Abstract
Mechanosensitive (MS) neurons in the periodontal ligament (PDL) pass information to the trigeminal ganglion when excited by mechanical stimulation of the tooth. During occlusal tooth trauma of PDL tissues, MS neurons are injured, resulting in atrophic neurites and eventual degeneration of MS neurons. Nerve growth factor (NGF), a neurotrophic factor, serves important roles in the regeneration of injured sensory neurons. In the present study, the effect of pro-inflammatory cytokines, including interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α), on transforming growth factor β1 (TGF-β1)-induced NGF expression was evaluated in rat PDL-derived SCDC2 cells. It was observed that TGF-β1 promoted NGF expression via Smad2/3 and p38 mitogen-activated protein kinase (MAPK) activation. IL-1β and TNF-α suppressed the TGF-β1-induced activation of Smad2/3 and p38 MAPK, resulting in the abrogation of NGF expression. NGF secreted by TGF-β1-treated SCDC2 cells promoted neurite extension and the expression of tyrosine hydroxylase, a rate-limiting enzyme in dopamine synthesis in rat pheochromocytoma PC12 cells. These results suggested that pro-inflammatory cytokines suppressed the TGF-β-mediated expression of NGF in PDL-derived fibroblasts through the inactivation of TGF-β-induced Smad2/3 and p38 MAPK signaling, possibly resulting in the disturbance of the regeneration of injured PDL neurons.
Keywords: interleukin 1β, tumor necrosis factor α, transforming growth factor β, nerve growth factor, Smad2/3, p38 mitogen-activated protein kinase, periodontal ligament fibroblast
Introduction
Periodontal ligament (PDL) is a connective tissue that anchors the tooth root to the alveolar bone (1). PDL contains primary mechanosensitive PDL (PM-PDL) neurons running into the trigeminal mesencephalic nucleus (Vmes) (2). Following their excitation by mechanical stimulation of the tooth, PM-PDL neurons transmit information to the trigeminal ganglion, resulting in the excitement of the ventral posteromedial nucleus of the thalamus (3,4). PM-PDL neuron innervation comprises Ruffini-like endings, which are partially covered by special lamellar Schwann cells and complex basal lamina (5,6). Thus, Ruffini-like endings function as a mechanosensor of the PDL tissue, collecting information on the mechanical stress of PDL influenced by the dental occlusal force and exciting the sense perception of chewing and crunching in the brain. Uemura et al (7) previously reported that afferent neurons of jaw muscles running into Vmes also function as a mechano-sensor of stress information during chewing and crunching. In addition, immunohistological analysis revealed that PDL neurons exhibit phosphorylated extracellular signal-regulated kinases 1 and 2 (ERK1/2), which are found in particular around blood vessels, suggesting that PDL neurons regulate peripheral blood supply in PDL tissue in an ERK1/2-dependent manner (8).
During occlusal trauma of PDL tissues, mechanosensitive (MS) neurons are injured, resulting in atrophic neurites and eventual degeneration of MS neurons (9). Nerve growth factor (NGF) is a neurotrophic factor that is known to serve important roles in neurite extension and regeneration in injured sensory neurons (10,11). NGF serves as a potential guidance cue for the axon outgrowth of dorsal root ganglion neurons through tropomyosin receptor kinase A (TrkA). The primary subunit of NGF is composed of 118 amino acids, as first identified in the mouse submaxillary gland, and the native protein comprises two subunits (12). Two types of receptors with high and low affinities for NGF have been identified, including TrkA, which is a receptor tyrosine kinase on the cell membrane and has a high affinity for NGF, and p75 neurotrophin receptor that has a low affinity for NGF (13).
Transforming growth factor β (TGF-β) is known to play important roles in immunosuppression (14). In particular, TGF-β1 is involved in the inhibition of renal inflammation progression in vitr and in viv in a Smad7-dependent manner (15). TGF-β is mainly synthesized by macrophages and secreted by these cells homing into inflammatory tissues (16), and directly binds to its type II receptors on the cell membrane. The type II receptor kinase activates the type I receptor kinase following the formation of a tetrameric complex composed of two type I and two type II receptors. The activated type I receptor then induces intracellular signal transduction through the phosphorylation of receptor-regulated Smads (R-Smads) (17-19). Smads are major signaling molecules of the TGF-β superfamily and comprise three groups as follows: i) R-Smads, including Smad1, Smad5 and Smad8 that are primarily activated by the bone morphogenetic protein-specific type I receptors, as well as Smad2 and Smad3 that are activated by TGF-β-specific type I receptors; ii) common mediator Smad (Co-Smads), such as Smad4; and iii) inhibitory Smads (I-Smads), such as Smad6 and Smad7. The activated R-Smads form complexes with Co-Smad, which enter the nucleus and regulate the transcription of specific target genes. Furthermore, I-Smads suppress the activation of R-Smads by competing with R-Smads for type I receptor interaction and recruiting specific ubiquitin ligases, resulting in their proteasomal degradation.
TGF-β is also known to induce NGF expression in various types of cells (20,21). In particular, TGF-β1 promotes NGF expression in chondrocytes in a Smad2/3-dependent manner (22). By contrast, TGF-β relays its intracellular signaling through non-Smad signaling pathways, such as c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) signaling (23). TGF-β1 promotes NGF expression in dental pulp cells through JNK and p38 MAPK signal transduction (24). However, whether TGF-β induces the expression of NGF in PDL fibroblasts through Smad and/or non-Smad signaling pathways is questionable.
Inflammatory cytokines, including interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α), are known to modulate TGF-β-induced NGF expression. TGF-β1 and IL-1β cooperatively and additively promote NGF production/secretion in the astroglial cell line RC7 (25). A previous study reported that IL-1β or TNF-α alone promoted NGF expression in the mouse fibroblast cell line Swiss 3T3, and notably, these cytokines synergistically promoted NGF expression in the cells (26). Intracellular signaling induced by IL-1β and TNF-α activates nuclear factor-κB (NF-κB), JNK and p38 MAPK signaling pathways (27,28). Hengerer et al (29) reported that the c-Fos/c-Jun heterodimer activator protein 1 activated the promoter of NGF gene, while Lee et al (30) demonstrated that cyclosporine A induced NGF expression in the human corneal epithelial HCECL cell line in a p38 MAPK-dependent manner. A study by Heese et al (31) further reported that NF-κB activation was a key positive regulatory event for NGF expression in B cells. These results suggest that IL-1β- and TNF-α-induced activation of JNK, p38 MAPK and NF-κB positively regulates NGF expression. However, the effect of IL-1β or TNF-α on NGF expression in PDL fibroblasts remains to be elucidated.
In the present study, the effects of the anti-inflammatory cytokine TGF-β1, the inflammatory cytokines IL-1β or TNF-α, and the combination of inflammatory and anti-inflammatory cytokines on the expression of NGF in PDL fibroblasts were investigated. In addition, the study evaluated the effect of NGF secreted by PDL fibroblasts on the status of neurite extension and the expression of tyrosine hydroxylase (TH), which is a rate-limiting enzyme in dopamine synthesis (32), in rat pheochromocytoma PC12 cells. The current study clarified the molecular mechanisms underlying the TGF-β-mediated regenerative effect of PDL fibroblasts on injured and inflamed PDL neurons, and the findings may aid in identifying drug targets for the treatment of PDL nerve injury at a molecular level.
