Abstract
Introduction
All Department of Defense (DoD) guidance documents recommend cefazolin or clindamycin as post-trauma antibiotic prophylaxis for open soft-tissue injuries. Although not advocated, some patients with open soft-tissue injuries also received expanded Gram-negative coverage (EGN) prophylaxis based on the judgment of front-line trauma providers. During the study period, revised guidelines in 2011/2012 re-emphasized recommendations for using cefazolin or clindamycin, and stewardship efforts in the DoD trauma community aimed to reduce the practice of adding EGN to guideline-recommended antibiotic prophylaxis. Our objective was to examine antibiotic utilization among wounded military personnel with open extremity soft-tissue injuries over a 5-yr period and assess the impact on infectious outcomes in patients who received EGN prophylaxis versus guideline-directed prophylaxis.
Methods
The study population included military personnel with open extremity soft-tissue injuries sustained in Iraq and Afghanistan (2009–2014) who transferred to participating hospitals in the USA following medical evacuation. The analysis was restricted to patients who were hospitalized for at least seven days at a U.S. facility and excluded those who sustained open fractures. Post-trauma antibiotic prophylactic regimens were defined as narrow if they followed recommended guidance (e.g., IV cefazolin or clindamycin) or EGN coverage when the narrow regimen also included fluoroquinolones and/or aminoglycosides. Intravenous amoxicillin-clavulanate, which is commonly used at non-U.S. coalition theater hospitals, was also classified as narrow because it conformed to coalition antibiotic prophylaxis guidelines. This study was approved by the Infectious Disease Institutional Review Board of the Uniformed Services University of the Health Sciences.
Results
A total of 287 wounded personnel with open soft-tissue injuries were assessed, of which 212 (74%) received narrow prophylaxis and 75 (26%) received EGN coverage (p < 0.001). Among patients in the narrow prophylaxis group, 81% were given cefazolin and/or clindamycin, while 19% received amoxicillin-clavulanate. In the EGN group, 88% and 12% received a fluoroquinolone and aminoglycoside, respectively. Use of EGN coverage significantly declined during the study period from 39% in 2009–2010 to 11% in 2013–2014 (p < 0.001). Approximately 3% of patients who received a narrow regimen developed an extremity skin and soft-tissue infection, while there were no skin and soft-tissue infections among patients in the EGN coverage group. Nonetheless, this was not a significant difference (p = 0.345). In addition, the proportion of non-extremity infections was not significantly different between narrow and EGN regimen groups (11% and 15%, respectively). There were also no significant differences between the narrow and EGN regimen groups related to duration of hospitalization (median of 19 versus 20 d).
Conclusion
Use of non-guideline directed EGN-based post-trauma antibiotic prophylaxis does not improve infectious outcomes nor does it shorten hospital stay.
Keywords: clinical practice guidelines, antimicrobial prophylaxis, combat-related infections, extremity infections, open soft-tissue injuries
BACKGROUND
Antimicrobial prophylaxis is an important adjunct to debridement in the prevention of post-trauma infections.1–5 With the goal of standardizing guidance for combat-related trauma antibiotic prophylaxis, clinical practice guidelines for the prevention and management of combat trauma-related infections were developed by an expert consensus panel convened by the Department of Defense (DoD) in 2008 and later revised in 2011.6,7 In addition, internal guidance documents with post-trauma prophylactic recommendations were also developed by the Joint Trauma System (JTS) following the publication of the guidelines in 2010 (no longer publically available) and 2012.8,9 Despite changes in recommendations for post-combat trauma antibiotic prophylaxis in Type III open fractures and hollow viscus injuries, use of IV cefazolin or clindamycin along with thorough debridement following combat-related open soft-tissue injury was/is uniformly recommended for the prevention of infections in all published and internal DoD and JTS guidance documents.
