Abstract
Background
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) has been shown as a promising technique for assessing lung lesions. However, DCE-MRI often suffers from motion artifacts and insufficient imaging speed. Therefore, highly-accelerated free-breathing DCE-MRI is of clinical interest for lung exams.
Purpose
To test the performance of rapid free-breathing DCE-MRI for simultaneous qualitative and quantitative assessment of pulmonary lesions using Golden-angle RAdial Sparse Parallel (GRASP) imaging.
Study Type
prospective
Population
26 patients (17 males, mean age=55.1±14.4) with known pulmonary lesions.
Field Strength /Sequence
3T MR scanner; a prototype fat-statured, T1-weighted stack-of-stars golden-angle radial sequence for data acquisition and a Cartesian breath-hold volumetric-interpolated examination (BH-VIBE) sequence for comparison.
Assessment
After a dual-mode GRASP reconstruction, one with 3-second temporal resolution (3s-GRASP) and the other one with 15-second temporal resolution (15s-GRASP), all GRASP and BH-VIBE images were pooled together for blind assessment by two experienced radiologists, who independently scored the overall image quality, lesion delineation, overall artifact level and diagnostic confidence of each case. Perfusion analysis was performed for the 3s-GRASP images using a Tofts model to generate the volume transfer coefficient (Ktrans) and interstitial volume (Ve).
Statistical Tests
Nonparametric paired two-tailed Wilcoxon signed-rank test; Cohen’s kappa; unpaired student t-test.
Results
15s-GRASP achieved comparable image quality with conventional BH-VIBE (P>0.05), except for the higher overall artifact level in the pre-contrast phase (P=0.018). The Ktrans and Ve in inflammation were higher than those in malignant lesions (Ktrans: 0.78±0.52 min−1 verse 0.37±0.22 min−1, P=0.020; Ve: 0.36±0.16 verse 0.26±0.1, P=0.177). Meanwhile, the Ktrans and Ve in malignant lesions were also higher than those in benign lesions (Ktrans: 0.37±0.22 min−1 verse 0.04±0.04 min−1, P=0.001; Ve: 0.26±0.12 verse 0.10±0.00, P=0.063).
Conclusion
This feasibility study has demonstrated the performance of high spatiotemporal resolution free-breathing DCE-MRI of the lung using GRASP for qualitative and quantitative assessment of pulmonary lesions.
Keywords: Dynamic contrast-enhanced MRI, Free-breathing, GRASP, Golden-angle radial, Lung neoplasm, Lung cancer
Introduction
Lung cancer remains the leading cause of cancer deaths worldwide, and its incidence rate has risen significantly over the past few years (1). Tumor angiogenesis is critical for its growth, invasion, and its subsequent metastasis. Similar to other solid tumors, the role of angiogenesis is well established in the progression of lung cancer, and high micro-vessel density has been shown as a prognostic indicator of metastasis and poor survival (2,3). Since newly formed vessels can be characterized by structural and functional abnormalities, imaging techniques, particularly dynamic contrast-enhanced computed tomography (DCE-CT) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), are valuable diagnostic tools that enable non-invasive assessment of tumor vascular perfusion and angiogenesis in lung cancer (4,5).
DCE-CT is widely used for clinical lung cancer screening due to its high spatial resolution, high sensitivity and fast imaging speed (5). However, a major limitation of CT is cumulative ionizing radiation associated with repeated CT exams, which are often needed to monitor the development of lesions in longitudinal studies. On the other hand, although lung MRI has been more challenging compared to other MR exams due to the presence of air, respiratory/cardiac motion, and the short T2* of the lung parenchyma, remarkable technological advances have been achieved during the past many years to address these challenges; and multiple studies have demonstrated convincing imaging performance of lung MRI with appropriate scan times (6–9). However, one of the main challenges of conventional MRI using Cartesian acquisitions is the sensitivity to motion (i.e., heart beating or respiration) and the requirement of breath-holds in clinical routine. This requirement restricts acquisition times and often leads to compromised spatial resolution, temporal resolution, and/or volumetric coverage (10–16). Therefore, free-breathing DCE-MRI with high spatiotemporal resolution is highly-desirable for chest and abdominal exams, including assessment of lung cancer. Moreover, it is also desired that imaging approaches can provide simultaneous evaluation of both perfusion dynamics and anatomical morphology of lung tumors (17).
Recently, a novel free-breathing MRI technique known as Golden-angle RAdial Sparse Parallel (GRASP) imaging has been introduced for rapid free-breathing MR acquisitions (18,19). GRASP combines compressed sensing, parallel imaging and a golden-angle radial sampling scheme in a synergistic framework to enable continuous free-breathing data acquisitions and allow flexible reconstruction of an image-series with arbitrary temporal resolution for different clinically needs. Thus, it offers benefits for patients who suffer from impaired breath-hold capacity and/or capabilities, and allows simultaneous qualitative and quantitative assessment. During the past few years, GRASP has been demonstrated for free-breathing DCE-MRI of many organs that are sensitive to motion-induced artifacts in conventional Cartesian imaging, such as the liver (20,21), the prostate (22), the kidney (23), and the bowel (24).
Based on these prior studies, we hypothesized that GRASP can also be useful in free-breathing DCE-MRI of the lung for assessment of pulmonary lesions. Thus, the purpose of this study was to evaluate the performance of GRASP for simultaneous assessment of the lung both qualitatively and quantitatively in a cohort of patients with known lung lesions. The results were compared with conventional Cartesian breath-hold volumetric-interpolated examination (BH-VIBE) images and were correlated with corresponding histological findings.
Materials and Methods
Patients
This prospective study was approved by the Institutional Medical Ethic Committee. Between January 2017 and June 2017, twenty-six consecutive patients (17 males, mean age=55.1±14.4 years) with known lung lesions confirmed in previous CT scans were recruited. The experiment procedure was fully explained to all participants, and written informed consent was obtained from each of them prior to the MRI scan. The patient inclusion criteria included: (i) the lesion size was larger than 15mm in any of the spatial dimensions; (ii) no metal implants was found; and (iii) the patient was able to cooperate with the MR exam.
