Abstract
Kushui rose (R. Setat x R. Rugosa) (KR) is a traditional Chinese medicine proven to be a potent antioxidant, and used for thousands of years. Approximately 30% of all human cancers relevant to mutational activated Ras, and over-activated Ras are accompanied by increased accumulation of reactive oxygen species (ROS). Thus, one way of developing anticancer drugs is to reduce ROS accumulation. Therefore, KR was predicted to have potential to combat over-activated Ras-related cancer. C. elegan with let-60(gf)/ra mutant, which exhibited tumor-like symptoms of the multivulva phenotype, was employed to determine the effect of KR on Ras/MAPK pathway. Other strains of worms and H2DCF-DA dye were also applied to study the antioxidant stress capacity of KR. This study was aimed to determine whether KR has a potential effect on combat over-activated Ras-related cancer through resistance to oxidative stress. Our results showed that Kushui rose decoction (KRD) has potent antioxidant activity in vitro, and can inhibit over-activated Ras in vivo Further, KRD significantly suppressed over-activated Ras/MAPK pathway by regulating oxidative stress-related proteins, such as forkhead transcription factor (DAF-16), glutathione S-transferase-4 (GST-4), superoxide dismutases (SODs) and heat shock protein-16.2 (HSP-16.2). However, essential oil and hydrosol of KR had no effect on over-activated Ras. Thus these results reminded us that people usually soak rose in hot water to prepare 'rose tea' as an effective way for health care. Thus, KRD was demonstrated to be a potential drug candidate for combating over-activated Ras-related cancer as an antioxidant.
Keywords: Ras/MAPK pathway, anti-oxidant, oxidative stress, Kushui rose (R. Setat x R. Rugosa), C. elegan
Introduction
Rosa genus (family Rosaceae) contains over 150 species that are widespread in Asia, Europe, Middle East and North America. Historically, roses were cultivated and used for medicinal purpose by the ancient Chinese 5,000 years ago (1,2). Recently, roses were used extensively in perfume, cosmetic, pharmaceutical and food industries as rose oil, rose water, rose concrete, rose hydrosol and dried petals (3).
Rose has served as folk medicine and raw material of perfume in China for thousands of years (4). There are many species of roses in China, R. Setat x R. Rugosa Yu et Ku is known as Kushui rose (KR), which is a hybrid plant and was large-scale cultivated in Kushui, Yongdeng country of Gansu Province for over 200 years (5). There are more than 100 kinds of active ingredients in KR, and the contents of essential oil and total flavonoids (rutin) reach 0.04% and 0.48/100 g, respectively. Especially, the content of citronellol is >50% in essential oil, which is the highest content among the rose around the world (6–8). But up to now, KR is still directly used as raw material for sauces and essential oil. Comprehensive utilization of KR is relatively insufficient and more attentions should to be paid to its biological activities and mechanisms (9).
Ras proteins are mutationally activated in as many as 30% of all human tumors (10). The over-activated Ras is a decisive factor in the formation of human malignant tumors (11). Mutation of ra gene leads to persistently activating Ras protein, increasing intracellular level of Ras-GTP, excessive proliferating of cells and finally leading to the occurrence of cancer (12). Thus, Ras protein has become a universally accepted target for drug screening (10). Although recent studies found certain compounds can specifically target mutational Ras, the clinical application of these compounds continues to be a long way off (13).
Cancers usually contain elevated levels of reactive oxygen species (ROS), and over-activated Ras is closely related to the increased ROS accumulation as well (14–16). Furthermore, free radical theory postulates that ROS is the main determinant for the promotion of cancer (17). One approach for developing anticancer drugs is to target ROS production and accumulation (18). For this reason, many anticancer drugs are screened mainly focusing on their ROS scavenging capacity (19). So it is reasonable to infer that antioxidants are potential drug candidates for treating over-activated Ras related cancer. Although numerous antioxidants have been synthesized, many of them suffered from toxic and side effects when tested as drug candidates (20–24). Thus there is an increasing interest in testing natural antioxidants as novel therapeutic agents (25–29). Only very few natural antioxidants were shown effective to combat cancers related to over-activated Ras, such as antroquinonol, which is a cytotoxicity agent from Antrodia camphorata (30,31). Interestingly, KR has been shown to be a potent antioxidant (26,32,33). Hence we hypothesize that KR could suppress over-activated Ras due to its antioxidant activity.
In Caenorhabditis elegans (C. elegans), let-6 is a homologous gene to ra in mammals, and Ras/MAPK signaling pathway determines the development of worm vulva (34). The over-activated Ras/MAPK pathway produces an abnormal multivulva (Muv) phenotype, which can be reversed by antitumor drug candidates (35). C. elegan is recognized as a powerful tool for screening antitumor drug candidates to suppress over-activated Ras/MAPK pathway (36–38). In this study, we applied the model organism C. elegan to determine whether KR can suppress over-activated Ras/MAPK pathway.
Therefore, the aim of this study was to determine the antioxidant activity of Kushui rose decoction (KRD), evaluating the inhibition activity on over-activated Ras of KR extracts, identifying new applications of KR and finally to improve the availability of KR and the local economics. Our results provide evidence to substantiate that KR can serve as a potential drug candidate for combating over-activated Ras-related cancer.
