and AVP ligand variation in NWMs raise two questions regarding genetic variability among species in the genes coding for these two peptide hormones. First, in spite of the widespread structural stability of OT throughout mammalian evolution, why do NWM represent a ‘hot-spot’ for genetic variation among placental mammals? As we have seen, multiple NS substitutions in OXT among these species lead to structural and potentially functional differences in the OT molecule. One other taxon – the cartilaginous sharks, skates, and rays – have also evolved multiple oxytocin-like peptides (Acher et al. 1995). This group of marine fishes utilize urea rather than salts for fluid homeostasis, and it has been argued that a conserved structure in OT-like peptides is not necessary for these osmoregulatory functions, thus stabilizing selection has been relaxed (Gimpl & Fahrenholz 2001). No comparable hypothesis has yet to be formulated for OT ligand diversity in NWMs, and features like body size, geographic distribution, and feeding ecology (Hawes & Peres 2014) fail to account for differences in OT structure. The second outstanding question derives from the observation that despite considerable variation in the OXT gene in NWM, there is no evidence for positive selection and hence variability in AVP.
Ligand-Receptor Coevolution: Macro- and Microscale
To exert their effects, OT and AVP must interact with the appropriate G protein-coupled receptors to effect downstream changes in cell, and ultimately, organismal, function. Markov et al. (2008) have argued that the analyses of ligand families and the corresponding structure of their receptors are among the best tests of coevolutionary processes. This is certainly the case with OT/AVP ligands and their receptors, given the centrality of their functions to survivorship and reproductive success, the early appearance of these signaling systems in vertebrates, and the diversity in nonapeptide ligand structures across different scales of evolutionary time. A single receptor has been identified that binds OT with high affinity (OTR), and it has wide distribution in the central nervous system (Vaidyanathan & Hammock 2017). Three receptor subtypes have been characterized for AVP (V1aR, V1bR, and V2R) in mammals. V2R is primarily expressed in the kidney, but both V1aR and V1bR are expressed widely throughout the brain (Song & Albers 2017).
When using a broad phylogenetic filter, nonapeptide receptor structure based on amino acid sequences in invertebrates are statistically distinct from all vertebrate receptors, and among vertebrates, AVP-like receptors are structurally distinct from all OT-like receptors. Distinct clusters also form on the basis of OTR structure among taxa that have isotocin, mesotocin, and oxytocin as the major OT ligand (Koehbach et al. 2013). It is clear that genetic variation in nonapeptide receptors maps on to large-scale evolutionary changes that are linked to differences in the signaling ligand structure.
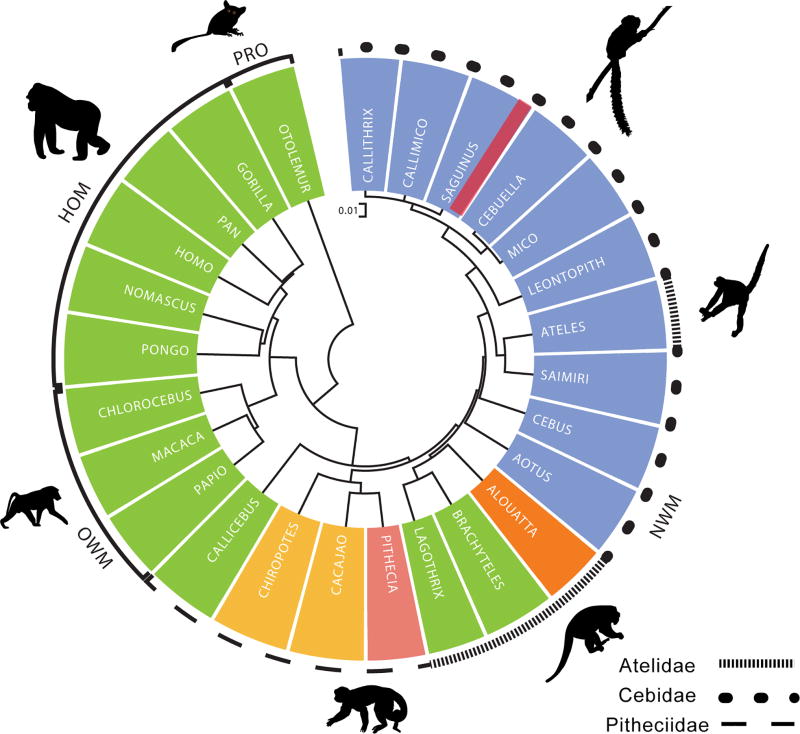
We conducted a similar phylogenetic analysis of OTR variability within a more restricted taxon, the Order Primates, with specific attention to nucleotide variation in the OTR gene among the NWMs (French et al. 2016). Fig 2 portrays a phylogenetic analysis for Simiiformes primates (monkeys and hominoids, with a prosimian as the reference out-group). Taxa can be differentiated according to consensus primate phylogeny, with OTR structures that are clearly distinct among prosimians, NWMs, Old World monkeys, and hominoids. Among NWMs, the three major clades also tend to cluster together on the basis of OTR structure. It is also apparent that OTR structure among species is significantly predicted by OT ligand structure (Ren et al. 2015; Vargas-Panilla et al. 2015). OTR structure in one genus of NWM (Ateles) stands out from the consensus primate phylogeny, in which the OTR structure is more like those of the small-bodied Cebidae. Notably, Ateles is the only NWM outside of the Cebids to express Pro8-OT (Fig 2). In this case, then, the OT ligand variant is a better predictor of OTR structure than consensus phylogenies. Ren et al. (2015) confirmed this trend by a comparing pairwise evolutionary distances among OT and OTR structure, and provided evidence for significant coevolution for species-specific ligands and changes in OTR structure among primates.
Figure 2.
Phylogeny constructed from OTR sequence across primates demonstrating significant coevolution of OT ligand structure and OTR sequences across primates. OT ligands across genera are indicated by colors matched from Figure 1. Figure modified from Ren et al. 2015.
There are tantalizing results that link changes in OT and OTR structure with social phenotypes among NWMs. Vargas-Pinilla et al. (2015) found a significant association between the OT ligand and litter size, with species possessing Pro8-OT more commonly producing more than one offspring at birth and extensive paternal care (a common trait among Callitrichid primates). Ren et al. (2015) also demonstrated a significant association between species classified as socially monogamous and OTR variability among primates. This finding was also demonstrated between social monogamy and V1aR variability among primates (Ren et al. 2014). Notably, V1aR receptor structure in the socially-monogamous and biparental titi monkey (Bales et al. 2017) is highly similar to Cebid species that also share these traits, despite considerable phylogenetic distance between titi monkeys and the Cebids (Ren et al. 2014).
In the remainder of this review, we expand upon these statistical associations among behavioral traits (social monogamy and biparental care) and nonapeptide and receptor variability in two distinct ways. First, we examine ligand-receptor pharmacology and downstream intracellular signaling via in vitro receptor binding and intracellular signaling assays to ascertain whether there are consequences of nonapeptide ligand variation for these processes. Second, we summarize our in vivo behavioral pharmacology experiments with marmosets testing whether OT agonist and OTR antagonist manipulations alter social behavior in marmosets in ways that are consistent with the phylogenetically-derived statistical associations between nonapeptide systems and sociality.