Data Acquisition
All MR Imaging was performed on a 3.0T MR scanner (MAGNETOM Trio, Siemens Healthcare, Erlangen, Germany) equipped with a twelve-element body matrix coil array. Each participant underwent a coronal T2-weighted HASTE (Half-Fourier-Acquired Single-shot Turbo spin Echo) scan, an axial diffusion weighted imaging (DWI) scan, an axial Cartesian BH-VIBE scan, an axial GRASP scan and then another axial BH-VIBE scan. The total scan time was about 15 minutes for each patient.
The GRASP acquisitions were performed using a prototype stack-of-stars radial sequence, in which golden-angle radial sampling is employed in the kx-ky plane and Cartesian sampling is employed along the slice dimension (kz). A fat saturation module was periodically inserted right before the acquisition of each stack, as described by Block KT et al (25). All data were acquired during free breathing with the following imaging parameters: in-plane FOV=300×300 mm2, in-plane matrix=256×256, number of slice=40, acquired slice thickness=5mm, flip angle=12°, in-plane spatial resolution=1.25×1.25 mm2; TR/TE=3.40 ms/1.64 ms, and bandwidth =580 Hz/voxel. Twenty-two partitions were acquired in each dataset with 90% partial Fourier applied along the slice dimension, and images were interpolated to 40 partitions after reconstruction, resulting in an interpolated slice thickness of 3 mm. A total of 3000 radial spokes were acquired in each partition and the total scan time was 281 seconds. For each scan, the patient was instructed to breathe normally and consistently as best as they can and to avoid sudden deep breathing. A weight-based full dose injection (0.2 mmol/kg of body weight) of Magnevist (Bayer Schering Pharma, Berlin, Germany) was performed ~20 seconds after the start of data acquisition at a rate of 3 mL/sec, followed by a 20 mL saline flush at the same rate.
The BH-VIBE scan was performed using a routine clinical sequence with the following imaging parameters: in-plane FOV=300×300 mm2, in-plane matrix=288×384, number of slice=26, acquired slice thickness=6 mm, flip angle=12°, GRAPPA factor=2, and TR/TE=4.07 ms/1.93 ms. Two BH-VIBE acquisitions were performed in each patient, one immediately before and another one right after the GRASP acquisition. Each data was acquired under one breath-hold in ~15 seconds. Images were interpolated to 40 partitions after reconstruction, resulting in an interpolated slice thickness of 4 mm.
Image Reconstruction
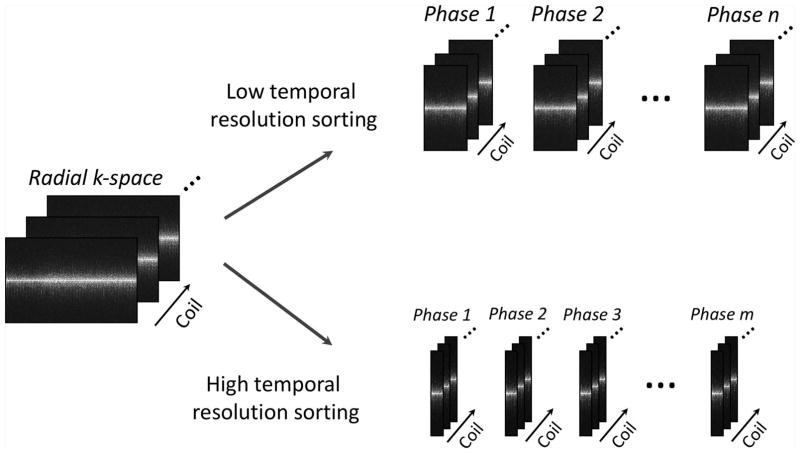
GRASP reconstruction was performed combining compressed sensing and parallel imaging as previously described in (19). Specifically, continuously acquired radial k-space was first sorted with two different resolution for different applications, as shown in Figure 1, followed by image reconstruction solving the following cost function:
| [1] |
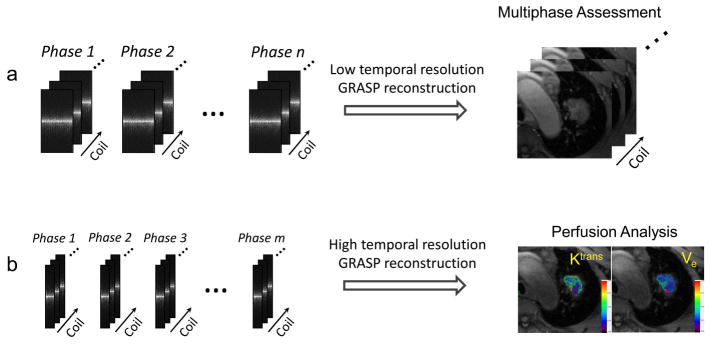
where F is the non-uniform FFT (NUFFT) operator, C represents coil sensitivities, y is the sorted dynamic multicoil radial k-space, x is the dynamic image-series to be reconstructed, and T is a sparsifying transform, which was chosen as the first-order finite differences along the dynamic dimension to minimize temporal total variation. The image reconstruction was performed off-line using a custom-developed implementation written in C++ on a 64-core Linux server equipped with 256 GB RAM. Two types of GRASP reconstruction were performed in each raw data, as shown in Figure 2. One reconstruction to generate an image-series a high temporal resolution (~3 seconds) by grouping every 34 consecutive spokes as one contrast phase, and the other one was reconstructed to generate an image-series with a low temporal resolution (~15 seconds) by grouping every 162 consecutive spokes as one contrast phase. Regularization parameter was empirically selected for each type of reconstruction separately, and was fixed for all datasets. BH-VIBE images were directly reconstructed on the MR scanner. For simplicity, the GRASP reconstruction with 3-second temporal resolution is referred to as 3s-GRASP and the GRASP reconstruction with 15-second temporal resolution is referred to as 15s-GRASP hereafter.