Materials and methods
Reagents
Recombinant human TGF-β1 was obtained from PeproTech, Inc. (Rocky Hill, NJ, USA), recombinant rat NGF was obtained from Alomone Labs (Jerusalem, Israel), and recombinant rat IL-1β and TNF-α were purchased from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany). SB-431542, a TGF-β type I receptor inhibitor, was supplied by Cell Signaling Technology, Inc. (Beverly, MA, USA). The p38 MAPK inhibitor SB203580, JNK inhibitor SP600125, and Smad3 inhibitor SIS3 (33) were obtained from Merck KGaA (Calbiochem; Darmstadt, Germany). Adenosine 5′-O-(3-thio) triphosphate (ATPγS; ab138911) was purchased from Abcam (Cambridge, UK). The TrkA inhibitor GW441756 was purchased from Selleck Chemicals (Houston, TX, USA), while the NF-κB inhibitor BAY 11-7085 was from Cayman Chemical (Ann Arbor, MI, USA).
Cell culture
The isolation of rat PDL-derived fibroblasts and the establishment of single cell-derived cultures (SCDCs) have been previously described (34). SCDC2 cells (1×105 cells) were cultured on type I collagen-coated plastic dishes (Sumilon Celltight Dishes with 90 mm diameters; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) in Ham's F-12 (Sigma-Aldrich; Merck KGaA) medium supplemented with 2 mM glutamine (100X solution; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10% fetal bovine serum (FBS), penicillin (50 units/ml)-streptomycin (50 µg/ml) (Invitrogen; Thermo Fisher Scientific, Inc.), 10 ng/ml fibroblast growth factor (FGF)-1 (R&D Systems Inc., Minneapolis, MN, USA), and 15 µg/ml heparin (Sigma-Aldrich; Merck KGaA) in a humidified atmosphere of 5% CO2 at 37°C. Rat pheochromocytoma PC12 cells obtained from RIKEN BioResource Research Center Cell Bank (Ibaragi, Japan) were cultured on type I collagen tissue culture plastic dishes in Dulbecco's modified Eagle's medium (Sigma-Aldrich; Merck KGaA) supplemented with 2 mM glutamine (100X solution), 5% FBS, 10% horse serum and penicillin (50 units/ml)-streptomycin (50 µg/ml) (Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 5% CO2 at 37°C.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from SCDC2 cells with ISOGEN I reagent (Nippon Gene Co., Ltd., Toyama, Japan) according to the manufacturer's protocol. Concentration/quality of total RNA was measured by using spectrophotometer Gene Spec III (Hitachi High-Tech Science Corp., Tokyo, Japan). First-strand complementary DNA (cDNA) was then synthesized from 1 µg of total RNA using the PrimeScript RT reagent kit (Takara Bio, Inc., Shiga, Japan). qPCR was subsequently performed on a Thermal Cycler Dice Real Time System (two-step cycle procedure for 40 cycles: Denaturation at 95°C for 5 sec and annealing and extension at 60°C for 30 sec; Takara Bio, Inc.) using SYBR Premix Ex Taq II (Takara Bio, Inc.) with specific oligonucleotide primers, as follows: Rat TH, 5′-GGAGCTGAAGGCTTATGGTG-3′ (forward) and 5′-CATTGAAGCTCTCGGACACA-3′ (reverse); rat NGF, 5′-TGCCAAGGACGCAGCTTTC-3′ (forward) and 5′-TGAAGTTTAGTCCAGTGGGCTTCAG-3′ (reverse); and rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-GGCACAGTCAAGGCTGAGAATG-3′ (forward) and 5′-ATGGTGGTGAAGACGCCAGTA-3′ (reverse). The mRNA levels of NGF and TH were normalized to the level of GAPDH, and the relative expression levels of genes are expressed as the fold increase or decrease relative to the control (35).
Effects of SB-431542, SB203580, SP600125, and SIS3 on the TGF-β1-promoted expression of NGF mRNA in SCDC2 cells
The cells were seeded onto type I collagen-coated 12-well tissue culture plates at a density of 7×104 cells/well in SCDC2 growth medium and maintained for 24 h. The growth medium was replaced with Ham's F-12 without FBS, FGF-1, and heparin for 24 h for cell starvation. The cells were starved and treated with or without TGF-β1 (10 ng/ml) for 24 h. Some cells were pretreated with SB-431542 (10 µM), SB203580 (10 µM), Smad3 inhibitor SIS3 (10 µM), or SP600125 (10 µM) for 30 min before TGF-β1 administration. The cells without pretreatment were incubated with DMSO as a vehicle control. The relative expression level of NGF was evaluated using RT-qPCR analysis, as described above.
Effect of IL-1β and TNF-α on TGF-β1-promoted expression of NGF mRNA in SCDC2 cells
The cells were seeded onto type I collagen-coated 12-well tissue culture plates at a density of 7×104 cells/well in SCDC2 growth medium and maintained for 24 h. The growth medium was replaced with Ham's F-12 without FBS, FGF-1, and heparin for 24 h for cell starvation. The cells were starved and treated with or without TGF-β1 (10 ng/ml) alone, TGF-β1 (10 ng/ml) plus IL-1β (10 ng/ml), or TGF-β1 (10 ng/ml) plus TNF-α (10 ng/ml) for 24 h. Some cells were pretreated with BAY 11-7085 (10 µM) for 30 min before IL-1β or TNF-α administration. The cells without pretreatment were incubated with dimethyl sulfoxide as a vehicle control. The relative expression level of NGF was evaluated using RT-qPCR analysis, as described above.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay buffer [containing 50 mM Tris-HCl (pH 7.2), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulfate (SDS); Sigma-Aldrich; Merck KGaA] or lysis buffer [including 20 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EDTA and 1% Triton X-100] containing protease and phosphatase inhibitor cocktails (Sigma-Aldrich; Merck KGaA). The protein content of the samples was measured using a bicinchoninic acid reagent (Pierce; Thermo Fisher Scientific, Inc.). Samples containing equal amounts of protein were separated on a 10% SDS-polyacrylamide gel by electrophoresis and then transferred onto polyvinylidene difluoride membranes (EMD Millipore, Bedford, MA, USA). Subsequent to blocking with 1% bovine serum albumin or 1% skim milk in TBS/Tween-20 [containing 50 mM Tris-HCl (pH 7.2), 150 mM NaCl and 0.05% Tween-20], the membranes were incubated with the appropriate primary antibody for 24 h in 4°C. The following antibodies were used: Anti-Smad2/3 purified mouse monoclonal antibody (1:1,000; cat. no. 610842; BD Transduction Laboratories™; BD Biosciences, Franklin Lakes, NJ, USA), as well as anti-phospho-Smad2/3, anti-p38 MAPK, anti-phospho-p38 MAPK, anti-MAPK-activated protein kinase 2 (MAPKAPK-2), anti-phospho-MAPKAPK-2 (Thr222), anti-JNK and anti-phospho-JNK rabbit antibodies (1:1,000; cat. nos. 8828, 9212, 9211, 12155, 3316, 9252S and 9251S, respectively; Cell Signaling Technology, Inc.). Next, the blots were incubated with alkaline phosphatase (AP)-conjugated secondary antibodies [anti-mouse IgG-AP donkey antibody or anti-rabbit IgG-AP goat antibody (1:2,000; cat. nos. sc-2320 and sc-2057, respectively; Santa-Cruz Biotechnology, Inc.)] for 1 h at room temperature. Signals were detected using an alkaline phosphatase substrate kit (BCIP/NBT Substrate kit; Vector Laboratories, Inc., Burlingame, CA, USA), and then images of the detected bands were captured. The expression level of the phosphorylated target protein was compared with that of the total target protein for evaluation of the phosphorylation status of the target protein.
Enzyme-linked immunosorbent assay (ELISA)
SCDC2 cells were seeded onto a type I collagen-coated 6-well plate at a cell density of 1.4×105 cells/well with the aforementioned growth medium for SCDC2 cells and cultured for 24 h. The growth medium was then replaced with Ham's F-12 without any supplementation, and SCDC2 cells were stimulated with or without 10 ng/ml of TGF-β1, IL-1β or TNF-α for 5 days. The amount of NGF secreted into the medium was measured using Rat Beta-NGF ELISA kit (ab193736; Abcam) according to the manufacturer's protocol.