While antibiotic prophylaxis for open soft-tissue injury recommendations remained constant, the addition of fluoroquinolones and/or aminoglycosides (i.e., expanded gram-negative [EGN] coverage) to narrow prophylaxis was common. For example, approximately 20% of patients with open soft-tissue injuries received EGN coverage between 2009 and 2011.10 The reason for this deviation from guideline recommendations is unclear, but it likely reflected concern for inoculation of gram-negative bacteria in grossly contaminated wounds. Of note, use of EGN coverage was also common in other types of injuries. In particular, 48% of patients who sustained combat-related open fracture injuries between 2009 and 2010 received EGN-based prophylaxis.11 Following internal and published assessments of antibiotic utilization,12 and with the goal of reducing use of broad-spectrum antibiotics, the JTS re-emphasized adherence to DoD and JTS guidance with the publication of the 2011 guideline and subsequent 2012 internal JTS document.7,9 Correspondingly, the use of EGN coverage with open soft-tissue injuries declined to approximately 6% in 2013–2014.10 Herein, we further examine patterns of antibiotic utilization among wounded military personnel with open soft-tissue extremity injuries and assess infectious outcomes related to use of EGN prophylaxis.
METHODS
Study Population and Data Collection
Wounded military personnel were eligible for inclusion in this retrospective analysis if they sustained at least one combat-related open extremity soft-tissue injury in either Iraq or Afghanistan between June 1, 2009 and May 31, 2014, which required medical evacuation from the operational theater to Landstuhl Regional Medical Center (Germany) before transfer to a participating military hospital in the USA. An additional requirement was that patients were hospitalized for at least 7 d in the USA based on previous data related to the timing of wound infections.13 Patients with open extremity fractures were excluded from this analysis and assessed separately.14 Data were collected as part of the Trauma Infectious Disease Outcomes Study (TIDOS), which is an observational, multisite cohort study implemented to examine the short- and long-term infectious complications related to deployment-related traumatic injuries.13 This study was approved by the Infectious Disease Institutional Review Board of the Uniformed Services University of the Health Sciences (Bethesda, MD, USA).
Patient demographics and injury characteristics were obtained from the DoD Trauma Registry.15 The injury data were standardized into Abbreviated Injury Scale-defined codes16 using an injury coding software system (Tri-Code, Digital Innovations, Inc., Forest Hill, MD, USA), which allowed for categorization of injury types by specific body regions. Infection-related data (e.g., infection syndromes and antimicrobial treatment) were retrieved from the supplemental TIDOS infectious disease module.13 Clinical findings and laboratory test results obtained from medical record abstraction were used to identify skin and soft-tissue infections (SSTIs), which were classified using the National Healthcare Safety Network (NHSN) standardized definitions.13,17 An infection was excluded if there was an alternate diagnosis and discontinuation of antimicrobial therapy. Multidrug-resistant organisms (MDROs) were identified using standardized NHSN definitions18 and collected through infection control-based surveillance groin swabs at hospital admission and clinical infection work-ups.
Antimicrobial Prophylaxis Classification
Antibiotic usage was ascertained through medical chart review, as described in detail in prior publications.10,11 Post-trauma antibiotic prophylaxis regimens were classified as narrow if there was any use of cefazolin or clindamycin. The use of IV amoxicillin-clavulanate (or ampicillin-sulbactam) was also considered a narrow regimen due to the transition of many patients through coalition treatment facilities where these antibiotics were used as a substitute for cefazolin.19,20 Classification of antibiotic prophylaxis as EGN coverage required the patient to receive a narrow regimen in addition to a fluoroquinolone (e.g., levofloxacin) and/or aminoglycoside (e.g., gentamicin). Patients who received either no antibiotics or any of the following were excluded from the analysis: macrolides, antifungals (prescribed due to concern for invasive fungal infections), oral antibiotics (with the exception of oral levofloxacin), and/or antibiotics traditionally used to target MDROs (e.g., carbapenems, piperacillin-tazobactam, and vancomycin). Assessment of antimicrobial prophylaxis occurred in the immediate period after injury for up to 48 h (i.e., day of injury and day after the injury) to account for potential documentation omissions and numerous transitions of care associated with combat casualty medical evacuation.11,12 Antibiotics provided at the point of injury prior to admission to combat support hospitals were not considered due to a lack of documentation.