Figure 1.
A dual-mode k-space sorting scheme was performed in each dataset, one sorted with a high temporal resolution (~3 seconds) by grouping every 34 consecutive spokes as one contrast phase, and the other one sorted with a low temporal resolution (~15 seconds) by grouping every 162 consecutive spokes as one contrast phase.
Figure 2.
GRASP reconstruction was performed for both low temporal resolution and high temporal resolution image-series, respectively. The low temporal resolution images were used for qualitative multiphase assessment and the high temporal resolution images were used for quantitative perfusion analysis.
Image Quality Assessment
One pre-contrast phase, one arterial phase, and one delayed-enhancement phase were manually selected from each GRASP image-set (both 15s-GRASP and 3s-GRASP with matching contrast phases). All the selected images were pooled together with corresponding BH-VIBE images for visual image quality assessment by two broad-certificated radiologists (X.H. and D.L.) with 15 and 6 years of experience in body MRI, respectively. The readers were blinded to patients’ clinical history. For each image-set, the readers independently scored the overall image quality, the delineation of lesions, the overall artifact level and the diagnostic confidence.
The overall image quality was scored using the following 4-point Likert-type scale: 4=good; 3=adequate; 2=borderline; and 1=non-diagnostic. The delineation of lesions (including lymph nodes if available) was assessed using the following 4-point Likert scale: 4=lesion with sharp margins; 3=lesion with slight blurring; 2=lesion with moderate blurring; and 1=non-diagnostic and lesion barely visualized because of severe blurring. The overall artifact level was scored on a 4-point Likert-type scale as follows: 4=none artifact; 3=mild artifact; 2= significant artifact; 1=unreadable. The diagnostic confidence was assessed using the following 3-point Likert scale: 3=good confidence; 2=moderate confidence; and 1=non-diagnostic. The scores from the two readers were averaged to represent mean ± standard deviation.
Perfusion Analysis
Perfusion analysis was performed for all 3s-GRASP results using the FireVoxel software that is available for free download online (https://files.nyu.edu/hr18/public/projects.html). Analyses were performed by a radiologist (L.C.) with 5 years of experience in MRI blinded to the pathologic findings.
First, a regions of interest (ROI) was manually drawn on the descending aorta to derive an individual arterial input function (AIF). Then, three free-hand ROIs were placed on the solid part of lesion in three consecutive slices. A Tofts model (26) was then applied to calculate the volume transfer coefficient (Ktrans) and the interstitial volume (Ve) for each ROI on a pixel-by-pixel basis using the following equation:
| [2] |
where Ktrans is the rate transfer constant of gadolinium between plasma and tissue interstitium, Ve is the extracellular-extravascular volume, Cp(t) is the gadolinium concentration in the arterial blood plasma, and C(t) is the tissue gadolinium concentration. Similar as that described in (21), concentrations were calculated as C(t)=(S(t)/S0–1)/HCT, where S(t) represents the post-contrast signal intensity and S0 represents the baseline (pre-contrast) signal intensity. A fixed hematocrit level (HCT) of 40% was assumed. The values of Ktrans and Ve were then averaged across the three ROIs to yield final results, which were then correlated with the histologic findings if available.
Statistical Analyses
A nonparametric paired two-tailed Wilcoxon signed-rank test was used to compare the readers’ scores i) between each type of GRASP and BH-VIBE, and ii) between 15s-GRASP and 3s-GRASP. A P value less than 0.05 was considered statistical significance. The inter-observer agreement in different image quality assessment category was evaluated using Cohen’s kappa. The measured mean Ktrans and Ve were compared between patients with different histologic findings using an unpaired student t-test. Statistical analyses were performed using SPSS (version 18.0; SPSS Inc, Chicago, IL, USA).
Results
The size of the pulmonary lesions across all patients was 32±9.0 mm, ranging from 15 to 57 mm. Among all participants, 23 had known pathologic findings, including 3 patients with histological confirmation on the surgical specimen after resection and 20 patients with CT-guided biopsy, both of which were performed after the MR exams. The histologic type of all 23 patients is described in Table 1.
Table 1.
Demographic and Clinical Indications of Patients.
| Patient and clinical information | |
|---|---|
| Total number of patients | 26 |
| Male/female | 17/9 |
| Age (years) | 55.1±14.4 |
| Size (cm) | 3.2 ± 0.9 |
| Number of patients with pathologic findings | 23 |
| Malignant (lung cancer) | 17 |
| Inflammation | 4 |
| Pneumoconiosis | 1 |
| Tuberculoma | 1 |
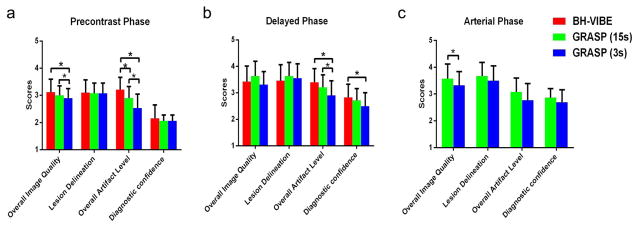
Figure 3 shows bar plots comparing visual image quality scores between different acquisition and reconstruction schemes. 15s-GRASP achieved comparable imaging performance with conventional BH-VIBE in all contrast phases, with P>0.05 in all the assessment categories except for the lower scores in overall artifact level in the pre-contrast phase (P=0.018). 3s-GRASP received relatively lower image quality scores than both 15s-GRASP and BH-VIBE, and significance was reached in the overall image quality and artifact level (P<0.001). However, 3s-GRASP provided additional temporal information that can be used for quantitatively analysis of the lesions. For the inter-observer agreement was good, the Kappa value was 0.76 in the overall image quality, 0.80 in the delineation of lesions, 0.77 in the overall artifact level and 0.94 in the diagnostic confidence, suggesting good agreement throughout all the assessment categories.