Immunofluorescence analysis of cultured cells
For immunofluorescence analysis of cultured cells, SCDC2 cells (2×104 cells) and PC12 cells (1×104 cells) were subcultured on non-coated 4-well glass culture slides and maintained in Ham's F-12 supplemented with 2 mM glutamine, penicillin-streptomycin and 10% FBS. Cells were cultured with or without TrkA inhibitor (10 µM), SB-431542 (10 µM), TGF-β1 (10 ng/ml), TNF-α (10 ng/ml) and IL-1β (10 ng/ml) for 4 days. Following incubation, cells were fixed in 4% paraformaldehyde (Nacalai Tesque, Inc., Kyoto, Japan) for 15 min and permeabilized with Triton X-100 (Sigma-Aldrich; Merck KGaA). Following background reduction with normal goat serum, the cells were incubated with the anti-Smad2/3 purified mouse monoclonal antibody (1:200), as well as the anti-NF-κB p65 (D14E12) rabbit and anti-neurofilament H (RMdO 20) mouse monoclonal antibodies (1:200 and 1:1,000, respectively; cat. nos. 8242 and 2836S, respectively; Cell Signaling Technology, Inc.) at room temperature for 1 h. After washing with phosphate-buffered saline (PBS) to remove any excess primary antibody, the cells were incubated with Alexa Fluor 568-conjugated goat anti-rabbit IgG or Alexa Fluor 568-conjugated goat anti-mouse IgG, as appropriate (1:400; A-11011 and A-11031, respectively; Molecular Probes; Thermo Fisher Scientific, Inc.). Subsequent to washing further with PBS to remove any excess secondary antibody, certain cells were stained with Alexa Fluor 488-conjugated phalloidin (1:1,000; A12379; Thermo Fisher Scientific, Inc.), which specifically detects F-actin. Next, nuclei were stained with the 4′,6-diamidino-2-phenylindole (DAPI)-containing mounting medium, DAPI Fluoromount-G (SouthernBiotech, Birmingham, AL, USA). The fluorescent signal was detected using an Olympus IX70 fluorescence microscope with the LCPIanFI 20 objective lens (Olympus Corp., Tokyo, Japan).
Evaluation of neurite extension from PC12 cells co-cultured with SCDC2 cells and TH expression in the PC12 cells
SCDC2 cells (2×104) and PC12 cells (1×104) were mixed and seeded into each well of a non-coated 4-well glass culture slide (Thermo Fisher Scientific, Inc.) with the aforementioned PC12 cell growth medium. The cells were subsequently incubated with or without TGF-β1 (10 ng/ml), IL-1β (10 ng/ml), TNF-α (10 ng/ml), SB-431542 (10 µM) and TrkA inhibitor GW441756 (2 nM) for 5 days. In the ATPγS-treated PC12 cell group, ATPγS (100 µM) was added to all cultures from the beginning of the co-culture. PC12 cells in the co-culture were specifically and immunofluorescently labeled with anti-neurofilament H antibody. The status of neurite extensions emerging from PC12 cells was observed using an Olympus IX70 fluorescence microscope with the LCPIanFI 20 objective lens (Olympus Corp.). In addition, statistical assessment of neurite extension was performed as follows: Cells bearing neurites longer than the length of the cell body were counted as cells with neurite extension. Neurite-extended and non-extended PC12 cells were counted in eight different fluorescent microscopic fields. The ratio of the number of neurite-extended cells to the number of total PC12 cells in the fluorescent microscopic field was statistically analyzed (n=8).
In order to evaluate the TH expression status at the mRNA level in the PC12 cells co-cultured with SCDC2 cells as described earlier, mRNA was extracted from the co-culture without separating PC12 and SCDC2 cells. TH is known as a specific marker for the identification of dopaminergic neuron (32), therefore it was hypothesized that PDL-derived fibroblasts SCDC2 cells are unlikely to express TH.
Statistical analysis
The data are presented as the mean ± standard deviation (n=6 or 8) and were statistically analyzed by Tukey's multiple comparison test. A value of P<0.05 was considered to indicate a difference that was statistically significant. The results presented are representative of at least three separate experiments.
Results
TGF-β1 promotes the expression of NGF mRNA in SCDC2 cells through its type I receptor kinase activity in a dose-dependent manner
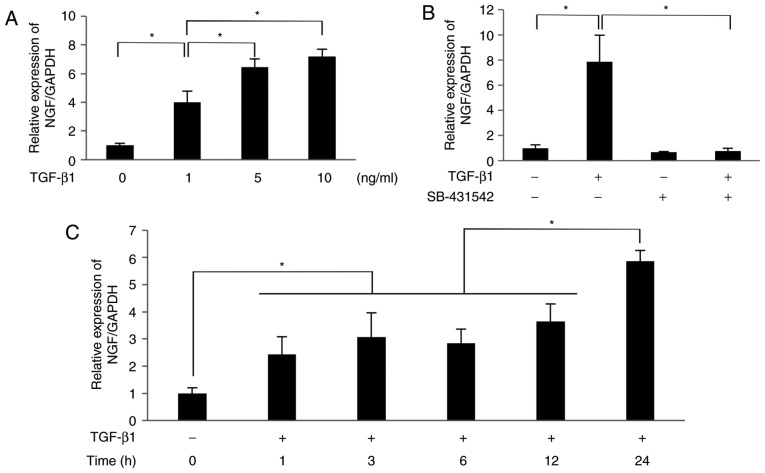
The results of RT-qPCR analysis revealed that TGF-β1 (1-10 ng/ml) significantly increased the mRNA expression level of NGF in SCDC2 cells in a dose-dependent manner (1 ng/ml, 4.0-fold increase; 5 ng/ml, 6.5-fold increase; 10 ng/ml, 7.2-fold increase; Fig. 1A). The selective inhibitor of TGF-β type I receptor SB-431542 (10 µM) completely suppressed NGF mRNA expression induced by 10 ng/ml TGF-β1 (Fig. 1B). These results indicate that TGF-β1 upregulated the mRNA expression of NGF in PDL-derived fibroblasts through its type I receptor kinase activity. As shown in Fig. 1C, TGF-β1 (10 ng/ml) significantly increased the mRNA level of NGF in SCDC2 cells in a time-dependent manner, with a 2.4-fold, 3.1-fold, 2.8-fold, 3.7-fold and 5.9-fold increase observed at 1, 3, 6, 12 and 24 h.
Figure 1.
TGF-β1 promoted the mRNA expression of NGF in SCDC2 cells through its type I receptor in a dose-dependent manner. After 24-h culture in growth medium, SCDC2 cells were starved for 24 h. The starved cells were then treated with (A) TGF-β1 at various concentrations for 24 h, or (B) pretreated with or without TGF-β type I receptor inhibitor SB-431542 (10 µM) for 30 min and then with or without TGF-β1 (10 ng/ml) for 24 h. (C) Starved cells were treated with or without TGF-β1 (10 ng/ml) for the indicated times. The relative expression level of NGF was evaluated using reverse transcription-quantitative polymerase chain reaction. Data represent the mean ± standard deviation (n=6). *P<0.05. TGF, transforming growth factor; NGF, nerve growth factor; SCDC, single cell-derived culture.