Materials and methods
Preparation of KR extracts
The decoction of KR (KRD) was extracted with distilled water. Fifty milliliters of decoction was obtained from 4 g dried rose buds, defined as RD (80 mg/ml). The concentration is presented as the content of the crude drug in solution (w/v). The KRD were diluted to 0.1 mg/ml (RDL), 0.2 mg/ml (RDM), 0.4 mg/ml (RDH), 0.8 mg/ml (RDHH), 1.6 mg/ml (RDHHH), respectively. The KR essential oil and hydrosol were obtained from Dongfang Tianrun (Tianjin, China) company as a gift, and the gradient was diluted by the solvent (0.1% DMSO and 2% PEG-400, final concentration) 50-fold.
Maintenance conditions
MT2124, let-60 (n1046sd, gf) IV was a gift from Howard Hughes Medical Institute. TJ356, zIs356 [daf-16p::daf-16a/b::GFP + rol-6]; CF1553, muIs84 [(pAD76) sod-3p::GFP + rol-6]; AM263, rmIs175 [unc-54p::Hsa-sod-1 (WT)::YFP]; TJ375, gpIs1[hsp-16-2::GFP]; CL2166, dvIs19 [(pAF15)gst-4p::GFP::NLS] III and Escherichia coli OP50 were provided by the Caenorhabditis Genetics Center (CGC), which is funded by the NIH National Center for Research Resources. Worms were maintained at 20°C by standard methods (39). Escherichia coli OP50 were used as standard food source.
Drug treatment
The drug treatments were performed in 96-well plates. All drugs except the rose essential oil were diluted by S buffer. Around 80–100 synchronized worms at L1 larvae cultured to adult in 180 µl of S buffer containing different treatment substances were transferred to 96-well plates, and 1 mg/ml freshly grown OP50 were added as a standard food resource. N-Acety-L-Cysteine (NAC) was purchased from TCI (Shanghai, China). Paraquat (PQ) was from Sigma. 2.5 mM NAC and 0.5 mM PQ were used as positive and negative control, respectively.
Quantification of the wild-type phenotype of let-60(gf) mutants
let-60(gf) mutants were treated as previously described and cultured to adults after 3–4 days, and the percentage of wild-type phenotype of let-60(gf) mutants were scored by an inverted microscope (SY-057). The percent of wild-type phenotype worms were calculated according to the formula: PW(%)=100% × NW/(NW+NM); where PW is the percentage of wild-type phenotype of worms, NW is the number of wild-type phenotype of worms, NM is the number of Muv phenotype of worms (40,41).
2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) radical scavenging activity of KRD
The capacity of KRD on scavenging ABTS radical in vitr was measured by the Total Antioxidant Capacity assay kit with the ABTS method (Beyotime, Shanghai, China) (42).
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of KRD
Anti-radical activities of KRD was examined by comparing to the known antioxidant ascorbic acid by DPPH (43). Briefly, 20 µl of rose decoction or different concentration of ascorbic acid (10, 5, 2.5, 1.25, 0.625, 0.3125 and 0.15625 mM) were mixed with 580 µl methanolic solution of DPPH (50 µM). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then the absorbance was measured at 517 nm against methanol as the blank in a spectrophotometer. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. The percent of DPPH discolorations of the samples was calculated according to the formula: I(%)=100% × (AB−AS)/AB; where AB is the absorbance of the control reaction (containing all reagents except the test sample), and AS is the absorbance of the extracts/reference.
Superoxide anion, hydroxyl radicals and hydrogen peroxide scavenging activity of KRD
The capacity of KRD on scavenging superoxide anion, hydroxyl radicals and hydrogen peroxide in vitro, respectively, by nitrotetrazolium blue chloride (NBT) method, Fenton reaction and luminol-H2O2 method as described (44).
Statistical analysis
The fluorescence signal intensity was quantified using ImageJ software. The results are presented as the average of three biological replicates. The data are analyzed by one-way ANOVA and Tukey multiple comparison using SPSS 17.0. The significant difference was set at a level of 0.05 among groups.
Results
KRD significant scavenging of the radicals in vitro
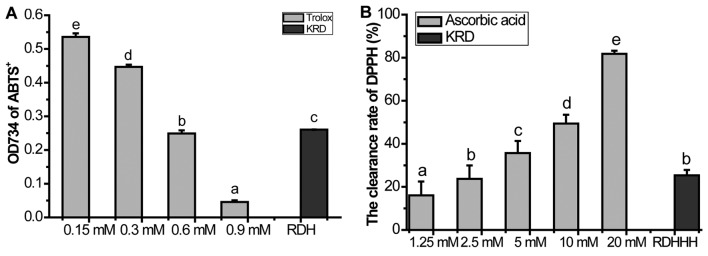
In ABTS radical scavenging activity test, Trolox was used as a calibration reader with concentrations ranging from 0.15 to 0.9 mM and the obtained calibration was Y (scavenging rate) = −0.6627 X (Trolox concentration, mM) + 0.6430 (r=0.9996). The additive amounts of the samples were calculated based on the obtained formula. Results were expressed in terms of mM Trolox per 1 mg/ml KRD. We found that 1 mg/ml KRD was equivalent to 1.1760 mM Trolox (Fig. 1A).
Figure 1.
Kushui Rose decoction (KRD) significantly scavenging free radicals in vitro. (A) The effect of KRD on 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) radical scavenging activity. (B) The effect of KRD on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity. RDH refers to 0.4 mg/ml KRD, RDHHH refers to 1.6 mg/ml KRD. Data are presented as the mean ± SD of at least three independent experiments. Bars with different letters indicate that there was a significant difference at a level of 0.05 among groups.
In the DPPH radical scavenging activity test, ascorbic acid was used as a calibration reader with concentrations ranging from 0.625 to 20 mM and the obtained calibration was Y (scavenging rate) = 0.0333 X (ascorbic acid concentration, mM) + 0.1532 (r=0.9964). The additive amounts of the samples were calculated based on the obtained formula. Results were expressed in terms of mM ascorbic acid per 1 mg/ml KRD. We found that 1 mg/ml KRD was equivalent to 1.8844 mM ascorbic acids (Fig. 1B).
