Abstract
Background
Molecular imaging of carotid plaque vulnerability to atheroembolic events is likely to lead to improvements in patient selection for carotid endarterectomy (CEA). The aims of this study were to assess the relative value of endothelial inflammatory markers for this application and develop molecular ultrasound contrast agents for their imaging.
Methods
Human CEA specimens were obtained prospectively from asymptomatic (30) and symptomatic (30) patients. Plaques were assessed by semi-quantitative immunohistochemistry (IHC) for vascular cell adhesion molecule-1 (VCAM-1), lectin-like oxidized LDL receptor-1 (LOX-1), P-selectin and von Willebrand Factor (vWF). Established small peptide ligands to each of these targets were then synthesized and covalently conjugated to the surface of lipid-shelled microbubble ultrasound contrast agents, which were then evaluated in a flow chamber for binding kinetics to activated human aortic endothelial cells (HAECs) under variable shear conditions.
Results
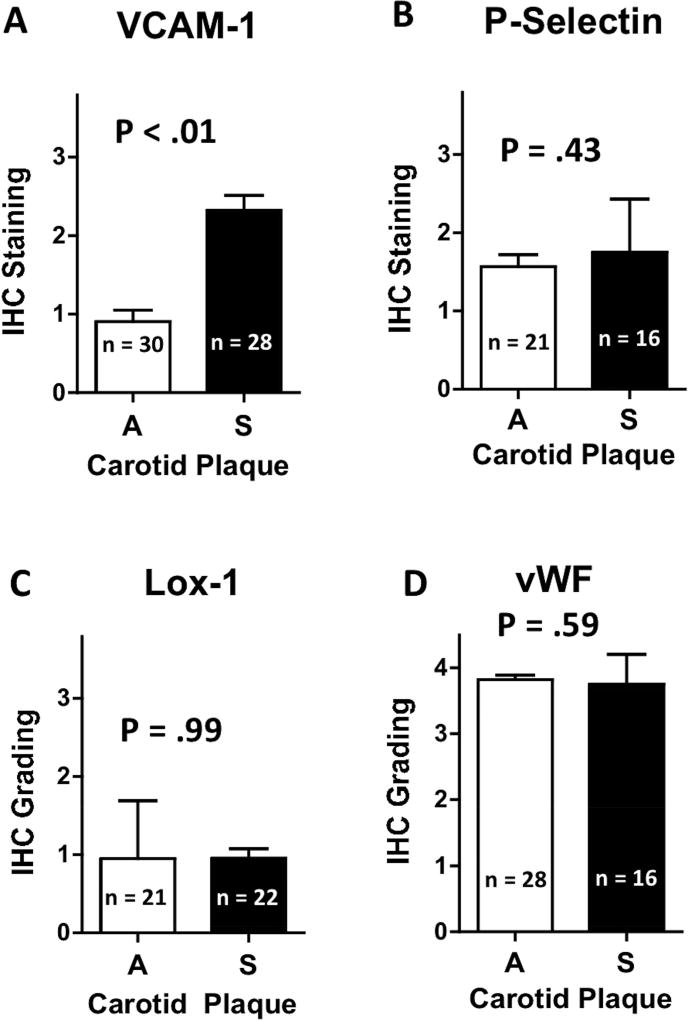
Expression of VCAM-1 on the endothelium of CEA specimens from symptomatic patients was 2.4-fold greater than that from asymptomatic patients (P < .01). Expression was not significantly different between groups for P-selectin (P = .43), vWF (P = .59) or LOX-1 (P = .99). Although most plaques from asymptomatic patients displayed low VCAM-1 expression, approximately 1 in 5 expressed high VCAM-1 similar to those of plaques from symptomatic patients. In vitro flow chamber experiments demonstrated that VCAM-1 targeted microbubbles bind cells that express VCAM-1, even under high-shear conditions that approximate those found in human carotid arteries, whereas binding efficiency was lower for the other agents.
Conclusion
VCAM-1 displays significantly higher expression on high-risk (symptomatic) vs. low-risk (asymptomatic) carotid plaques. Ultrasound contrast agents bearing ligands for VCAM-1 can sustain high-shear attachment and may be useful for identifying patients in whom more aggressive treatment is warranted.