Overview of OTR Signaling
OTRs are members of the G-protein coupled receptor (GPCR) Group-A family, which are among the largest and most diverse mammalian protein families. GPCRs consist of seven membrane-spanning helices, an extracellular N-terminus, and an intracellular C-terminus, which interacts with G-protein messenger complexes. GPCRs ultimately function to transduce extracellular stimuli (release of neurotransmitter or hormone) into intracellular signaling events (changes in cell function and regulation). These signaling events are mostly mediated by receptor intracellular coupling to a heterotrimeric G-protein complex (Gα, Gβ, Gγ), which upon activation changes conformational states that lead to specific changes in downstream cell activity. Much attention has focused on the Gα subunit due to the Gα subunit consisting of a number of isoforms (Gq, Gi/o, Gs), any of which can be preferentially activated by specific ligands. GPCRs possess multiple active conformation states that preferentially couple to isoforms, and these responses lead to a variety of distinct intracellular responses including stimulating adenynyl cyclase and production of cAMP (Gs), mobilizing intracellular calcium ions (Ca2+) from the endoplasmic reticulum and regulating potassium ion (K+) channels (Gq/11), inhibiting production of cAMP and also regulating ion channels (Gi/o), and all isoforms are involved in regulating a number of protein kinases which have many diverse functions (Simon et al. 1991). Through preferential activation of specific G-proteins, GPCR responses can exert a high degree of specificity in eliciting functional cellular responses (Maudsley et al. 2005).
When activated by OT, OTRs preferentially couple to both Gq/11 and Gi/o-proteins (Gimpl & Fahrenholz 2001; Busnelli et al. 2012). A number of factors can influence OTR activation including circulating concentrations of OT ligand near OTRs and the density of OTR expression. For example, at lower OT concentrations [1–10 nM], OTRs preferentially couples to Gq, while at higher OT concentrations [11–100 nM] OTRs couple to both Gq and Gi/o (Busnelli et al. 2012). Overall, OTRs primarily couple to Gq proteins, but this OT concentration gradient governing preferential OTR coupling to G-proteins is also evident within specific Gi isoform subtypes (Gi1, Gi2, and Gi3), and the Go subtypes (GoA and GoB). Specifically, OTR activation via OT induces Gi3 coupling at lower concentrations and Gi1, and GoB coupling only at higher concentrations (Busnelli et al. 2012). This functional selectivity of coupling to Gq or Gi/o has differential cellular outcomes. It has been shown in human embryonic kidney cells (HEK293) stably expressing OTRs that Gq coupling stimulates cell growth while Gi/o coupling inhibits cell growth (Rimoldi et al. 2003). Moreover, in olfactory neuron cell lines (GN11), OTR coupling to Gq was shown to decrease K+ inward rectifying currents, while coupling to Gi/o increased these currents (Gravati et al. 2010). These data demonstrate that the coupling of OTR to specific G-proteins produce differential cellular changes depending on a variety of cellular contexts, and that OTR selectivity to specific G-proteins is physiologically relevant.
OTR coupling to Gq is most widespread because endogenous concentrations of OT are usually in the 1–5 nM range, but it is possible that endogenous OT concentrations vary across the brain. OT concentrations in the brain can be modulated by a number of neuronal features including the number of OT fiber projections across various regions, local axonal release from these fibers, and distance from passive diffusion of OT from dendrites of OT fibers (Grinevich et al. 2016). Moreover, OT concentrations can change in response to external stimuli. Some regions of the brain show up to 4-fold increases in basal OT concentrations in response to social stimuli (Zoicas et al. 2014), particularly in areas where levels of OTR expression are high (e.g., lateral septum, nucleus accumbens) (Ross et al. 2009), and this is an important mechanism by which OT modulates social behavior. Thus, the relationship between OT release in response to external stimuli and the heterogeneous distribution of OTRs across the brain are all key factors that can moderate specific cellular processes in response of OTR G-protein coupling.
The heterogeneous distribution in the brain of OTRs and G-proteins are an important mechanism by which OT system can finely tune cellular and behavioral responses. The distribution of OTRs in the brain varies across species that display differential patterns of social behavior (e.g., pair-living, biparental care, cooperation; (Freeman & Young 2016), and OTR distribution also differs between males and females of the same species (Dumais & Veenema 2016). Data on OTR expression in nonhuman primates is currently available for marmosets (Schorscher-Petcu et al. 2009), titi monkeys (Freeman et al. 2014), and macaques (Freeman et al. 2014). Marmosets show high OTR expression in the nucleus accumbens but not in the hippocampus. Conversely, titi monkeys show no OTR expression in the nucleus accumbens, but high OTR expression in the hippocampus (Freeman & Young 2016). Overall, OTR expression levels across broader taxa (i.e., primates and rodents) demonstrate two key phenomena. First, OTR expression is high for primates and rodents in areas involved in visual and olfactory processing respectively such as nucleus basalis of Meynert and superior colliculus in primates and olfactory bulb, lateral septum, and hippocampus in rodents (Freeman & Young 2016). Second, OTR expression is more variable in limbic structures like the nucleus accumbens and the bed nucleus of the stria terminalis (Freeman & Young 2016). The overlap between OT fibers and OTRs within the brain is also region-specific. In rodents, many regions that are innervated by OT neurons show prevalent OTR expression (Knobloch & Grinevich 2014; Knobloch et al. 2012), but this isn’t always the case. Regions including the preoptic area, olfactory bulbs, ventral pallidum, and the ventromedial hypothalamic nucleus have high OTR expression but do not receive direct OT projections (Grinevich et al. 2016). Overall, this variable OTR expression in limbic structures may underlie species- and experience-dependent OTR regulation of social behavior, while high OTR expression in sensory processing areas is likely a conserved phenomenon.
Our current knowledge regarding OTR signaling points to an important intersection between variability in OTR induced cellular responses and variability in OTR expression across the brain. This intersection is critical toward advancing our understanding of variability in OT-mediated physiological and behavioral responses to social stimuli across and within species. Like OTRs, many neurotransmitter receptor distributions in the brain are also variable both across and within species. Importantly, unlike the OT system, in nearly all neurotransmitter systems, the structure of the ligand is strictly conserved; yet we have shown the OT peptide has undergone structural changes many times in the recent evolutionary history of NWMs, despite its pivotal physiological functions. How changes in OT/OTR structure-function relationships facilitate or respond to unique environmental conditions or stimuli will provide new insights into OTR signaling at multiple levels of biological organization.
Do Changes in OT Structure Correspond to Changes in OTR Signaling?
Here, we highlight how evolutionary changes in the structure of OT ligands can subtly tune OTR function through a variety of pharmacological properties (Table 1). Pharmacological data are just now emerging from work both in our lab and others demonstrating that the specific OT variants Leu8-OT, Pro8-OT, and Val3-Pro8-OT, and the related nonapeptide AVP, show distinct functional fingerprints upon activating cells stably expressing OTRs. We will focus on how these variants affect four functional cellular outcomes including binding affinity, intracellular Ca2+ mobilization, G-protein functional selectivity, and receptor internalization, and highlight how these outcomes elucidate physiological mechanisms that underlie the OT regulation of social behavior (Table 1).
Table 1.
Description of potential functional pharmacological outcomes associated with OTR signaling
|
|
|
|
|
|
|
|
1) OT Binding Affinity at OTRs
The physiological responses induced by OT release in the brain generally require OT interaction with OTRs. Importantly, the likelihood of OT induced OTR activation can vary based on a variety of biochemical and biophysical properties associated with the ligand-receptor interaction. Changes in the structure of the OT ligand can potentially enhance or diminish the ligand’s ability to bind to the receptor and induce a cellular response. We currently know that both OT and AVP can bind to OTRs (Manning et al. 2012), but OT has a much higher affinity than AVP at least partially due to the differences in AVP structure and AVP’s higher polarity. Conversely, AVP and OT both bind to vasopressin receptors with AVP showing higher affinity (Manning et al. 2012).
Data are currently available for NWM OT ligand variants and binding affinity at human (hOTR) and marmoset OTRs (mOTR). Pro8-OT has a stronger binding affinity than Leu8-OT at the mOTR, where Pro8-OT is the native ligand, and Pro8-OT also has stronger affinity than Leu8-OT at the hOTR, where Pro8-OT is not the native ligand. [Taylor et al. unpubl data]. Additionally Pro8-OT and Leu8-OT both have stronger binding affinities than AVP at each of these OTRs, which is consistent with previous findings of binding affinities between Leu8-OT and AVP at the hOTR and rodent OTRs (Manning et al. 2012). Of the OTRs tested thus far, Pro8-OT shows a higher OTR binding affinity than Leu8-OT regardless of whether it is the species’ native ligand. Further work should explore whether this finding translates more broadly to other NWM OTRs and whether these differences persist at V1a and V1b receptors via OT crosstalk.