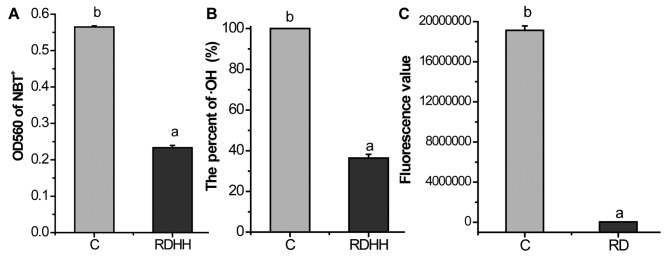
Additionally, we also tested the scavenging capacity of KRD on hydroxyl radical, superoxide anion and hydrogen peroxide. As showed in Fig. 2, KRD can mop up 58.76% superoxide anion (Fig. 2A), 63.64% hydroxyl radical (Fig. 2B) and 99.80% hydrogen peroxide (Fig. 2C), respectively. These results showed that KRD possesses remarkable scavenging effect against radicals in vitro.
Figure 2.
Kushui Rose decoction (KRD) significantly scavenging (A) superoxide anion, (B) hydroxyl radicals and (C) hydrogen peroxide in vitro. RDHH refers to 0.8 mg/ml KRD. RD refers to 80 mg/ml KRD. RDHH can clear 58.76% superoxide anion of 75 µmol/l NBT, 63.64% hydroxyl radical. RD can mop up 99.80% H2O2 (0.3%). Data are presented as the mean ± SD of at least three independent experiments. Bars with different letters indicate that there was a significant difference at a level of 0.05 among groups.
KRD significantly suppresses over-activated Ras/MAPK pathway, but not the essential oil and hydrosol
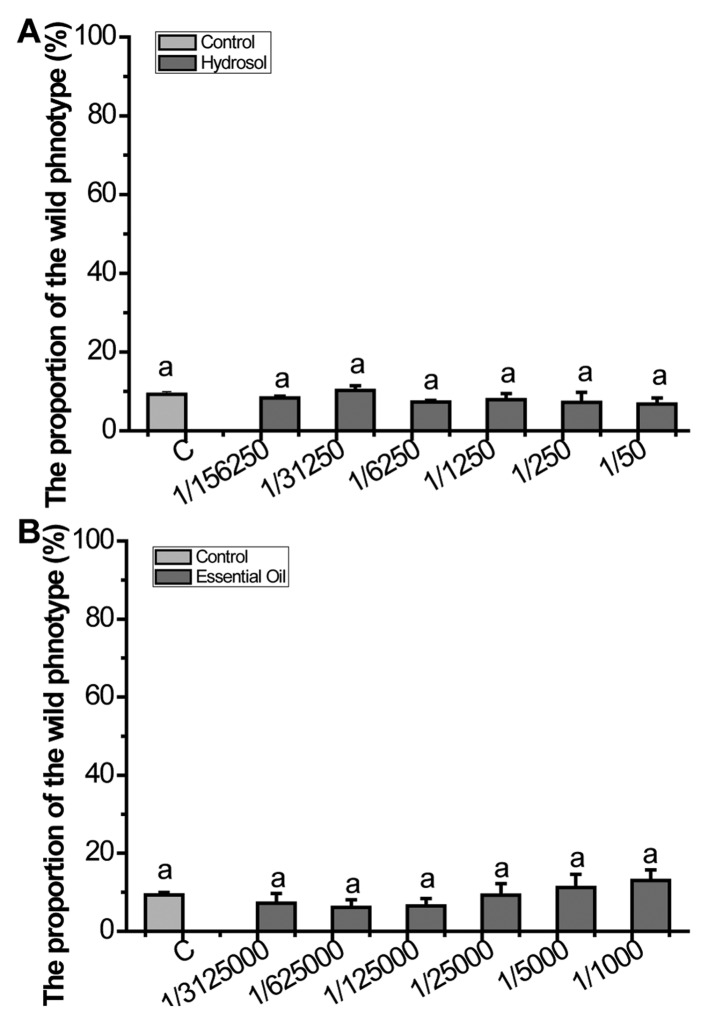
KRD inhibited the Muv phenotype of let-6 mutants strongly in a dose-dependent manner (Fig. 3). RDH increased the percent of wild-type phenotype of let-60(gf) mutants to 74.58%. Even at RDL as low as 0.05 mg/ml, the decoction still has efficacy (33.83%). The essential oil is known to have high biological activity, so we applied essential oil and hydrosol to assess whether the essential oil can inhibit over-activated ras. The results showed that neither of them can suppress over-activated Ras (Fig. 4).
Figure 3.
