Abstract
Tongue squamous cell carcinoma (TSCC) is highly malignant and poorly differentiated, resulting in a high frequency of local recurrence and distant metastases. Sox2 (Sry-box2), an important factor in embryonic development and cell differentiation, has been shown to associate with malignant phenotypes and epithelial-mesenchymal transition (EMT) progression in numerous types of human tumors. However, the clinical relevance and molecular mechanisms of Sox2 in TSCC remain unclear. In the present study, the expression levels of Sox2 were assessed in 61 pairs of TSCC samples and corresponding adjacent non-cancerous tissues using immunohistochemical methods. Associations between Sox2 expression and clinicopathological features were evaluated. Furthermore, Sox2 was overexpressed and inhibited using full-length Sox2 cDNA and short hairpin RNA (shRNA) transfection in UM2 and Cal27 cell lines, respectively. The malignant phenotypes were assessed by plate clone formation assays, wound-healing assays and Transwell assays. EMT markers (E-cadherin, vimentin, Twist, Slug and Snail) and β-catenin were detected by reverse transcription-polymerase chain reaction and western blot analysis following the alterations of Sox2 expression. The results indicated that Sox2 expression was markedly upregulated in TSCC samples and was significantly associated with tumor growth (pT stage), cell differentiation, lymphatic metastasis (pN stage) and clinical stage (pTNM stage). Cal27-shRNA-Sox2 cells not only exhibited a decreased capacity for cell proliferation, but also suppressed cell migration and invasion, and an attenuated colony formation capacity. By contrast, UM2-Sox2 cells exhibited accelerated cell malignant phenotypes and EMT progression. Moreover, when the expression of Sox2 was decreased by shRNA transduction, β-catenin expression was attenuated. An opposing phenomenon was observed in UM2-Sox2 cells. In conclusion, this study suggests that Sox2 expression serves a role in TSCC malignant phenotypes and EMT progression, and that β-catenin may act as a modulated factor in this progression.
Keywords: Sry-box2, tongue squamous cell carcinoma, epithelial-mesenchymal transition, β-catenin
Introduction
Tongue squamous cell carcinoma (TSCC) is the most common form of head and neck cancer, and is a major cause of cancer-related mortality worldwide (1). Although the overall survival rate of patients with TSCC can reach 5 years or more, numerous patients experience metastatic disease and a poor prognosis (2). Declines in mortality rates have been attributed to earlier detection and improvements in therapy. However, the precise underlying mechanisms of metastasis remain unclear.
Cancer invasiveness and metastasis are tightly linked with the acquisition of a migratory phenotype that enables cancer cells to invade adjacent tissues. Epithelial-mesenchymal transition (EMT) is known to be a central mechanism in charge of the invasiveness and metastasis of cancer via the loss of the cell to cell contact of epithelial cells and the acquisition of migratory properties (3). It has been confirmed that the acquisition of the expression of mesenchymal markers, including vimentin, Slug, Snail and Twist, but the reduction of the expression of epithelial markers, including E-cadherin, endows cells with high potential for dissemination (4). Cell movement is regulated by members of the regulatory factors and signaling pathways. The Wnt/β-catenin signaling pathway has been certified to regulate cell motility and confer cellular metastasis in cancer (5,6). It has also been proven that β-catenin participates in the regulation of E-cadherin, Snail and Twist, and that it induces epithelial cells to become invasive in colon cancer (7).
The transcription factor Sox2 (Sry-box2), a high-mobility group DNA binding protein, serves an important role in various phases of embryonic development, and affects cell fate and differentiation (8). Sox2 has been studied in several types of human solid tumors, such as breast cancer, prostate cancer and glioblastoma tumor (9-11). Li et al (12) suggested that Sox2 could mediate EMT by inducing β-catenin in breast and prostate cancer. While the aforementioned cancer types are each adenocarcinoma, it was uncertain whether β-catenin would be a key factor in squamous cell carcinoma, particularly TSCC. Sox2 is overexpressed in cancerous tissues compared with that in para-tumoral tissues in oral squamous cell carcinoma (OSCC) (13), and the high expression of Sox2 in the primary tissues of OSCC has been significantly correlated with the poor prognosis accompanying lymph node metastasis (14). However, OSCC is an umbrella term that included TSCC, buccal mucosa squamous cell carcinoma, mouth floor squamous cell carcinoma, gingival carcinoma and carcinoma of the palate. Heterogeneity among these types of squamous cell carcinoma may exist. The present study aimed to investigate the function of Sox2 expression with a focus on TSCC.