Figure 3.
Bar plots comparing visual image quality scores between different acquisition and reconstruction schemes. 15s-GRASP achieved comparable imaging performance with conventional BH-VIBE in all contrast phases, with P>0.05 in all the assessment categories except for the lower scores in overall artifact level in the pre-contrast phase (P=0.018). 3s-GRASP received relatively lower image quality scores than both 15s-GRASP and BH-VIBE, and significance was reached in the overall image quality and artifact level (P<0.001). A star in the plot indicates statistical significance.
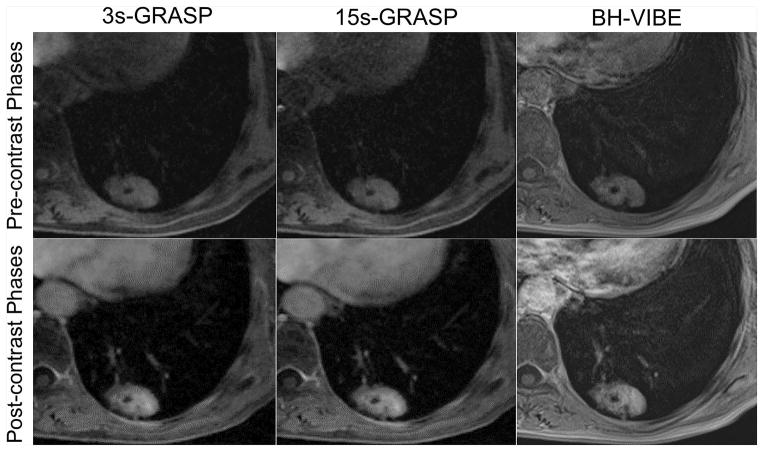
Figure 4 shows pre-contrast and post-contrast phases from one representative patient with adenocarcinoma comparing 15s-GRASP, 3s-GRASP and BH-VIBE. All images show adequate image quality with comparable delineation of the pulmonary lesion.
Figure 4.
Representative pre-contrast (top row) and post-contrast (bottom row) phases from a patient diagnosed as adenocarcinoma comparing 15s-GRASP, 3s-GRASP and BH-VIBE. All images show adequate image quality and comparable delineation of the pulmonary lesion.
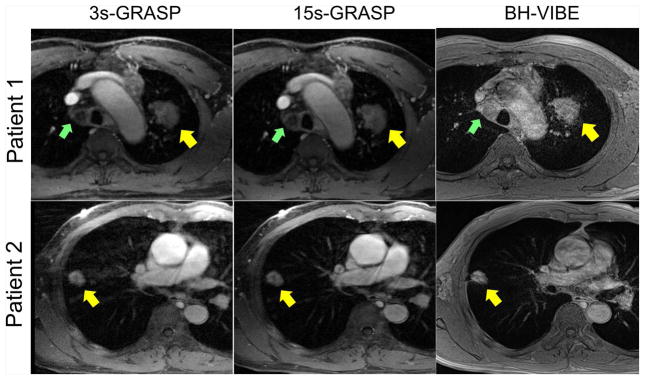
Figure 5 shows post-contrast phases comparing different types of GRASP with BH-VIBE images in two patients, one was diagnosed as squamous cell carcinoma and the other one as adenocarcinoma. Although the 3s-GRASP images show certain residual artifacts with relatively lower image quality due to higher acceleration rate, the 15s-GRASP images yielded comparable overall image quality with BH-VIBE with less motion artifacts. In particular, the mediastinal lymph node in patient 1 (indicated by green arrows) was not clearly delineated in the BH-VIBE images due to motion-induced artifacts in great vessels.
Figure 5.
Post-contrast phases in two patients comparing GRASP with BH-VIBE images. One patient (top row) was diagnosed as squamous cell carcinoma and the other patient (bottom row) was diagnosed as adenocarcinoma. Although the 3s-GRASP images show certain residual artifacts with relatively lower image quality due to high acceleration rate, the 15s-GRASP images yielded overall image quality that is comparable with BH-VIBE and with less motion artifacts. In particular, the mediastinal lymph node in patient 1 (indicated by green arrows) was not clearly delineated in the BH-VIBE images due to motion-induced artifacts in great vessels.
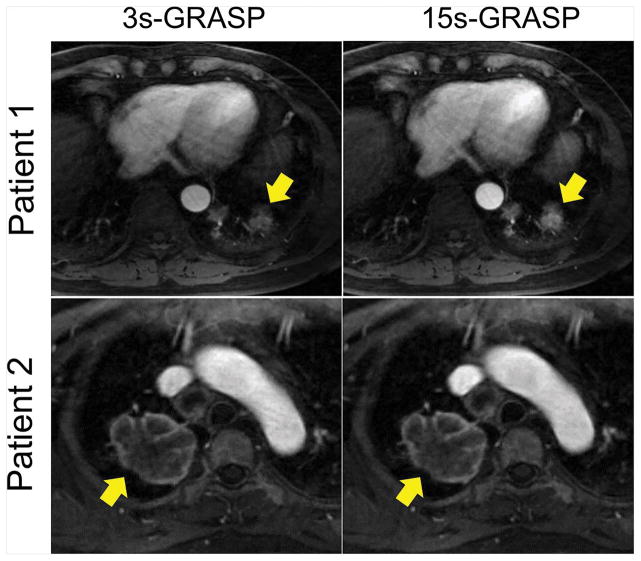
Figure 6 shows a similar comparison of the arterial phase between 15s-GRASP and 3s-GRASP in two patients. In patient 1, the lesion (yellow arrows) is small and is close to the diaphragm, and thus the 15s-GRASP image shows better lesion delineation than the 3s-GRASP image. However, they both show good delineation of the mass in patient 2, whose lesion is far from the diaphragm.