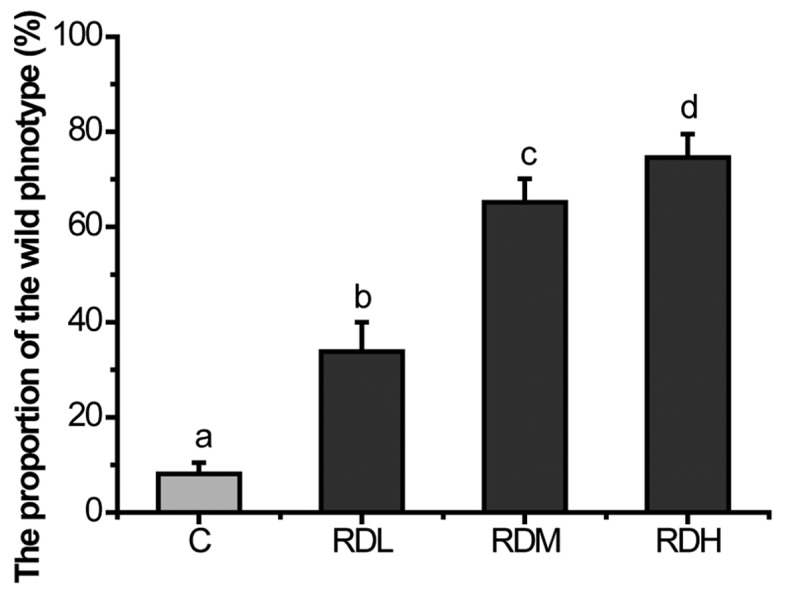
Kushui Rose decoction (KRD) significantly inhibits the multivulva (Muv) phenotype of the let-60(gf) mutants in a dose-dependent manner. The Muv phenotype of let-60(gf) mutants was reversed to the wild-type phenotype after treated with KRD for 3–4 days. The proportion of wild-type of let-60(gf) was the percents of wild phenotype worms in tested worms (N=80–100). C, control. RDL, RDM, RDH refer to 0.1, 0.2 and 0.4 mg/ml KRD, respectively. Data are presented as the mean ± SD of at least three independent experiments. Bars with different letters indicate that there was a significant difference at a level of 0.05 among groups.
Figure 4.
(A and B) Both essential oil and hydrosol of KR had no effect on let-60(gf) mutants (N=80–100). Data are presented as the mean ± SD of at least three independent experiments. Bars with different letters indicate that there was a significant difference at a level of 0.05 among groups.
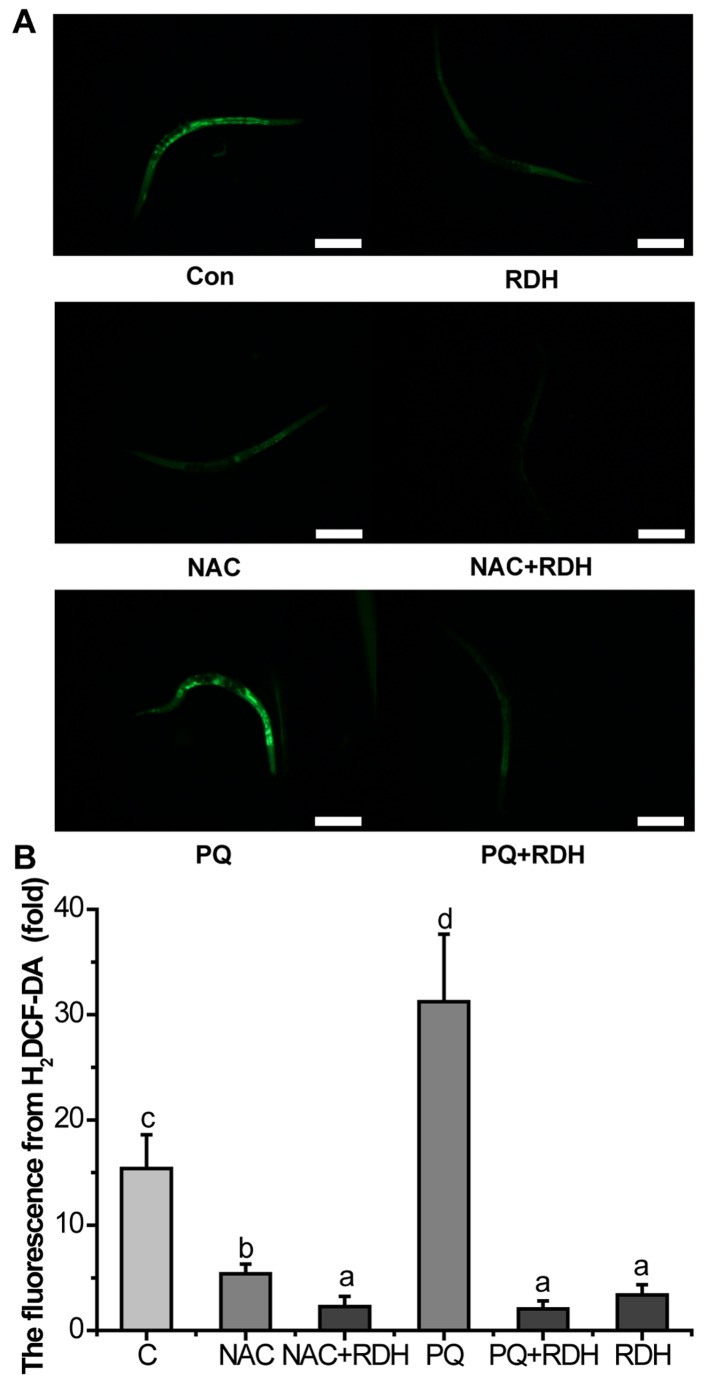
KRD significantly decreases the ROS level in C. elegans
The level of ROS in let-60(gf) mutants was measured by using the ROS probe H2DCF-DA. KRD can strongly reduce ROS in vivo. NAC is an antioxidant that can scavenge all kinds of ROS. NAC decreased the level of ROS of let-60(gf) mutants, and KRD combined with NAC further scavenged the ROS. PQ is a well-known pro-oxidant. PQ greatly increased the accumulation of ROS, and KRD can almost completely eliminate the ROS induced by PQ (Fig. 5).
Figure 5.