TGF-β1 promotes the expression of NGF mRNA in SCDC2 cells in Smad2/3-dependent and p38 MAPK-dependent manners
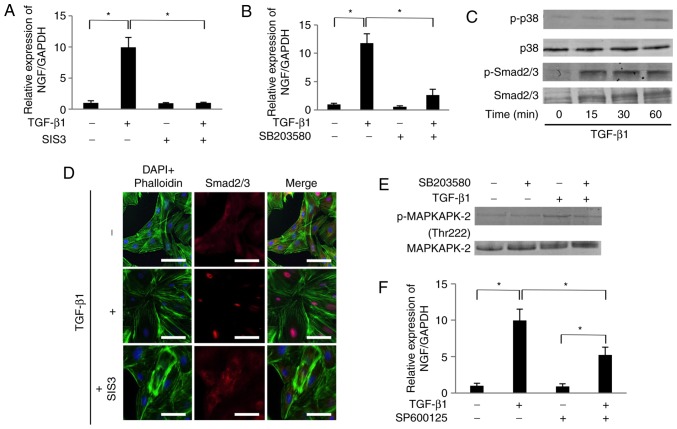
Treatment with TGF-β1 (10 ng/ml) significantly increased the mRNA expression level of NGF in SCDC2 cells by 10-fold (Fig. 2A). The TGF-β1-mediated increase in NGF mRNA was completely suppressed by the Smad3 inhibitor SIS3 (10 µM; Fig. 2A). In addition, TGF-β1-mediated increase in NGF mRNA expression was markedly suppressed by the p38 MAPK inhibitor SB203580 (10 µM; Fig. 2B). It was also confirmed that TGF-β1 upregulated the phosphorylation of Smad2/3 and p38 MAPK in SCDC2 cells within 15 and 30 min of administration, respectively (Fig. 2C). Furthermore, the inhibitor SIS3 completely abrogated TGF-β1-induced nuclear translocation of Smad2/3, as evident from the immuno-fluorescence analysis (Fig. 2D). The results further confirmed the effect of SB203580 on the phosphorylation status of MAPKAPK-2, which is known as the direct target of p38 MAPK (Fig. 2E). The phosphorylation level of MAPKAPK-2 was upregulated at 30 min following 10 ng/ml TGF-β1 administration. SB203580 inhibited the TGF-β1-induced MAPKAPK-2 phosphorylation, indicating that this inhibitor attenuated p38 MAPK-mediated signaling activated by TGF-β1. Although the phosphorylation status of JNK was not at a detectable level following TGF-β1 stimulation (data not shown), the JNK inhibitor SP600125 (10 µM) partially and significantly suppressed the TGF-β1-mediated increase in NGF mRNA expression (Fig. 2F).
Figure 2.
TGF-β1 promoted the mRNA expression of NGF in SCDC2 cells in Smad2/3-dependent and p38 MAPK-dependent manners. Effects of (A) SIS3 (10 µM), and (B) SB203580 (10 µM) on expression of NGF mRNA were evaluated as described in Materials and methods. Data represent the mean ± standard deviation (n=6). *P<0.05. (C) Phosphorylation status of Smad2/3 and p38 MAPK in cells stimulated with TGF-β1 (10 ng/ml) for the indicated times, evaluated using western blot analysis. (D) After 24-h starvation, cells were pretreated with Smad3 inhibitor SIS3 (10 µM) for 30 min and then treated with or without TGF-β1 (10 ng/ml) for 30 min, and the status of nuclear translocation of Smad2/3 following TGF-β1 stimulation was examined using immunofluorescence analysis (×200 magnification; scale bar, 50 µm). (E) Phosphorylation status of MAPKAPK-2 evaluated using western blot analysis in cells stimulated with TGF-β1 (10 ng/ml) and/or with the inhibitor SB203580. (F) Effect of SP600125 (10 µM) on expression of NGF mRNA was evaluated as described in Materials and methods. Data represent the mean ± standard deviation (n=6). *P<0.05. TGF, transforming growth factor; NGF, nerve growth factor; SCDC, single cell-derived culture; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; MAPK, mitogen-activated protein kinase; MAPKAPK-2, MAPK-activated protein kinase 2.
IL-1β and TNF-α suppress the TGF-β1-induced secretion of NGF in SCDC2 cells by abrogating the activities of Smad2/3 and p38 MAPK
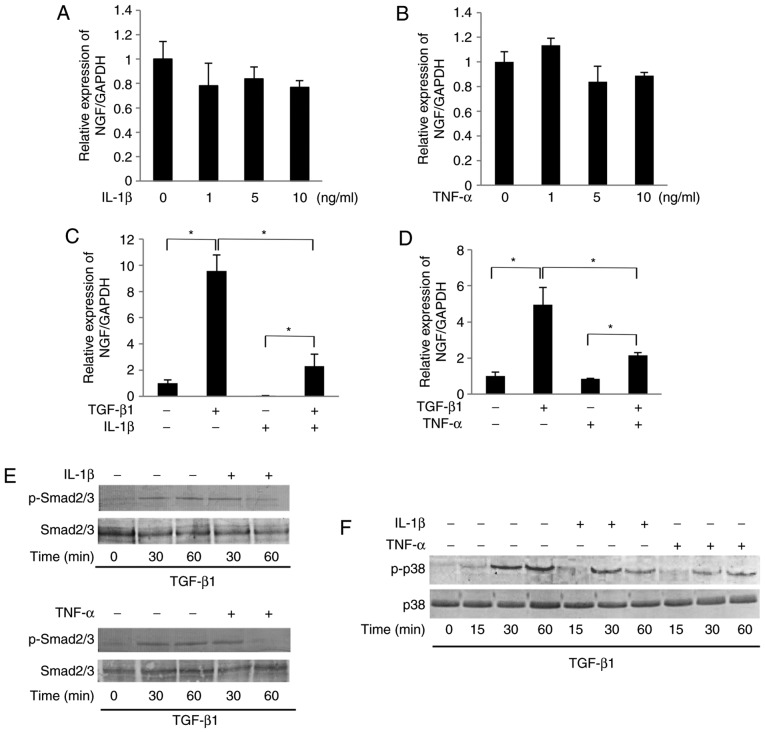
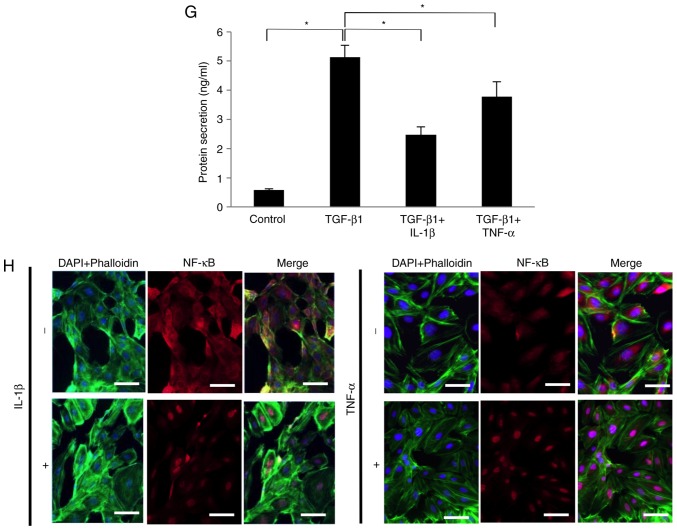
As shown in Fig. 3A and B, IL-1β (1-10 ng/ml) or TNF-α (1-10 ng/ml) failed to significantly affect the mRNA expression level of NGF in SCDC2 cells in the absence of TGF-β1. However, both IL-1β (10 ng/ml) and TNF-α (10 ng/ml) were able to significantly suppress the TGF-β1-mediated NGF mRNA expression (Fig. 3C and D, respectively). Additionally, NF-κB inhibitor BAY 11-7085 (10 µM) did not reverse the IL-1β-induced and TNF-α-induced suppression of the TGF-β1-promoted NGF mRNA expression (data not shown). Intriguingly, IL-1β (10 ng/ml) and TNF-α (10 ng/ml) significantly suppressed the TGF-β1-induced phosphorylation of Smad2/3 and p38 MAPK, particularly at 60 min after stimulation (Fig. 3E and F, respectively). The study also evaluated the protein level of NGF in the conditioned medium of SCDC2 cells using ELISA. TGF-β1 (10 ng/ml) treatment increased the concentration of NGF from 0.5 to 5 ng/ml in SCDC2 conditioned medium. However, IL-1β (10 ng/ml) and TNF-α (10 ng/ml) significantly decreased the TGF-β1-mediated increase in the NGF protein level from 5 ng/ml to 2.5 and 3.8 ng/ml, respectively, in the conditioned medium (Fig. 3G). Immunofluorescence analysis further revealed that IL-1β (10 ng/ml) and TNF-α (10 ng/ml) induced the nuclear trans-location of NF-κB from the cytoplasm (Fig. 3H), indicating the induction of signal transduction in SCDC2 cells following stimulations with IL-1β and TNF-α.