Figure 6.
A comparison of the arterial phase between 15s-GRASP and 3s-GRASP in two patients. In patient 1, the lesion (yellow arrows) is relatively smaller and closer to the diaphragm, and thus the 15s-GRASP image shows better lesion delineation than the 3s-GRASP image. However, they both show good delineation of the mass in patient 2.
Table 2 summarizes the perfusion parameters obtained in different patient groups. Among all lesions presented in the recruited patient group, the Ktrans in inflammation was significantly higher than those in malignant lesions (0.78±0.52 min−1 versus 0.37±0.22 min−1, P=0.020). While Ve was also higher in inflammation compared to malignant lesions, significance was not reached (0.36±0.16 versus 0.26±0.1, P=0.177). The Ktrans in malignant lesions was significantly higher than those in benign lesions (pneumoconiosis and tuberculoma) (0.37±0.22 min−1 versus 0.04±0.04 min−1, P=0.001). The Ve was higher in malignant lesions compared to benign lesions but statistical significance was not reached (0.26±0.12 versus 0.10±0.00, P=0.063).
Table 2.
Summary of Ktrans and Ve measured in different patient subgroups. Among all lesions, the Ktrans in inflammation was significantly higher than those in malignant lesions (P=0.020). Ve was also higher in inflammation compared to malignant lesions without statistical significance (P=0.177). The Ktrans in malignant lesions was significantly higher than those in benign lesions (pneumoconiosis and tuberculoma) (P=0.001). The Ve was higher in malignant lesions compared to benign lesions without statistical significance (P=0.063).
(−): standard deviation not applicable.
| Group | No. of patients (%) | Mean Ktrans | Mean Ve |
|---|---|---|---|
| Malignant lesions | 17 (73.9) | 0.37±0.22 | 0.26±0.12 |
| Adenocarcinoma | 10 (43.5) | 0.41±0.28 | 0.29±0.14 |
| Squamous cell carcinoma | 5 (21.7) | 0.33±0.07 | 0.24±0.06 |
| Small cell lung cancer | 1 (4.3) | 0.24(−) | 0.18(−) |
| Non-small cell carcinoma | 1 (4.3) | 0.30(−) | 0.16(−) |
| Benign lesions | 2 (8.7) | 0.07±0.01 | 0.10±0.00 |
| Pneumoconiosis | 1 (4.3) | 0.07(−) | 0.10(−) |
| Tuberculoma | 1 (4.3) | 0.06(−) | 0.10(−) |
| Inflammation | 4 (17.4) | 0.78±0.52 | 0.36±0.16 |
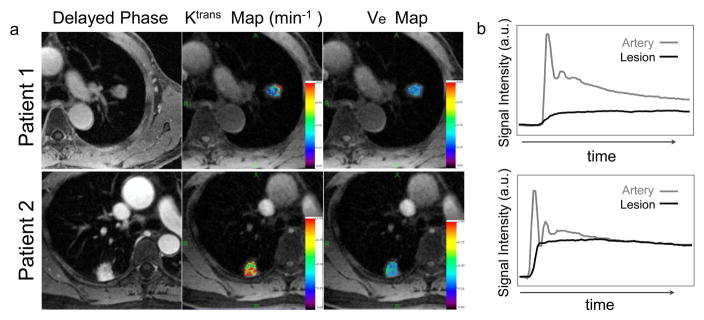
Figure 7a shows two examples of the Ktrans and Ve maps in two patients, one with adenocarcinoma and the other one with inflammation. Both the Ktrans and Ve of inflammation were higher than those in adenocarcinoma (Ktrans: 0.65 min−1 versus 0.22 min−1 and Ve: 0.32 versus 0.19). Figure 7b shows corresponding signal enhancement ratio along time in the lesion and the artery in these two patients.
Figure 7.
(a) Exemplary Ktrans and Ve maps in two patients, one patient diagnosed as adenocarcinoma (top row) and the other patient diagnosed as inflammation (bottom row). The Ktrans and Ve in inflammation are higher than those of adenocarcinoma (Ktrans: 0.65 min−1 versus 0.22 min−1 and Ve: 0.32 versus 0.19). (b) Corresponding signal enhancement ratio along time for the arteries and the lesions.
Discussion
DCE-MRI is a promising imaging technique for measuring the perfusion of lesions by imaging the process that a contrast agent passes through the vascular system to different organs and then wash out. In order to obtain dynamic images that can be used to generate reliable perfusion parameters, a sufficient temporal resolution up to 3–5 seconds is required to capture the passage of contrast agent through the capillary bed (27,28). In this study, we tested the feasibility and performance of a highly-accelerated free-breathing DCE-MRI approach for simultaneous qualitative and quantitative assessment of pulmonary lesions using the GRASP technique. The acceleration capabilities of multicoil compressed sensing reconstruction, in combination with golden-angle radial acquisitions, enabled free-breathing dynamic lung imaging with a temporal resolution up to 3 seconds per volume. The results suggested that adequate visual image quality and diagnostic confidence, together with quantitative perfusion information, can be obtained with the proposed approach.
In conventional Cartesian DCE-MRI of the lung, patients are often required to hold their breath during data acquisitions to avoid motion-induced artifacts. Thus, acquisition times are normally restricted to a breath-hold duration, thus leading to compromised spatiotemporal resolution and volume coverage. The trade-off that must be made between spatial and temporal resolution is currently one of the main barriers for technical standardization in DCE-MRI of the lung for routine clinical use. We have shown that GRASP is a promising imaging technique allowing patient to breath normally during MR scans without significantly compromising image quality and diagnostic confidence.
