Abstract
Morin, a natural flavonol, exhibits antioxidative, anti-inflammatory and anti-apoptotic effects in various pathological and physiological processes. However, whether morin exerts a protective effect on myocardial ischemia-reperfusion injury (MIRI) is unknown. The present study aimed to determine the effect of morin on MIRI in cultured cardiomyocytes and isolated rat hearts, and to additionally explore the underlying mechanism. The effect of morin on the viability, lactate dehydrogenase (LDH) activity and apoptosis of H9c2 cardiomyocytes subjected to hypoxia/reoxygenation, and cardiac function and infarct size of rat hearts following ischemia/reperfusion in an animal model were measured. Furthermore, the mitochondrial permeability transition pore (MPTP) opening, mitochondrial membrane potential (ΔΨm), and the change in the expression levels of B-cell lymphoma 2 (Bcl2)-associated X protein (Bax), Bcl-2 and mitochondrial apoptosis-associated proteins following MPTP opening were also detected. The results indicated that morin treatment significantly increased cell viability, decreased LDH activity and cell apoptosis, improved the recovery of cardiac function and decreased the myocardial infarct size. Furthermore, morin treatment markedly inhibited MPTP opening, prevented the decrease of ΔΨm, and decreased the expression of cytochrome c, apoptotic protease activating factor-1, caspase-9, caspase-3 and the Bax/Bcl-2 ratio. However, these beneficial effects were reversed by treatment with atractyloside, an MPTP opener. The present study demonstrated that morin may prevent MIRI by inhibiting MPTP opening and revealed the possible mechanism of the cardioprotection of morin and its acting target. It also provided an important theoretical basis for the research on drug interventions for MIRI in clinical applications.
Keywords: myocardial ischemia-reperfusion injury, morin, atractyloside, mitochondrial permeability transition pore, apoptosis
Introduction
Acute myocardial infarction (AMI) is one of the most severe fatal conditions worldwide (1). AMI is caused by the complete blockage of the coronary artery which can lead to serious complications following myocardial necrosis, including malignant ventricular arrhythmia, heart failure, rupture of the heart and even sudden cardiac death. Thus, AMI patients remain at a higher risk of long-term mortality following discharge from hospitals (2,3). Restoration of the blood supply to the heart, termed reperfusion, is considered the most effective treatment for patients with AMI (4,5). However, reperfusion itself may aggravate the extent of myocardial injury, which is termed myocardial ischemia-reperfusion injury (MIRI) (6). The manifestations of MIRI include myocardial stunning, severe fatal ventricular arrhythmia, no reflow in coronary artery or even enlargement of myocardial infarct size (4,7). Although several methods, including ischemic preconditioning, ischemic post-conditioning and remote ischemic preconditioning, have been demonstrated to alleviate MIRI (8-10), these procedures are limited in clinical practice due to medical ethics. Therefore, identification of a novel drug to prevent and mitigate MIRI that is more viable in clinical practice has become the focus of clinical studies.
The process of MIRI is complex and involves multiple mechanisms, including oxidative stress, inflammatory response and apoptosis (11-13). The mitochondrial membrane permeability transition pore (MPTP) is a transmembrane structure of mitochondria. MPTP has been identified as a key modulator of MIRI (14). Several studies have identified that MPTP remains closed during myocardial ischemia, but becomes open in the early stage of reperfusion (15). MPTP opening is usually induced by increased cytosolic and mitochondrial matrix Ca2+ levels, accumulation of reactive oxygen species (ROS) and oxidative stress (16-18). Blocking the opening of MPTP with its specific inhibitor (cyclosporin A) prior or subsequent to ischemia may attenuate MIRI in animals and humans (19,20). Therefore, MPTP is an important therapeutic target for preventing MIRI.
Morin, or 3,5,7,2′,4′-pentahydroxyflavone, is a natural flavonol compound (21). Morin has multiple pharmacological effects, including antioxidative, anti-inflammatory and anti-apoptotic functions, scavenging of oxygen free radicals, inhibition of lipid peroxidation and improving the activities of various oxidases (22,23). Furthermore, morin is able to scavenge reactive oxygen species (ROS) and decrease the level of ROS (23). Therefore, we hypothesized that morin may protect against MIRI by inhibiting the MPTP opening. Taken together, the aim of the present study was to determine the protective effect of morin on MIRI in cultured H9c2 cardiomyocytes and isolated rat hearts, and to additionally explore the critical role of MPTP in the cardioprotective effects of morin.
Materials and methods
Animals
A total of 135 healthy male SPF Wistar rats, weighing 300±20 g, were purchased from Liaoning Changsheng Biotechnology Co., (Shenyang, China). The rats were housed in a quiet animal room maintained at 20–25°C with a relative humidity of 50–65%. The rats were provided fresh food and water ad libitum and a 12-h light/dark cycle. The food was also purchased from Liaoning Changsheng Biotechnology Co., Ltd. All rats were treated and used according to the Guide for the Care and Use of Laboratory Animals (Federal Register Doc. 2011-11490; National Institutes of Health, Bethesda, MD, USA) (24). The experimental protocol was approved by the Institutional Ethics Committee of China Medical University.
Drugs
Morin (CAS, 480-16-0; purity, ≥98%) and atractyloside (ATR; CAS, 102130-43-8, purity: ≥98%) were purchased from Nantong Feiyu Biological Technology Co. Ltd. (Nantong, China). The 2,3,5-triphenyltetrazolium chloride (TTC) was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Morin and ATR powder used in the animal experiments were dissolved in 10% polyethylene glycol 400 (PEG400; Beijing SolarBio Technology Co., Ltd., Beijing, China) and normal saline, respectively, to prepare solutions for intraperitoneal injections. Morin and ATR powders used in the cell experiments were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA).
Establishment of MIRI model in isolated rat hearts
Preparation of the heart tissue was performed as described previously (25). In brief, rats were anesthetized with intraperitoneal injection of pentobarbital sodium at a dose of 30 mg/kg. Each rat heart was rapidly isolated following opening of the chest, and then placed into an iced heparinized K-H solution (NaCl 0.15 mol/l, KCl 0.006 mol/l, CaCl2 0.002 mol/l, NaHCO3 0.002 mol/l), which was saturated with 100% oxygen. Subsequent to removal of the redundant connective tissue of the heart, each isolated rat heart was swiftly connected to the Langendorff apparatus through the aorta with a 7-0 surgical suture and continuously perfused using a K-H solution saturated with a mixed gas solution containing 95% O2 and 5% CO2 at the pressure of 75 mmHg at 37°C. All isolated rat hearts were perfused with the K-H solution using the perfusion apparatus and stabilized for 10 min prior to the induction of ischemia, and then subjected to global ischemia by stopping perfusion for 30 min, followed by the induction of a 60 min reperfusion to induce MIRI.