Figure 3.
IL-1β and TNF-α suppressed the TGF-β1-induced mRNA expression of NGF in SCDC2 cells by abrogating Smad2/3 and p38 MAPK activities. The effects of IL-1β and TNF-α on TGF-β1-induced mRNA expression of NGF in SCDC2 cells were evaluated using RT-qPCR. The cells were treated with or without (A) IL-1β alone or (B) TNF-α alone at indicated concentrations, (C) TGF-β1 (10 ng/ml) and/or IL-1β (10 ng/ml), and (D) TGF-β1 (10 ng/ml) and/or TNF-α (10 ng/ml). Data represent the mean ± standard deviation (n=6). *P<0.05. Phosphorylation status of (E) Smad2/3 and (F) p38 MAPK was evaluated using western blot analysis in cells treated with or without TGF-β1 (10 ng/ml) alone, TGF-β1 (10 ng/ml) + IL-1β (10 ng/ml), or TGF-β1 (10 ng/ml) + TNF-α (10 ng/ml) for the indicated times. (G) NGF protein concentration secreted into the culture medium was determined using ELISA in cells cultured with or without TGF-β1 (10 ng/ml) alone, TGF-β1 (10 ng/ml) + IL-1β (10 ng/ml), or TGF-β1 (10 ng/ml) + TNF-α (10 ng/ml) for 5 days. (H) Nuclear translocation status of NF-κB p65 (red) was evaluated using immunofluorescence analysis (blue, nuclei; green, filamentous actin) in SCDC2 cells treated with or without IL-1β (10 ng/ml) or TNF-α (10 ng/ml) for 24 h (×200 magnification; scale bar, 50 µm). IL, interleukin; TNF, tumor necrosis factor; TGF, transforming growth factor; NGF, nerve growth factor; SCDC, single cell-derived culture; MAPK, mitogen-activated protein kinase.
NGF secreted by SCDC2 cells upon stimulation with TGF-β1 promotes neurite extension from the surface of PC12 cells treated with ATPγS
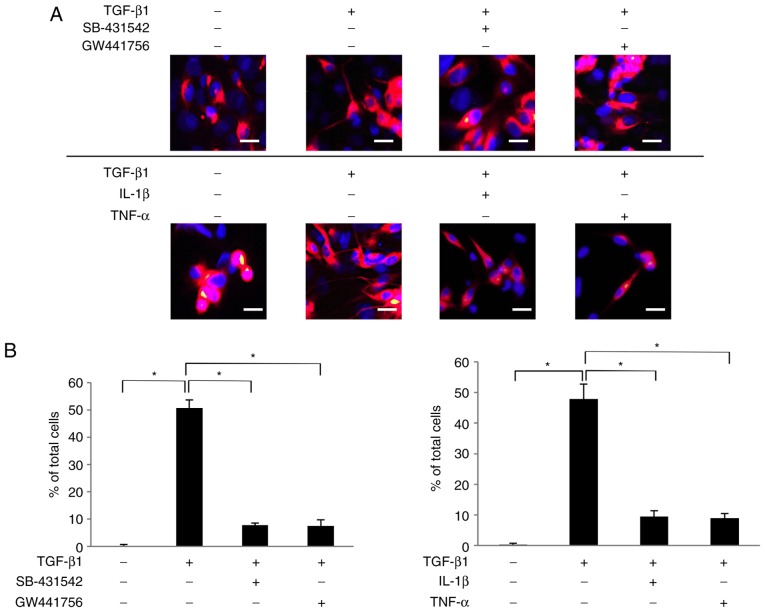
The present study then examined whether NGF secreted by the TGF-β1-treated SCDC2 cells retained the neurotrophic activity to induce neurite extension from the surface of PC12 cells. Arthur et al (36) reported that ATPγS enhanced the sensitivity of PC12 cells to NGF stimulation through P2Y2 receptor-mediated activation of ERK1/2 and p38 MAPK. As a consequence, NGF-induced neurite extension from the surface of PC12 cells was accelerated (36). In the current study, the neurogenerative effect of NGF secreted by SCDC2 cells upon TGF-β1 stimulation was evaluated, and whether the secreted NGF promoted neurite extension from the surface of PC12 cells was investigated using a co-culture system. ATPγS-treated PC12 cells co-cultured with non-treated SCDC2 cells failed to exhibit neurite extension (Fig. 4A). However, ATPγS-treated PC12 cells co-cultured with TGF-β1-treated SCDC2 cells clearly exhibited neurite outgrowth (Fig. 4A). In addition, SB-431542 and GW441756 suppressed both the morphological change of PC12 cells from round shape to elongated shape and the neurite extension from the surface of PC12 cells induced by the SCDC2-secreted NGF (Fig. 4A). By contrast, IL-1β and TNF-α did not completely suppress the morphological changes of PC12 cells from round shape to elongated shape, whereas they clearly suppressed neurite extension from the surface of ATPγS-treated PC12 cells induced by the SCDC2-secreted NGF (Fig. 4A). In addition, the results confirmed that ATPγS itself did not affect the expression level of NGF mRNA in the SCDC2 cells using RT-qPCR analysis (data not shown). Statistical assessment of neurite extension in PC12 cells co-cultured with SCDC2 cells was also performed. Approximately 50% of ATPγS-treated PC12 cells co-cultured with TGF-β1-stimulated SCDC2 cells exhibited neurite extension, whereas none of the ATPγS-treated PC12 cells co-cultured with non-stimulated SCDC2 cells exhibited neurite growth (Fig. 4B). Furthermore, SB-431542 (10 µM) and TrkA inhibitor GW441756 (2 nM) significantly reduced the ratio of neurite-extended PC12 cells to total PC12 cells, which were activated by treatment with ATPγS (100 µM) and co-cultured with TGF-β1-treated SCDC2 cells (from 50.7% to 7.8 and 7.5., respectively). Furthermore, IL-1β and TNF-α significantly reduced the ratio of neurite-extended PC12 cells to total PC12 cells, which were activated by treatment with ATPγS (100 µM) and co-cultured with TGF-β1-stimulated SCDC2 cells (from 47.9% to 9.4 and 8.9%, respectively).
Figure 4.