The motion robustness of GRASP is mainly attributed to the repeated sampling of the center of k-space in radial acquisitions, thus resulting in dramatically reduced sensitivity to motion-induced phase shifts compared to conventional Cartesian acquisitions (29). In addition, the inherently presented incoherent undersampling property provided in radial sampling is also well-suited for the application of sparsity-based image reconstruction approaches. However, in several previous studies (18,19,30), it has been shown that GRASP can suffer from motion blurring, particularly in the cases of deep breathing. Thus, additional motion compensation would be desired to further improve imaging performance. This will be further explored in the next step using techniques such as XD-GRASP (31) or RACER-GRASP(30).
With a golden-angle sampling scheme, GRASP not only enables a continuous data acquisition, but also allows flexible reconstruction with any temporal resolution defined by users. In this study, two types of GRASP reconstruction were performed, one with a low temporal resolution (15 seconds per volume) for morphological evaluation, and another one with a high temporal resolution (3 seconds per volume) for perfusion quantification. The 15s-GRASP achieved image quality that is comparable with conventional breath-hold imaging, while the 3s-GRASP can be used for additional perfusion quantification. Moreover, given the flexibility in GRASP reconstruction, it would also be interesting to compare perfusion quantification from images reconstructed with different temporal resolutions. This is inaccessible with most traditional imaging approaches, which often require one to pre-define temporal frames/temporal resolution.
In this work, The perfusion parameters generated with 3s-GRASP were within a range that reported in a prior paper (32). However, we noticed that the previously reported Ktrans values were different across studies, ranging from 0.09 to 0.65 min−1 (14,15,32–34). One potential cause of this variation might be the motion effects introduced by both heart beating and respiration, which in turn lead to intra-frame ghosting and blurring effects and inter-frame misalignments. Although inter-frame misalignments may be corrected with a dedicated registration algorithm, the intra-frame motion effect is difficult to correct retrospectively and thus can yield bias in the quantification of pharmacokinetic parameters. GRASP would be a more useful imaging technique to overcome these issues, and the imaging performance is expected to be further improved with additional motion suppression or compensation (31).
Multiple studies have previously used DCE-MRI to quantitatively assess lung lesions by deriving different perfusion parameters, such as relative signal intensity increase (35), maximum enhancement ratio (Max ER) (36,37), slope of enhancement (37), maximum peak (38), enhancement curve (39) and washout ratio (40). Kono et al., reported that malignant nodules yielded a higher Max ER than tuberculomas/hamartomas, and active inflammatory nodules resulted in an even higher Max ER compared to malignancy (36). On the other hand, Zhang et al., demonstrated that the maximum peak of malignant nodules and inflammatory nodules were both higher than benign nodules, while no statistically significant differences were found in the maximum peak between malignant nodules and inflammatory nodules (38). These results are in consistent with the perfusion parameters reported in our study, in which Ktrans and Ve were higher in the malignant and inflammatory lesions than the benign lesions (pneumoconiosis and tuberculoma). This finding can be explained in part by the fact that the passage of contrast media through the area of an acutely inflammatory nodule or granulomas is quite different from that in either normal lung tissue or malignant/benign nodules. The alterations in vascular caliber increase blood flow and the permeability of vessels, which is depended on the inflammatory activity (4). Therefore, there is an overlap of perfusion characteristic between inflammatory nodule and malignant/benign nodules.
This study has several limitations that warrant acknowledgements. First, the performance of GRASP was only demonstrated in a small number of patients with the size of lesions larger than 15mm. Although our results suggested convincing imaging performance, the usefulness and efficiency of this technique still need to be further validated in a large number of patient population with different lesion sizes. Second, we only performed conventional BH-VIBE imaging in the beginning and the end of GRASP scans. Thus, perfusion imaging was not available using BH-VIBE. Although it is desired to compare GRASP and BH-VIBE in patients with two separate visits and two contrast injections, this is very challenging to pursue in a patient cohort, particularly given the lack of lung MR imaging volume in current clinical routine. Third, this study assumed a linear relation between contrast concentration and signal intensity. Although it is known that a separate T1 map is usually required for this conversion, it was previously suggested that this assumption is adequate for demonstrating the feasibility and for comparison of perfusion parameters between different clinical indications (21). Fourth, the temporal resolution used for perfusion quantification was set as the mean transit times of contrast agent in the capillary bed (3–5 seconds). Given that GRASP can retrospectively reconstruct images with different temporal resolutions, it would be particularly interesting to compare perfusion parameters generated from image-series with a range of different temporal resolutions. Fifth, the DCE lung images were not compared with other imaging modalities such as DCE-CT. Such a comparison will be explored more in future work.
In conclusion, this study has demonstrated the performance of qualitative and quantitative assessment of pulmonary lesions using free-breathing DCE-MRI in combination with the GRASP technique. This technique offers a flexible alternative way to study solitary pulmonary nodules and masses. The results have suggested that GRASP may be a promising complementary quantitative tool for assessing pulmonary lesions in patients.
Supplementary Material
An example displaying different dynamic frames of 3s-GRASP images from a representative patient diagnosed as squamous-cell carcinoma.
Acknowledgments
This work was supported in part by the Combat Trauma Treatment New Clinical Technology (SWH2016JSTSYB-44) and the Wuxi Young Medical Talents (QNRC061).
The reconstruction part of this work was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R), a NIBIB supported Biomedical Technology Resource Center (P41 EB017183).
The authors would like to thank Dr. Henry Rusinek and Dr. Kai Tobias Block from the New York University School of Medicine for their support in using the Firevoxel software and implementing the image reconstruction, respectively.
Footnotes
Minimization of Potential Conflict of Interest: This study was performed on a Siemens clinical MR scanner using a prototype working in progress (WIP) sequence. Xiaoyue Zhou, an employee of Siemens Healthcare Ltd., Shanghai, China, supported the implementation and optimization of this sequence. Robert Grimm, an employee of Siemens Healthcare GmbH, Erlangen, Germany, supported the GRASP image reconstruction tasks.