Statistical Analysis
Primary endpoints include SSTI, isolation of MDROs, and Clostridium difficile infections. Susceptibility of gram-negative organisms to fluoroquinolones and aminoglycosides was also examined. Categorical variables were compared using Fisher’s exact and Chi square tests, while Kruskal–Wallis test was used to compare overall distribution of continuous variables. Statistical analysis was performed with SAS version 9.3 (SAS, Cary, NC, USA). Significance was defined as p < 0.05.
RESULTS
Study Population
Between June 2009 and May 2014, a total of 2,564 wounded military personnel were transferred to a participating U.S. hospital. Patients who did not meet the inclusion criteria based on U.S. hospitalization of less than seven days (N = 419), lack of qualifying extremity trauma (N = 504), sustaining an open extremity fracture (N = 1,044), and ineligible antibiotic prophylactic regimen (no antibiotics, N = 99; broad-spectrum antibiotics, N = 191; macrolides, N = 2; antifungals, N = 3; oral antibiotics [other than oral levofloxacin], N = 15) were excluded. Therefore, the study population was comprised of 287 military personnel with an open extremity soft-tissue injury.
Patients included in the study population were predominantly young (median of 24 yr) men (99%) who were injured in support of operations in Afghanistan (95%; Table 1). The primary mechanism of injury was blast (65%), followed by gunshot (27%). In general, injury severity was minor (44% with Injury Severity Score21 of 1–9) with a median hospitalization of 19 d. Two patients (0.7%) in the study population died.
Table I.
Characteristics of Patients with Open Extremity Soft-Tissue Injuries by Post-Trauma Antibiotic Prophylaxisa .
| Characteristics, No. (%) | Total (N = 287) | Narrow (N = 212) | EGN (N = 75) | p-Value |
|---|---|---|---|---|
| Male | 283 (98.6) | 208 (98.1) | 75 (100) | 0.576 |
| Age, median years (IQR) | 24 (21, 28) | 24 (22, 28) | 23 (21, 28) | 0.764 |
| Injured in Afghanistan | 273 (95.1) | 200 (94.3) | 73 (97.3) | 0.532 |
| Injury mechanism | ||||
| Gunshot | 76 (26.5) | 62 (29.3) | 14 (18.7) | 0.074 |
| Blast | 187 (65.2) | 132 (62.3) | 55 (73.3) | 0.084 |
| Motor vehicle crash | 17 (5.9) | 13 (6.1) | 4 (5.3) | ~1.00 |
| Injury Severity Scoreb | ||||
| Median (IQR) | 11 (6, 24) | 11 (6, 24) | 12 (6, 26) | 0.206 |
| 1–9 (minor) | 126 (43.9) | 96 (45.3) | 30 (40.0) | 0.868c |
| 10–15 (moderate) | 45 (15.7) | 32 (15.1) | 13 (17.3) | |
| 16–25 (severe) | 45 (15.7) | 32 (15.1) | 13 (17.3) | |
| ≥26 (critical) | 71 (24.7) | 52 (24.5) | 19 (25.3) | |
| ICU admission | ||||
| None | 174 (60.6) | 134 (63.2) | 40 (53.3) | 0.322 |
| LRMC only | 32 (11.1) | 22 (10.4) | 10 (13.3) | |
| LRMC ± U.S. hospital | 81 (28.2) | 56 (26.4) | 25 (33.3) | |
| RBC units 24 h post-injury | ||||
| None or missing | 208 (72.5) | 155 (73.1) | 53 (70.7) | 0.341 |
| 1–9 | 64 (22.3) | 45 (21.2) | 19 (25.3) | |
| 10–20 | 9 (3.1) | 6 (2.8) | 3 (4.0) | |
| ≥21 | 6 (2.1) | 6 (2.8) | 0 | |
EGN, expanded gram-negative coverage; ICU, intensive care unit; IQR, interquartile range; LRMC, Landstuhl Regional Medical Center; RBC, red blood cell.
aExpanded gram-negative (i.e., addition of a fluoroquinolone and/or aminoglycoside to narrow coverage).
bInjury Severity Score21 is an overall measure calculated for each patient based on the top three maximum Abbreviated Injury Scale anatomical region values.
cp-Value is for the comparison of the Injury Severity Score profile between the regimen groups.