Nerve growth factor secreted by SCDC2 cells following TGF-β1 stimulation promoted neurite extension from the surface of ATPγS-treated PC12 cells. (A) Neurite extension of PC12 cells was visualized by immunostaining (×200 magnification; scale bar, 50 µm) with anti-neurofilament H antibody (red) and nuclei were stained with DAPI (blue). SCDC2 cells (2×104 cells) and rat pheochromocytoma cells PC12 (1×104 cells) were co-cultured and treated with or without TGF-β1 (10 ng/ml) for 4 days. Cells were also treated with TGF-β type I receptor inhibitor SB-431542 (10 µM), TrkA inhibitor GW441756 (2 nM), IL-1β (10 ng/ml), or TNF-α (10 ng/ml) from the beginning of the co-culture. In addition, ATPγS (100 µM) was added to all cultures during cell seeding. Dimethyl sulfoxide was added to cell cultures as a vehicle control for SB-431542 and GW441756, respectively. (B) Statistical assessment of neurite extension in PC12 cells co-cultured with SCDC2 cells. Data represent the mean ± standard deviation (n=8). *P<0.05. TGF, transforming growth factor; SCDC, single cell-derived culture; IL, interleukin; TNF, tumor necrosis factor; ATPγS, adenosine 5′-O-(3-thio)triphosphate; TrkA, tropomyosin receptor kinase A.
NGF secreted by SCDC2 cells upon TGF-β1 stimulation promotes mRNA expression of TH in PC12 cells
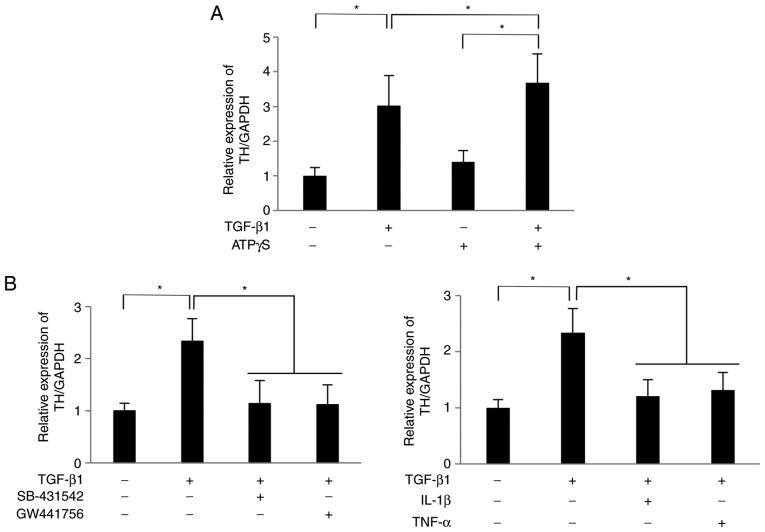
The study further evaluated how NGF secreted by TGF-β1-treated SCDC2 cells affected the expression status of TH, which is a rate-limiting enzyme in dopamine synthesis (32), in PC12 cells. As shown in Fig. 5A, the mRNA expression level of TH in PC12 cells co-cultured with TGF-β1 (10 ng/ml)-treated SCDC2 cells was 3.0 times higher than that in PC12 cells co-cultured with non-treated SCDC2 cells at 24 h after initiation of the co-culture. In addition, ATPγS (100 µM) significantly enhanced the TH expression in PC12 cells co-cultured with TGF-β1-treated SCDC2 cells compared with cells cultured with TGF-β1 or ATPγS alone (Fig. 5A). Furthermore, treatment with the TGF-β type I receptor inhibitor SB-431542, TrkA inhibitor GW441756, IL-1 or TNF-α significantly suppressed the TGF-β1-promoted expression of TH (Fig. 5B). Thus, NGF secreted from SCDC2 cells stimulated with TGF-β1 exerted a neurofunctional activity in PC12 cells.
Figure 5.
Nerve growth factor secreted by SCDC2 cells subsequent to TGF-β1 stimulation promoted the expression of TH mRNA in PC12 cells. SCDC2 cells (7×104 cells) and rat pheochromocytoma cells PC12 cells (3.5×104 cells) were co-cultured and stimulated with or without TGF-β1 (10 ng/ml) for 24 h. The relative expression level of TH was evaluated using reverse transcription-quantitative polymerase chain reaction in cells also treated with (A) ATPγS (100 µM), and with (B) TGF-β type I receptor inhibitor SB-431542 (10 µM), TrkA inhibitor GW441756 (2 nM), IL-1β (10 ng/ml) or TNF-α (10 ng/ml) during the co-culture. Dimethyl sulfoxide was added to cell cultures as a vehicle control for SB-431542 and GW441756, respectively. Data represent the mean ± standard deviation (n=6). *P<0.05. TGF, transforming growth factor; SCDC, single cell-derived culture; IL, interleukin; TNF, tumor necrosis factor; ATPγS, adenosine 5′-O-(3-thio)triphosphate; TrkA, tropomyosin receptor kinase A; TH, tyrosine hydroxylase.
Discussion
The present study demonstrated that TGF-β1 significantly enhanced the expression levels of NGF mRNA and protein in rat PDL fibroblasts SCDC2 (Figs. 1 and 3G, respectively). In addition, TGF-β1 upregulated the phosphorylation levels of Smad2/3 and p38 MAPK (Fig. 2C). It was also reported that the Smad3 inhibitor SIS3 and the p38 MAPK inhibitor SB203580 (10 µM) significantly abrogated TGF-β1-induced upregulation of NGF expression in SCDC2 cells (Fig. 2A and B). These results strongly suggest that TGF-β1 upregulated NGF expression in PDL fibroblasts in Smad2/3-dependent and p38 MAPK-dependent manners. Next, the effect of the inflammatory cytokine on TGF-β-mediated expression of NGF was evaluated. Hattori et al (26) previously reported that IL-1β and TNF-α promoted NGF expression in mouse fibroblasts, whereas IL-1β or TNF-α alone failed to exhibit any effect on NGF expression in SCDC2 cells in the current study (Fig. 3A and B). In addition, Hahn et al (25) demonstrated that TGF-β1 and IL-1β cooperatively and additively promoted NGF production/secretion in astroglial cells. The present study revealed that the pro-inflammatory cytokines IL-1β and TNF-α presented a partial but significant inhibition of TGF-β1-mediated NGF expression (Fig. 3C and D, respectively). These results indicate that the differential effects of pro-inflammatory cytokines on NGF expression may vary according to the cell type. In addition, IL-1β and TNF-α downregulated the phosphorylation levels of Smad2/3 and p38 MAPK (Fig. 3E and F, respectively). These results strongly suggest that IL-1β and TNF-α suppressed the TGF-β1-induced expression of NGF mRNA in SCDC2 cells by abrogating Smad2/3 and p38 MAPK activities. It was also confirmed that TGF-β1-mediated secretion of NGF by SCDC2 cells was partially but significantly inhibited by IL-1β and TNF-α (Fig. 3G).
NF-κB signaling is known to induce Smad7, a negative regulator of TGF-β signaling (37). Therefore, the current study examined the effect of the NF-κB inhibitor BAY 11-7085 on IL-1β-induced and TNF-α-induced suppression of the TGF-β1-promoted expression of NGF. BAY 11-7085 (10 µM) did not reverse the IL-1β-induced and TNF-α-induced suppression of the TGF-β1-promoted expression of NGF (data not shown). These results suggested that IL-1β and TNF-α suppressed the TGF-β1-promoted expression of NGF through the activation of signal transduction molecules other than NF-κB.
The present study also investigated how JNK affected the TGF-β1-mediated NGF expression. Although JNK was not phosphorylated following TGF-β1 stimulation (data not shown), JNK inhibitor SP600125 significantly and partially suppressed the TGF-β1-mediated expression of NGF mRNA (Fig. 2F), suggesting that the basal level of JNK activity may be important for the TGF-β1-mediated NGF expression in SCDC2 cells.