It is currently unknown whether Val3-Pro8-OT has a stronger or weaker affinity at hOTR and mOTRs. The structural changes of Val3-Pro8-OT may offer interesting insights into how conformational changes in the ligand can affect binding affinity at hOTRs and mOTRs given that the Val3-Pro8-OT variant has substitutions at both residue positions that differentiate mammalian OT from AVP. Thus it seems plausible that Val3-Pro8-OT may show a distinct binding affinity profile compared to the other ligands and future work should explore whether these changes result in enhanced or inhibited affinities to human and marmoset OTRs and AVP receptors.
2) OT Induced Ca2+ Mobilization
Gq-mediated Ca2+ cellular responses are an important second messenger system that is involved in numerous processes of signal transmission. Alterations in the efficacy or potency of Ca2+ mobilization in the cell and corresponding disruption of ion channel regulation via Ca2+ or K+ –dependent ion channels can have significant effects on physiological function. While OT ligand changes can alter OTR binding affinities, OT ligand changes can also alter corresponding OTR/G-protein coupling by enhancing or inhibiting the magnitude and selectivity of OTR/Gq-coupling and downstream Ca2+ mobilization. While binding affinity provides insight into the sensitivity of OTRs to respond to OT, the magnitude and selectivity of the intracellular processes must be examined to evaluate meaningful physiological changes associated with OT signaling.
Studies have begun to evaluate whether changes in the OT ligand produce changes in the magnitude/efficacy (i.e., maximal calcium mobilization response) and potency (i.e., OT concentration required to produce 50% of the maximal Ca2+ response) of the Ca2+ mobilization response upon activating OTRs. In HEK293T cell lines stably expressing hOTRs, Parreira-e-Silva et al. (2017) tested the efficacy and potency of Pro8-, Val3-Pro8-, and Leu8-OT at inducing Ca2+ mobilization responses. They found that all three ligands produced similar efficacy and potencies suggesting that all the OT ligands function as full agonists at hOTRs via the classic OTR activated Gq pathway. These findings are similar to that found by collaborative work in our lab exploring whether Leu8-OT and Pro8-OT produced differences in Ca2+ mobilization in CHO cell lines stably expressing hOTRs (Pierce et al. unpubl data). However, at mOTRs, there are some notable differences in Ca2+ mobilization responses, where Pro8-OT produced a larger maximal intracellular Ca2+ mobilization response compared to Leu8-OT, but the potency of this effect did not differ between OT ligands (Pierce et al. unpubl data) and matched measures of potencies at hOTRs (Busnelli et al. 2012; Parreira-e-Silva et al. 2017). These findings show that both Pro8-OT and Leu8-OT activation elicits similar patterns of Ca2+ mobilization triggered by OTR/Gq-coupling at hOTRs, while Pro8-OT activation induces a stronger Ca2+ response compared to Leu8-OT at mOTRs. Overall, Pro8-OT has a higher binding affinity and induces larger Gq-mediated Ca2+ mobilization responses compared to Leu8-OT in mOTRs. Given these changes are limited to mOTRs, these pharmacological responses to Pro8-OT could have important physiological consequences for underlying OT regulation of marmoset behavior.
3) Functional Selectivity of OT Ligands
OT and many synthetic OT analogues can activate specific G-proteins in a manner that suggests OTRs display levels of functional selectivity. For example, carbetocin, atosiban, D-Nal-OVT show distinct functional selectivity for specific forms OTR/G-protein coupling, which have been used as important research tools and have important therapeutic ramifications for targeted OT signaling (Busnelli et al. 2012; Busnelli et al. 2013). OT can produce a variety of outcomes related to sociality, as well as changes in other processes including pain modulation, appetite regulation, and reproduction. The variability in OT induced functional selectivity can serve as a source of diversity in how OT signaling events at OTRs enhance or inhibit many behavioral and physiological responses. Thus, demonstrating that OT variants show functional selectivity of OTR/G-protein coupling can identify potential mechanisms underlying coevolved behavioral processes in NWMs.
While OTRs predominately couple to Gq, OTRs also couple to Gi/o proteins, which results in reduced cellular cAMP production and also regulates Ca2+ and K+ dependent ion channels, potentially mediating cell growth, pro-inflammatory effects, and neuronal excitability of OT (Rimoldi et al. 2003; Zhou et al. 2007; Gravati et al. 2010; Busnelli et al. 2012;). The regulation of these ion channels is important for signal transmission and altering cellular membrane potentials that are critical components of neural transmission. This functional selectivity of activating Gi/o or Gq more or less favorably is referred to as ‘biased agonism’ (Kenakin 2007; Busnelli et al. 2012), and can have important cellular consequences based on the diversity of G-protein mediated responses.
OTR coupling to Gi/o can be measured in a number of ways. Coupling can be measured directly via measuring bioluminescence resonance energy transfer (BRET) between OTRs and G subunits (Busnelli et al. 2012; Parreira-e-Silva et al. 2017), or indirectly by measuring changes in membrane potential (e.g., cell hyperpolarization) following pharmacologic disruption of Gi/o coupling via pertussis toxin PTX (Pierce et al. unpubl data). Parreira-e-Silva et al. (2017) showed that OT ligand induced OTR coupling varies by the specific Gi subunit. Gi1 and Gi3 subunits show similar levels of hOTR coupling following OT activation regardless of the OT ligand. On the other hand Gi2 showed stronger coupling to hOTRs, but only when activated by Leu8-OT, suggesting that Leu8-OT shows a lower degree of functional selectivity at inducing G-coupling (Parreira-e-Silva et al. 2017). However, OT ligand induced Gi coupling was significantly lower than Gq coupling for all ligands, suggesting that OTRs mainly couple to Gq regardless of OTR activation by Leu8, Pro8, or Val3-Pro8-OT. Whether these results are similar in mOTRs have yet to be evaluated using BRET. However, recent work from our lab collaborators shows that when disrupting Gi/o coupling via PTX, mOTRs showed a significant reduction in efficacy of Leu8-OT-mediated hyperpolarization, while PTX treatment did not affect Pro8-OT-induced hyperpolarization. Similarly at hOTRs, PTX treatment reduced the efficacy of Leu8-OT-mediated but did not significantly affect Pro8-OT-mediated hyperpolarization (Pierce et al. unpubl data). Taken together, these data suggest that hyperpolarization responses following Pro8-OT activation of OTRs are almost completely Gq-mediated, and future work should explore potential downstream functional consequences of via functional selectivity of Leu8-OT and Pro8-OT at OTRs.
Differences in the ways that Pro8-OT and Leu8-OT induce Gi/o coupling may have important consequences on regulating Ca2+ and K+ dependent ion channels. Leu8-OT and Pro8-OT produced concentration-dependent decreases in hyperpolarization at both hOTRs and mOTRs, and Leu8-OT displayed greater potency than Pro8-OT with regard to changes in membrane potential (Pierce et al. unpubl data). Given that Gq and Gi/o coupling are important for regulating ion channels, future work should examine the degree to which these OT ligands directly regulate Ca2+ and K+ -dependent inward rectifying and intermediate conductance (IK) channels (Gravati et al. 2010) as a potential source for functional selectivity of OT activation via OTR/G-protein coupling.