Among the 287 trauma patients, 212 (74%) and 75 (26%) received narrow and EGN post-trauma antibiotic prophylaxis, respectively (p < 0.001). There was no significant difference between the patients who received the narrow regimen and EGN coverage regarding injury severity, mechanism of injury, transfusion requirements, and admission to the intensive care unit (Table 1). Nonetheless, there appeared to be a non-significant trend towards EGN patients being less likely to have gunshot wounds and more likely to have blast as their mechanism of injury.
Antimicrobial Use Patterns
The narrow regimen contained cefazolin (or clindamycin) for 172 (81% of 212) patients, while 40 (19%) received a beta-lactam beta-lactamase inhibitor (e.g., IV amoxicillin-clavulanate). For patients who received EGN coverage, the majority were prescribed a fluoroquinolone (N = 66; 88% of 75) compared with aminoglycosides (N = 9; 12%). No patients received both a fluoroquinolone and aminoglycoside. For patients who received a regimen classified as narrow, they had a significantly shorter duration of antibiotic use (median: 3 d; interquartile range: 2–5 d) when compared with the duration of narrow-spectrum antibiotics used among patients in the EGN group (median: 4 d; interquartile range: 2–5 d; p = 0.023). Over the study period, use of EGN coverage declined (p < 0.001) with the highest proportion occurring between 2009 and 2011 (34–39%), followed by a decline to 9% in 2011–2012, increase to 18% in 2012–2013, and, finally, a decrease to 11% in 2013–2014.
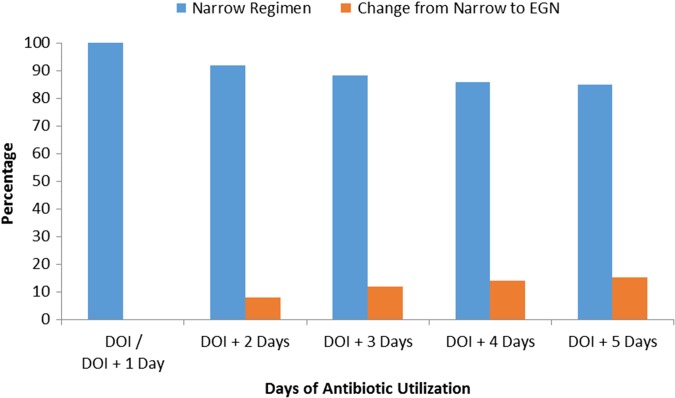
Thirty-two patients (15% of 212) had their prophylactic regimen changed to include EGN coverage after receiving a narrow regimen for at least 48 h (day of injury plus day after injury) (Fig. 1). The majority of patients (17; 53% of 32) had the change occur 1 d after the start of the narrow regimen with small increments of patients continuing to transition to EGN coverage as time following the initiation of the narrow regimen progressed.
FIGURE 1.
Proportion of patients with narrow prophylaxis (N = 212) who had their regimen changed to include EGN coverage. DOI, day of injury.
Non-Infection Outcomes
There was no significant difference in the number of operating room visits (75% of both groups had less than two visits) or duration of hospitalization (median of 20 versus 19 d) between the patients who received EGN coverage and a narrow regimen, respectively (Table 2). There was also no difference in mortality between the two groups, and the deaths that occurred were in very severely injured soldiers and not the result of a SSTI.
Table II.