The rat pheochromocytoma PC12 cell line has been established and used as a model for the growth and differentiation of neural crest cells (38). Furthermore, the neural crest cells are known to differentiate into various types of cells, which construct the neural network in the orofacial region during the mouse embryonic development, such as sensory and autonomic nerves, ganglia, Schwann cells and cells of the dental pulp involving odontoblasts (39). Therefore, the neurotrophic effect of the secreted NGF from PDL fibroblasts on the status of neurite extension in PC12 cells may reflect the NGF effect on PM-PDL neurons running into Vmes. The present study reported that ~50% of ATPγS-treated PC12 cells exhibited neurite extension when co-cultured with TGF-β1-stimulated SCDC2 cells, whereas this was not detected in the ATPγS-treated PC12 cells that were co-cultured with non-stimulated SCDC2 cells (Fig. 4A and B). In addition, TGF-β type I receptor inhibitor SB-431542 clearly abrogated the neurite extension from the ATPγS-treated PC12 cells co-cultured with TGF-β1-stimulated SCDC2 cells. However, TGF-β1 alone failed to promote neurite extension from the ATPγS-treated PC12 cells in the absence of co-culture (data not shown). The inhibitor of TrkA (GW441756) clearly abrogated the neurite extension from the ATPγS-treated PC12 cells co-cultured with TGF-β1-stimulated SCDC2 cells. Thus, NGF secreted from TGF-β1-treated SCDC2 cells appeared to retain a physiological activity as a neurotrophic factor.
By contrast, the mRNA expression level of TH in PC12 cells co-cultured with TGF-β1 (10 ng/ml)-treated SCDC2 cells was 3.0-fold higher in comparison with that in PC12 cells co-cultured with non-treated SCDC2 cells (Fig. 5A). Furthermore, treatment with TGF-β type I receptor inhibitor SB-431542, TrkA inhibitor GW441756, IL-1 or TNF-α significantly suppressed the TGF-β1-promoted expression of TH (Fig. 5B). These results suggested that NGF secreted from the TGF-β1-treated SCDC2 cells upregulated the mRNA expression of TH in PC12 cells, which was not inconsistent with the findings of a previous study reporting that NGF induced TH expression at the transcriptional level in PC12 cells (40). Thus, NGF secreted from SCDC2 cells stimulated with TGF-β1 exerted a neurofunctional activity in PC12 cells.
During occlusal trauma of PDL tissues, MS neurons are injured, and their neurites undergo atrophy and eventually degenerate. As an inflammatory response, the injured PDL tissues recruit inflammatory blood cells involving pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages that advance and complete inflammation, respectively (41). M1 macrophages synthesize and secrete pro-inflammatory cytokines, including IL-1β and TNF-α, whereas M2 macrophages synthesize and secrete anti-inflammatory cytokines, such as TGF-β1 and IL-10 (42). TGF-β1 derived from M2 macrophages stimulates PDL fibroblasts in the inflammatory PDL tissue, resulting in the upregulation of NGF synthesis and secretion by PDL fibroblasts. NGF secreted from TGF-β1-stimulated PDL fibroblasts subsequently and neurotrophically regenerates the injured MS neurons in the damaged PDL tissue. IL-1β and TNF-α derived from M1 macrophages suppress TGF-β1-mediated secretion of NGF from PDL fibroblasts. Therefore, the inhibition of IL-1β and TNF-α signaling in the fibroblasts from the inflamed PDL tissue may be an efficient therapeutic strategy for NGF-mediated regeneration of injured PDL neurons. Neutralizing agents against IL-1β (such as canakinumab) (43) and TNF-α (including infliximab, adalimumab, golimumab and etanercept) (44) are clinically used for the treatment of chronic inflammatory diseases and may be available for the regenerative therapy of injured PDL neurons. Furthermore, Li et al (45) reported that the transplantation of PDL-derived mesenchymal cells exhibited a potential value in the repair of the crush-injured left mental nerve in rats. Hence, the possibility of the establishment of regenerative medicine for the damaged neurons by transplantation of PDL-derived fibroblasts treated with TGF-β1 following the administration of neutralizing agents against IL-1β and TNF-α is proposed.
In conclusion, the present study findings partly clarified the molecular mechanisms underlying the regeneration of neuronal tissues by PDL-derived fibroblasts and may benefit future research on neuronal regenerative medicine by transplantation of PDL-derived fibroblasts.
Acknowledgments
Not applicable.
Abbreviations
- MS
mechanosensitive
- PDL
periodontal ligament
- NGF
nerve growth factor
- IL-1β
interleukin 1β
- TNF-α
tumor necrosis factor α
- TGF-β
transforming growth factor β
- MAPK
mitogen-activated protein kinase
- Vmes
trigeminal mesencephalic nucleus
- ERK1/2
extracellular signal-regulated kinases 1/2
- TrkA
tropomyosin receptor kinase A
- R-Smad
receptor-regulated Smad
- co-Smad
common mediator Smad
- I-Smad
inhibitory Smad
- JNK
c-Jun N-terminal kinase
- NF-κB
nuclear factor-κB
- TH
tyrosine hydroxylase
- ATPγS
adenosine 5′-O-(3-thio)triphosphate
- SCDC
single cell-derived culture
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- MAPKAPK-2
MAPK-activated protein kinase 2
- PBS
phosphate-buffered saline
Funding
This study was supported in part by the JSPS KAKENHI (grant nos. JP22592076 and JP17K11851 awarded to MK, JP25463053 and JP16K11654 awarded to NC, JP26462823 awarded to SK, and JP26670852 and JP16H05534 awarded to AI) and the Grant-in-Aid for Strategic Medical Science Research Centre (awarded to AI), both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MO performed and interpreted data from RT-qPCR, western blot analysis and immunofluorescence analysis, and was a contributor in writing the manuscript. NC analyzed and interpreted ELISA, and was a contributor in writing the manuscript. SK performed RNA isolation and protein extraction from cells, and was a contributor in writing the manuscript. SY, NO, AN, and MK were contributors in asisting MO in performing ELISA, western blot analysis, and immunofluorescence analysis. SJ and KS were contributors in designing the experimental plan and writing manuscript. AI made substantial conributions to the conception and design of the study, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Jiang N, Guo W, Chen M, Zheng Y, Zhou J, Kim SG, Embree MC, Songhee Song K, Marao HF, Mao JJ. Periodontal ligament and alveolar bone in health and adaptation: Tooth movement. Front Oral Biol. 2016;18:1–8. doi: 10.1159/000351894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shienaga Y, Doe K, Suemune S, Mitsuhiro Y, Tsuru K, Otani K, Shirana Y, Hoshi M, Yoshida A, Kagawa K. Physiological and morphological characteristics of periodontal mesencephalic trigeminal neurons in the cat-intra-axonal staining with HRP. Brain Res. 1989;505:91–110. doi: 10.1016/0006-8993(89)90119-4. [DOI] [PubMed] [Google Scholar]
- 3.Karita K, Tabata T. Response properties of periodontal mechanosensitive units in the cat's thalamus. Exp Brain Res. 1991;86:341–346. doi: 10.1007/BF00228957. [DOI] [PubMed] [Google Scholar]
- 4.Tabata T, Takahashi Y, Hayashi H. Response properties of mechanosensitive neurons in the rat trigeminal sensory complex projecting to the posteromedial ventral nucleus of the thalamus. Arch Oral Biol. 2001;46:881–889. doi: 10.1016/S0003-9969(01)00059-0. [DOI] [PubMed] [Google Scholar]
- 5.Byers MR. Sensory innervation of periodontal ligament of rat molars consists of unencapsulated Ruffini-like mechanoreceptors and free nerve endings. J Comp Neurol. 1985;231:500–518. doi: 10.1002/cne.902310408. [DOI] [PubMed] [Google Scholar]