4) OTR Desensitization and Internalization
Receptor desensitization and internalization are important processes that can greatly affect receptor sensitivity and responsiveness to long-term ligand stimulation. These processes are regulated by a number of G-protein receptor kinases (GRK) and arrestins (e.g., β-arrestin1 and 2), which prevent G-protein coupling with the receptor and signal receptor endocytosis. An important consideration for OTR function is the extent to which OT ligands show stronger or weaker efficacy or biases at activating GRKs and β-arrestin recruitment leading to receptor desensitization and internalization.
Thus far, one study has evaluated and demonstrated that OT ligands produce differential effects on OTR desensitization and internalization. Parreira-e-Silva et al. (2017) showed that Pro8-OT activation of OTRs resulted in a marked reduction in potency for both β-arrestin1 and β-arrestin2 recruitment compared to Leu8-OT in hOTRs, and Val3-Pro8-OT showed an even greater reduction in potency compared to Leu8- and Pro8-OT. Pro8-OT showed a significant reduction in β-arrestin1 recruitment efficacy, producing only 72% of the maximal response induced by Leu8-OT at β-arrestin1, but Pro8-OT showed no significant difference in β-arrestin2 recruitment efficacy compared to Leu8-OT at hOTRs. Val3-Pro8-OT was associated with a significant reduction in recruitment efficacy at both β-arrestin1 and 2 producing only 62% and 70% of the Leu8-OT response, respectively. Overall these results reveal that both Pro8-OT and Val3-Pro8-OT induce significantly diminished OTR desensitization compared to Leu8-OT at hOTRs, suggesting potential biases in OTR recruitment of β-arrestins. Notably, Val3-Pro8-OT, shares a similar pharmacological profile with AVP in regard to hOTR β-arrestin recruitment at OTRs.
Similarly to desensitization, Pro8-OT and Val3-Pro8-OT are both much weaker at inducing hOTR internalization (Parreira-e-Silva et al. 2017). Leu8-OT induced a robust and time-dependent loss of hOTRs from the plasma membrane (and thus a loss in OTR signaling), and Pro8-OT only moderately induced hOTR loss from the membrane (about half as potent). Val3-Pro8-OT induced no internalization of hOTRs at all. These findings show that Leu8-OT robustly desensitizes and internalizes hOTRs, Pro8-OT does so to a lesser extent, and Val3-Pro8-OT induces very little desensitization and no internalization. When activated by Pro8-OT or Val3-Pro8-OT, hOTRs are likely to remain more responsive to OT activation. These OT differences are quite remarkable, and further experiments examining desensitization, internalization, and the recruitment of other trafficking and recycling signalers may be a fruitful area for future investigations.
Summary of OT Ligands and OTR Pharmacology
OT ligands clearly produce a number of different functional consequences in OTR binding, signaling, and internalization. First, Pro8-OT appears to bind with higher affinity and, at least in mOTRs, induces stronger Ca2+ mobilization responses. Second, Pro8-OT appears to induce OTR coupling almost exclusively through Gq, while Leu8-OT shows less selective G-protein coupling. Certainly more work is needed to evaluate whether Pro8-OT shows true functional selectivity or ‘biased agonism’. Finally, Pro8-OT and Val3-Pro8-OT activation of hOTRs show lower affinities for β-arrestin recruitment, which likely results in more responsive OTR functioning. Taken together, these pharmacological data suggest mechanisms through which OT/OTR coevolution may lead to functional differences in physiological and behavioral outcomes. Much more work is needed to chart out the pharmacological cartography through which these ligands may directly modify signal transduction. Such approaches should include directly measuring neural function to evaluate whether the in vitro findings translate to native tissues including neuronal cultures and other neuronal cell contexts. Moreover, exploring pharmacological profiles in other primate species expressing Leu8-OT with similar behavioral repertoires (e.g., titi monkeys), or Pro8-OT expressing species with differing behavioral repertoires (e.g., capuchin or spider monkeys), and the functional consequences of OT crosstalk at V1a and V1b receptors (Bales et al. 2007; Gupta et al. 2008; Schorscher-Petcu et al. 2010; Song & Albers 2017) will advance our understanding of the physiological importance of these signaling responses in OTRs.
Finally, recent attention has shifted toward potential signaling impacts related to OTR homo- and heterodimerization with promising results. For instance, synthetic bivalent OT ligands have been shown to display super-potent properties (~1000 times greater potency) to activate OTR/Gq coupling compared to OT (Busnelli et al. 2016). This increased potency is believed to be the result of bivalent OT ligands specifically targeting OTR dimers. Additionally these bivalent OT ligands produce significant behavioral consequences exhibiting 100 times greater potency at promoting sociability in mice compared to endogenous OT and 40 times greater potency at promoting sociability in zebrafish compared to endogenous isotocin (Busnelli et al. 2016). OTRs also form heterodimers with other Class A GPCRs. One compelling example includes the formation of heterodimers between OTRs and Dopamine (DA) 2 receptors (D2Rs), which, in turn, exhibit reciprocal interactions (Romero-Fernandez et al. 2013; de la Mora et al. 2016). Specifically OT activation increases D2R affinity for DA and DA agonists, while DA activation of D2Rs enhances OT-induced signaling. With recently converging evidence for direct synergistic effects of OT neuronal release modulating DA neural activity and social behavior (Hung et al. 2017; Xiao et al. 2017), OTR/D2R heterodimers may represent an important physiological mechanism through which these behavioral outcomes can be modulated (De La Mora et al. 2016). Whether OT ligand variants enhance or inhibit the conformational changes necessary to facilitate or impair OTR homo- and heterodimerization is an important area for future investigation.
Do Changes in OT Structure Correspond to Changes in Social Behavior?
We have demonstrated there are considerable structural differences in OT ligands and OTRs in NWM. We have also demonstrated these OT ligands induce differences in the pharmacological properties of OTRs. The final arbiter of the importance of these structural and functional changes from the perspective of social behavior is the degree to which Pro8-OT ligand variants selectively modify behavioral and social processes in NWM. This taxonomic group represents a ‘hot-spot’ for OT ligand variation among placental mammals, and this group corresponds with a ‘hot-spot’ for many uncommon behavioral phenotypes among mammals including biparental care and social monogamy (Lukas & Clutton-Brock 2013; French et al. 2017). Currently, data examining the impact of exogenous manipulation with OT ligand variants on social behavior are available for marmoset monkeys (Callithrix spp.). The social and mating systems of marmosets have been characterized variously as socially monogamous, polyandrous, polygynous, and ‘flexible’ (Díaz-Muñoz & Bales 2016). Marmosets express many of the specific individual behavioral traits that are commonly associated with social monogamy (French et al. 2017). For example, adult male and female marmosets are successfully housed in long-term pair-living contexts, engage in high levels of affiliative behavior, and maintain proximity with pairmates, all of which are hallmarks of species that are traditionally viewed as socially monogamous (Agmo et al. 2012; Smith et al. 2010; Cavanaugh et al. 2014). Marmosets also live in extended family groups and exhibit cooperative breeding with both parents and siblings providing caregiving to offspring (Digby 1995; Snowdon 1996). Finally, marmosets display prosocial preferences including ‘altruistic’ food provisioning (Burkart et al. 2007; Mustoe et al. 2015). In this section, we evaluate whether OT ligand variants (Pro8-OT and Leu8-OT) influence behavioral outcomes across several components of sociality (mate-directed behavior, stranger-directed behavior, and parental behavior). To this end, we can determine whether specific behavioral components show 1) OT-specific effects where treatment with Pro8-OT and/or Leu8-OT produce different behavioral outcomes, 2) OT-generic effects where both OT ligand treatments have similar effects on behavioral outcomes, or 3) no OT effect where behavioral measures are not altered by OT treatment.