Open Extremity Soft-Tissue Injury Outcomes by Post-Trauma Antibiotic Prophylaxis.
| Outcome, No. (%) | Total (N = 287) | Narrow (N = 212) | EGN (N = 75) | p-Value |
|---|---|---|---|---|
| Any infection at LRMC | 17 (5.9) | 10 (4.7) | 7 (9.3) | 0.145 |
| Any infection at U.S. hospital | 43 (15.0) | 32 (15.1) | 11 (14.7) | 0.929 |
| Extremity SSTI | 6 (2.1) | 6 (2.8) | 0 | 0.345 |
| Non-extremity Infections | 35 (12.2) | 24 (11.3) | 11 (14.7) | 0.447 |
| Occurrence within 2 wk of injury | ||||
| Extremity SSTI | 5 (1.7) | 5 (2.4) | 0 | 0.331 |
| MDRO isolation | 54 (18.8) | 38 (17.9) | 16 (21.3) | 0.516 |
| MRSA isolation | 3 (1.1) | 2 (0.9) | 1 (1.3) | ~1.00 |
| VRE isolation | 0 | 0 | 0 | NA |
| Occurrence within 4 wk of injury | ||||
| Extremity SSTI | 6 (2.1) | 6 (2.8) | 0 | 0.345 |
| Clostridium difficile during initial hospitalization | 2 (0.7) | 2 (0.9) | 0 | ~1.00 |
| EGN-resistant gram-negative organism isolationa | 68 (23.7) | 41 (19.3) | 27 (36.0) | 0.001 |
| Median visits to OR (IQR) | 0 (0, 2) | 0 (0, 2) | 0 (0, 2) | 0.621 |
| Total Hospitalization, median days (IQR)b | 19 (14, 28) | 19 (14, 26) | 20 (15, 34) | 0.069 |
| Death | 2 (0.7) | 2 (0.9) | 0 | 1.000 |
EGN, expanded gram-negative coverage; IQR, interquartile range; LRMC, Landstuhl Regional Medical Center; MDRO, multidrug-resistant organism; MRSA, methicillin-resistant Staphylococcus aureus; OR, operating room; SSTI, skin and soft-tissue infection; VRE, vancomycin-resistant Enterococcus.
aRecovery of gram-negative organisms from any site that were resistant to fluoroquinolones and/or aminoglycosides.
bInjury to first discharge from U.S. participating hospital.
Gram-negative organisms (from any site) not susceptible to fluoroquinolones and/or aminoglycosides were isolated more commonly from patients receiving EGN coverage versus a narrow regimen (36% and 19%, respectively; p < 0.001; Table 2). When the population was restricted to the subset of 159 patients who had gram-negative organisms isolated (37 patients with EGN coverage and 122 patient in the narrow regimen group), the proportion of resistant organisms increased to 73% and 34%, respectively.
Infection Outcomes
Six patients (2.1%) developed an extremity SSTI, of which all were in the narrow regimen group (2.8% of 212; Table 2). Four of the patients were diagnosed with a deep SSTI (i.e., involves deep soft tissues, such as fascia and muscle layers of the wound), while the remaining two had superficial SSTIs (i.e., involves only skin and subcutaneous tissue). The frequency of non-extremity infections was comparable between the narrow regimen and EGN coverage groups (11% and 15%, respectively). There was also no significant difference related to isolation of MDROs and methicillin-resistant Staphylococcus aureus within the first two weeks of injury between the groups. When the definition of EGN coverage was expanded to include patients who received fluoroquinolones or aminoglycosides on day of injury plus two days, the results related to infectious outcomes remained the same (six SSTIs in narrow group and zero in EGN coverage group; p = 0.182). Furthermore, a higher proportion of EGN coverage patients recovered Gram-negative organisms resistant to fluoroquinolones/aminoglycosides (32% versus 19%; p < 0.001). A restricted analysis removed 32 patients who had EGN added after the initial prophylaxis period and the results demonstrated no difference from the full population (data not shown).
Given the high frequency of blast injuries coupled with a trend towards higher rates of EGN prophylaxis among blast patients, the frequency of SSTIs among the 187 blast patients (132 narrow and 55 EGN) was evaluated. No benefit in SSTI rates was observed with use of EGN prophylaxis. Specifically, there were three patients in the narrow group with extremity SSTIs compared with zero in the EGN group (p = 0.557). Blast injury patients also had a higher risk of isolating a Gram-negative organism resistant to fluoroquinolones and/or aminoglycosides when EGN was used (36% versus 17%; p = 0.001). Further sensitivity analysis excluded 59 patients who received a beta-lactam beta-lactamase inhibitor (e.g., IV amoxicillin-clavulanate). Among the remaining 171 patients in the narrow regimen group, 3 (1.8%) developed an extremity SSTI (all classified as deep). As with the other subgroup analyses, there were no significant differences between the narrow and EGN groups regarding infectious outcomes, while a higher proportion of fluoroquinolone and/or aminoglycoside-resistant Gram-negative organisms were recovered from patients who received EGN coverage (35% versus 19%; p < 0.001).