Materials and methods
Patients and tissue samples
Specimens were obtained from the patients (35 male and 26 female patients; mean age, 53.25 years; median age, 54 years) following radical surgery (open operation for the excision of lesion and part of normal tongue surrounding) between January 2005 and December 2015 at Guanghua Hospital of Stomatology of Sun Yat-sen University (Guangdong, China). The patients selected had primary squamous cell carcinoma of the tongue, had not been subjected to radiotherapy or chemotherapy preoperatively and had no history of other systemic diseases (e.g. diabetes, hypertension, etc.). The confirmed diagnosis was tongue squamous cell carcinoma by pathological examination. Informed consent was obtained on the use of surgically resected specimens for research purposes, according to the guidelines for research for human tissues and samples set by the Institution Review Board (IRB) of Sun Yat-sen University, who also approved the study. No patients received any form of adjuvant therapy prior to surgery. These tissues included 61 pairs of TSCC samples and corresponding adjacent non-cancerous tissues. Of the 61 TSCC samples, there were 31 (50.8%) well-differentiated and 30 (49.2%) moderately or poorly differentiated TSCCs, plus 26 (42.6%) tissues with lymph node metastases. The clinical characteristics of all these patients are summarized in Table I. Clinical stages were classified according to the Union for International Cancer Control (2002) (15).
Table I.
Association between Sox2 expression and clinicopathological features in tongue squamous cell carcinoma patients.
| Clinicopathological features | No. of cases | Sox2 expression, n (%)
|
P-valuea | |
|---|---|---|---|---|
| High | Low | |||
| Sex | ||||
| Male | 35 | 23 (65.7) | 12 (34.3) | 0.737 |
| Female | 26 | 16 (61.5) | 10 (39.5) | |
| Age, years | ||||
| ≥55 | 29 | 16 (53.3) | 13 (46.7) | 0.175 |
| <55 | 32 | 23 (74.2) | 9 (25.8) | |
| Differentiation | ||||
| Well | 31 | 16 (51.6) | 15 (48.4) | 0.042b |
| Moderately + poorly | 30 | 23 (76.7) | 7 (23.3) | |
| pT stage | ||||
| T1-2 | 46 | 26 (56.5) | 20 (43.5) | 0.035b |
| T3-4 | 15 | 13 (86.7) | 2 (13.3) | |
| pN stage | ||||
| N0 | 38 | 20 (52.6) | 18 (47.4) | 0.018b |
| N+ | 23 | 19 (82.6) | 4 (17.4) | |
| pTNM stage | ||||
| I-II | 31 | 14 (45.2) | 17 (54.8) | 0.002b |
| III-IV | 30 | 25 (83.3) | 5 (16.7) | |
P-value of χ2 test is shown;
P<0.05. Sox2, Sry-box2; TNM, Tumor-Node-Metastasis.
Immunohistochemistry
Expression of Sox2 was measured by immunohistochemical staining. Paraffin-embedded specimens were cut into 4-μm thick sections at room temperature. These paraffin sections were deparaffinized and rehydrated, and then immersed in 3% H2O2 to quench endogenous peroxidase activity. Subsequent to being treated with 0.01 M citrate buffer, the sections were blocked with 5% goat serum followed by incubation with anti-Sox2 antibody (dilution, 1:100; rabbit polyclonal anti-human; catalog no. 3579; Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 40°C. After washing three times in phosphate-buffered saline (PBS), the slides were incubated with ChemMate™ EnVision secondary antibody/horseradish peroxidase (HRP) for 1 h at room temperature and then visualized with diaminobenzidine (Dako; Agilent Technologies GmbH, Waldbronn, Germany). The slides were counterstained with hematoxylin at room temperature. To confirm the specificity of the immunostaining, a negative control was included in each run by substituting PBS for the primary antibody.
Evaluation of immunohistochemical staining
Five random fields of each section were viewed under a light microscope (Axioskop 40; Zeiss GmbH, Jena, Germany) at ×400 magnification. The sections were independently examined and scored by three investigators, who were blinded to the clinical features and outcomes. The expression of Sox2 was scored by multiplication of the mean signal intensity (on a scale of 0-3: 0, no staining; 1, light staining; 2, moderate staining; 3, high staining) and the percentage of positively stained tumor cells (on a scale of 0-4: 0, 0%; 1, 0-25%; 2, 26-50%; 3, 51-75%; 4, 76-100%). The final immunoreactive score reported is the mean of the scores from the three investigators. By receiver operating characteristic analysis, the cases with a final score >4 were classified as exhibiting high Sox2 expression (sensitivity 82.9%, specificity 91.6%), whereas the cases with a score ≤4 were classified as exhibiting low Sox2 expression.