In our work on nonapeptide modulation of social behavior in marmosets, we have utilized intranasal administration of nonapeptides (Pro8-OT, Leu8-OT, and/or AVP), and oral administration of a non-peptide OTR antagonist (OTA: L368,899; Boccia et al. 2007). Subsequent to these treatments, social behavior in marmosets was recorded in a number of behavioral paradigms. First, we routinely record spontaneous social interactions that occur in normal home environments. The most common experimental paradigm is the partner preference test, in which an OT-treated marmoset can preferentially investigate and socially interact with pairmates or strangers. In a novel environment stressor, OT-treated marmosets were removed from their home enclosure and either separated with or isolated from their pairmate. This task allows for the investigation of social buffering (Smith & French 1997; Rukstalis & French 2005; Hennessy et al. 2009), a phenomenon in which stressors are experienced as less severe (measured in animals physiologically or behaviorally) when a social partner is present compared to when a social partner is absent. In a food-sharing task, treated marmosets were given sole access to food and permitted to allocate to others (e.g. mate, stranger, and offspring). This allows for the investigation of prosociality, altruism, inequity aversion, and offspring provisioning. In cases when marmosets were removed from their home environment and separated from their mates, we observed behavior during reunion with their mate in their home enclosure. Finally, in an infant interest test, treated marmosets were exposed to simulated infant stimuli and control stimuli. Much like in the partner preference test, these stimuli are separated into different compartments, so marmosets can investigate stimuli independently.
One can view how OT may influence social relationships through two distinct behavioral processes. The first mechanism involves OT enhancing strong social attachment relationships that are ‘endogenous’ to bonded marmoset pairs. The second mechanism is ‘exogenous’ to the pairs, and derives from OT promoting disinterest toward unfamiliar strangers of the opposite sex or by increasing aggressive exclusion of strangers by one or both members of the marmoset pair. We review the evidence for each of these mechanisms for maintaining social relationships among partners (Anzenberger 1985), first by discussing the impact of OT manipulations on mate-directed behavior, followed by an evaluation of these manipulations on stranger-directed behavior.
Mate-Directed Behavior
If OT is critical for regulating mate-directed behaviors, and Pro8-OT produces enhanced mOTR signaling, then we would expect that Pro8-OT would show greater enhancement of mate-directed affiliative behavior than Leu8-OT. It is clear that OT is involved in mate-directed behavior in marmosets because evidence from OTA treated marmosets show a reduction in mate-directed social approach in a variety of contexts, including reduced proximity during partner preference tests (Smith et al. 2010; Cavanaugh et al. 2014), reduced proximity during and after a novel environment stressor (Cavanaugh et al. 2016; Cavanaugh et al. unplubl data), and reduced food sharing behavior in the home cage (Smith et al. 2010); though OTA doesn’t always have this effect (Cavanaugh et al. 2014; Mustoe et al. 2015; Mustoe et al. 2016).
Studies examining marmoset partner-preference tests only showed modest effects of OT in the enhancement mate-directed behavior (Smith et al. 2010; Cavanaugh et al., 2014). With regard to OT ligand specificity, Cavanaugh et al. (2014) found that Pro8-OT enhanced the amount of time spent in close proximity with their mate during a partner-preference test but in females only, while Leu8-OT had no effect for both males and females. However, the vast majority of findings in marmosets suggest that both OT ligands produce little to no effects on mate-directed behaviors including social approach, grooming, or huddling behaviors (all affiliative behaviors associated with pair-bond maintenance) (Cavanaugh et al. 2014; Cavanaugh et al. 2015); cooperative food sharing in a experimental prosocial food-sharing tasks (Mustoe et al. 2015; Mustoe et al. 2016); and affiliative behaviors (time in proximity, grooming, huddling) upon reunion following a long-term social separation (Cavanaugh et al. unpubl data). While it would seem most likely that Pro8-OT treatments would produce larger effects on mate-directed behavior than Leu8-OT in marmosets, the data suggest there are minimal OT-specific effects. Furthermore, OT in general (either ligand) does not appear to strongly enhance mate-directed behaviors in marmosets, but OT does affect marmoset social behavior in a variety of other important ways.
One way in which OT does produce behavioral changes between marmoset mates is inducing changes in how untreated marmosets interact with their OT-treated mates. Individuals who are treated with OT received increased affiliative behavior from their mates (Cavanaugh et al. 2015). In some cases, the effects of OT are similar for both ligands. For instance, females treated with either Pro8-OT or Leu8-OT received more grooming from their pairmates. Males treated with either Pro8-OT or Leu8-OT are approached by their mates more frequently (Cavanaugh et al. 2015), suggesting these are OT-general effects. In other cases, the effect of OT appears to be specific to Pro8-OT, where Pro8-OT treated individuals received increased duration of gaze from their mate (Cavanaugh et al. unpubl data). Overall, OT treatments may be subtly changing some characteristic(s) in marmosets that make them more likely to receive increased affiliation from their mate, and these OT changes appear to be both OT-specific and OT-general effects depending on the behavior.
Stranger-Directed Behavior
Our data reveal that OT manipulations produce more robust behavioral changes with regard to interactions with opposite-sex strangers compared to interactions with their pairmates. Marmosets gregariously interact with strangers when given the opportunity (Cavanaugh et al. 2014; Mustoe et al. 2015; Smith et al. 2010). Activation of the OT system significantly reduces these stranger-directed behaviors, and these effects are specific to Pro8-OT. Pro8-OT treated marmosets spent less time with opposite sex strangers in the partner preference test, and were slower to engage in sexual solicitation displays with strangers, but these measures were unaffected by Leu8-OT treatments (Cavanaugh et al. 2014). Similarly, untreated marmosets preferentially donated food rewards to strangers vs. pairmates in a prosocial food-sharing task (Mustoe et al. 2015). Marmosets treated with Pro8-OT showed a significant reduction in stranger-directed food sharing, but Leu8-OT treatment did not alter stranger-directed food sharing. In two experimental contexts, then, Pro8-OT reduces stranger-directed behavior while Leu8-OT had no effect, which supports the view that there is greater OT-specificity for stranger-directed behavior than mate-directed behavior.
Parental Behavior
Experimental treatment with OT alters several components of parental behavior in marmosets. Saito and Nakamura (2011) examined the rates fathers shared food with young offspring under two conditions: intracerebroventricular administration of Leu8-OT or saline treatment. Leu8-OT treatment lead to greater transfer of food from fathers to offspring, by decreasing the rates of food sharing refusals (Saito & Nakamura 2011). In contrast, our lab showed that Pro8-OT treated male marmosets were less likely to engage in alloparental care via sharing food with their younger siblings (Taylor et al. 2017). Importantly, many contextual and procedural features between these studies differed, making a direct comparison between the opposing Leu8- and Pro8-OT effects difficult. A recent study addresses whether OT variants differentially affect parental behavior utilizing a rodent model (a Leu8-OT species) (Parreiras-e-Silva et al. 2017). Administration of NWM OT ligand variants (Pro8-OT and Val3-Pro8-OT) to adult male and female rats (intranasally) produced differential effects on caregiving behavior toward pups. Female rats treated with Val3-Pro8-OT and Pro8-OT they were faster to retrieve separated pups compared to females treated with Leu8-OT or saline. Male rats, not normally parental, retrieved pups more rapidly when treated with any OT ligand (Pro8-OT, Leu8-OT or Val3Pro8-OT) compared to saline (Parreiras-e-Silva et al. 2017). This indicates that even in a Leu8-OT rodent species, Pro8-OT is as effective or more effective (in females at least) at enhancing parental behavior as Leu8-OT. As is the case with mate- and stranger-directed behavior, Pro8-OT tends to produce stronger behavioral effects.
Data comparing differences in parental behavior following either Pro8-OT or AVP treatments are also of interest due to the potential for OT and AVP cross-activation at OTRs. Taylor and French (2015) measured responses of adult male and female marmosets to acoustic and visual stimuli associated with infants. Both Pro8-OT and AVP treatments enhanced responsiveness to infant stimuli (reduced latency to approach and increased investigation of infant stimuli), but the effects were sex-specific. Pro8-OT enhanced responsiveness to infant stimuli in males, and AVP enhanced responsiveness to infant stimuli in females. Pro8-OT and AVP do not produce the same effects on responses to infant and juvenile stimuli (Taylor & French 2015).