Kushui Rose decoction (KRD) decreased significantly the level of reactive oxygen species (ROS) accumulation in let-60(gf) mutants. (A) Fluorescence image of worms stained by H2DCF-DA, which were stained by 10 µM H2DCF-DA at 20°C for 20 min (N=20). Con and C, control. NAC, 2.5 mM N-Acety-L-Cysteine; PQ, 0.5 mM paraquat. Scale bar, 200 µm. (B) Quantified fluorescence intensity of H2DCF-DA of each group. 2.5 mM NAC and 0.5 mM PQ were used as positive and negative control, respectively. Data are presented as the mean ± SD of at least three independent experiments. Bars with different letters indicate that there was a significant difference at a level of 0.05 among groups.
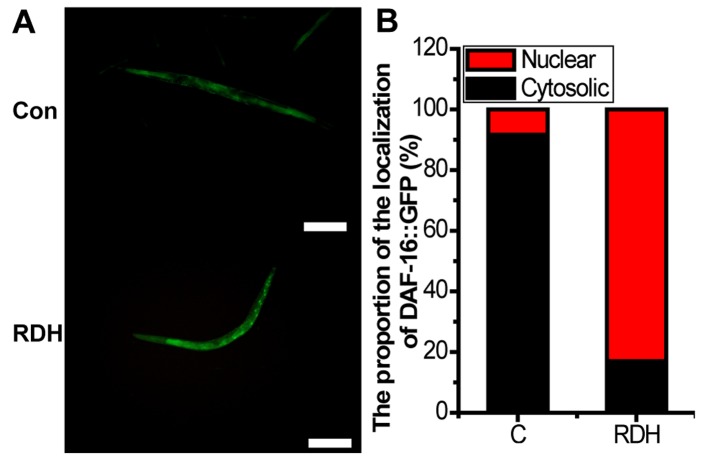
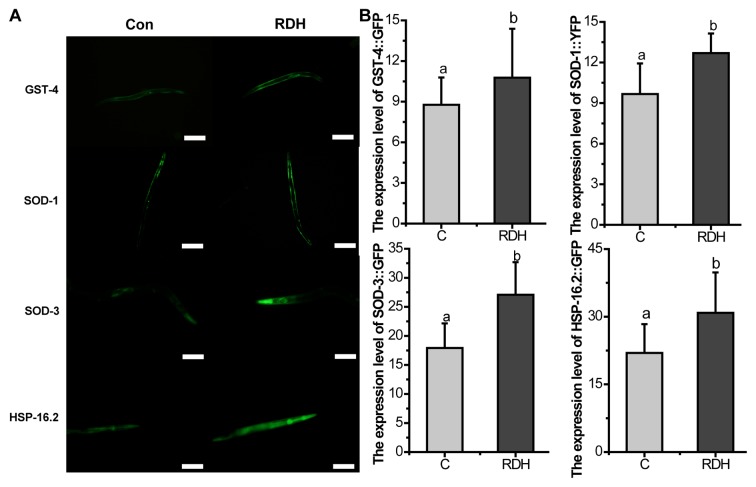
KRD significantly increases the oxidative stress related proteins in C. elegans
DAF-16 is necessary for stress resistance (45). GSTs are main cellular detoxification enzymes, and GST can protect the worms against oxidative stress damage (46). SODs avoid the formation of highly aggressive ROS, such as peroxynitrite or the hydroxyl radical (47). HSP-16.2 is also a stress-sensitive reporter (48). The results of the study showed that the proportion of DAF-16::GFP nuclear translocation and the expression of GST-4, SOD-1, SOD-3 were all significantly upregulated by KRD (Figs. 6 and 7).
Figure 6.
Kushui Rose decoction (KRD) promotes forkhead transcription factor (DAF-16)::GFP nuclear translocation in transgenic strain TJ356. (A) Fluorescence image of worms with or without DAF-16::GFP nuclear translocation (N=20). Con and C, control. Scale bar, 100 µm. (B) Quantified GFP intensity of each group. Data are presented as the mean ± SD of at least three independent experiments. Bars with different letters indicate that there was a significant difference at a level of 0.05 among groups.
Figure 7.
Kushui Rose decoction (KRD) increased expression of glutathione S-transferase-4 (GST-4)::GFP, superoxide dismutase-1 (SOD-1)::GFP, SOD-3::GFP and heat shock protein-16.2 (HSP-16.2)::GFP in transgenic worms (N=20). (A) Fluorescence image of worms with expression of GST-4::GFP and SOD-1::GFP, Scale bar, 200 µm. SOD-3::GFP and HSP-16.2::GFP. Scale bar, 100 µm. Con and C, control. (B) Quantified GFP intensity of each group. Data are presented as the mean ± SD of at least three independent experiments. Bars with different letters indicated that there was a significant difference at a level of 0.05 among groups.
Discussion
The free radical theory postulates that the production of intracellular reactive oxygen species is the main determinant and promotion of cancer (17). This is supported by findings from a variety of studies, suggested that reduction of oxidative stress is associated with the treatment of cancer (18). It is reasonable to conclude that antioxidants are potential drug candidates for cancer therapy. Further, over-activated Ras is closely related to the increased ROS accumulation as well (14–16). In C. elegans, let-6 is a homologous gene to ra in mammals; let-60(gf) could cause the multivulva phenotype. Thus, we employed C. elegan with let-60(gf) mutant to determine whether KRD and its extracts can suppress over-activated Ras/MAPK pathways thought resistance to oxidative stress.