Keywords: carotid plaque, carotid endarterectomy, stoke, atherosclerosis, inflammation, VCAM-1, ultrasound contrast agent, molecular medicine
INTRODUCTION
Stroke is the leading cause of disability in the United States and the fourth leading cause of death. Approximately 20–25% of strokes are attributable to carotid atherosclerotic disease.1 Although carotid endarterectomy (CEA) reduces stroke rate, its benefit is substantially less in asymptomatic patients compared to symptomatic patients.2–5 Treatment of patients with carotid atherosclerosis is limited by our ability to define individualized stroke risks based on specific patient or carotid plaque characteristics. Thus, the number needed to treat to reduce 1 stroke over 5 years is 20.2,5
There is increasing interest in using non-invasive imaging to better identify which asymptomatic patients with carotid disease may benefit from surgical therapy. One promising strategy is to apply technologies for imaging that reveal the molecular or cellular profile of carotid plaques. Pathologic processes that have been associated with rapid plaque progression and susceptibility to atheroembolic events include plaque inflammatory burden, oxidative stress, protease activity, neovascularization, and loss of normal anti-platelet functions.6,7 Contrast-enhanced ultrasound (CEU) is a promising technique for molecular imaging of plaque phenotype based on practical issues of cost, speed, and convenience.8,9 However, CEU relies on imaging targeted microbubbles that are confined within the vascular space and cannot reveal interior plaque components.
The aims of this study were to identify useful endothelial biomarkers that can differentiate plaque stability and to test clinically-feasible microbubble targeting strategies for CEU. To accomplish our aims, specific immunohistochemistry of human CEA specimens were compared from symptomatic versus asymptomatic subjects. Microbubbles targeted to candidate markers were prepared using small molecular targeting moieties and surface conjugation strategies that are feasible for human use. Attachment efficiency was assessed by in vitro flow chamber experiments with cultured activated endothelial cells.
MATERIALS AND METHODS
Carotid Endarterectomy Specimens
Studies were approved by the Investigational Review Board of the University of Arizona. CEA specimens from 30 consecutive asymptomatic and 30 consecutive symptomatic patients treated for carotid atherosclerotic disease between 2014–2017 were evaluated. We considered symptomatic patients those with transient ischemic attacks (TIAs) or strokes with motor or speech symptoms that were lateralizing to the contralateral side of the carotid lesion or amarousis fugax on the ipsilateral side within 6 months of surgery; of note, all patients in this study had surgery within 4 weeks of symptoms. Patients operated on for atypical “symptoms” were excluded from the study. Patients who had no relevant symptoms within 6 months of surgery were considered asymptomatic. Specimens were fixed in formalin for 24 hours, transferred to 70% EtOH and decalcified (ImmunoCal™). Plaques were partitioned lengthwise into ~5 mm segments and embedded in paraffin on end to obtain cross-sections sampled throughout the length of the plaque. Four partitions were embedded per block requiring 1–2 blocks per plaque. Sections were cut to 4 µm thickness.
Immunohistochemistry
CEA sections were rehydrated with graded ethanol mixtures and antigen retrieval was performed using a borate buffer for 60 minutes. Immunohistochemistry (IHC) was performed in uniform fashion using the automated high-throughput Ventana Medical Stainer (VMS™) with monoclonal or polyclonal antibodies (Abcam) against human VCAM-1 (ab106777), vWF (ab6994), LOX-1 (ab60178) and P-selectin (ab135792). Staining was optimized using dilutions of 1:50, 1:100 and 1:200 in a TRIS-based diluent (Ventana Medical Systems). After antigen retrieval using CC1 borate buffer at 100° C for 64 minutes (Ventana Medical Systems), the primary antibodies were added and the samples incubated for 60 minutes and then washed. The same antigen retrieval strategy was used for all samples. Peroxidase staining was performed using a diaminobenzadine detection kit (OptiView DAB kit, Ventana Medical Systems) that is an indirect, biotin-free system for detecting mouse IgG, mouse IgM and rabbit primary antibodies. Detection was performed using a HQ universal linker and a horseradish peroxidase multimer. Hematoxylin II and bluing reagent was used for 4 minutes each for counter staining. Samples were processed in 1–3 total groups (20–30 samples per group) to optimize standardization with asymptomatic and symptomatic plaques processed as specimens were obtained. Each staining batch had positive control tissue stained simultaneously (human lymph node for VCAM-1 and P-selectin and normal human aorta for vWF and LOX-1). Optimization protocols resulted in the use of dilutions of 1:150, 1:100, 1:50 and 1:100; for VCAM-1, vWF, LOX-1 and P-selectin, respectively. IHC staining was evaluated by two independent physicians in a blinded fashion using 4×, 10× and 20× objectives. For grading purposes, a section was evaluated from each segment (~5mm) throughout the plaque (4–6 sections per plaque). Antibody staining was quantified on a 0–4 scale (0 = minimal, 4 = high) based on intensity of endothelial staining. Uniform endothelial staining throughout all segments of plaque is not expected and was not observed; as such, the plaque was given the grade of the highest staining observed through the plaque. For example, if one section (of the 4–6 sections evaluated) had grade 4, but the others all had 1, then the overall plaque grade was 4. Grading was done in batches to optimize uniformity of scale and representative samples of each grade were available throughout grading process for reference. Discrepancies in grading were discussed and the two blinded physicians agreed on a single grade.