Summary of Pro8-OT and Leu8-OT Effects on Social Behavior
Treatment effects that are mixed and/or context dependent make summarizing the collection of results from these experiments challenging but useful. As such we have taken a “bird’s eye view” of the published work comparing Pro8-OT, Leu8-OT, OTA, and AVP, and a summary is shown in Table 2. Here we highlight a few patterns that are apparent. First, OTRs must be involved in augmenting social behavior, since blocking the OTR with an antagonist reduces measures of sociality across a wide variety of experimental contexts. Second, when Pro8-OT treatments produce an effect on behavior it is almost always in the expected direction; Pro8-OT enhances mate-directed or mate-received behavior and reduces stranger-directed behavior. Two notable exceptions are the findings that young male marmosets treated with Pro8-OT reduce food sharing directed toward siblings (Taylor et al. 2017), and adult marmosets treated with Pro8-OT increased time spent alone and reduced time spent with the mate (Cavanaugh et al. 2014). Third, Leu8-OT treatments produced mixed effects on whether social behavior was enhanced or impaired, and Leu8-OT never enhanced a social behavior that Pro8-OT did not also enhance. Differences between Pro8-OT and Leu8-OT across behavioral contexts may be explained by the observations that Leu8-OT functions as a partial agonist with lower efficacy than Pro8-OT for mOTRs, and that Leu8-OT leads to increased OTR internalization (Pierce et al. unpubl data; Parreiras-e-Silva et al. 2017), assuming full activation of OTRs are necessary to induce these behaviors. It is also possible that the lack of effects or the mixed results may be due to inconsistent and poor penetrance of OT into brain regions with receptors following intranasal delivery (Leng & Ludwig 2016) or potential differences in Pro8-OT penetrance. Finally, the behaviors that consistently appear most sensitive to OT treatments (stranger-directed behaviors) also show the greatest degree of Pro8-OT specificity.
Table 2.
Oxytocin ligand variants and social behavior in marmosets
| Behavior | Details | Pro8-OT | Leu8-OT | AVP | OTA |
|---|---|---|---|---|---|
| Social Approach | |||||
| Partner preference test (3 weeks cohabitation)a | -↑ | ↓ | |||
| Proximity (undisturbed)c | - | - | - | ||
| Partner preference Test (8+ weeks cohabitation)b | ↑F,↓M | - | - | ||
| Proximity during novel environment stressorf | ↑ | - | ↓ | ||
| Proximity during reunioni | - | - | ↓ | ||
| Responsiveness to infant Stimulie | ↑M,-F | -M,↑F | - | ||
| Social Attention | |||||
| Grooming(undisturbed)c | - | - | - | ||
| Huddling(undisturbed)c | - | - | - | ||
| Solicit grooming(undisturbed, reunion)c,i | - | - | - | ||
| Gazei | - | - | - | ||
| Social Attractiveness | |||||
| Received Grooming (undisturbed)c | ↑F,-M | ↑F,-M | - | ||
| Received Grooming (reunion)i | - | ↓- | ↓- | ||
| Approached by matei | - | - | - | ||
| Received gazei | ↑ | ↓- | ↓ | ||
| Food Sharing | |||||
| Mate-Directed prosocial food sharing taskd,g | - | - | - | ||
| Mate-Directed food sharing (undisturbed)a | - | ↓ | |||
| Sibling directed food sharingh | ↓M | ||||
| Offspring directed food sharingh,j | ↑M | ↓M | |||
| Stranger-Directed Behavior | |||||
| Stranger-Directed prosocial food sharing taskd,g | ↓- | - | ↓- | ||
| Sexual solicitationb | ↓ | - | - | ||
| Gazei | - | - | - | ||
| Anxiety &Stress | |||||
| Social Buffering of stress responsef | - | - | - | ||
| Cortisol reactivityf | - | - | ↑ | ||
| Contact Callinge,f | - | - | - | - | |
| Locomotione,f | - | - | - | - | |
| Aggressive Behavior | |||||
| Scent Markingc,f | - | - | - | ||
| Infant-directed aggressive vocalizationh | - | - | ↑ | ||
Note. ↑ indicates that behavior was increased after treatment, ↓ indicates that behavior was decreased after treatment, - indicates no effect of treatment. Results noted with ↑- or ↓- indicates at least one significant treatment effect for that behavior and one no effect. F indicates effect was present in females, M indicates effect was present in males. Parenthetical “(undisturbed)” indicates observations were conducted in home cage without any other behavioral tasks.
Superscripts indicate references: aSmith, et al. 2010;
Cavanaugh, et al unpubl data;
Concluding Remarks
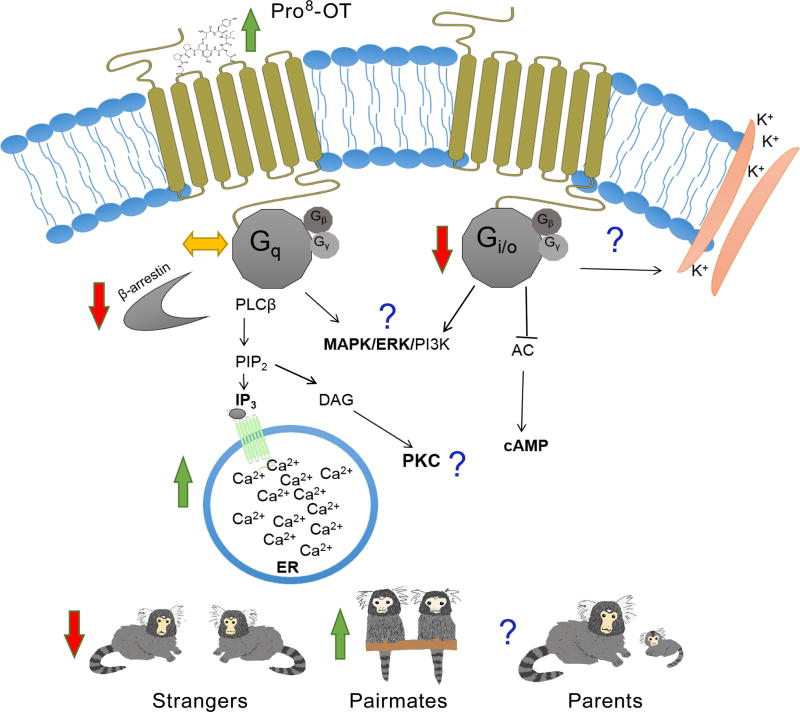
It is clear that OT is among one of the most pervasive signaling systems modulating physiology and social behavior in mammals, and changes to this signaling molecule are likely to result in meaningful and targeted consequences. Pro8-OT as a signaling molecule at OTRs shows increased binding affinity, produces increased intracellular Ca2+ mobilization, and induces reduced receptor internalization compared to the ancestral mammalian Leu8-OT molecule. Importantly, Pro8-OT also elicits specific behavioral changes in pair-bonded marmosets in some contexts, especially with regard to reducing social interest directed toward opposite-sex strangers. Together, these findings demonstrate that the unique OTR pharmacological properties associated with activation by the Pro8-OT ligand result in potentially important functional consequences (Fig 3). The extent to which these OT structural changes modify neuronal processes, enhance or diminish OT/OTR interactions with other neuronal systems, or possess targeted therapeutic potential has yet to be thoroughly explored. However, our observations that naturally occurring modifications in the OT/OTR system among NWMs lead to significant functional consequences provides an intriguing ‘natural experiment’ to explore OT under a new lens. Our approach should be viewed as complementary to efforts that explore laboratory-induced changes in the OT molecule as a means to discover super potent and efficacious synthetic ligands as potential candidates for clinically relevant endpoints (e.g., Busnelli et al. 2016; Muttenthaler et al. 2017). Overall, these findings from OT pharmacology to OT-induced behavioral changes clearly demonstrate that OT/OTR structural modifications in NWM that arise from natural selection can orchestrate important changes in OT-mediated physiological and behavioral outcomes.