KRD had a very potent antioxidant activity in vitro (Fig. 1). KRD (1 mg/ml) was equivalent to 1.1760 mM Trolox or 1.8844 mM ascorbic acids, respectively. As the results show in Fig. 2, KRD can mop up 58.76% superoxide anion (Fig. 2A), 63.64% hydroxyl radical (Fig. 2B) and 99.80% hydrogen peroxide (Fig. 2C), respectively. Thus, KRD can remarkable scavenge the radicals in vitro.
In recent years, increased attention has been given to rose, especially the essential oils (49) and rose essential oil is one of the most expensive essential oils. The antioxidant activity of the rose essential oil was previously studied, and high antioxidant capacity was observed (26). Since KRD, rose essential oil and its hydrosol all had potent antioxidant capacity, the effect of KRD, rose essential oil and its hydrosol on the over-activated Ras was further investigated. In this study, KRD inhibited the Muv phenotype significantly in a dose-dependent manner in let-6 mutants (Fig. 3). In Fig. 4, the results show that neither rose essential oil nor hydrosol can suppress the over-activated Ras, thus indicating that the active ingredient which inhibit the over-activated Ras mainly exist in KRD. Therefore, reminding us that soaking rose in hot water to prepare 'rose tea' is an effective way for health care.
Since KRD can significantly inhibit the over-activated Ras (Fig. 3), and have a potent antioxidant activity in vivo (Figs. 1 and 2), we attempted to illuminate whether the mechanism of KRD is built on its antioxidant activity. The results showed that KRD can strongly reduce ROS in vivo (Fig. 5). NAC can decrease the level of ROS of let-60(gf) mutants. KRD combined with NAC can further scavenge ROS. PQ can substantially increase the accumulation of ROS, and KRD can almost completely eliminate the ROS induced by PQ. These phenomena indicated that KRD scavenged ROS like NAC and resisted the oxidative stress induced by PQ in let-60(gf) mutants (Fig. 5).
DAF-16/forkhead transcription factor, GSTs, SODs, HSP-16.2 all can serve as stress-sensitive reporters. The proportion of DAF-16::GFP nuclear translocation and the expression of GST-4, SOD-1, SOD-3, HSP-16.2 were all increased by KRD treatment (Figs. 6 and 7). These anti-oxidative stress related factors were all upregulated by KRD, suggest that KRD inhibit over-activated Ras via a mechanism that is based on its antioxidant capacity and upregulating the anti-oxidative stress related factors. So the antitumor effect of KRD in C. elegan is shown by downregulation of Ras/MAPK pathway and resistance to oxidative stress.
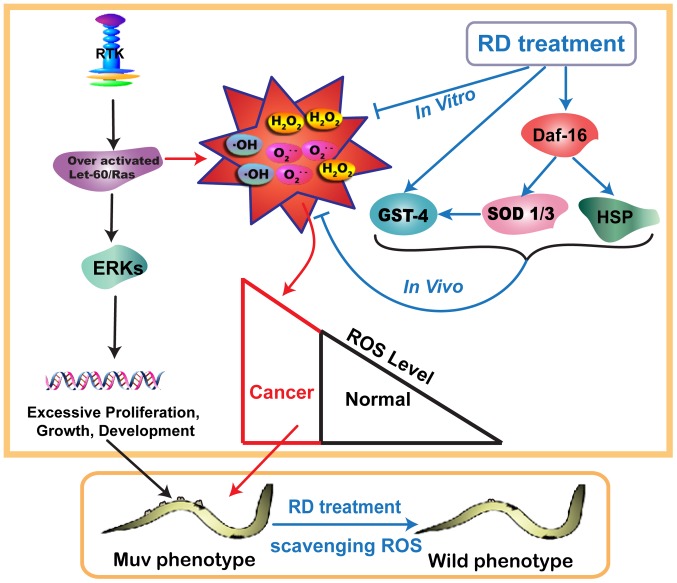
In conclusion, the mechanism of KRD that significantly suppressed the over-activated Ras/MAPK pathway is mainly dependent on the process as showed in Fig. 8. First of all, over-activated Ras/MAPK pathway led to the excessive accumulation of ROS. Then worms treated with KRD were able to scavenge ROS. Next, KRD triggered the nuclear translocation of DAF-16 and the expression of GST-4, SOD-1, SOD-3 and HSP-16.2 to protect the worms against oxidative stress. Finally, the level of ROS returned to normal and the Muv phenotype in let-60(gf) mutants was reverted by KRD. The above indicates KRD can serve as a potential drug candidate for combating over-activated Ras-related cancer.
Figure 8.
Schematic diagram of mechanism of Kushui Rose decoction (KRD) suppressing multivulva (Muv) phenotype induced by over-activated Ras. First, over-activated Ras/MAPK pathway led to the excessive accumulation of reactive oxygen species (ROS), and then worms were treated with KRD which can scavenge ROS. Next, KRD triggered the nuclear translocation of forkhead transcription factor (DAF-16) and the expression of glutathione S-transferase-4 (GST-4), superoxide dismutase-1 (SOD-1), SOD-3, heat shock protein-16.2 (HSP-16.2) to protect the worms against oxidative stress. Finally, the level of ROS returned to normal and the Muv phenotype in let-60 (gf) mutants was reverted by KRD.
Acknowledgments
The authors wish to thank Dr Kuang Yu Chen, Rutgers University, and Dr Li Ming Hao, Harvard Medical School, for their valuable suggestions and kind help in checking the English presentation of this manuscript. MT2124 was kindly provided by Howard Hughes Medical Institute.