Microbubble Preparation
Small peptide targeting ligands with specific affinity for human VCAM-1, LOX-1, P-selectin and von Willebrand Factor (vWF) were prepared by NuxOx Pharma (Tucson, AZ).9–12 The amino acid sequences for each of the targeting moieties are presented in Table I. Phospholipid-coated microbubbles (MB) entrapping perfluorocarbon gas were prepared. Lipids used in the formulation included dipalmitoylphosphatidylcholine (DPPC), dipalmitoyl-sn-glycerophosphatidylethanolamine-polyethyleneglycol-2000-OMe (DPPE-MPEG2000) and a phospholipid-PEG2000–ligand bioconjugate comprised of either DPPE-PEG2000-NH- linked to the ligand via a suberoyl linker (Sub) or DPPE-PEG2000-C(=O)-ligand linked via an amide bond. Control microbubbles contained DPPE-MPEG2000 in lieu of the phospholipid-PEG2000-peptide conjugate. A dissolved suspension of DPPC (90 mol%), DPPE-MPEG2000 (9 mol%) and phospholipid-PEG2000-linker-peptide conjugate (1%) was produced in propylene-glycol at 50–65°C. Dioctadecyl tetramethylindocarbocyanine (DiI) was added to the suspension for fluorescent labeling of the microbubble shell. The warm solution of phospholipids in propylene glycol was then added in several aliquots to a solution of phosphate buffered saline containing 5% glycerol by volume with stirring at 50–65°C; this solution was stirred 5–10 minutes. The solution was then transferred to a serum vial, which was immediately stoppered and crimp capped. The solution was allowed to come to ambient temperature and then stored at 4°C. A tranche of 25–50 2 mL nominal capacity serum vials were filled with 1.5 mL aliquots of the chilled phospholipid solution followed by application of light vacuum and purging with perfluorobutane gas; those operations were followed by rapid stoppering and crimp capping of the vial. Vials were stored at 4°C until use, whereupon they were allowed to warm to ambient temperature and agitated on a Bristol Myers Squibb Vial Mix apparatus for 45 seconds at 75 Hz (4500 rpm) to form the microbubbles.
Table I. Targeting peptides.
Biotinylated peptides were designed to bind inflammatory molecules of interest. Peptide purity was ≥95% purity (reverse phase HPLC analysis).
| Target | Sequence | Mol. Wt. | Observed Mass/Charge |
|---|---|---|---|
| LOX1 | Btn-JJYDPWTPS-NH2 | 1380.47 | 1381.29 (M+H)+ |
| LOX1 | Ac-YDPWTPSJJ-K(Btn)-NH2 | 1549.68 | 1548.55 (M−H)− |
| LOX1 | Btn-JJLTPATAI-NH2 | 1201.38 | 1202.28 (M+H)+, 1224.23 (M+Na)+ |
| LOX1 | Ac-LTPATAIJJ-K(Btn)-NH2 | 1372.60 | 698.25 (M+H+Na)2+, 706.19 (M+K+H)2+, 1372.85 (M+H)+, 1394.86 (M+Na)+ |
| LOX1 | Btn-JJFQTPPQL-NH2 | 1345.72 | 1346.42 (M+H)+ |
| LOX1 | Ac-FQTPPQLJJ-K(Btn)-NH2 | 1516.73 | 1517.94 (M+H)+, 759.66 (M+2H)2+ |
| P-Sel | Btn-JJLVSVLDLEPLDAAWL-NH2 | 2169.72 | 1086.31 (M+2H)2+ |
| P-Sel | Ac-LVSVLDLEPLDAAWLJJ-K(Btn)-NH2 | 2340.73 | 1171.74 (M+2H)2+, 1182.79 (M+Na+H)2+, 1190.59 (M+K+H)2+ |
| VCAM1 | Btn-JJ-RANLRILARY-NH2 | 1762.15 | 881.74 (M+2H)2+ |
| VCAM1 | Ac-RANLRILARYJJ-K(Btn)-NH2 | 1932.28 | 967.59 (M+2H)2+ |
| vWF | Btn-JJRVVCEYVFGRGAVCS-NH2 | 2159.57 | 1080.54 (M+2H)2+ |
| vWF | Ac-RVVCEYVFGRGAVCSJJ-K(Btn)-NH2 | 2327.69 | 1165.20 (M+2H)2+ |
Btn = Biotinyl; J = 8-amino-3,6-dioxaoctanoyl linker. Mass/charge ratio is observed using electrospray mass spectrometry where M denotes the neutral peptide molecule, H denotes a proton, Na is a sodium cation, K is a potassium cation.