Figure 3.
A schematic summary of this review of the main findings based on OTR functional pharmacology outcomes such as receptor binding and G-protein signaling, and social behavior outcomes (in marmosets) following activation of Pro8-OT. Arrows indicate direction of effect compared to Leu8-OT induced activation of OTRs at h- and mOTRs. “?” represent findings that have yet to be determined or evaluated. The pharmacological outcomes associated with OT activation are known to be involved in neuronal membrane/cell excitability (Gong et al. 2015), long-term potentiation (Lin et al. 2012), and regulating many facets of social behavior (Blume et al. 2008; Jurek et al. 2012; Wang et al. 2015). For a full representation of the OT signaling network see Chatterjee et al. 2016.
Acknowledgments
The authors thank Dongren Ren, Thomas Murray, Marsha Pierce, and Myron Toews for colleagueship and collaboration on the research findings reviewed in this contribution, and for future directions in our work on nonapeptide ligand/receptor variation. The work reported here, and the preparation of this manuscript, are supported in part by funds from the NIH (HD042882, HD089147).
References
- Acher R, Chauvet J, Chauvet MT. Man and the chimaera. Selective versus neutral oxytocin evolution. Advances in Experimental Medicine and Biology. 1995;395:615–27. [PubMed] [Google Scholar]
- Agmo A, Smith AS, Birnie AK, French JA. Behavioral characteristics of pair bonding in the black tufted-ear marmoset (Callithrix penicillata) Behaviour. 2012;149:3–4. doi: 10.1163/156853912X638454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Hormones and Behavior. 2012;6:283–92. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Anzenberger G. How stranger encounters of common marmosets (Callithrix jacchus jacchus) are influenced by family members: the quality of behavior. Folia Primatologica. 1985;45:204–24. [Google Scholar]
- Arnold C, Matthews LJ, Nunn CL. The 10kTrees website: a new online resource for primate phylogeny. Evolutionary Anthropology: Issues, News, and Reviews. 2010;19:114–18. [Google Scholar]
- Arrowsmith S, Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. Journal of Neuroendocrinology. 2014;26:356–69. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- Bales KL, del Razo RA, Conklin QA, et al. Focus: Comparative medicine: Titi monkeys as a novel non-human primate model for the neurobiology of pair bonding. The Yale journal of Biology and Medicine. 2017;90:373. [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, et al. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beets I, Temmerman L, Janssen T, Schoofs L. Worm. Taylor & Francis: 2013. Ancient neuromodulation by vasopressin/oxytocin-related peptides; p. e24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, et al. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. European Journal of Neuroscience. 2008;27:1947–56. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Goursaud APS, Bachevalier J, et al. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non-human primates. Hormones and Behavior. 2007;52:344–51. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart JM, Fehr E, Efferson C, van Schaik CP. Other-regarding preferences in a non-human primate: Common marmosets provision food altruistically. Proceedings of the National Academy of Sciences. 2007;104:19762–6. doi: 10.1073/pnas.0710310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnelli M, Chini B. Molecular basis of oxytocin receptor signalling in the brain: What we know and what we need to wnow. Berlin, Heidelberg: Springer Berlin Heidelberg; 2017. [DOI] [PubMed] [Google Scholar]
- Busnelli M, Kleinau G, Muttenthaler M, et al. Design and characterization of superpotent bivalent ligands targeting oxytocin receptor dimers via a channel-like structure. Journal of Medicinal Chemistry. 2016;59:7152–66. doi: 10.1021/acs.jmedchem.6b00564. [DOI] [PubMed] [Google Scholar]
- Busnelli M, Sauliere A, Manning M, et al. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. Journal of Biological Chemistry. 2012;287:3617–29. doi: 10.1074/jbc.M111.277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK. Oxytocin and vasopressin: powerful regulators of social behavior. The Neuroscientist. 2017 doi: 10.1177/1073858417708284. 1073858417708284. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS. Vasopressin: Behavioral roles of an “original” neuropeptide. Progress in Neurobiology. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Young WS. Handbook of neurochemistry and molecular neurobiology. Springer; 2006. Oxytocin and vasopressin: genetics and behavioral implications; pp. 573–607. [Google Scholar]
- Cavanaugh J, Carp SB, Rock CM, French JA. Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology. 2016;66:22–30. doi: 10.1016/j.psyneuen.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Huffman MC, Harnisch AM, French JA. Marmosets treated with oxytocin are more socially attractive to their long-term mate. Frontiers in Behavioral Neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology. 2014;49:1–10. doi: 10.1016/j.psyneuen.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee O, Patil K, Sahu A, et al. An overview of the oxytocin-oxytocin receptor signaling network. Journal of Cell Communication and Signaling. 2016;10:355–360. doi: 10.1007/s12079-016-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Mouillac B, Balestre MN, et al. Two aromatic residues regulate the response of the human oxytocin receptor to the partial agonist arginine vasopressin. FEBS Letters. 1996;397:201–6. doi: 10.1016/s0014-5793(96)01135-0. [DOI] [PubMed] [Google Scholar]
- Crowley WR. Neuroendocrine regulation of lactation and milk production. Comprehensive Physiology. 2015;5:255–91. doi: 10.1002/cphy.c140029. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz SL, Bales KL. “Monogamy” in primates: Variability, trends, and synthesis: Introduction to special issue on primate monogamy. American Journal of Primatology. 2016;78:283–7. doi: 10.1002/ajp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby LJ. Social organization in a wild population ofCallithrix jacchus: II. Intragroup social behavior. Primates. 1995;36:361–75. [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Frontiers in Neuroendocrinology. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. The neurobiology of human attachments. Trends in Cognitive Sciences. 2017;21:80–99. doi: 10.1016/j.tics.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, et al. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–41. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, et al. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. Journal of Neuroendocrinology. 2016;28 doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Cavanaugh J, Mustoe AC, Carp SB, Womack S. Social monogamy in nonhuman primates: Phylogeny, phenotype, and physiology. The Journal of Sex Research. 2017;13:1–25. doi: 10.1080/00224499.2017.1339774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Taylor JH, Mustoe AC, Cavanaugh J. Neuropeptide diversity and the regulation of social behavior in New World primates. Frontiers in Neuroendocrinology. 2016;42:18–39. doi: 10.1016/j.yfrne.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler I, Chmielewski J. Cationic amphiphilic polyproline helices: Side-Chain variations and cell-specific internalization. Chemical Biology & Drug Design. 2009;73:39–45. doi: 10.1111/j.1747-0285.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological Reviews. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gupta J, Russell RJ, Wayman CP, Hurley D, Jackson VM. Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. British Journal of Pharmacology. 2008;155:118–26. doi: 10.1038/bjp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Gao F, Li J, et al. Oxytocin-induced membrane hyperpolarization in pain-sensitive dorsal root ganglia neurons mediated by Ca2+/nNOS/NO/K ATP pathway. Neuroscience. 2015;289:417–28. doi: 10.1016/j.neuroscience.2014.12.058. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology. 2013;38:465–78. doi: 10.1016/j.psyneuen.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Gravati M, Busnelli M, Bulgheroni E, et al. Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. Journal of Neurochemistry. 2010;114:1424–35. doi: 10.1111/j.1471-4159.2010.06861.x. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, et al. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biological Psychiatry. 2016;79:155–64. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Gruber CW. Physiology of invertebrate oxytocin and vasopressin neuropeptides. Experimental Physiology. 2014;99:55–61. doi: 10.1113/expphysiol.2013.072561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CW, Koehbach J, Muttenthaler M. Exploring bioactive peptides from natural sources for oxytocin and vasopressin drug discovery. Future. 2012;4:1791–98. doi: 10.4155/fmc.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes JE, Peres CA. Ecological correlates of trophic status and frugivory in neotropical primates. Oikos. 