Abbreviations
- ABTS
2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid
- C. elegans
Caenorhabditis elegans
- DAF-16
forkhead transcription factor
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- GST-4
glutathione S-transferase-4
- HSP-16.2
heat shock protein-16.2
- KR
Kushui Rose (R. Setat x R. Rugosa)
- KRD
Kushui Rose decoction
- NAC
N-acetyl cysteine
- NBT
nitrotetrazolium blue chloride
- PQ
paraquat
- ROS
reactive oxygen
- SOD
superoxide dismutase
Funding
This study was supported by the Ministry of Science and Technology New Drug Project of MOST of China (2015ZX09501-004-003-008); the National Natural Science Foundation of China (31772147 and 31571989); and the Fundamental Research Funds for the Central Universities (lzujbky-2018-40 and lzujbky-2017-206).
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YLiu performed all the experiments and was a major contributor to the writing of the manuscript. DF and ZZ prepared the KR decoction. YLiu, DZ, XW and ZW analyzed the data. HL, YLi and PC designed the experiments. XW, ZW, YLi and PC revised the manuscript critically. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Slavov A, Kiyohara H, Yamada H. Immunomodulating pectic polysaccharides from waste rose petals of Rosa damascena Mill. Int J Biol Macromol. 2013;59:192–200. doi: 10.1016/j.ijbiomac.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 2.Davis PH. Flora of Turkey and the East Aegean Islands. Edinburgh University Press; Edinburgh: 1965. p. 567. [Google Scholar]
- 3.Kazaz S, Baydar H, Erbas S. Variations in Chemical Compositions of Rosa damascena Mill. and Rosa canina L. Fruits. Czech J Food Sci. 2009;27:178–184. [Google Scholar]
- 4.Jin J. The comprehensive development of rose. Chin Wild Plant Resour. 2000;19:21–23. [Google Scholar]
- 5.Cai F, He Y, Li X. A Survey of Rosa rugos germplasm resource. World Sci Technology-Modernization Tradit Chin Med Materia Med. 2007;3:75–80. In Chinese. [Google Scholar]
- 6.Gao Y, Mao XL, Wang WS, Chen Z, Huang JY. Analysis of active components in kushui roses. Flavour Fragrance Cosmetics. 2014:22–26. [Google Scholar]
- 7.Zhai YL, Xie CJ. Kushui rose in Lanzhou. Forestry of China. 2010:44–44. [Google Scholar]
- 8.Zhou W, Zhou XP, Zhao GH, Liu HW, Ding L, Chen LR. Studies of aroma components on essential oil of Chinese kushui rose. Se Pu. 2002;20:560–564. In Chinese. [PubMed] [Google Scholar]
- 9.Yu C, Zhao Y, Xu Q, Yu P, Zhang X. The thinking of kushui rose (R. Setat x R. rugosa) industry development. Gansu Sci Technol. 2012;28:14–15. [Google Scholar]
- 10.Grunwald A, Gottfried I, Cox AD, Haklai R, Kloog Y, Ashery U. Rasosomes originate from the Golgi to dispense Ras signals. Cell Death Dis. 2013;4:e496. doi: 10.1038/cddis.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo A, Ficili B, Candido S, Pezzino FM, Guarneri C, Biondi A, Travali S, McCubrey JA, Spandidos DA, Libra M. Emerging targeted therapies for melanoma treatment (review) Int J Oncol. 2014;45:516–524. doi: 10.3892/ijo.2014.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friday BB, Adjei AA. K-ras as a target for cancer therapy. Biochim Biophys Acta. 2005;1756:127–144. doi: 10.1016/j.bbcan.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg F, Chandel NS. Mitochondrial metabolism and cancer. Ann NY Acad Sci. 2009;1177:66–73. doi: 10.1111/j.1749-6632.2009.05039.x. [DOI] [PubMed] [Google Scholar]
- 14.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Lu B, Xu L, Yin LH, Wang XN, Peng JY, Liu KX. The antioxidant activity and hypolipidemic activity of the total flavonoids from the fruit of Rosa laevigata Michx. Nat Sci. 2010;2:175–183. [Google Scholar]
- 16.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 17.Bagchi D, Swaroop A, Preuss HG, Bagchi M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat Res. 2014;768:69–73. doi: 10.1016/j.mrfmmm.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Bhandary B, Marahatta A, Kim HR, Chae HJ. Mitochondria in relation to cancer metastasis. J Bioenerg Biomembr. 2012;44:623–627. doi: 10.1007/s10863-012-9464-x. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui IA, Adhami VM, Saleem M, Mukhtar H. Beneficial effects of tea and its polyphenols against prostate cancer. Mol Nutr Food Res. 2006;50:130–143. doi: 10.1002/mnfr.200500113. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Hameed el-SS, Bazaid SA, Salman MS. Characterization of the phytochemical constituents of Taif rose and its antioxidant and anticancer activities. Biomed Res Int. 2013;2013:345465. doi: 10.1155/2013/345465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bairati I, Meyer F, Gélinas M, Fortin A, Nabid A, Brochet F, Mercier JP, Têtu B, Harel F, Mâsse B, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97:481–488. doi: 10.1093/jnci/dji095. [DOI] [PubMed] [Google Scholar]
- 22.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;3:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinonen OP, Albanes D. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. 1994 N Engl J Med;330:1029–1035. [Google Scholar]
- 24.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 25.Velázquez E, Tournier HA, Mordujovich de Buschiazzo P, Saavedra G, Schinella GR. Antioxidant activity of Paraguayan plant extracts. Fitoterapia. 2003;74:91–97. doi: 10.1016/S0367-326X(02)00293-9. [DOI] [PubMed] [Google Scholar]
- 26.Baydar NG, Baydar H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind Crops Prod. 2013;41:375–380. doi: 10.1016/j.indcrop.2012.04.045. [DOI] [Google Scholar]
- 27.Cociancich E, Pace R. Process for the extraction of taxol and derivatives thereof from roots of plants of the genus Taxus. 5744333 A. US Patent US. Filed 28 April, 1998; issued, 31 January, 1992.