Flow Chamber Assessment of Microbubble Binding
HAECs were cultured with vascular cell basal medium (ATCC, PCS-100-030, Manassas, VA) and an endothelial cell growth kit-VEGF (ATCC, PCS-100-041, Manassas, VA). This growth kit included recombinant human (rh) vascular endothelial growth factor (VEGF) (5ng/mL), rh epidermal growth factor (EGF) (5ng/mL), rh FGF basic (5ng/mL), rh (IGF-1) (15ng/mL), L-glutamine (10mM), heparin sulfate (0.75 units/mL), hydrocortisone (1 µg/mL), ascorbic acid (50 µg/mL), and fetal bovine serum (2%). HAECs used were primary cell lines for which all experiments were completed with no more than four passages. For experiments, HAECs were stained with 5 microliters of calcein (green) dye and transferred to fibronectin-coated dishes. Calcein was used to evaluate the viability of the cells as it will only stain the cytoplasm of living cells. Culture dishes were mounted on a parallel plate flow chamber kit (Glycotech, Gaithersburg, MD) with a gasket thickness of 0.0254 cm and channel width of 0.25 cm. The parallel plate flow chamber was placed on an inverted microscope (Eclipse E600, Nikon) with a 40× objective. The DiI labeled microbubbles were infused through the chamber with a peristaltic pump (Econo, BIO-RAD, Hercules, CA) at a dilution of 1:1000 (microbubbles : volume of PBS). A variety of flow rates (mL/min) were tested, and the flow rate was converted to shear-stress (dynes/cm2). The flow rates tested ranged from 1.0 – 6.4 mL/min which converted to shear-stress ranging from 4.34–27.78 dynes/cm2, respectively. Imaging was performed using low level transillumination with fluorescent epi-illumination (Nikon Y-FL). Videos were captured by camera (PIXIS:2048 Princeton Instruments, Trenton, NJ) interfaced with the microscope and were viewed with Q-Capture Pro 7 and analyzed with ImageJ. Shear stress was calculated using the following equation: τw = μγ = [(6μQ/(a2b)]/60. τw = wall shear stress, dynes/cm2; γ = shear rate, 1/sec; μ = apparent viscosity of media (PBS= 0.0076P); a = channel height (gasket thickness, = 0.0254 cm); b = channel width (gasket width, = 0.25cm); Q = volumetric flow rate, mL/sec.13
Statistical Analysis
Regarding patient characteristics, group differences between asymptomatic and symptomatic patients were determined using a non-parametric Wilcox-Mann-Whitney test for the continuous data (age) and chi-square tests for categorical data. Fisher’s Exact test was used in cases where one of the cell size in frequency distribution was less than 5. Differences between IHC staining were analyzed using the unpaired Student’s t-test. Analysis of microbubbles binding to the HAECs was performed using two sample Student’s t-tests assuming unequal variances. An alpha value of < .05 was used for significance. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient Characteristics
A total of 60 CEA specimens were obtained from consecutive individuals who were symptomatic (30) and asymptomatic (30). The demographic and patient characteristics for the two different cohorts are presented in Table II. Symptomatic subjects had a greater prevalence of diabetes mellitus. Smoking and lack of anti-platelet therapy were more common in patients with symptomatic disease compared to asymptomatic disease but this did not reach statistical significance.
Table II. Patient demographics.
Carotid plaque specimens were collected from patients undergoing carotid endarterectomy from 2014–2017.
| A | S | |||
|---|---|---|---|---|
| n = 30 | n = 30 | |||
| p | ||||
| Age (mean, range) | 71.5, 48–89 | 72.3, 57–89 | 0.63 | |
| Sex (male) | 59.6 | 69.2 | 0.41 | |
| Race | 0.05 | |||
| Caucasian | 97.9 | 84.6 | ||
| Hispanic | 2.1 | 15.4 | ||
| Smoking | 0.15 | |||
| Current | 25.5 | 26.9 | ||
| Quit | 49 | 65.4 | ||
| Never | 25.5 | 7.7 | ||
| Anti-Platelet | ||||
| None | 0 | 19.2 | * | |
| Aspirin | 72.3 | 50 | ||
| Plavix | 6.4 | 3.8 | ||
| Both | 21.3 | 26.9 | ||
| Anti-coagulation | 8.5 | 7.7 | 0.9 | |
| Statin | 85.1 | 69.2 | 0.11 | |
| DM | 12.8 | 38.5 | 0.01 | |
| Afib | 10.6 | 3.8 | 0.39 | |
| Stenosis | ||||
| >50% | 0 | 19.2 | * | |
| >70% | 100 | 80.8 | ||
A = asymptomatic. S = symptomatic. Data are given as percentage unless otherwise noted.
denotes no statistics performed because zero numerical value.
CEA Plaque Molecular Expression
CEA samples were processed in sequential order, but stained in batches for standardization. Using antibodies specific for Lox-1 (1:50), P-selectin (1:100), vWF (1:100) and VCAM-1 (1:150) IHC staining of carotid specimens was performed to evaluate sections throughout the plaque (Figure 1). Representative images of 40× and 100× magnification are shown for each antibody. There was minimal staining in the no primary antibody control; although negative controls were run for each antibody, we only show one negative control sample because IHC protocol for each antibody was identical. Although our focus was endothelial cell staining, staining with these antibodies throughout the intima and media was observed as expected. We noted robust vWF staining throughout all samples, mild Lox-1 staining throughout all samples and variable VCAM-1 and P-selectin staining.