2014;123:365–77. [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30:470–82. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357:1406–11. doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–79. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Maloumby R, et al. Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. Plos One. 2012;7:e37060. doi: 10.1371/journal.pone.0037060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Social functions of individual vasopressin–oxytocin cell groups in vertebrates: What do we really know? Frontiers in Neuroendocrinology. 2014;35:512–29. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehbach J, Stockner T, Bergmayr C, et al. Insights into the molecular evolution of oxytocin receptor ligand binding. Biochemical Society Transactions. 2013;41:197–204. doi: 10.1042/BST20120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC, et al. A novel form of oxytocin in New World monkeys. Biology Letters. 2011;7:584–7. doi: 10.1098/rsbl.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biological Psychiatry. 2016;79:243–50. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. Social bonding: regulation by neuropeptides. Frontiers in Neuroscience. 2014;8 doi: 10.3389/fnins.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Huang CC, Hsu KS. Oxytocin promotes long-term potentiation by enhancing epidermal growth factor receptor-mediated local translation of protein kinase Mζ. Journal of Neuroscience. 2012;32:15476–488. doi: 10.1523/JNEUROSCI.2429-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature Reviews Neuroscience. 2006;7:126–36. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341:526–30. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology. 2012;24:609–28. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley S, Martin B, Luttrell LM. The origins of diversity and specificity in G protein-coupled receptor signaling. Journal of Pharmacology and Experimental Therapeutics. 2005;314:485–494. doi: 10.1124/jpet.105.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Pérez-Carrera D, Crespo-Ramírez M, et al. Signaling in dopamine D2 receptor-oxytocin receptor heterocomplexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the rat. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2016;1862:2075–85. doi: 10.1016/j.bbadis.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Mustoe AC, Cavanaugh J, Harnisch AM, Thompson BE, French JA. Do marmosets care to share? Oxytocin treatment reduces prosocial behavior toward strangers. Hormones and Behavior. 2015;71:83–90. doi: 10.1016/j.yhbeh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe AC, Harnisch AM, Hochfelder B, Cavanaugh J, French JA. Inequity aversion strategies between marmosets are influenced by partner familiarity and sex but not by oxytocin. Animal Behaviour. 2016;115:69–79. doi: 10.1016/j.anbehav.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muttenthaler M, Andersson A, Vetter I, et al. Subtle modifications to oxytocin produce ligands that retain potency and improved selectivity across species. Science Signaling. 2017;10 doi: 10.1126/scisignal.aan3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother–infant bonding and pair bonding: similarities, differences, and broader implications. Hormones and Behavior. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreiras-e-Silva LT, Vargas-Pinilla P, Duarte DA, et al. Functional New World monkey oxytocin forms elicit an altered signaling profile and promotes parental care in rats. Proceedings of the National Academy of Sciences. 2017;114:9044–49. doi: 10.1073/pnas.1711687114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelman P, Johnson WE, Roos C, et al. A molecular phylogeny of living primates. Plos Genetics. 2011;7:e1001342. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Chin KR, French JA. Molecular variation in AVP and AVPR1a in New World Monkeys (Primates, Platyrrhini): Evolution and implications for social monogamy. Plos One. 2014;9:e111638. doi: 10.1371/journal.pone.0111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Lu G, Moriyama H, et al. Genetic diversity in oxytocin ligands and receptors in New World Monkeys. Plos One. 2015;10:e0125775. doi: 10.1371/journal.pone.0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–76. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi V, Reversi A, Taverna E, et al. Oxytocin receptor elicits different EGFR/MAPK activation patterns depending on its localization in caveolin-1 enriched domains. Oncogene. 2003;22:6054. doi: 10.1038/sj.onc.1206612. [DOI] [PubMed] [Google Scholar]
- Romero-Fernandez W, Borroto-Escuela DO, Agnati LF, Fuxe K. Evidence for the existence of dopamine d2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor–receptor interactions. Molecular Psychiatry. 2013;18:849–50. doi: 10.1038/mp.2012.103. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, et al. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Hormones and Behavior. 2005;47:1–7. doi: 10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Nakamura K. Oxytocin changes primate paternal tolerance to offspring in food transfer. Journal of Comparative Physiology A. 2011;197:329–37. doi: 10.1007/s00359-010-0617-2. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupré A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neuroscience Letters. 2009;461:217–22. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. The Journal of Neuroscience. 2010;30:8274–84. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–8. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57:255–62. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiology & Behavior. 1997;62:225–32. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. Infant care in cooperatively breeding species. Advances in the Study of Behavior. 1996;25:643–89. [Google Scholar]
- Song Z, Albers HE. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Frontiers in Neuroendocrinology. 2017 doi: 10.1016/j.yfrne.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Larkin TE, Albers HE. Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus) Hormones and Behavior. 2016;81:20–7. doi: 10.1016/j.yhbeh.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–59. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Current Opinion in Neurobiology. 2014;29:187–93. doi: 10.1016/j.conb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Taylor JH, French JA. Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Hormones and Behavior. 2015;75:154–59. doi: 10.1016/j.yhbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Intorre AA, French JA. Vasopressin and oxytocin reduce food sharing behavior in male, but not female marmosets in family groups. Frontiers in Endocrinology. 2017;81 doi: 10.3389/fendo.2017.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan R, Hammock EA. Oxytocin receptor dynamics in the brain across development and species. Developmental Neurobiology. 2017;77:143–57. doi: 10.1002/dneu.22403. [DOI] [PubMed] [Google Scholar]
- Vargas-Pinilla P, Paixão-Côrtes VR, Paré P, et al. Evolutionary pattern in the OXT-OXTR system in primates: Coevolution and positive selection footprints. Proceedings of the National Academy of Sciences. 2015;112:88–93. doi: 10.1073/pnas.1419399112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis M. Molecular evolution of the neurohypophysial hormone precursors in mammals: Comparative genomics reveals novel mammalian oxytocin and vasopressin analogues. General and Comparative Endocrinology. 2012;179:313–18. doi: 10.1016/j.ygcen.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao S, Wu Z, Feng Y, Zhao C, Zhang C. Oxytocin in the regulation of social behaviours in medial amygdala-lesioned mice via the inhibition of the extracellular signal-regulated kinase signalling pathway. Clinical and Experimental Pharmacology and Physiology. 2015;42:465–74. doi: 10.1111/1440-1681.12378. [DOI] [PubMed] [Google Scholar]
- Wesley VJ, Hawtin SR, Howard HC, Wheatley M. Agonist-specific, high-affinity binding epitopes are contributed by an arginine in the N-terminus of the human oxytocin receptor. Biochemistry. 2002;41:5086–92. doi: 10.1021/bi015990v. [DOI] [PubMed] [Google Scholar]
- Wyttenbach T, Liu D, Bowers MT. Interactions of the hormone oxytocin with divalent metal ions. Journal of the American Chemical Society. 2008;130:5993–6000. doi: 10.1021/ja8002342. [DOI] [PubMed] [Google Scholar]
- Xiao L, Priest MF, Nasenbeny J, et al. Biased oxytocinergic modulation of midbrain dopamine systems. Neuron. 2017;95:368–84. doi: 10.1016/j.neuron.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Rates of conservative and radical nonsynonymous nucleotide substitutions in mammalian nuclear genes. Journal of molecular evolution. 2000;50(1):56–68. doi: 10.1007/s002399910007. [DOI] [PubMed] [Google Scholar]
- Zingg HH, Laporte SA. The oxytocin receptor. Trends in Endocrinology & Metabolism. 2003;14:222–7. doi: 10.1016/s1043-2760(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology. 2014;39:3027–35. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]