Animal experimental protocol
The animal experiment consisted of two phases. In the first phase, 75 rats were randomly divided into 5 groups with 15 rats/group as follows: i) the ischemia-reperfusion group (IR); ii) the solvent group (vehicle): Rats were treated with 1.5 ml 10% PEG400 by intraperitoneal injection once/day for a total of 5 days prior to surgery; iii) the 10 mg/kg morin pretreatment group (morin10): Rats were treated with 10 mg/kg morin by intraperitoneal injection once/day for a total of 5 days prior to surgery; iv) the 20 mg/kg morin pretreatment group (morin20): Rats were treated with 20 mg/kg morin by intraperitoneal injection as aforementioned for the morin10 group; and v) the 40 mg/kg morin pretreatment group (morin40): Rats were treated with 40 mg/kg morin by intraperitoneal injection as aforementioned for the morin10 group.
Based on the results at the first phase, morin at a dose of 20 mg/kg was used to additionally explore the mechanism of action. Therefore, in the second phase, 60 rats were randomly divided into 4 groups with 15 rats per group, as follows: i) The IR group; ii) the vehicle group; iii) the morin20 group; and iv) the morin20 combined with ATR group (morin20+ATR): Following treatment with 20 mg/kg morin the rats were additionally treated with ATR by intraperitoneal injection 30 min prior to surgery at a dose of 5 mg/kg.
Cell culture
H9c2 cardiomyocytes (Shanghai Institutes for Biological Sciences, China, Chinese Academy for Sciences, Shanghai, China) were cultured in Low Glucose Dulbecco’s Modified Eagle’s Medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; TBD, Tianjin, China) and 1% penicillin/streptomycin (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) under humid conditions in incubators with 5% CO2 at 37°C.
Cell experimental protocol
The H9c2 cells were subjected to hypoxia/reoxygenation to mimic the MIRI model in vitro. Specifically, cells were treated when the confluence of cells was 80-90%. The culture medium was removed and changed to Earle’s medium (CaCl2 0.18 mmol/l, MgSO4, 7H2O 0.08 mmol/l, KCl 0.05 mmol/l, NaCl 11.43 mmol/l, NaHCO3 2.62 mmol/l and NaH2PO4 0.10 mmol/l) without glucose and FBS, and cells were cultured in a tri-gas incubator with 90% N2, 5% CO2 and 5% O2 at 37°C for 12 h to induce hypoxia. Subsequently, Earle’s medium was removed, and the cells were cultured for 1 h with normal medium in incubators with 5% CO2 at 37°C to re-oxygenate the samples.
Similar to the aforementioned animal experimental protocol, cells were divided into six groups during the first phase as follows: i) The control group (control): Cardiomyocytes were cultured under normal conditions for 13 h; ii) the hypoxia/reoxygenation group (HR): Cardiomyocytes were subjected to hypoxia for 12 h followed by reoxygenation for 1 h; iii) the solvent group (vehicle): Cardiomyocytes were pretreated with 0.1% DMSO for 12 h followed by hypoxia/reoxygenation as described above for the HR group; iv) the 12.5 µM morin pretreatment group (morin12.5): Cardiomyocytes were pretreated with morin at a dose of 12.5 µM for 12 h, followed by hypoxia/reoxygenation as described above for the HR group; v) the 25 µM morin pretreatment group (morin25): Cardiomyocytes were pretreated with morin at a dose of 25 µM for 12 h, followed by hypoxia/reoxygenation as aforementioned for the HR group; and vi) the 50 µM morin pretreatment group (morin50): Cardiomyocytes were pretreated with morin at a dose of 50 µM for 12 h followed by hypoxia/reoxygenation as aforementioned for the HR group.
According to the result of the first phase, morin at a dose of 25 µM was used to additionally explore the mechanism of action. Cells were subsequently divided into four groups in the second phase, as follows: i) The control group; ii) the HR group; iii) the morin25 group; and iv) the 25 µM morin combined with 20 µM ATR pretreatment group (morin25+ATR): Cardiomyocytes were pretreated with morin at a dose of 25 µM and ATR at a dose of 20 µM for 12 h followed by hypoxia/reoxygenation as aforementioned for the HR group.
Cardiac function monitoring
Cardiac function parameters, including heart rate and coronary flow, were measured at 10 min of stabilization, and at 30 and 60 min following reperfusion.
Hematoxylin & eosin (HE) staining
The hearts were removed from the perfusion apparatus at the end of reperfusion. The heart tissues were fixed in 4% paraformaldehyde at room temperature for 24~72 h. Then, the tissues were washed with flowing water for 4 h, and immersed in 70% ethanol for 2 h, 80% ethanol overnight, 90% ethanol for 2 h, anhydrous ethanol I for 1 h, and anhydrous ethanol II for 1 h at room temperature to be dehydrated. Following dehydration the tissues were paraffin embedded and the paraffin blocks cut into 5-µm-thick paraffin sections. The paraffin sections were immersed in xylene I for 15 min, xylene II for 15 min, absolute ethanol I for 5 min, absolute ethanol II for 5 min, 95% ethanol for 2 min, 85% ethanol for 2 min, 75% ethanol for 2 min, and distilled water for 2 min at room temperature to be de-waxed and rehydrated. They were then immersed in hematoxylin solution for 5 min, 1% hydrochloric acid alcohol for 3 sec, and eosin solution for 3 min at room temperature for staining. The pathological changes in heart tissues were observed under a light microscope and images were captured at magnification, ×400.
Measurement of the infarct size
The myocardial infarct size was measured by TTC staining. The hearts were harvested as aforementioned, and the heart samples were incubated at −80°C for 30 min. The frozen hearts were cut into 1-2 mm thick sections from the apex to the bottom of the hearts and incubated in a 1% TTC solution at 37°C for 30 min. Subsequently, heart slices were washed with 1X PBS and fixed in 4% paraformaldehyde at room temperature overnight. Images of the stained slices were captured using a digital camera and analyzed using Image J2x analysis software (National Institutes of Health). The severity of the myocardial infarction was indicated by the ratio of the infarct size to the total size.
Measurement of cell viability
Cell viability was determined by CCK-8 assay using the Cell Counting Kit-8 (Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer’s protocol. The absorbance value was detected at a wavelength of 450 nm by ultramicro microporous plate spectrophotometer (Biotek Instruments, Inc., Winooski, UT, USA).
Measurement of lactate dehydrogenase (LDH) activity
LDH released from cardiomyocytes into the culture medium was determined using the LDH assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. The absorbance value was detected at a wavelength of 450 nm by ultramicro microporous plate spectrophotometer (Biotek Instuments, Inc.).
Measurement of apoptosis
Myocardial apoptosis was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using and In Situ Cell Death Detection kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s protocol. The apoptotic cells were observed under a light microscope and ≥3 fields of view at lease were captured at ×400 magnification. Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used for cell counting, and SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) used for data analysis.
Cell apoptosis was determined by flow cytometry analysis (LSRFortessa, BD Biosciences, Franklin Lakes, NJ, USA) using the Annexin V-Fluorescein isothiocyanate (FITC)/Propidium Iodide (PI) kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) to label the cells according to the manufacturer’s protocol. SPSS version 17.0 (SPSS, Inc.) was used for data analysis.