Cell culture
TSCC cell lines, UM1, UM2, Cal27 and SCC9 (preserved and managed in Guangdong Provincial Key Laboratory of Stomatology, Guangzhou, China), and one human oral mucosa epithelial cell immortalized by insertion of the human telomerase reverse transcriptase (hTERT) gene (hTERT+-OME) (16) were used in this study. UM1, UM2, Cal27 and hTERT+-OME cells were maintained in Dulbecco's modified Eagle's medium (DMEM)/F12 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10% fetal bovine serum (FBS; Gibco, New York, NY, USA) at 37°C with 5% CO2. SCC9 cells were cultured in DMEM/F12 containing 1% hydrocortisone and 10% FBS in the same conditions as aforementioned. The cells were confirmed to be free from mycoplasma by testing of the cell lines once every 3 months.
Establishment of Sox2-overexpressing TSCC cells
Human full-length Sox2 cDNA was cloned into pSin-EF2-Sox2-Pur (plasmid no. 16577; Addgene, Inc., Cambridge, MA, USA). Using co-transfection of plasmid DNA with lentivector plus helper plasmids (pRSV-REV, pMDLg-pRRE and pMD2.G) (isoconcentration), transfected into 293T cells occurred using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). In order to improve the transfection efficiency, 6 μg/ml polybrene was added in the culture medium incubated with TSCC cells and lentivirus. Stable Sox2-overexpressing TSCC cells were purified using the antibiotic puromycin.
Construction of Sox2-silenced TSCC cells
Sox2 short hairpin RNA (shRNA) transduction plasmids were purchased from Sigma-Aldrich; Merck KGaA (catalog no. TRCNOO000231641). The sequence of this region was as follows: 5′-CCGGCAACGGCAGCTACAGCATGATCTCGAGATCATGCTGTAGCTGCCGTTGTTTTTG-3′. MISSION® non-target shRNA control (shRNA-NC) transduction particles (Sigma-Aldrich; Merck KGaA) were utilized for the experimental control. Lentivirus production was performed by co-transfection of plasmid Sox2 shRNA and helper plasmid (pCM-VSV-G) (isoconcentration) using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Procedures of cell transfection and purification were as aforementioned.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the cultured cells was prepared using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. Total RNA (1 μg) was reverse transcribed to cDNA using a reverse transcription kit (Toyobo Life Science, Osaka, Japan) according to the manufacturer's protocols. The amplification was performed in 96-well plates, each with a total volume of 20 μl, containing 100 nM forward and reverse primers, 4 mM MgCl2, LightCycler SYBR-Green I and 2 μl cDNA template. Amplification was performed with an initial denaturation step at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, 55°C for 30 sec and 72°C for 25 sec. The reference gene used was β-actin and the primer sequences used were forward, 5′-TGAAGTGTGACGTGGACATC-3′ and reverse, 5′-GGAGGAGCAATGATCTTGAT-3′. The quantification method used was the 2−∆∆Cq method (17). All reactions were performed in duplicate three times.
Western blot analysis
Proteins (20 μg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (10% separation gel and 5% spacer gel). RIPA lysis buffer was the protein extraction buffer used. BCA was used for protein determination. Subsequent to blocking using 5% skimmed milk powder blended with TBST at room temperature for 2 h, the membranes were then incubated with anti-human antibodies overnight at 40°C. Target proteins were detected by enhanced chemiluminescence reagents (Thermo Fisher Scientific, Inc.) following incubation with secondary antibodies conjugated to HRP [HRP-labeled goat anti-rabbit IgG (H+L) (A0208) and HRP-labeled goat anti-mouse IgG (H+L) (A0216) (dilution, 1:1,000; Beyotime Institute of Biotechnology, Shanghai, China)] for 1 h at room temperature. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control.
Cell proliferation assay
Cell proliferation was analyzed using a colorimetric (MTT) assay kit. Cells (2×104) were seeded in each well of a 96-well plate and cultured for 24-72 h at 37°C with 5% CO2. Next, 10 μl MTT solution was added into the culture medium, followed by incubation for 4 h at 37°C with 5% CO2. Dimethyl sulfoxide (100 μl) was added to dissolve the formazan and the color reaction was analyzed with a microplate reader set at 560 nm.