Figure 1. IHC staining for Lox-1, P-selectin, vWF and VCAM-1.
Carotid plaques were evaluated by IHC using antibodies specific for Lox-1 (1:50), P-selectin (1:100), vWF (1:100) and VCAM-1 (1:150) as described in Materials and Methods. Representative images of each are shown in either 40× or 100× magnification. Control images show IHC with no primary antibody used; as staining protocols were the same for all four antibodies, we only show one representative control image. L indicates the carotid lumen in each section. Black bars represent 500 µm in 40× images and 100 µm in 100× images.
To quantify endothelial-specific expression, we evaluated expression using a semiquantitative scoring scale with a scoring range of 0 – 4. As noted, specimens were evaluated throughout the entire length of the plaque. Staining was assessed by two blinded experts as described in Materials and Methods. Representative examples of IHC for VCAM-1 and the semiquantitative scoring scale for endothelial staining is illustrated in Figure 2. We found a significantly greater degree of staining (P < .01) for endothelial VCAM-1 in plaques from symptomatic versus asymptomatic subjects (Figure 3). Although endothelial staining of Lox-1, P-selectin and vWF, there were no significant differences between the patient cohorts (Figure 3). However, it should be noted that IHC does not necessarily differentiate P-selectin or vWF that is surface expressed from that which is stored within intracellular Weibel-Palade bodies. Expression of LOX-1 was relatively lower compared to other candidate molecules evaluated, but similar between cohorts. Of particular importance when considering whether there may be an at-risk population of asymptomatic patients, we found that although VCAM-1 expression was significantly higher in symptomatic compared asymptomatic patients, 22% of those with asymptomatic plaques had VCAM-1 staining scores of grade 2 or greater.
Figure 2. IHC Grading scale.
Carotid plaque sections processed such that cross sections every 5mm throughout the length of the plaque were evaluated by IHC using the four antibodies shown in Figure 1. Endothelial staining throughout the plaque was evaluated by two fully blinded physicians and graded from 0 (minimal staining) to 4 (highest staining), as described in Materials and Methods. Sections here show representative specimens for each of those grades from VCAM-1-stained sections (40×); arrows point to carotid lumen endothelium. Black bars represent 100 µm.
Figure 3. VCAM-1 expression differentiates carotid plaques from asymptomatic vs. symptomatic patients.
Carotid plaques from symptomatic (S) and asymptomatic (A) patients were evaluated by immunohistochemistry (IHC) using antibodies specific for VCAM-1 (A), P-selectin (B), Lox-1 (C) and vWF (D). Endothelial staining intensity was graded blindly by two observers (per grading scale seen in Fig. 1). Graphs show mean staining intensity; error bars represent standard deviation (SD); P values were calculated using non-paired student t-test.
Targeted Microbubble Attachment to HAECs
Microbubbles targeted to each of the molecular epitopes present on atherosclerotic plaques in humans (VCAM-1, P-selectin, vWF and LOX-1) using peptide ligands were prepared. HAECs that were used to test targeted microbubbles were consistently grown to confluence and were found to express the target molecules except P-selectin; level of expression was not quantified. For microbubble binding quantification, flow chambers were used with plated HAECs and bound microbubbles were counted under varying mechanical shear stress conditions (experiments were repeated 3–5 times per point). Irrespective of the shear stress, there was not permanent attachment of control non-targeted microbubbles (Figure 4). At low shear stress that are typically found in the larger microcirculation (4–5 dynes/cm2), attachment occurred for all targeted agents, although attachment was greatest for VCAM-1-targeted microbubbles (P < .05). Attachment efficiency decreased with increased shear stress for all agents but remained higher for VCAM-1. At high continuous shear stresses like that found in the human carotid artery (>20 dynes/cm2) attachment was only observed for VCAM-1-targeted microbubbles.
Figure 4. Targeted microbubbles bind cultured human aortic endothelial cells (HAECs).
Targeted microbubbles were exposed to HAEC cells in flow chambers and cell-bound microbubbles were counted. VCAM-1-targeted microbubbles had significantly increased binding to HAEC cells compared to control (no-peptide) and other microbubble preparations (P < .05). Error bars show standard deviation for 3–5 experiments. Inset displays a screenshot of video of VCAM-targeted microbubbles (red) bound to a HAEC (green).
DISCUSSION
The current approach for selecting patients with asymptomatic carotid vascular disease for surgical intervention is based largely on randomized trials performed over a decade ago (ACAS, ACST I.2,3,5 There remains significant controversy on which and whether asymptomatic patients with carotid atherosclerosis benefit from CEA. Accordingly, there is interest in the creation of non-invasive approaches using blood tests or imaging that add predictive information on risk of future atheroembolic events to basic information of percent stenosis of the internal carotid artery and symptomatic status. The lack of accounting for patient genetics, carotid plaque molecular expression profiles, or even advanced imaging techniques indicate the need for improvements in both diagnosis and treatment of this disease.