Sensitivity of MPTP to calcium
The mitochondria were isolated from the heart tissues using the Mitochondrial Extract kit (BestBio Co., Shanghai, China) according to the manufacturer’s protocol. The sensitivity of MPTP to calcium was determined using the Purified Mitochondrial Membrane Pore Channel Colorimetric Assay kit (Shanghai Genmed Pharmaceutical Technology Co., Ltd., Shanghai, China) according to the manufacturer’s protocol.
Measurement of mitochondrial membrane potential (ΔΨm)
The change in ΔΨm was determined using the Mitochondrial Membrane Potential Assay kit with 5,5′,6,6′-Tetrachloro-1,1′, 3,3′-tetraethyl-imidacarbocyanine iodide (JC-1; Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer’s protocol. JC-1 is an ideal fluorescent probe widely used for the detection of ΔΨm. When ΔΨm is high, JC-1 accumulates in the matrix of mitochondria and forms J-aggregates that produce red fluorescence. However, when ΔΨm decreases, JC-1 cannot aggregate in the matrix of the mitochondria and maintains a monomer that produces green fluorescence. The fluorescence was detected under a fluorescence microscope and images were captured at ×400 magnification. Image-Pro Plus version 6.0 (Media Cybernetics, Inc.) was used for cell counting.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from myocardium and cardiomyocytes using TRIzol® reagent (Life Technologies; Thermo Fisher Scientific, Inc.). RNA was transcribed into cDNA using PrimeScript RT reagent kit with gDNA Eraser (Takara Bio, Inc., Otsu Japan) according to the manufacturer’s protocol. The RNA was heated at 37°C for 15 min and at 85°C for 5 sec, and then cooled at 4°C to obtain cDNA. SYBR Premix Ex Taq II (Takara Bio, Inc.) was used for qPCR amplification, according to the manufacturer’s protocol. The cDNA was amplified under the following four segments: Segment 1: 95°C for 5 min; segment 2: 8 cycles of 95°C for 30 sec, 60°C for 45 sec, 72°C for 20 sec; segment 3: 35 cycles of 95°C for 30 sec, 56°C for 45 sec, 72°C for 20 sec; segment 4: 95°C for 1 min, 55°C for 30 sec, 95°C for 30 sec. All oligonucleotide primer pairs and the reference primers (β-actin) were designed by Sangon Biotech Co., Ltd. (Shanghai, China) (Table I). Relative gene expression was analyzed using the 2ΔΔCq method (26).
Table I.
Primer sequences in the present study.
| Gene | Primer sequences (5′-3′) |
|---|---|
| Bax forward | GGCGATGAACTGGACAACAA |
| Bax reverse | CAGTTGAAGTTGCCGTCTGC |
| Bcl-2 forward | CACGGTGGTGGAGGAACTCT |
| Bcl-2 reverse | TCCACAGAGCGATGTTGTCC |
| Cytochrome c forward | AGGGTGTCGCCTCAAACCTA |
| Cytochrome c reverse | ACTGAAGCACGGGTGAGTCT |
| APAF-1 forward | CAAGGACACAGACGGTGGAA |
| APAF-1 reverse | TGAATCGCACTGACCAGCTT |
| Caspase-9 forward | CAGGTGGAGGTCAGGTGTGA |
| Caspase-9 reverse | TCCGTGAGAGAGGATGACCA |
| Caspase-3 forward | CCATCCTTCAGTGGTGGACA |
| Caspase-3 reverse | TTGAGGCTGCTGCATAATCG |
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; APAF-1, apoptotic protease activating factor-1.
Western blot analysis
The myocardium and cardiomyocytes were homogenized with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). Following 15 min of centrifugation of 13,000 × g at 4°C, the supernatants containing proteins were collected. Protein concentrations were measured using the BCA protein concentration kit (Beyotime Institute of Biotechnology). Subsequently, 30 µg protein was denatured at 100°C for 10 min, and then SDS-PAGE (12% separation gel and 5% stacking gel) electrophoresis was performed for protein separation, and then the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was blocked with 5% skim milk for 1 h at room temperature, and then incubated with specific antibodies, including anti-B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax; cat. no. WL01637; 1:1,000; Shenyang Wan Biotechnology Co., Ltd., Shenyang, China), anti-Bcl-2 (cat. no. WL01556; 1:1,000; Shenyang Wan Biotechnology Co., Ltd.), anti-cytochrome c (cat. no. ab90529; 1:1,000; Abcam, Cambridge, UK), anti-apoptotic protease activating factor-1 (APAF-1; cat. no. ab2001; 1:1,000; Abcam), anti-cleaved caspase-9 (cat. no. 40503-1; 1:1,000; Signalway Anitbody LLC, College Park, MD USA), anti-cleaved caspase-3 (cat. no. ab2302; 1:1,000; Abcam) and anti-β-actin (cat. no. TA-09; 1:1,000; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China), at 4°C overnight. The following day, the PVDF membrane was incubated with horseradish peroxidase labeled goat anti-rabbit immunoglobulin G (cat. no. E030120-01; 1:4,000; EarthOx Life Sciences, Millbrae, CA, USA) or goat anti-mouse immunoglobulin G (cat. no. E030110-01; 1:4,000; EarthOx Life Sciences) at room temperature for 30 min. Detection of protein bands was performed using an enhanced chemiluminescence for western blotting kit (Beyotime Institute of Biotechnology) according to the manufacturer’s protocol. Relative densitometry was calculated using Image J2x analysis software (National Institutes of Health).
Statistical analysis
All data were presented as the mean ± standard deviation and analyzed using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA). Differences among ≥ three groups were firstly evaluated using one-way analysis of variance, and if the differences were significant, multiple comparison analysis was performed using Fisher’s Least Significant Difference test. Differences between two groups were evaluated using independent sample t-test. P<0.05 were considered to indicate a statistically significant difference.
Results
Effect of morin on cardiac function
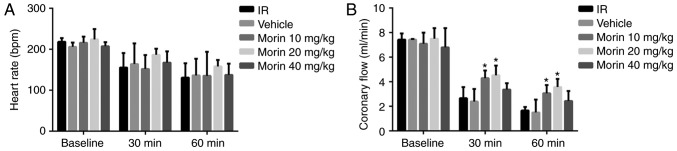
No difference was observed in the heart rate across all the groups (Fig. 1A). Coronary circulation at 30 and 60 min of reperfusion in the morin10 and morin20 groups was significantly increased compared with the IR group (Fig. 1B).
Figure 1.
Cardiac function prior to and during reperfusion. (A) Heart rate. (B) Coronary circulation. Values are presented as the mean ± standard deviation; n=3-15; *P<0.05 vs. the IR group. IR, ischemia/reperfusion.