- 6.Maeda T, Sato O, Kobayashi S, Iwanaga T, Fujita T. The ultrastructure of Ruffini endings in the periodontal ligament of rat incisors with special reference to the terminal Schwann cells (K-cells) Anat Rec. 1989;223:95–103. doi: 10.1002/ar.1092230114. [DOI] [PubMed] [Google Scholar]
- 7.Uemura T, Yasuda K, Ishihara K, Yasuda H, Okayama M, Hasumi-Nakayama Y, Furusawa K. A comparison of the postnatal development of muscle-spindle and periodontal-ligament neurons in the mesencephalic trigeminal nucleus of the rat. Neurosci Lett. 2010;473:155–157. doi: 10.1016/j.neulet.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 8.Korkmaz Y, Bloch W, Klinz FJ, Kübler AC, Schneider K, Zimmer S, Addicks K, Raab WH. The constructive activation of extracellular signal-regulated kinase 1 and 2 in periodontal ligament nerve fibers. J Periodontol. 2009;80:850–859. doi: 10.1902/jop.2009.080550. [DOI] [PubMed] [Google Scholar]
- 9.Atsumi Y, Imai T, Matsumoto K, Sakuda M, Maeda T, Kurisu K, Wakisaka S. Effects of different types of injury to the inferior alveolar nerve on the behavior of Schwann cells during the regeneration of periodontal nerve fibers of rat incisor. Arch Histol Cytol. 2000;63:43–54. doi: 10.1679/aohc.63.43. [DOI] [PubMed] [Google Scholar]
- 10.Webber CA, Xu Y, Vanneste KJ, Martinez JA, Verge VM, Zochodne DW. Guiding adult mammalian sensory axons during regeneration. J Neuropathol Exp Neurol. 2008;67:212–222. doi: 10.1097/NEN.0b013e3181654972. [DOI] [PubMed] [Google Scholar]
- 11.Donnerer J. Regeneration of primary sensory neurons. Pharmacology. 2003;67:169–181. doi: 10.1159/000068405. [DOI] [PubMed] [Google Scholar]
- 12.Angeletti RH, Bradshaw RA. Nerve growth factor from mouse submaxillary gland: Amino acid sequence. Proc Natl Acad Sci USA. 1971;68:2417–2420. doi: 10.1073/pnas.68.10.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirose M, Kuroda Y, Murata E. NGF/TrkA signaling as a therapeutic target for pain. Pain Pract. 2016;16:175–182. doi: 10.1111/papr.12342. [DOI] [PubMed] [Google Scholar]
- 14.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, Wang XL, Lan HY. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: Role of Smad7. J Am Soc Nephrol. 2005;16:1371–1383. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]
- 16.Korns D, Frasch SC, Fernandes-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front Immunol. 2011;2:57. doi: 10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed S, Bradshaw AD, Gera S, Dewan MZ, Xu R. The TGF-β/Smad4 signaling pathway in pancreatic carcinogenesis and clinical significance. J Clin Med. 2017;6:E5. doi: 10.3390/jcm6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldin CH, Moustakas A. Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol. 2016;8:a022053. doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macias MJ, Martin-Malpartida P, Massagué J. Structural determinations of Smad function in TGF-β signaling. Trends Biochem Sci. 2015;40:296–308. doi: 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu G, Fahnestock M. Differential expression of nerve growth factor transcripts in glia and neurons and their regulation by transforming growth factor-beta1. Mol Brain Res. 2002;105:115–125. doi: 10.1016/S0169-328X(02)00399-6. [DOI] [PubMed] [Google Scholar]
- 21.Lindholm D, Hengerer B, Zafra F, Thoenen H. Transforming growth factor-beta 1 stimulates expression of nerve growth factor in the rat CNS. Neuroreport. 1990;1:9–12. doi: 10.1097/00001756-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Blaney Davidson EN, van Carm APM, Vitters EL, Bennink MB, Thijssen E, van der Berg WB, Koenders MI, van Lent PLEM, van deLoo FAJ, van der Kraan PM. TGF-β is a potent inducer of nerve growth factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarth Cartil. 2015;23:478–486. doi: 10.1016/j.joca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 24.Yongchaitracl T, Pavasant P. Transforming growth factor-beta1 up-regulates the expression of nerve growth factor through mitogen-activated protein kinase signaling pathways in dental pulp cells. Eur J Oral Sci. 2007;115:57–63. doi: 10.1111/j.1600-0722.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- 25.Hahn M, Lorez H, Fischer G. The immortalized astrogrial cell line RC7 is a new model system for the study of nerve growth factor (NGF) regulation: Stimulation by interleukin-1 beta and transforming growth factor-beta 1 is additive and affected differently by dibutyryl cyclic AMP. Glia. 1994;10:286–295. doi: 10.1002/glia.440100407. [DOI] [PubMed] [Google Scholar]
- 26.Hattori A, Iwasaki S, Murase K, Tsujimoto M, Sato M, Hayashi K, Kohno M. Tumor necrosis factor is markedly synergistic with interleukin 1 and interferon-gamma in stimulating the production of nerve growth factor n fibroblasts. FEBS Lett. 1994;340:177–180. doi: 10.1016/0014-5793(94)80132-0. [DOI] [PubMed] [Google Scholar]
- 27.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3 doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 28.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hengerer B, Lindholm D, Heumann R, Rüther U, Wagner EF, Thoenen H. Lesion-induced increase in nerve growth factor mRNA is mediated by c-fos. Proc Natl Acad Sci USA. 1990;87:3899–3903. doi: 10.1073/pnas.87.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Kim JW, Im YS, Seong GJ, Lee HK. Cyclosporine A induces nerve growth factor expression via activation of p38 and NFAT5. Cornea Suppl. 2011;1:S19–S24. doi: 10.1097/ICO.0b013e3182281028. [DOI] [PubMed] [Google Scholar]
- 31.Heese K, Inoue N, Sawada T. NF-κB regulates B-cell-derived nerve growth factor expression. Cell Mol Immunol. 2006;3:63–66. [PubMed] [Google Scholar]
- 32.White RB, Thomas MG. Moving beyond tyrosine hydroxylase to defined dopaminergic neurons for use in cell replacement therapies for Parkinson's disease. CNS Neurol Disord Drug Targets. 2012;11:340–349. doi: 10.2174/187152712800792758. [DOI] [PubMed] [Google Scholar]
- 33.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 34.Okubo N, Ishisaki A, Iizuka T, Tamura M, Kitagawa Y. Vascular cell-like potential of undifferentiated ligament fibroblasts to construct vascular cell-specific marker-positive blood vessel structures in a PI3K-activation-dependent manner. J Vasc Res. 2010;47:369–383. doi: 10.1159/000277724. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA. 2005;102:19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Böttinger EP. A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA. Genes Dev. 2000;15:187–197. [PMC free article] [PubMed] [Google Scholar]
- 38.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chigero DJ., Jr The early distribution and possible role of nerves during odontogenesis. Int J Dev Biol. 1995;39:191–194. [PubMed] [Google Scholar]
- 40.Gizang-Ginsberg E, Ziff EB. Nerve growth factor regulates tyrosine hydroxylase gene transcription through a nucleoprotein complex that contains c-Fos. Genes Dev. 1990;4:477–491. doi: 10.1101/gad.4.4.477. [DOI] [PubMed] [Google Scholar]
- 41.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–529. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle) 2012;1:10–16. doi: 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gram H. Preclinical characterization and clinical development of ILARIS(®) (canakinumab) for the treatment of autoinflammatory diseases. Curr Opin Chem Biol. 2016;32:1–9. doi: 10.1016/j.cbpa.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Billmeier U, Dieterich W, Neurath MF, Atreya R. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol. 2016;22:9300–9313. doi: 10.3748/wjg.v22.i42.9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li B, Jung HJ, Kim SM, Kim MJ, Jahng JW, Lee JH. Human periodontal ligament stem cells repair mental nerve injury. Neural Regen Res. 2013;8:2827–2837. doi: 10.3969/j.issn.1673-5374.2013.30.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.