Pre-clinical and clinical studies have suggested that novel forms of diagnostic imaging could aid in stratifying patients for stroke risk. Plaque assessment by MRI has been a popular approach owing to the ability to use different imaging sequences to discern plaque composition. MRI has been used to detect the presence of intra-plaque hemorrhage (IPH) as well as to differentiate between fibrous versus fatty/necrotic plaques. Aside from cost, there are some drawbacks in the MRI diagnosis of IPH to select high-risk patients with asymptomatic carotid vascular disease. Based on our data (unpublished) and those of others,14,15 50–85% of patients with asymptomatic carotid disease have IPH based on histological evaluation. Some studies find no difference in IPH presence in plaques from symptomatic and asymptomatic patients. Other experimental imaging modalities that bear mentioning include MRI evaluation for prior subclinical events;16,17 transcranial doppler to detect microemboli;18 and various strategies of plaque characterization with ultrasound tissue characterization or vasa vasorum detection.19 Larger trials evaluating these experimental technologies will be needed to validate the wide-spread adoption these new technologies.
A more accurate method for evaluating susceptibility to atheroembolic events and selection for surgical management could be to image the molecular events that predispose to plaque instability. The potential targets for this purpose are very broad and include endothelial activation, plaque inflammatory cell content or activity, protease activity, apoptosis, and oxidative lipid accumulation.20,21 Clinical studies have been performed primarily with 18F-FDG PET as a putative marker for plaque inflammatory activity.22 CEU methods that have been developed are based on the idea of targeting intravascular events at the plaque surface that provide information on the presence of more unstable plaque.23 These markers include expression of endothelial cell adhesion molecules that are responsible for monocyte recruitment (VCAM-1, selectins, ICAM-1), thrombosis-related factors (platelets, dysregulated vWF, fibrin, tissue factor), LOX-1, and general markers of endothelial activation or angiogenesis.24–28
The primary purpose of this study was to assess the relative endothelial expression profile on human carotid plaque of some of the targets that have appeared most promising in pre-clinical studies for defining high-risk plaques. Other groups have performed similar experiments to evaluate human carotid plaque molecular expression profiles, but we have seen no data for evaluation for endothelial expression for the markers we felt were most promising.29,30 Of the four candidate molecules evaluated, VCAM-1 was the most useful to differentiate high-risk (symptomatic) vs. low-risk (asymptomatic) carotid plaques (P < .01). This contradicts Schneiderman et. al. because they found similar or lower VCAM-1 expression in symptomatic plaques; however, their manuscript does not show VCAM-1staining for evaluation, they do not evaluate the full carotid plaque and most importantly they were evaluating total VCAM-1 expression as opposed to only endothelial expression.31 vWF had the highest expression in carotid plaque endothelium, but did not differentiate between plaques from symptomatic and asymptomatic patients. Although P-selectin, vWF and LOX-1 have key roles in atherosclerotic plaque biology, our data indicate that these molecules do not differentiate high-risk from low-risk carotid plaques in humans based on endothelial expression. However, IHC does not necessarily reflect the activated molecular form of vWF that targeted microbubbles bind in vivo.24 Our data also show that although most carotid plaques from asymptomatic patients have low (grade 0 or 1) VCAM-1 expression, 22% have moderate or high expression (grade 2–4). This is key because if all plaques from asymptomatic patients had low expression, there would be no potential utility of VCAM-1 expression to identify asymptomatic patients who may be at higher risk for atheroembolic events similar to symptomatic patients.
These IHC-based studies are limited by their quantification methodology. Although staining classification was done in a fully blinded fashion with multiple steps to ensure full and consistent evaluation throughout the process, the grading methodology is still based on a subjective scale. An alternative would have been quantification with immunofluorescence (IF) measurement. However, because the candidate molecules expression is not limited to the blood vessel endothelium, expression would still need to be quantified with significant human input. As such, we did not see a benefit of IF over IHC. In addition, we feel plaque specimen processing and full-length evaluation is more reproducible using IHC methodology. These experiments are also limited because no in vivo carotid plaque imaging in humans could be done and we have no way in this study to know whether VCAM-1 or the other markers will be predictive of atheroembolic events. Detection of VCAM-1 expression in asymptomatic plaques as a marker of high-risk phenotype, however, is intriguing. Prospective human studies will be needed to determine if VCAM-1 expression correlates with increased risk of subsequent atheroembolic events in patients who present with asymptomatic disease.