Effect of morin on myocardial damage
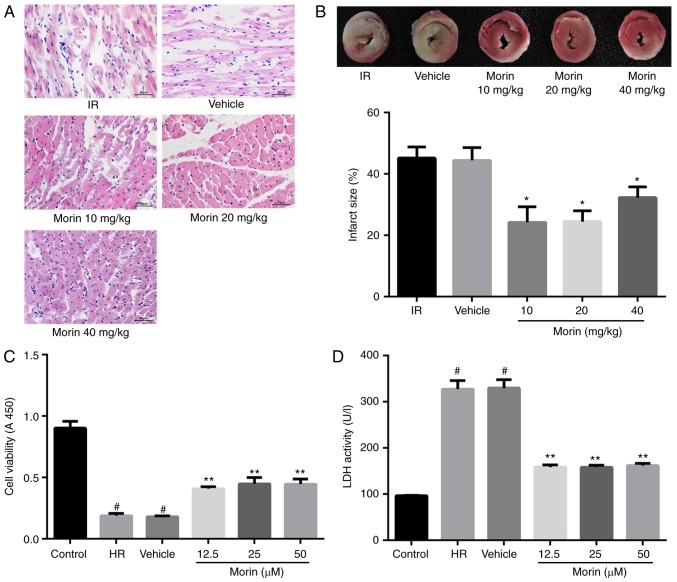
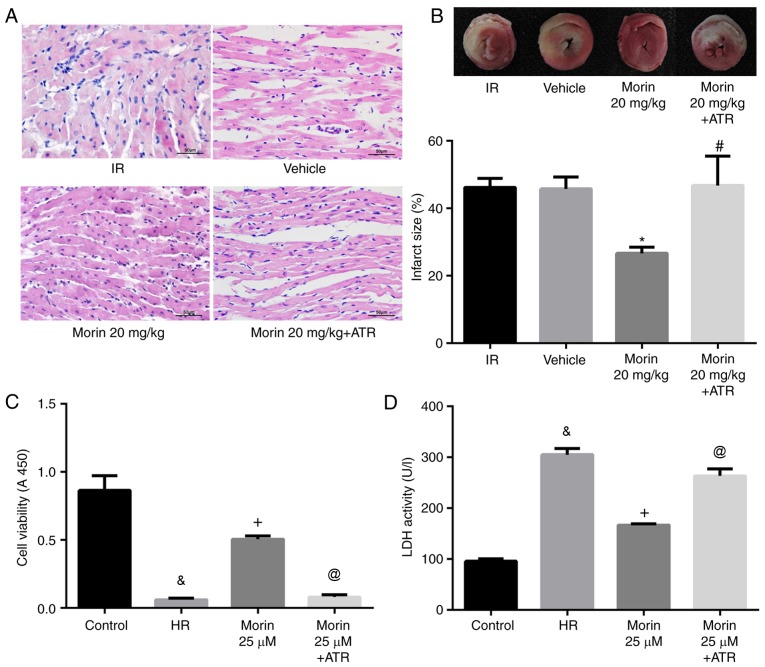
The results of the HE staining indicated that myocardial damage was ameliorated following treatment with morin at different doses, as evidenced by less vacuolation in cytoplasm and a decreased number of cells exhibiting nuclear condensation, dissolution and fragmentation compared with the IR group. Compared with the morin10 group, fewer instances of severe myocardial damage were observed in the morin20 group (Fig. 2A). In addition, the myocardial infarct sizes following treatment with morin at different doses were significantly decreased compared with the IR group (Fig. 2B). Although there was no significant difference in myocardial infarct size between morin20 and morin10 groups, it was identified visually that the extent of myocardial damage indicated in morin20 group was less compared with that in morin10 group through the result of HE staining. Therefore, the dose of 20 mg/kg was selected for the additional animal experiments.
Figure 2.
Protective effect of morin on myocardial ischemia-reperfusion injury in vitro and in vivo. (A) Photomicrographs were captured at magnification, ×400. (B) Results of myocardial 2, 3, 5-triphenyltetrazolium chloride staining and the percentage infarct size. The non-infarction areas were stained red or pink, whereas the infarction areas were white or gray. (C) Cell viability following hypoxia/reoxygenation. (D) Cell lactate dehydrogenase activity. Values are presented as the mean ± standard deviation; n=3-6; *P<0.01 vs. the IR group; #P<0.01 vs. the control group; and **P<0.01 vs. the HR group. IR, ischemia-reperfusion; HR, hypoxia/reoxygenation.
The damage of cardiomyocytes caused by hypoxia/reoxygenation was evaluated by determining cell viability and LDH activity. The results demonstrated that cell viability was significantly increased and LDH activity was markedly decreased in different morin treatment groups (morin12.5, morin25 and morin50) compared with those in the HR group. However, there was no difference in cell viability and LDH activity among groups treated with different doses of morin (Fig. 2C and D). Although different doses of morin treatment groups exhibited no statistical difference in cell viability, the cell viability in morin 25 µM group was increased by almost 10% compared with that in morin 12.5 µM group (0.449±0.052 vs. 0.409±0.017; P=0.213). Considering that the sample size was relatively small (n=3), we hypothesized that the statistical difference between these two groups may be observed if the sample size became larger. Therefore, the dose of 25 µM was selected for additional cell experimentation.
Effect of morin on the alterations in MPTP opening and ΔΨm
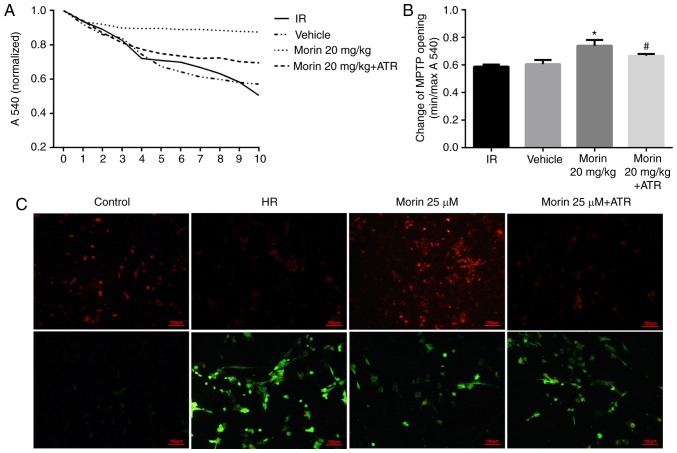
Calcium may induce MPTP opening (16). The sensitivity of mitochondria to calcium is a marker for MPTP opening (27). The results indicated that the sensitivity of mitochondria to calcium was significantly attenuated in the morin treatment group compared with the IR group, suggesting that morin inhibited the extent of MPTP opening (Fig. 3A and B). In addition, the results of ΔΨm assay indicated that in the cardiomyocytes, the red fluorescence intensity was increased and green fluorescence intensity was decreased in the morin treatment group compared with the HR group, indicating that morin significantly inhibited the decrease of the ΔΨm induced by hypoxia/reoxygenation (Fig. 3C).
Figure 3.
Changes in mitochondrial function. (A) Sensitivity of MPTP opening to calcium. (B) Alterations in MPTP opening. (C) Alterations in mitochondrial membrane potential. The presence of red fluorescence indicated normal ΔΨm and state of the cells. The green fluorescence indicated that the ΔΨm decreased, and the cells were most likely to be in the early stage of apoptosis. Values are presented as the mean ± standard deviation; n=3; *P<0.01 vs. the IR group; #P<0.05 vs. the morin20 group. MPTP, mitochondrial permeability transition pore; IR, ischemia-reperfusion; HR, hypoxia/reoxygenation; ATR, atractyloside; ΔΨm, mitochondrial membrane potential.
