Catastrophic epileptic encephalopathy of unclear etiology following a mild metabolic insult generally has a poor outcome. Here, we present 2 such unrelated individuals in whom whole-exome sequencing identified the same de novo recurrent mutation (c.1207C>T p.Arg403Cys) in the gene encoding the guanosine triphosphatase (GTPase) Dynamin-1 like Protein (DNM1L) (reference sequence NM_012062.4).
The dynamic fission and fusion of the intracellular mitochondrial network are essential to facilitate mitophagy and thus mitochondrial quality and function.1 During mitochondrial division, the GTPase DNM1L forms multimeric collars at specific fission sites, constricting portions of the mitochondrial reticulum and generating fragments for engulfment and degradation.2
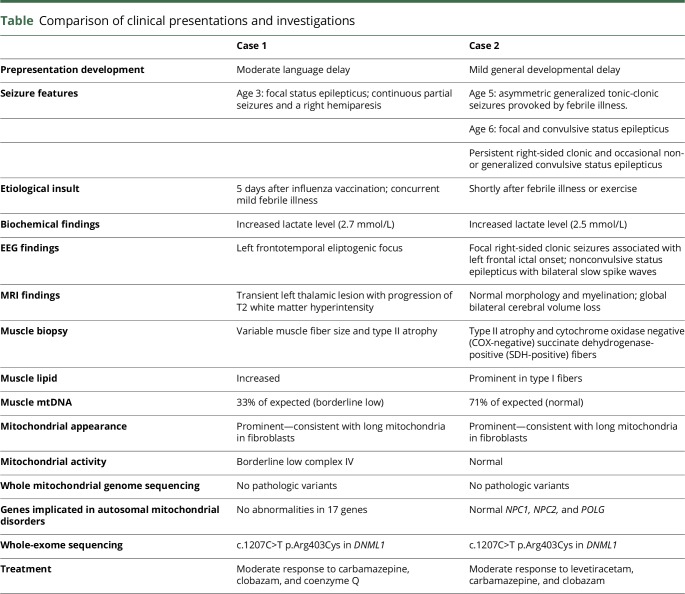
DNM1L has been implicated in several presentations of refractory epilepsy.3 Both of our patients exhibited signs of preexisting developmental delay and presented with epilepsy during, or recently following, a febrile illness or exercise. Elevated lactate levels, epilepsia partialis continua, nonspecific imaging, and evidence of lipid storage myopathy all support mitochondrial dysfunction (See table for presentation summary and e-case report for details, links.lww.com/NXG/A63). This evidence supports an etiological role for DNM1L in mitochondrial epilepsy syndrome with fever sensitivity (MEFS).
Table.
Comparison of clinical presentations and investigations
Methods
We used IN Cell Analyzer 1000 (IN Cell 1000), a previously validated high-throughput imaging method for quantifying mitophagy and mitochondrial DNA (mtDNA) in cultured fibroblasts from patients compared with cultures derived from karyotypically normal controls. Cells were immunostained for the autophagy marker Light Chain 3 (LC3) and the mitochondrial import receptor, translocase of outer membrane 20 (TOM20) and analyzed with IN Cell1000. The readout for mitophagy was colocalization of LC3 puncta with TOM20-positive mitochondria.
Results
We showed that the mitochondria in fibroblasts from both patients are lengthened and hyperpolarized (figure e-1, A and B, links.lww.com/NXG/A62). Total mitophagic flux was increased, showing that mitophagy is activated (figure e-1C, i). This is consistent with the mtDNA depletion documented in fibroblasts (figure e-1C, iii) and the borderline low mtDNA content in skeletal muscle from patient 1 (table).
Discussion
In 2 patients, we identified a de novo dominant mutation in DMN1L, the same mutation having now been identified in 4 unrelated patients with refractory epilepsy.3 Presentation features and investigation findings supported an underlying mitochondrial pathology. Altered mitochondrial dynamics are now a well-established cause of disease (see supplementary information for reference, links.lww.com/NXG/A63), and defective mitochondrial fission may both cause synaptic dysfunction1 and impair responses to infection.4
In mice, constitutive homozygous knockouts of Drp1 (the murine homolog of DNM1L) do not survive embryogenesis, while conditional ablation leads to developmental defects, both associated with abnormal mitochondrial fission.5 Individuals heterozygous for DNM1L p.Arg403Cys display a milder phenotype, both in terms of mitochondrial structural and functional abnormalities and symptom severity.