Transwell assay
Briefly, 24-well plates containing 8-μm pore Transwell inserts (EMD Millipore, Billerica, MA, USA) were coated with Matrigel (BD Biosciences, San Jose, CA, USA). The upper chambers were filled with a suspension of 1×104 cells cultured in serum-free DMEM/F12, while the lower chambers were filled with DMEM/F12 plus 10% FBS. Following 48 h of incubation at 37°C, the filters were fixed with methanol for 30 min and stained with crystal violet for 20 min at room temperature. The number of cells which had invaded through the filter pores was counted under a phase-contrast microscope.
Wound-healing assay
Cultured cells (2×105) were seeded in each well of a 6-well plate. When the cells reached 90-100% confluence, a wound was scratched in the central area of the confluent culture, followed by careful washing to remove detached cells. Next, the cells were cultured at 37°C with 5% CO2. Images of the wounded areas were captured using a phase-contrast microscope at 0 and 24 h.
Plate clone formation assay
Each well of the culture plate was seeded with 200 cells and incubated at 37°C for 2 weeks. The cells were then fixed with 4% paraformaldehyde for 15 min and then stained with Giemsa for 15 min at room temperature. The number of visible colonies with a diameter ≥100 μm was counted under a light microscope (magnification, ×40; Axioskop 40; Zeiss GmbH).
Statistical analysis
Experimental results presented in the figures are representative of at least three different repetitions. The data are presented as the mean ± standard deviation. The associations between Sox2 expression and clinicopathological parameters were determined using the χ2 test. Patient survival was evaluated using the Kaplan-Meier method, and the statistical significance was examined using the log-rank test. Assessment of the survival time compared with the clinicopathological variables was performed by univariate and multivariate analyses using the Cox regression model. Comparisons between groups were evaluated using Student's t-test and analysis of variance (one-way ANOVA) followed by Student-Newman-Keuls (S-N-K) post hoc test. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS for Windows (version 22.0; IBM SPSS, Inc., Armonk, NY, USA).
Results
Expression of Sox2 in TSCC
The expression of Sox2 was evaluated by immunohistochemical staining in 61 pairs of TSCC samples and corresponding adjacent non-cancerous tissues. As shown in Fig. 1, significantly higher expression of Sox2 was observed in TSCC samples (83.6%, 51/61) compared with that in the corresponding adjacent non-cancerous tissues (63.9%, 39/61). In order to evaluate the role of Sox2 in TSCC, associations between Sox2 expression and the clinicopathological parameters were investigated (Table I). The results showed that Sox2 expression was significantly associated with tumor differentiation (P=0.042), pT stage (P=0.035), pN stage (P=0.018) and pTNM stage (P=0.002). No significant association was found between Sox2 expression and sex (P=0.737) or age (P=0.175).
Figure 1.
Expression of Sox2 in TSCC. (A-a and b) High expression of Sox2 in TSCC; (A-c and d) low expression of Sox2 in corresponding adjacent non-cancerous tongue tissue; (B-a and b) low expression of Sox2 in well differentiated TSCC; (B-c and d) high expression of Sox2 in moderately/poorly differentiated TSCC; (C-a and b) low expression of Sox2 in pN0 stage TSCC; (C-c and d) high expression of Sox2 in pN+ stage TSCC. (a and c) ×100 magnification, and (b and d) corresponding rectanglular regions at ×200 magnification. TSCC, tongue squamous cell carcinoma; Sox2, Sry-box2.
Association between Sox2 expression and survival in TSCC patients
To investigate the association between Sox2 expression and the clinical outcome of TSCC patients, associations between patient survival status and Sox2 expression were analyzed. The results showed that patients with tumors with high Sox2 expression had a significantly worse prognosis than those with low Sox2 expression (P=0.006; Fig. 2).
Figure 2.
Keplan-Meier survival analysis for TSCC patients. The P-value was determined using the log-rank test. Survival time was calculated based on the date of surgery and the last follow-up. Comparison of the overall survival between TSCC patients with tumors with low and high Sox2 expression. TSCC, tongue squamous cell carcinoma; Sox2, Sry-box2.
To further assess whether Sox2 expression represents a prognostic parameter in patients with TSCC, regression analysis using Cox proportional hazards model was performed. Upon univariate analysis, low Sox2 expression and pN+ stage were associated with a poor prognosis. Moreover, multivariate analysis was performed using the significant variables observed in univariate analysis. The results showed that Sox2 expression was the only independent prognostic predictor (P=0.033) (Table II). These results strongly indicated that the downregulated Sox2 expression in TSCC patients is closely associated with a poor prognosis.
Table II.