Effect of morin on the expression levels of Bax and Bcl-2
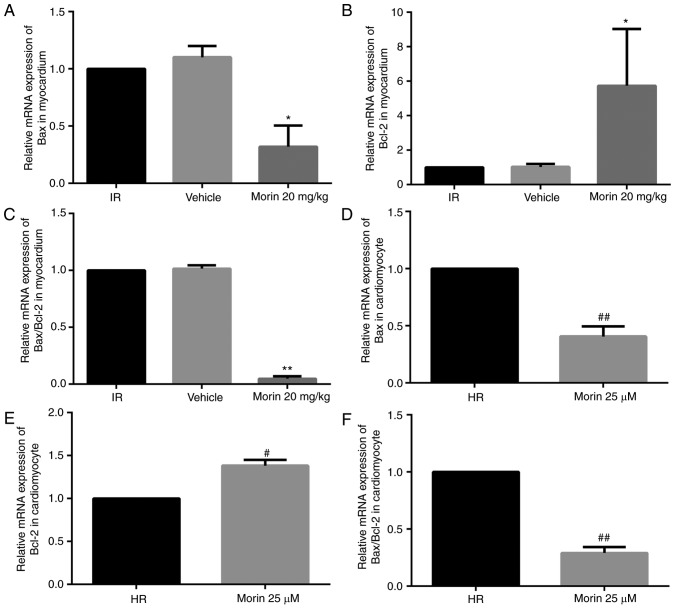
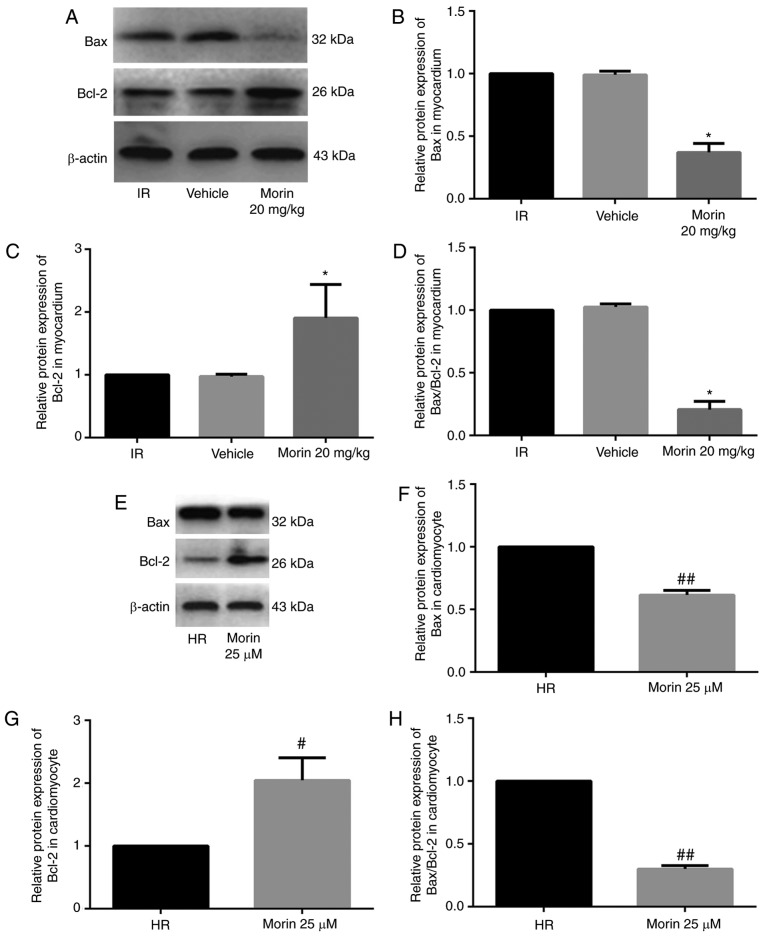
Morin treatment significantly downregulated the mRNA expression of Bax, upregulated the mRNA expression of Bcl-2 and decreased the Bax/Bcl-2 ratio in the myocardium and in cardiomyocytes (Fig. 4). Consistent with the changes in mRNA expression levels, morin treatment also markedly downregulated the protein expression of Bax, upregulated the protein expression of Bcl-2 and decreased the Bax/Bcl-2 ratio in the myocardium and in cardiomyocytes (Fig. 5).
Figure 4.
Relative mRNA expression levels of Bax and Bc12, and the Bax/Bcl-2 ratio. (A) Relative mRNA expression level of Bax in the heart tissue. (B) Relative mRNA expression level of Bcl-2 in the heart tissue. (C) Relative Bax/Bcl2 mRNA ratio in the heart tissue. (D) Relative mRNA expression level of Bax in cardiomyocytes. (E) Relative mRNA expression level of Bcl-2 in cardiomyocytes. (F) Relative Bax/Bcl-2 mRNA ratio in cardiomyocytes. Values are presented as the mean ± standard deviation; n=3; *P<0.05 vs. the IR group; **P<0.01 vs. the IR group; #P<0.05 vs. the HR group; ##P<0.01 vs. the HR group. IR, ischemia-reperfusion; HR, hypoxia/reoxygenation; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
Figure 5.