Previously described cases similarly display several years of relatively normal development followed by severe, refractory epilepsy following a mild metabolic insult, vaccinations, or low-grade fever, resulting in profound global developmental delay.3 As in case 1, nonspecific thalamic hyperintensities were seen on MRI scans on the 2 previously reported probands with the p.Arg403Cys mutation.3 Case 2 demonstrated diffuse cerebral volume atrophy. These changes are in keeping with previously reported nonspecific T2 MRI hyperintensities and cerebral or cerebellar atrophy seen in other mitochondrial disorders. It remains to be seen whether MEFS is part of the same clinical spectrum of other conditions associated with status epilepticus related to febrile illnesses, i.e., new-onset refractory status epilepticus or febrile illness–related epilepsy syndrome.6
DNM1L is required for division of mitochondria and peroxisomes, interacting with receptor Mff and endoplasmic reticulum (ER) components. The missense mutation shared in our cases lies in the middle domain of DNM1L,3 impairing oligomerization and recruitment to mitochondria3 consistent with our findings of elongated mitochondria in fibroblasts from both patients (figure e-1A, links.lww.com/NXG/A62). Furthermore, knockdown of the DNM1L ortholog Drp1 in mouse cells and its ligand Mff can each cause mtDNA depletion and mitochondrial dysfunction,7 consistent with the borderline low mtDNA content and COX activity in skeletal muscle we demonstrated in case 1. This is likely due to increased mitophagic flux (figure e-1C, i).
Our findings suggest that DNM1L is implicated as a genetic contributor to MEFS. DNM1L p.Arg403Cys mutation screening could therefore be useful in patients with similar presentations and in identifying impaired mitochondrial fission causing synaptic dysfunction1 and defective response to infection.
Author contributions
E. Ladds wrote the initial case reports and manuscript draft, was part of the editing and submissions process, and prepared the cases for presentation at the British Paediatric Neurology Association Conference 2018. A. Whitney was the pediatrician responsible for identifying SP and summarizing his case. E. Dombi and M. Hofer performed the laboratory tests and analysis of the data. G. Anand was the pediatrician responsible for initially summarizing MN's clinical presentation with the key learning points, bringing together the coauthors, and initiating and supervising case presentation at the National British Paediatric Neurology Association Conference 2018. He also contributed to the final editing process. V. Harrison initiated the laboratory tests and analysis of the data. C. Fratter, J. Carver, and I.A. Barbosa performed the laboratory tests and analysis of the data. M. Simpson performed the whole-exome sequencing. S. Jayawant was the pediatric neurology consultant responsible for MN's clinical care and identifying the case for publication. J. Poulton performed conceptualization of the study and analysis of the data and revised the manuscript.
Study funding
The authors acknowledge Kate Sergeant and Charu Deshpande for their involvement in whole-exome sequencing and parental testing of case 1, which was supported by the Lily Foundation.
Disclosure
E. Ladds reports no disclosures. A Whitney has served on the scientific advisory board of Zogenix and has received funding for speaker honoraria from Zogenix. E. Dombi, M. Hofer, G. Anand, V. Harrison, C. Fratter, and J. Carver report no disclosures. I.A. Barbosa has received research funding from the Lily Foundation. M. Simpson is employed by Genomics Plc. S. Jayawant reports no disclosures. J. Poulton receives research support from the Lily Foundation, the Wellcome Trust, and the UK Medical Research Council. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NG.
References
- 1.Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol 2013;23:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 2001;12:2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahrner JA, Liu R, Perry MS, Klein J, Chan DC. A novel de novo dominant negative mutation in DNM1L impairs mitochondrial fission and presents as childhood epileptic encephalopathy. Am J Med Genet A 2016;170:2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahni R, Cale CM, Anderson G, et al. Signal transducer and activator of transcription 2 deficiency is a novel disorder of mitochondrial fission. Brain 2015;138:2834–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahill TJ, Leo V, Kelly M, et al. Resistance of dynamin-related protein 1 oligomers to disassembly impairs mitophagy, resulting in myocardial inflammation and heart failure. J Biol Chem 2015;290:25907–25919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaspard N, Hirsch LJ, Sculier C, et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia 2018;59:745–752. [DOI] [PubMed] [Google Scholar]
- 7.Parone PA, Da Cruz S, Tondera D, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One 2008;3:e3257. [DOI] [PMC free article] [PubMed] [Google Scholar]