The authors did not receive any financial support from the Siemens for this study.
Lihua Chen, Daihong Liu, Jiuquan Zhang, Bing Xie, Xuequan Huang, Jian Wang do not have any conflicts of interest to disclose.
Together with a few other investigators (not involved in this study), Li Feng has a pending patent application (Publication number: US20150077112 A1) for the GRASP MRI technique.
The authors tried to minimize potential conflict of interest without involving and mentioning any commercial content/information in the paper.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Goh V, Padhani AR, Rasheed S. Functional imaging of colorectal cancer angiogenesis. The Lancet Oncology. 2007;8(3):245–255. doi: 10.1016/S1470-2045(07)70075-X. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(14):3243–3256. doi: 10.1200/JCO.2005.18.853. [DOI] [PubMed] [Google Scholar]
- 4.Ohno Y, Nishio M, Koyama H, et al. Dynamic contrast-enhanced CT and MRI for pulmonary nodule assessment. Ajr American Journal of Roentgenology. 2014;202(3):515. doi: 10.2214/AJR.13.11888. [DOI] [PubMed] [Google Scholar]
- 5.Miles KA, Lee TY, Goh V, et al. Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. European radiology. 2012;22(7):1430–1441. doi: 10.1007/s00330-012-2379-4. [DOI] [PubMed] [Google Scholar]
- 6.Ohno Y, Koyama H, Yoshikawa T, Matsumoto S, Sugimura K. Lung Cancer Assessment Using MR Imaging: An Update. Magnetic resonance imaging clinics of North America. 2015;23(2):231–244. doi: 10.1016/j.mric.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Koyama H, Ohno Y, Seki S, et al. Magnetic resonance imaging for lung cancer. Journal of thoracic imaging. 2013;28(3):138–150. doi: 10.1097/RTI.0b013e31828d4234. [DOI] [PubMed] [Google Scholar]
- 8.Kauczor HU. MRI of the Lung. Springer; 2009. [Google Scholar]
- 9.Ingrisch M, Maxien D, Meinel FG, Reiser MF, Nikolaou K, Dietrich O. Detection of pulmonary embolism with free-breathing dynamic contrast-enhanced MRI. Journal of Magnetic Resonance Imaging. 2016;43(4):887–893. doi: 10.1002/jmri.25050. [DOI] [PubMed] [Google Scholar]
- 10.Ohno Y, Nishio M, Koyama H, et al. Solitary pulmonary nodules: Comparison of dynamic first-pass contrast-enhanced perfusion area-detector CT, dynamic first-pass contrast-enhanced MR imaging, and FDG PET/CT. Radiology. 2015;274(2):563. doi: 10.1148/radiol.14132289. [DOI] [PubMed] [Google Scholar]
- 11.Mamata H, Tokuda J, Gill RR, et al. Clinical application of pharmacokinetic analysis as a biomarker of solitary pulmonary nodules: dynamic contrast-enhanced MR imaging. Magnetic resonance in medicine. 2012;68(5):1614–1622. doi: 10.1002/mrm.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coolen J, Vansteenkiste J, De KF, et al. Characterisation of solitary pulmonary lesions combining visual perfusion and quantitative diffusion MR imaging. European radiology. 2014;24(2):531–541. doi: 10.1007/s00330-013-3053-1. [DOI] [PubMed] [Google Scholar]
- 13.Zou Y, Zhang M, Wang Q, Shang D, Wang L, Yu G. Quantitative Investigation of Solitary Pulmonary Nodules: Dynamic Contrast-Enhanced MRI and Histopathologic Analysis. Ajr American Journal of Roentgenology. 2008;191(1):252–259. doi: 10.2214/AJR.07.2284. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Chen L, Chen Y, et al. Tumor vascularity and glucose metabolism correlated in adenocarcinoma, but not in squamous cell carcinoma of the lung. PloS one. 2014;9(3):e91649. doi: 10.1371/journal.pone.0091649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan M, Zhang YD, Zhu C, et al. Comparison of intravoxel incoherent motion diffusion-weighted MR imaging with dynamic contrast-enhanced MRI for differentiating lung cancer from benign solitary pulmonary lesions. Journal of magnetic resonance imaging : JMRI. 2016;43(3):669–679. doi: 10.1002/jmri.25018. [DOI] [PubMed] [Google Scholar]
- 16.Chung J, Kim JH, Lee EJ, Kim YN, Yi CA. Semiautomatic determination of arterial input functions for quantitative dynamic contrast-enhanced magnetic resonance imaging in non-small cell lung cancer patients. Investigative radiology. 2015;50(3):129–134. doi: 10.1097/RLI.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 17.Koenigkam-Santos M, Optazaite E, Sommer G, et al. Contrast-enhanced magnetic resonance imaging of pulmonary lesions: description of a technique aiming clinical practice. European journal of radiology. 2015;84(1):185–192. doi: 10.1016/j.ejrad.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Chandarana H, Feng L, Block TK, et al. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Investigative radiology. 2013;48(1):10–16. doi: 10.1097/RLI.0b013e318271869c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic resonance in medicine. 2014;72(3):707–717. doi: 10.1002/mrm.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandarana H, Feng L, Ream J, et al. Respiratory Motion-Resolved Compressed Sensing Reconstruction of Free-Breathing Radial Acquisition for Dynamic Liver Magnetic Resonance Imaging. Investigative radiology. 2015;50(11):749–756. doi: 10.1097/RLI.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandarana H, Block TK, Ream J, et al. Estimating Liver Perfusion From Free-breathing Continuously Acquired Dynamic Gd-EOB-DTPA Enhanced Acquisition With Compressed Sensing Reconstruction. Investigative radiology. 2015;50(2):88–94. doi: 10.1097/RLI.