The six patients with diagnosed SSTIs in the narrow regimen group were further assessed. Three had minor injury severity (ISS < 10), one had severe injury severity (ISS = 24), and the remaining two had critical, life-threatening injury severity (ISS ≥26). None of the six patients with SSTIs died. In addition, the six SSTI events would not likely have been prevented by using EGN prophylaxis because the SSTIs either did not involve gram-negative organisms or, in cases with gram-negative organisms, the isolates were fluoroquinolone resistant.
DISCUSSION
Antibiotic prophylaxis targeting gram-positive skin flora and thorough debridement/irrigation are accepted, standard principles in the management of civilian and combat-related post-traumatic open soft-tissue injuries. Despite broad agreement between civilian and military guidelines,1,6–9 our findings reaffirm previous analyses showing that the use of EGN coverage in DoD combat-related injuries was prevalent in 2009–2011 and declined in the ensuing years.10,11 More importantly, our findings extend the body of literature by showing that adding a fluoroquinolone or aminoglycoside to post-traumatic antibiotic prophylaxis fails to reduce SSTI outcomes and comes at a cost of increased fluoroquinolone and/or aminoglycoside resistance to gram-negative organisms. These findings should reassure trauma clinicians that the practice of adding EGN antibiotics to post-trauma antibiotic prophylaxis regimens for soft-tissue injuries is unnecessary.
The main limitation of our analysis is that it is not a randomized trial, and instead is a retrospective observational study. During the study period, use of EGN was not uniform and declined following the dissemination and publication of the 2011 guideline.7 Nevertheless, the different usage patterns in the study years allowed us to assess outcomes in a period where injury severity, injury mechanism, and infection definitions remained constant. One factor our analysis did not control for is the possibility of varying approaches to the diagnosis of SSTI by different clinicians in different time periods; however, we feel it is unlikely that the approach to the clinical diagnosis of SSTI changed during the study period. Although not statistical significant, the patients who received EGN coverage had a high proportion of blast injuries. This may be due to the fact that use of EGN was more common in the years leading up to publication of the 2011 guideline7 when blast patients were more common relative to other injury types. Despite this trend, when the analysis was restricted to patients with blast injury, the findings of the study did not change. Another limitation is that some patients had EGN prophylactic antibiotics added more than 2 d following their traumatic injury. Despite this variance, when their prophylaxis category was re-categorized and when they were removed from the analysis, the results did not change. Duration of antibiotic prophylaxis was also slightly longer in the patients who received EGN prophylaxis; however, the median and ranges were similar and not clinically significant.
In summary, our findings provide evidence that the addition of EGN antibiotics to post-traumatic combat-related soft-tissue injuries does not improve infectious outcomes. Importantly, neither the guidelines nor this study should deter clinicians from using their clinical judgment and individualizing care.
Acknowledgements
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
Presentation
A portion of this data was presented at the 2016 Infectious Disease Society of America ID Week, October 26–30, 2016, New Orleans, LA, USA.
Funding
Support for this work (IDCRP-024) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Inter-Agency Agreement Y1-AI-5072, and the Department of the Navy under the Wounded, Ill, and Injured Program.