Relative protein expression levels of Bax and Bcl-2, and the Bax/Bcl-2 ratio. (A) Protein expression levels of Bax and Bcl-2 in the heart tissue detected by western blot analysis. (B) Relative protein expression level of Bax in the heart tissue. (C) Relative protein expression level of Bcl-2 in the heart tissue; (D) Relative Bax/Bcl-2 protein ratio in the heart tissue; (E) Protein expression levels of Bax and Bcl-2 in cardiomyocytes detected by western blot analysis. (F) Relative protein expression level of Bax in cardiomyocytes. (G) Relative protein expression level of Bcl-2 in cardiomyocytes. (H) Relative Bax/Bcl-2 protein ratio in cardiomyocytes. Values are presented as the mean ± standard deviation; n=3-6; *P<0.01 vs. the IR group; #P<0.05 vs. the HR group; ##P<0.01 vs. the HR group. IR, ischemia-reperfusion; HR, hypoxia/reoxygenation; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
Effect of morin on the mitochondrial apoptotic pathway and apoptosis
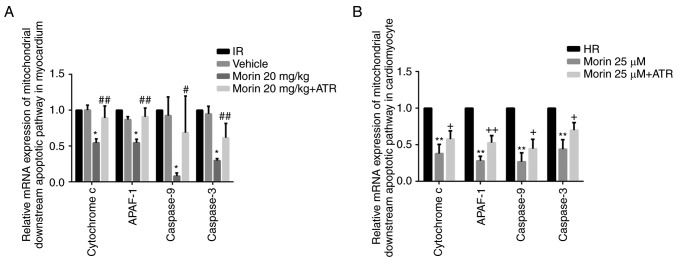
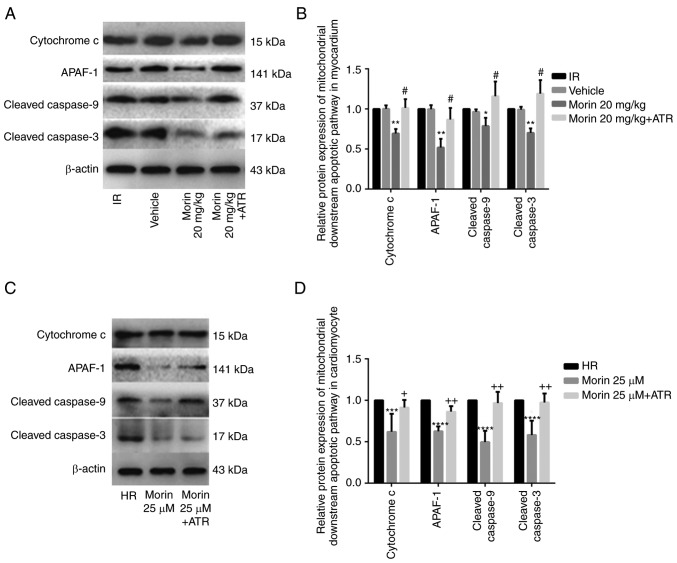
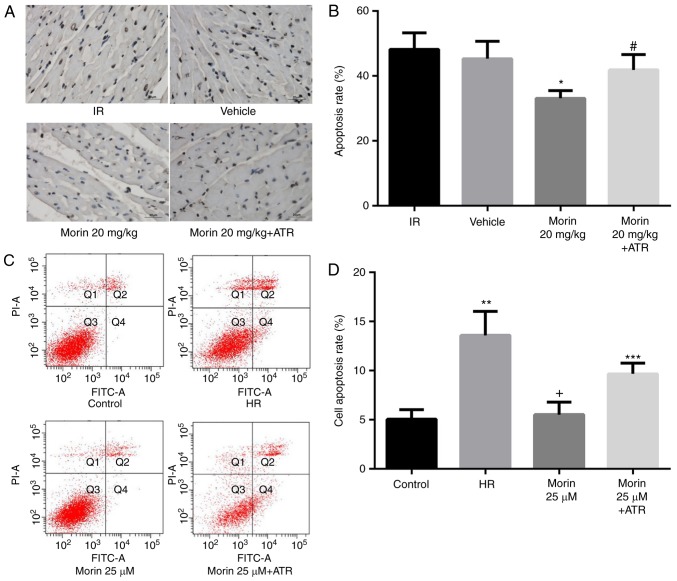
MPTP opening may activate the mitochondrial apoptotic pathway and induce apoptosis (15). Therefore, the present study explored the effect of morin on the apoptotic rate and on the expression of mitochondrial apoptosis-associated proteins. The results indicated that morin significantly decreased the mRNA and protein expression of cytochrome c, APAF-1, caspase-9 and caspase-3 in the myocardium and in cardiomyocytes (Figs. 6 and 7). In addition, morin also significantly decreased the apoptotic rate of treated cells compared with the HR cells (Fig. 8).
Figure 6.
Relative mRNA expression levels of downstream genes of the mitochondrial apoptotic pathway. (A) Relative mRNA expression levels of cytochrome c, APAF-1, caspase-9 and caspase-3 in the heart tissue; (B) Relative mRNA expression levels of cytochrome c, APAF-1, caspase-9 and caspase-3 in cardiomyocytes. Values are presented as the mean ± standard deviation; n=3-5; *P<0.01 vs. the IR group; #P<0.05 vs. the morin20 group; ##P<0.01 vs. the morin20 group; **P<0.01 vs. the HR group; +P<0.05 vs. the morin25 group; ++P<0.01 vs. the morin25 group. APAF-1, apoptotic protease activating factor-1; IR, ischemia-reperfusion; HR, hypoxia/reoxygenation.
Figure 7.
Relative protein expression levels of downstream genes of the mitochondrial apoptotic pathway. (A) Protein expression levels of cytochrome c, APAF-1, cleaved caspase-9 and cleaved caspase-3 in the heart tissue detected by western blot analysis. (B) Relative protein expression levels of cytochrome c, APAF-1, cleaved caspase-9 and cleaved caspase-3 in the heart tissue. (C) Protein expression levels of cytochrome c, APAF-1, cleaved caspase-9 and cleaved caspase-3 in cardiomyocytes detected by western blot analysis. (D) Relative protein expression levels of cytochrome c, APAF-1, cleaved caspase-9 and cleaved caspase-3 in cardiomyocytes. Values are presented as the mean ± standard deviation; n=3-5; *P<0.05 vs. the IR group; **P<0.01 vs. the IR group; #P<0.01 vs. the morin20 group; ***P<0.05 vs. the HR group; ****P<0.01 vs. the HR group; +P<0.05 vs. the morin25 group; ++P<0.01 vs. the morin25 group. APAF-1, apoptotic protease activating factor-1; IR, ischemia-reperfusion; HR, hypoxia/reoxygenation.
Figure 8.
Apoptosis following myocardial infarction. (A) Photomicrographs were captured at magnification, ×400. Apoptotic cardiomyocyte nuclear regions were stained brown, whereas terminal deoxynucleotidyl transferase dUTP nick end labeling-negative nuclear regions appeared blue. (B) The percentage apoptosis rate. (C) Cell apoptosis detected by flow cytometry. (D) Cell apoptosis rate. Values are presented as the mean ± standard deviation; n=3-8; *P<0.01 vs. the IR group; #P<0.01 vs. the morin20 group; **P<0.01 vs. the control group; +P<0.01 vs. the HR group; ***P<0.05 vs. the morin25 group. IR, ischemia-reperfusion; HR, hypoxia/reoxygenation; ATR, atractyloside; FITC, fluorescein isothiocyanate; PI, propidium iodide.
ATR abrogated the protective effects of morin
To additionally confirm whether morin prevented MIRI through inhibition of MPTP opening, the effect of ATR, a known MPTP opener, on the protective effect of morin was examined. As expected, the inhibitory effects of morin on MPTP opening (Fig. 3A and B), the decrease of ΔΨm (Fig. 3C), the expression of mitochondrial apoptosis-associated proteins (Figs. 6 and 7) and apoptosis were reversed by ATR (Fig. 8). In addition, it was observed that myocardial infarct size was increased, cell viability was decreased and LDH activity was increased in the morin combined with ATR pretreatment groups as compared with the morin pretreatment groups (Fig. 9). Considering that previous studies had demonstrated that ATR at a dose of 5 mg/kg does not aggravate myocardial IR injury (28,29), these results suggest that the protective effects of morin were abrogated by induction of MPTP opening using ATR.
Figure 9.