Prior strategies for localizing endothelial molecules with ultrasound contrast have utilized immunoglobulin (Ig) protein for microbubble targeting, which can be of limited utility in humans secondary to immunoreactivity. We created ultrasound contrast agents using peptides shown to be specific for VCAM-1 and other candidate biomolecules.9–12 Our data show that VCAM-1 targeted microbubbles bind human endothelial cells under shear conditions that approximate those found in the human carotid artery. Decreased binding of other targeted microbubbles could be from differential target expression on HAECs and/or peptide/ligand binding efficiency, but these hypotheses were not tested. Although decreased binding of microbubbles observed with increased shear stress could be secondary to changes in endothelial molecular expression profiles, we believe that it is more likely that binding efficiency of microbubbles decreases with the increased shear.32 Although our data indicate that vWF and LOX-1 expression did not differentiate high-risk from low-risk plaques, these targeted microbubbles may prove useful for studying development of atherosclerosis in animal models. These experiments are limited because of their in vitro nature and lack of evaluation for binding efficiency and specificity. As such, our proof-of-concept experiments will need further investigation. Utility of these agents for detection of murine endothelial expression patterns in atherosclerosis models will need to be evaluated as well as further pre-clinical trials before their usefulness in humans can be evaluated.
Summary and Conclusions
Our data are the first to show that VCAM-1 is significantly upregulated in symptomatic versus asymptomatic carotid plaques from humans. It may be that VCAM-1 expression represents a more sophisticated, biomolecular-based method to categorize high-risk carotid plaques. These data, if corroborated by others, are potentially major contributions to the field because they confirm molecular pathways associated with plaque instability and represent a promising molecular target for diagnostic, personalized medicine. Furthermore, our data are also the first to show that peptide-bearing targeted, clinically translatable microbubbles are tools for the study of dysfunctional vascular endothelium and hold a great potential to help differentiate high-risk from low-risk plaques in vivo. This imaging strategy would include a simple intravenous injection of an ultrasound contrast agent and evaluation of carotid plaque with duplex ultrasound. Such non-invasive imaging could greatly improve patient selection for surgical intervention resulting in improved cost/benefit ratios and prevention of associated morbidity and mortality of unneeded surgeries.
Take Home Message: VCAM-1 was significantly elevated in carotid plaques of symptomatic vs asymptomatic patients. Targeted microbubble anti-VCAM-1 was demonstrated in an in-vitro flow chamber in carotid plaques.
Recommendation: VCAM-1 may be a marker of activated endothelium in symptomatic carotid plaques and is a potential target for ultrasound imaging.
Non-invasive methods for stroke risk assessment in patients with asymptomatic carotid atherosclerosis is long overdue and key to improving treatment. Our data are the first to show Vascular Cell Adhesion Molecule-1 (VCAM-1) expression is significantly increased in high-risk compared to low-risk carotid plaques in humans (P = .0005) and they demonstrate a novel targeted ultrasound agent that could be used for non-invasive VCAM-1 evaluation in patients. Furthermore, these data build on our understanding of carotid plaque biology and define tools for in vivo molecular analysis of atherosclerosis in animal models as well as humans. This ultrasound technology would be easily translatable to a vascular outpatient setting.
Acknowledgments
The authors would like to acknowledge the generous assistance of Drs. Arthur Gmitro and Andrew Rouse of the University of Arizona Dept. of Medical Imaging for use of their microscope/fluorescence equipment. Research in this publication was supported by the National Cancer Center Support Grant P30 CA23074 and used the Tissue Acquisition and Cellular/Molecular Analysis Shared Resource (TACMASR). We would also like to thank the surgeons involved with the study: Wei Zhou, Scott Berman, Shonda Banegas, Kay Goshima, John D. Hughes, Luis R. Leon Jr., Joseph L. Mills Sr., Miguel Montero-Baker and John P. Pacanowski.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation Information:
Full presentation for Western Vascular Society (WVS) 31st Annual Meeting; Sep 24 – 27, 2016, Colorado Springs, Colorado.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 383:245–54. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 3.Goldstein MR. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;274:1505–6. doi: 10.1001/jama.1995.03530190019015. author reply 1506-7. [DOI] [PubMed] [Google Scholar]