- 28.Cragg GM, Newman DJ. Plants as a source of anticancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Sieber SM, Mead JA, Adamson RH. Pharmacology of antitumor agents from higher plants. Cancer Treat Rep. 1976;60:1127–1139. [PubMed] [Google Scholar]
- 30.Lee TH, Lee CK, Tsou WL, Liu SY, Kuo MT, Wen WC. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata. Planta Med. 2007;73:1412–1415. doi: 10.1055/s-2007-990232. [DOI] [PubMed] [Google Scholar]
- 31.Kumar VB, Yuan TC, Liou JW, Yang CJ, Sung PJ, Weng CF. Antroquinonol inhibits NSCLC proliferation by altering PI3K/mTOR proteins and miRNA expression profiles. Mutat Res. 2011;707:42–52. doi: 10.1016/j.mrfmmm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Ng TB, He JS, Niu SM, Zhao L, Pi ZF, Shao W, Liu F. A gallic acid derivative and polysaccharides with antioxidative activity from rose (Rosa rugosa) flowers. J Pharm Pharmacol. 2004;56:537–545. doi: 10.1211/0022357022944. [DOI] [PubMed] [Google Scholar]
- 33.Vinokur Y, Rodov V, Reznick N, Goldman G, Horev B, Umiel N, Friedman H. Rose petal tea as an antioxidant-rich beverage: Cultivar effects. J Food Sci. 2006;71:S42–S47. doi: 10.1111/j.1365-2621.2006.tb12404.x. [DOI] [Google Scholar]
- 34.Mattiasson G. Analysis of mitochondrial generation and release of reactive oxygen species. Cytometry A. 2004;62:89–96. doi: 10.1002/cyto.a.20089. [DOI] [PubMed] [Google Scholar]
- 35.Liu D, Zhi D, Zhou T, Yu Q, Wan F, Bai Y, Li H. Realgar bioleaching solution is a less toxic arsenic agent in suppressing the Ras/MAPK pathway in Caenorhabditis elegans. Environ Toxicol Pharmacol. 2013;35:292–299. doi: 10.1016/j.etap.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Dong Y, Guha S, Sun X, Cao M, Wang X, Zou S. Nutraceutical interventions for promoting healthy aging in invertebrate models. Oxid Med Cell Longev. 2012;2012:718491. doi: 10.1155/2012/718491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sternberg PW, Han M. Genetics of RAS signaling in C. elegans. Trends Genet. 1998;14:466–472. doi: 10.1016/S0168-9525(98)01592-3. [DOI] [PubMed] [Google Scholar]
- 38.Zhi J, Feng N, Liu DL, Hou RL, Wang MZ, Ding XX, Li HY. Realgar bioleaching solution suppress ras excessive activation by increasing ROS in Caenorhabditis elegans. Arch Pharm Res. 2014;37:390–398. doi: 10.1007/s12272-013-0182-7. [DOI] [PubMed] [Google Scholar]
- 39.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara M, Han M. Ras farnesyltransferase inhibitors suppress the phenotype resulting from an activated ras mutation in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:3333–3337. doi: 10.1073/pnas.92.8.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiner DJ, Gonzalez-Perez V, Der CJ, Cox AD. Use of Caenorhabditis elegan to evaluate inhibitors of Ras function in vivo. Methods Enzymol. 2008;439:425–449. doi: 10.1016/S0076-6879(07)00430-2. [DOI] [PubMed] [Google Scholar]
- 42.Kampkötter A, Pielarski T, Rohrig R, Timpel C, Chovolou Y, Wätjen W, Kahl R. The Ginkgo bilob extract EGb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacol Res. 2007;55:139–147. doi: 10.1016/j.phrs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Kaneria M, Kanani B, Chanda S. Assessment of effect of hydroalcoholic and decoction methods on extraction of antioxidants from selected Indian medicinal plants. Asian Pac J Trop Biomed. 2012;2:195–202. doi: 10.1016/S2221-1691(12)60041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li XT, Zhang YK, Kuang HX, Jin FX, Liu DW, Gao MB, Liu Z, Xin XJ. Mitochondrial protection and anti-aging activity of Astragalus polysaccharides and their potential mechanism. Int J Mol Sci. 2012;13:1747–1761. doi: 10.3390/ijms13021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Jie G, Zhang J, Zhao B. Significant longevity-extending effects of EGCG on Caenorhabditis elegan under stress. Free Radic Biol Med. 2009;46:414–421. doi: 10.1016/j.freeradbiomed.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 46.Leiers B, Kampkötter A, Grevelding CG, Link CD, Johnson TE, Henkle-Dührsen K. A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic Biol Med. 2003;34:1405–1415. doi: 10.1016/S0891-5849(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 47.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Wu H, Zhao Y, Guo Y, Xu L, Zhao B. Significant longevity-extending effects of a tetrapeptide from maize on Caenorhabditis elegan under stress. Food Chem. 2012;130:254–260. doi: 10.1016/j.foodchem.2011.07.027. [DOI] [Google Scholar]
- 49.Ge Q, Ma X. Composition and antioxidant activity of antho-cyanins isolated from Yunnan edible rose (An ning) Food Sci Hum Wellness. 2013;2:68–74. doi: 10.1016/j.fshw.2013.04.001. [DOI] [Google Scholar]