ATR abrogates the protective effects of morin. (A) Photomicrographs were captured at magnification, ×400. (B) Results of myocardial 2, 3, 5-triphenyltetrazolium chloride staining and the percentage infarct size. The non-infarction areas were stained red or pink, whereas the infarction areas were white or gray. (C) Cell viability. (D) Cell LDH activity. Values are presented as the mean ± standard deviation; n=3-6; *P<0.01 vs. the IR group; #P<0.01 vs. the morin20 group; &P<0.01 vs. the control group; +P<0.01 vs. the HR group; @P<0.01 vs. the morin25 group. IR, ischemia-reperfusion; HR, hypoxia/reoxygenation; ATR, atractyloside.
Discussion
In the present study, it was identified that morin treatment prior to ischemia markedly alleviated the extent of MIRI, as evidenced by the increased cell viability, decreased LDH activity and cell apoptosis, the improved recovery of cardiac function and the reduction of myocardial infarct size observed. Although Al-Numair et al (30) previously demonstrated that morin exhibited an antioxidative activity in an isoproterenol-induced model of myocardial infarction, they did not provide direct evidence of the protective effect of morin on myocardial ischemia injury. To the best of our knowledge, this is the first study to describe the protective effect of morin on MIRI in detail. Therefore, morin seems to be a promising compound that may be used for preventing MIRI. A number of drugs and chemical compounds, including rosuvastatin (31), tauroursodeoxycholic acid (32), tilianin (33) and phosphodiesterases (34), have been demonstrated to prevent MIRI by inhibition of MPTP opening. Woodman et al (35) confirmed that 3′,4′-dihydroxyflavonol, a flavonoid compound, may inhibit MPTP opening and preserve mitochondrial function. In the present study, the results indicated that morin decreased the sensitivity of MPTP to calcium, which is a marker of MPTP opening, therefore suggesting that morin inhibited the opening of MPTP. To the best of the authors’ knowledge, this is a novel result within this field of study. The irreversible opening of MPTP causes the loss of ΔΨm (36), release of cytochrome c to the cytoplasm and activation of mitochondrial apoptosis-associated proteins, including APAF-1, caspase-9 and caspase-3, subsequently resulting in apoptosis induction (37,38). As expected, the present study also identified that morin prevented the loss and preserved the stability of ΔΨm. In addition, the expression levels of cytochrome c, APAF-1, cleaved caspase-9 and cleaved caspase-3 were significantly decreased by morin treatment. Similar to the results of the present study, Chen et al (22) identified that morin decreased the expression of caspase3 in focal cerebral ischemic rats. In addition, the present study demonstrated that the beneficial effects of morin on MIRI were reversed by an MPTP opener (ATR), therefore providing additional evidence of morin-mediated inhibition of MPTP opening (39). Taken together, we hypothesize that morin may prevent MIRI by inhibiting MPTP opening.
MPTP opening may be regulated by Bax and Bcl-2 (40). The pro-apoptotic protein Bax may mediate MPTP opening through binding of adenine nucleotide translocase (ANT) or voltage-dependent anion channel (VDAC), while the anti-apoptotic protein Bcl-2 may directly inhibit the interaction between Bax and ANT/VDAC to inhibit MPTP opening by competing with ANT for the Bax binding site (41). The Bax/Bcl-2 balance is critical for maintaining cell homeostasis (42). Overexpression of Bcl-2 inhibits mitochondrial ROS production and the release of cytochrome c, and apoptosis-inducing factor. It may also increase mitochondrial calcium tolerance load, prevent caspase cascade activation and exert anti-apoptotic effects (43,44). In the present study, the results demonstrated that morin treatment may significantly downregulate the expression of Bax, upregulate the expression of Bcl-2 and decrease the ratio of Bax to Bcl-2. In addition, the Bax and Bcl-2 expression levels following ATR rescue were also examined, but the results indicated that ATR did not affect the expressions of Bax and Bcl-2 (data not shown). The primary explanation may be that ATR is an inducer of MPTP opening through the inhibition of adenine nucleotide translocator (45,46). It may induce MPTP opening and activate mitochondrial downstream apoptotic pathway proteins, but cannot affect the expressions of Bax and Bcl-2. Therefore, we hypothesized that morin may regulate the MPTP opening by controlling the expression of Bax and Bcl-2.
Due to the absence of neural and humoral regulation in the isolated rat heart models of the present study, the pathophysiological changes normally observed during MIRI were not able to be simulated completely, which is a limitation of the present study. Therefore, the protective effect of morin on MIRI requires additional verification in vivo.
In conclusion, to the best of our knowledge, this is the first study to demonstrate morin-mediated prevention of MIRI. The protective effect of morin on MIRI may be associated with inhibition of MPTP opening. Therefore, morin may be a promising compound for MIRI prevention in the future.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81670320) and the Natural Science Foundation of Liaoning Province (grant no. 201602826).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
DJ and NW conceived and designed the experiments. SL conducted the experiments. JM, ZH and XL participated in the completion of the experiments. SL, PJ and YG analyzed the data. SL wrote the paper. NW revised the manuscript. All the authors read and approved the final paper.
Ethics approval and consent to participate
The experimental protocol was approved by the Institutional Ethics Committee of China Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Guo X, Li Z, Vittinghoff E, Sun Y, Pletcher MJ. Trends in rate of acute myocardial infarction among patients aged <30 years. Nat Rev Cardiol. 2018;15:119. doi: 10.1038/nrcardio.2017.191. [DOI] [PubMed] [Google Scholar]
- 3.Plakht Y, Gilutz H, Shiyovich A. Excess long-term mortality among hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction (SAMI) project. Public Health. 2017;143:25–36. doi: 10.1016/j.puhe.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Bainey KR, Armstrong PW. Clinical perspectives on reperfusion injury in acute myocardial infarction. Am Heart J. 2014;167:637–645. doi: 10.1016/j.ahj.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Gerczuk PZ, Kloner RA. An update on cardioprotection: A review of the latest adjunctive therapies to limit myocardial infarction size in clinical trials. J Am Coll Cardiol. 2012;59:969–978. doi: 10.1016/j.jacc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Neri M, Riezzo I, Pascale N, Pomara C, Turillazzi E. Ischemia/reperfusion injury following acute myocardial infarction: A critical issue for clinicians and forensic pathologists. Mediators Inflamm. 2017;2017:7018393. doi: 10.1155/2017/7018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 10.Donato M, Evelson P, Gelpi RJ. Protecting the heart from ischemia/reperfusion injury: An update on remote ischemic preconditioning and postconditioning. Curr Opin Cardiol. 2017;32:784–790. doi: 10.1097/HCO.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 11.Zou R, Shi W, Tao J, Li H, Lin X, Yang S, Hua P. SIRT5 and post-translational protein modifications: A potential therapeutic target for myocardial ischemia-reperfusion injury with regard to mitochondrial dynamics and oxidative metabolism. Eur J Pharmacol. 2018;818:410–418. doi: 10.1016/j.ejphar.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Wang H, Li J. Inflammation and inflammatory cells in myocardial infarction and reperfusion injury: A double-edged sword. Clin Med Insights Cardiol. 2016;10:79–84. doi: 10.4137/CMC.S33164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badalzadeh R, Mokhtari B, Yavari R. Contribution of apoptosis in myocardial reperfusion injury and loss of cardioprotection in diabetes mellitus. J Physiol Sci. 2015;65:201–215. doi: 10.1007/s12576-015-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morciano G, Bonora M, Campo G, Aquila G, Rizzo P, Giorgi C, Wieckowski MR, Pinton P. Mechanistic role of mPTP in ischemia-reperfusion injury. Adv Exp Med Biol. 2017;982:169–189. doi: 10.1007/978-3-319-55330-6_9. [DOI] [PubMed] [Google Scholar]