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenkrantz AB, Geppert C, Grimm R, et al. Dynamic contrast-enhanced MRI of the prostate with high spatiotemporal resolution using compressed sensing, parallel imaging, and continuous golden-angle radial sampling: preliminary experience. Journal of magnetic resonance imaging : JMRI. 2015;41(5):1365–1373. doi: 10.1002/jmri.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riffel P, Zoellner FG, Budjan J, et al. “One-Stop Shop”: Free-Breathing Dynamic Contrast-Enhanced Magnetic Resonance Imaging of the Kidney Using Iterative Reconstruction and Continuous Golden-Angle Radial Sampling. Investigative radiology. 2016;51(11):714–719. doi: 10.1097/RLI.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 24.Ream JM, Doshi A, Lala SV, Kim S, Rusinek H, Chandarana H. High Spatiotemporal Resolution Dynamic Contrast-Enhanced MR Enterography in Crohn Disease Terminal Ileitis Using Continuous Golden-Angle Radial Sampling, Compressed Sensing, and Parallel Imaging. AJR American journal of roentgenology. 2015;204(6):663–669. doi: 10.2214/AJR.14.13674. [DOI] [PubMed] [Google Scholar]
- 25.Kai TB, Chandarana H, Milla S, et al. Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. Journal of the Korean Society of Magnetic Resonance in Medicine. 2014;18(2):87. [Google Scholar]
- 26.Koh TS, Bisdas S, Koh DM, Thng CH. Fundamentals of tracer kinetics for dynamic contrast-enhanced MRI. Journal of magnetic resonance imaging : JMRI. 2011;34(6):1262–1276. doi: 10.1002/jmri.22795. [DOI] [PubMed] [Google Scholar]
- 27.Østergaard L. Principles of cerebral perfusion imaging by bolus tracking. Journal of Magnetic Resonance Imaging. 2005;22(6):710–717. doi: 10.1002/jmri.20460. [DOI] [PubMed] [Google Scholar]
- 28.Sourbron S. Technical aspects of MR perfusion. European journal of radiology. 2010;76(3):304–313. doi: 10.1016/j.ejrad.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Chandarana H, Block KT, Winfeld MJ, et al. Free-breathing contrast-enhanced T1-weighted gradient-echo imaging with radial k-space sampling for paediatric abdominopelvic MRI. European radiology. 2014;24(2):320–326. doi: 10.1007/s00330-013-3026-4. [DOI] [PubMed] [Google Scholar]
- 30.Feng L, Huang C, Shanbhogue K, Sodickson DK, Chandarana H, Otazo R. RACER-GRASP: Respiratory-weighted, aortic contrast enhancement-guided and coil-unstreaking golden-angle radial sparse MRI. Magnetic resonance in medicine. 2017 doi: 10.1002/mrm.27002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magnetic resonance in medicine. 2016;75(2):775–788. doi: 10.1002/mrm.25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiuli T, Han O, Ning W, et al. [Preliminary study of semi-quantitative and quantitative dynamic contrast-enhanced MRI in evaluating the response to concurrent chemoradiotherapy in patients with non-small cell lung cancer] Zhonghua zhong liu za zhi [Chinese journal of oncology] 2015;37(4):272–277. [PubMed] [Google Scholar]
- 33.Wang LL, Lin J, Liu K, et al. Intravoxel incoherent motion diffusion-weighted MR imaging in differentiation of lung cancer from obstructive lung consolidation: comparison and correlation with pharmacokinetic analysis from dynamic contrast-enhanced MR imaging. European radiology. 2014;24(8):1914–1922. doi: 10.1007/s00330-014-3176-z. [DOI] [PubMed] [Google Scholar]
- 34.Chang YC, Yu CJ, Chen CM, et al. Dynamic contrast-enhanced MRI in advanced nonsmall-cell lung cancer patients treated with first-line bevacizumab, gemcitabine, and cisplatin. Journal of magnetic resonance imaging : JMRI. 2012;36(2):387–396. doi: 10.1002/jmri.23660. [DOI] [PubMed] [Google Scholar]
- 35.Hittmair K, Eckersberger F, Klepetko W, Helbich T, Herold CJ. Evaluation of solitary pulmonary nodules with dynamic contrast-enhanced MR imaging--a promising technique. Magnetic resonance imaging. 1995;13(7):923–933. doi: 10.1016/0730-725x(95)02010-q. [DOI] [PubMed] [Google Scholar]
- 36.Kono R, Fujimoto K, Terasaki H, et al. Dynamic MRI of solitary pulmonary nodules: comparison of enhancement patterns of malignant and benign small peripheral lung lesions. Ajr American Journal of Roentgenology. 2007;188(1):26–36. doi: 10.2214/AJR.05.1446. [DOI] [PubMed] [Google Scholar]
- 37.Ohno Y, Koyama HD, Nogami M, et al. Dynamic MRI, dynamic multidetector-row computed tomography (MDCT), and coregistered 2-(fluorine-18)-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET)/CT: comparative study of capability for management of pulmonary nodules. Journal of Magnetic Resonance Imaging Jmri. 2008;27(6):1284–1295. doi: 10.1002/jmri.21348. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology. 1997;205(2):471. doi: 10.1148/radiology.205.2.9356631. [DOI] [PubMed] [Google Scholar]
- 39.Ohno Y, Hatabu H, Takenaka D, Adachi S, Kono M, Sugimura K. Solitary pulmonary nodules: potential role of dynamic MR imaging in management initial experience. Radiology. 2002;224(2):503. doi: 10.1148/radiol.2242010992. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Kim HJ, Lee KH, Kim KH, Lee HL. Solitary pulmonary nodules: a comparative study evaluated with contrast-enhanced dynamic MR imaging and CT. Journal of computer assisted tomography. 2004;28(6):766–775. doi: 10.1097/00004728-200411000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An example displaying different dynamic frames of 3s-GRASP images from a representative patient diagnosed as squamous-cell carcinoma.