- 4.Irvine CD, Baird RN, Lamont PM, Davies AH. Endarterectomy for asymptomatic carotid artery stenosis. BMJ. 1995;311:1113–4. doi: 10.1136/bmj.311.7013.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 376:1074–84. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Inaba Y, Lindner JR. Molecular imaging of disease with targeted contrast ultrasound imaging. Transl Res. 159:140–8. doi: 10.1016/j.trsl.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–84. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 10.Ulrichts H, Depraetere H, Harsfalvi J, Deckmyn H. Selection of phages that inhibit vWF interaction with collagen under both static and flow conditions. Thromb Haemost. 2001;86:630–5. [PubMed] [Google Scholar]
- 11.Molenaar TJ, Twisk J, de Haas SA, Peterse N, Vogelaar BJ, van Leeuwen SH, et al. P-selectin as a candidate target in atherosclerosis. Biochem Pharmacol. 2003;66:859–66. doi: 10.1016/s0006-2952(03)00387-3. [DOI] [PubMed] [Google Scholar]
- 12.White SJ, Nicklin SA, Sawamura T, Baker AH. Identification of peptides that target the endothelial cell-specific LOX-1 receptor. Hypertension. 2001;37:449–55. doi: 10.1161/01.hyp.37.2.449. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence MB, McIntire LV, Eskin SK. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–90. [PubMed] [Google Scholar]
- 14.Milei J, Parodi JC, Ferreira M, Barrone A, Grana DR, Matturri L. Atherosclerotic plaque rupture and intraplaque hemorrhage do not correlate with symptoms in carotid artery stenosis. J Vasc Surg. 2003;38:1241–7. doi: 10.1016/s0741-5214(03)00910-8. [DOI] [PubMed] [Google Scholar]
- 15.Milei J, Parodi JC, Alonso GF, Barone A, Grana D, Matturri L. Carotid rupture and intraplaque hemorrhage: immunophenotype and role of cells involved. Am Heart J. 1998;136:1096–105. doi: 10.1016/s0002-8703(98)70169-3. [DOI] [PubMed] [Google Scholar]
- 16.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke. 2006;37:818–23. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Moody AR, Gladstone DJ, Leung G, Ravikumar R, Zhan J, et al. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology. 2009;252:502–8. doi: 10.1148/radiol.2522080792. [DOI] [PubMed] [Google Scholar]
- 18.Streifler JY, den Hartog AG, Pan S, Pan H, Bulbulia R, Thomas DJ, et al. Ten-year risk of stroke in patients with previous cerebral infarction and the impact of carotid surgery in the Asymptomatic Carotid Surgery Trial. Int J Stroke. doi: 10.1177/1747493016660319. [DOI] [PubMed] [Google Scholar]
- 19.Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol. 9:663–71. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paraskevas KI, Spence JD, Veith FJ, Nicolaides AN. Identifying which patients with asymptomatic carotid stenosis could benefit from intervention. Stroke. 45:3720–4. doi: 10.1161/STROKEAHA.114.006912. [DOI] [PubMed] [Google Scholar]
- 21.Lindner JR, Sinusas A. Molecular imaging in cardiovascular disease: Which methods, which diseases? J Nucl Cardiol. 20:990–1001. doi: 10.1007/s12350-013-9785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahrendorf M, Sosnovik DE, French BA, Swirski FK, Bengel F, Sadeghi MM, et al. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 62:909–17. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 24.Lindner JR. Molecular imaging of vascular phenotype in cardiovascular disease: new diagnostic opportunities on the horizon. J Am Soc Echocardiogr. 23:343–50. doi: 10.1016/j.echo.2010.01.025. quiz 450-2. [DOI] [PubMed] [Google Scholar]
- 25.Shim CY, Liu YN, Atkinson T, Xie A, Foster T, Davidson BP, et al. Molecular Imaging of Platelet-Endothelial Interactions and Endothelial von Willebrand Factor in Early and Mid-Stage Atherosclerosis. Circ Cardiovasc Imaging. 8:e002765. doi: 10.1161/CIRCIMAGING.114.002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Davidson BP, Yue Q, Belcik T, Xie A, et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging. 6:74–82. doi: 10.1161/CIRCIMAGING.112.975193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Patel AR, Klibanov AL, Kramer CM, Ruiz M, Kang BY, et al. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ Cardiovasc Imaging. 3:464–72. doi: 10.1161/CIRCIMAGING.109.896654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003;107:455–60. doi: 10.1161/01.cir.0000044916.05919.8b. [DOI] [PubMed] [Google Scholar]
- 29.Klegerman ME, Zou Y, McPherson DD. Fibrin targeting of echogenic liposomes with inactivated tissue plasminogen activator. J Liposome Res. 2008;18:95–112. doi: 10.1080/08982100802118482. [DOI] [PubMed] [Google Scholar]
- 30.Schneiderman J, Simon AJ, Schroeter MR, Flugelman MY, Konstantinides S, Schaefer K. Leptin receptor is elevated in carotid plaques from neurologically symptomatic patients and positively correlated with augmented macrophage density. J Vasc Surg. 2008;48:1146–55. doi: 10.1016/j.jvs.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher H, Kaiser E, Schnabel PA, Sykora J, Eckstein HH, Allenberg JR. Immunophenotypic Characterisation of Carotid Plaque: Increased Amount of Inflammatory Cells as an Independent Predictor for Ischaemic Symptoms. Eur J Vasc Endovasc Surg. 2001;21:494–501. doi: 10.1053/ejvs.2001.1362. [DOI] [PubMed] [Google Scholar]
- 32.Bailey KA, Haj FG, Simon SI, Passerini AG. Atherosusceptible Shear Stress Activates Endoplasmic Reticulum Stress to Promote Endothelial Inflammation. Scientific Reports. 2017;7 doi: 10.1038/s41598-017-08417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]