- 15.Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol. 2015;78:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Hurst S, Hoek J, Sheu SS. Mitochondrial Ca2+ and regulation of the permeability transition pore. J Bioenerg Biomembr. 2017;49:27–47. doi: 10.1007/s10863-016-9672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong JQ, Molkentin JD. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 2015;21:206–214. doi: 10.1016/j.cmet.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Šileikytė J, Forte M. Shutting down the pore: The search for small molecule inhibitors of the mitochondrial permeability transition. Biochim Biophys Acta. 18572016:1197–1202. doi: 10.1016/j.bbabio.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim WY, Messow CM, Berry C. Cyclosporin variably and inconsistently reduces infarct size in experimental models of reperfused myocardial infarction: A systematic review and meta-analysis. Br J Pharmacol. 2012;165:2034–2043. doi: 10.1111/j.1476-5381.2011.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 21.Rizvi F, Mathur A, Kakkar P. Morin mitigates acetaminophen-induced liver injury by potentiating Nrf2 regulated survival mechanism through molecular intervention in PHLPP2-Akt-Gsk3β axis. Apoptosis. 2015;20:1296–1306. doi: 10.1007/s10495-015-1160-y. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Li Y, Xu H, Li G, Ma Y, Pang YJ. Morin mitigates oxidative stress, apoptosis and inflammation in cerebral ischemic rats. Afr J Tradit Complement Altern Med. 2017;14:348–355. doi: 10.21010/ajtcam.v14i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Kang KA, Piao MJ, Maeng YH, Lee KH, Chang WY, You HJ, Kim JS, Kang SS, Hyun JW. Cellular protection of morin against the oxidative stress induced by hydrogen peroxide. Chem Biol Interact. 2009;177:21–27. doi: 10.1016/j.cbi.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Kastenmayer RJ, Moore RM, Bright AL, Torres-Cruz R, Elkins WR. Select agent and toxin regulations: Beyond the eighth edition of the Guide for the Care and Use of Laboratory Animals. J Am Assoc Lab Anim Sci. 2012;51:333–338. [PMC free article] [PubMed] [Google Scholar]
- 25.Wu N, Li W, Shu W, Jia D. Protective effect of picroside II on myocardial ischemia reperfusion injury in rats. Drug Des Devel Ther. 2014;8:545–554. doi: 10.2147/DDDT.S62355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Strutyns’ka NA, Dorofeieva NO, Vavilova HL, Sahach VF. Hydrogen sulfide inhibits Ca2+-induced mitochondrial permeability transition pore opening in spontaneously hypertensive rats. Fiziol Zh. 2013;59:3–10. doi: 10.15407/fz59.05.003. In Ukrainian. [DOI] [PubMed] [Google Scholar]
- 28.Nazari A, Sadr SS, Faghihi M, Azizi Y, Hosseini MJ, Mobarra N, Tavakoli A, Imani A. Vasopressin attenuates ischemia-reperfusion injury via reduction of oxidative stress and inhibition of mitochondrial permeability transition pore opening in rat hearts. Eur J Pharmacol. 2015;760:96–102. doi: 10.1016/j.ejphar.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Alizadeh AM, Faghihi M, Khori V, Sohanaki H, Pourkhalili K, Mohammadghasemi F, Mohsenikia M. Oxytocin protects cardiomyocytes from apoptosis induced by ischemia-reperfusion in rat heart: Role of mitochondrial ATP-dependent potassium channel and permeability transition pore. Peptides. 2012;36:71–77. doi: 10.1016/j.peptides.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Al-Numair KS, Chandramohan G, Alsaif MA, Veeramani C, El Newehy AS. Morin, a flavonoid, on lipid peroxidation and antioxidant status in experimental myocardial ischemic rats. Afr J Tradit Complement Altern Med. 2014;11:14–20. doi: 10.4314/ajtcam.v11i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CW, Yang F, Cheng SZ, Liu Y, Wan LH, Cong HL. Rosuvastatin postconditioning protects isolated hearts against ischemia-reperfusion injury: The role of radical oxygen species, PI3K-Akt-GSK-3β pathway, and mitochondrial permeability transition pore. Cardiovasc Ther. 2017;35:3–9. doi: 10.1111/1755-5922.12225. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y, He Y, Cai Z, Cai J, Xi M, Zhang Y, Xi J. Tauroursodeoxycholic acid inhibits endoplasmic reticulum stress, blocks mitochondrial permeability transition pore opening, and suppresses reperfusion injury through GSK-3β in cardiac H9c2 cells. Am J Transl Res. 2016;8:4586–4597. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Yuan Y, Wang X, Wang Y, Cheng J, Tian L, Guo X, Qin D, Cao W. Tilianin post-conditioning attenuates myocardial ischemia/reperfusion injury via mitochondrial protection and inhibition of apoptosis. Med Sci Monit. 2017;23:4490–4499. doi: 10.12659/MSM.903259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chanoit G, Zhou J, Lee S, McIntosh R, Shen X, Zvara DA, Xu Z. Inhibition of phosphodiesterases leads to prevention of the mitochondrial permeability transition pore opening and reperfusion injury in cardiac H9c2 cells. Cardiovasc Drugs Ther. 2011;25:299–306. doi: 10.1007/s10557-011-6310-z. [DOI] [PubMed] [Google Scholar]
- 35.Woodman OL, Long R, Pons S, Eychenne N, Berdeaux A, Morin D. The cardioprotectant 3′,4′-dihydroxyflavonol inhibits opening of the mitochondrial permeability transition pore after myocardial ischemia and reperfusion in rats. Pharmacol Res. 2014;81:26–33. doi: 10.1016/j.phrs.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 37.Hüttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J, Ramzan R, et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 18172012:598–609. doi: 10.1016/j.bbabio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: A common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463–470. doi: 10.1016/S0006-291X(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 39.Yang S, Li H, Tang L, Ge G, Ma J, Qiao Z, Liu H, Fang W. Apelin-13 protects the heart against ischemia-reperfusion injury through the RISK-GSK-3β-mPTP pathway. Arch Med Sci. 2015;11:1065–1073. doi: 10.5114/aoms.2015.54863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 41.Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA, Kinnally KW. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.e04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl2/Bax expression. Cell Res. 2003;13:385–391. doi: 10.1038/sj.cr.7290184. [DOI] [PubMed] [Google Scholar]
- 43.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 44.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 45.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 46.Javadov S, Karmazyn M, Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther. 2009;330:670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.