REFERENCES
- 1. Hoff WS, Bonadies JA, Cachecho R, Dorlac WC: East Practice Management Guidelines Work Group: update to practice management guidelines for prophylactic antibiotic use in open fractures. J Trauma 2011; 70(3): 751–4. [DOI] [PubMed] [Google Scholar]
- 2. Jaeger M, Maier D, Kern WV, Sudkamp NP: Antibiotics in trauma and orthopedic surgery – a primer of evidence-based recommendations. Injury 2006; 37(Suppl 2): S74–80. [DOI] [PubMed] [Google Scholar]
- 3. Gosselin RA, Roberts I, Gillespie WJ: Antibiotics for preventing infection in open limb fractures. Cochrane Database Syst Rev 2004; (1): CD003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane JC, Mabvuure NT, Hindocha S, Khan W: Current concepts of prophylactic antibiotics in trauma: a review. Open Orthop J 2012; 6: 511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hauser CJ, Adams CA Jr., Eachempati SR: Council of the Surgical Infection Society: Surgical Infection Society guideline: prophylactic antibiotic use in open fractures: an evidence-based guideline. Surg Infect (Larchmt) 2006; 7(4): 379–405. [DOI] [PubMed] [Google Scholar]
- 6. Hospenthal DR, Murray CK, Andersen RC, et al. : Guidelines for the prevention of infection after combat-related injuries. J Trauma 2008; 64(3): S211–20. [DOI] [PubMed] [Google Scholar]
- 7. Hospenthal DR, Murray CK, Andersen RC, et al. : Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma 2011; 71(2 Suppl 2): S210–34. [DOI] [PubMed] [Google Scholar]
- 8. Joint Theater Trauma System : Guidelines to prevent infection in combat-related injuries (Approved March 1, 2010). United States Army Institute of Surgical Research; 2010. [no longer publically available do to publication of revised guideline].
- 9. Joint Theater Trauma System : Guidelines to prevent infection in combat-related injuries (Approved April 2, 2012). United States Army Institute of Surgical Research; 2012. Available at http://www.usaisr.amedd.army.mil/cpgs/Infection_Control_2_Apr_12.pdf; accessed 7 August 2017.
- 10. Lloyd BA, Murray CK, Bradley W, et al. : Variation in post-injury antibiotic prophylaxis patterns over five years in a combat zone. Mil Med 2017; 182(S1): 346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lloyd BA, Weintrob AC, Hinkle MK, et al. : Adherence to published antimicrobial prophylaxis guidelines for wounded service members in the ongoing conflicts in southwest Asia. Mil Med 2014; 179(3): 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tribble DR, Lloyd B, Weintrob A, et al. : Antimicrobial prescribing practices following publication of guidelines for the prevention of infections associated with combat-related injuries. J Trauma 2011; 71(2 Suppl 2): S299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tribble DR, Conger NG, Fraser S, et al. : Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: trauma infectious disease outcome study. J Trauma 2011; 71(1 Suppl): S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lloyd BA, Murray CK, Shaikh FS, et al. : Early infectious outcomes following addition of fluoroquinolone or aminoglycoside to post-trauma antibiotic prophylaxis in combat-related open fracture injuries. J Trauma Acute Care Surg 2017; 83(5): 854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB: Trauma system development in a theater of war: experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 2006; 61(6): 1366–72. [DOI] [PubMed] [Google Scholar]
- 16. Champion HR, Holcomb JB, Lawnick MM, et al. : Improved characterization of combat injury. J Trauma 2010; 68(5): 1139–50. [DOI] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention : CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2014. Available at http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf; accessed 7 August 2017.
- 18. Division of Healthcare Quality Promotion : The National Healthcare Safety Network (NHSN) Manual. Patient Safety Component. Multidrug-Resistant Organism and Clostridium difficile Infection (MDRO/CDI) Module. Centers for Disease Control and Prevention; 2016. Available at http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf; accessed 7 August 2017.
- 19. Merens A, Rapp C, Delaune D, Danis J, Berger F, Michel R: Prevention of combat-related infections: antimicrobial therapy in battlefield and barrier measures in French military medical treatment facilities. Travel Med Infect Dis 2014; 12(4): 318–29. [DOI] [PubMed] [Google Scholar]
- 20. United Kingdom Ministry of Defence : Clinical Guidelines for Operations. Joint Service Publication 999. Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/79106/20121204-8-AVB-CGO_Online_2012.pdf; Accessed 7 August 2017.
- 21. Linn S: The injury severity score – importance and uses. Ann Epidemiol 1995; 5(6): 440–46. [DOI] [PubMed] [Google Scholar]