Cox proportional hazards model analysis of variables affecting survival in tongue squamous cell carcinoma patients.
| Variables | Categories | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Gender | Female/male | 0.735 (0.333-1.624) | 0.447 | ||
| Age, years | <55/≥55 | 1.614 (0.738-3.533) | 0.231 | ||
| Differentiation | Moderately + poorly/well | 1.349 (0.623-2.925) | 0.448 | ||
| pT stage | T3-4/T1-2 | 1.615 (0.714-3.653) | 0.250 | ||
| pTNM stage | III-IV/I-II | 2.129 (0.961-4.719) | 0.063 | ||
| pN stage | N+/N0 | 2.319 (1.055-5.095) | 0.036a | 1.582 (0.698-3.583) | 0.272 |
| Sox2 | High/low | 3.977 (1.365-11.582) | 0.011a | 3.366 (1.101-10.294) | 0.033a |
HR, hazard ratio; CI, confidence interval; TNM, Tumor-Node-Metastasis.
Sox2 regulates malignant phenotypes and EMT progression in TSCC
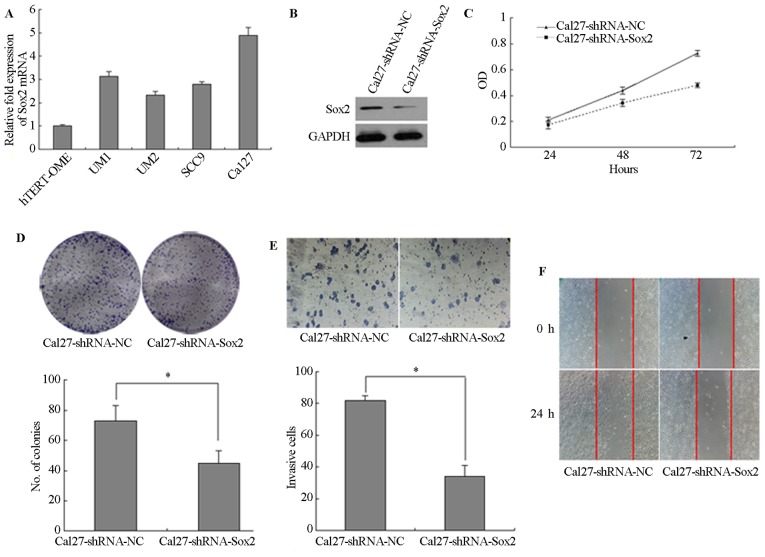
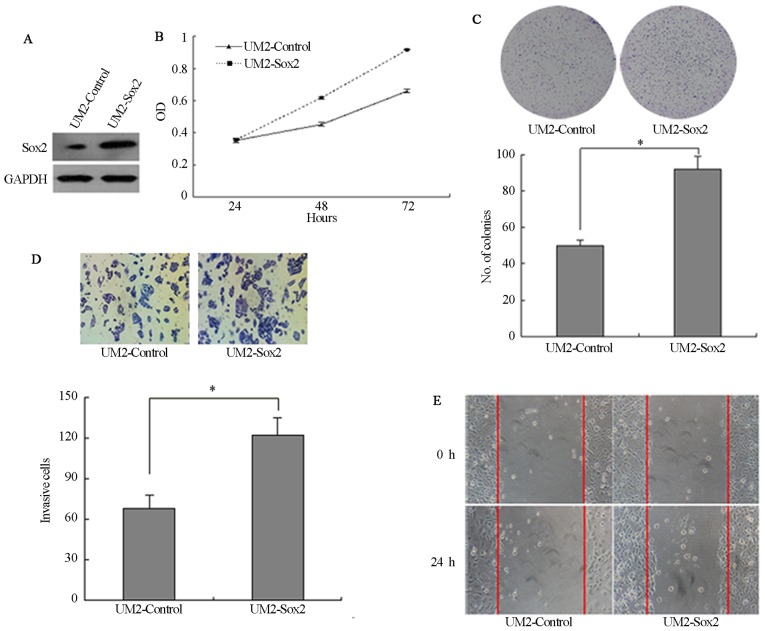
The expression of Sox2 was detected by RT-qPCR and the relative expression level of Sox2 was lower in immortalized oral mucosal epithelial cells (hTERT+-OME) than that in the 4 TSCC cells (UM1, UM2, SCC9 and Cal27) (Fig. 3A). To determine the role of Sox2 in TSCC cells, the expression of Sox2 was downregulated in Cal27 cells by transfection with lentiviral vector expressing shRNA and the expression of Sox2 was lower in Cal27-shRNA-Sox2 than that in Cal27-shRNA-NC (Fig. 3B). Silencing of Sox2 significantly inhibited the proliferation of the Cal27 cells (P<0.05; Fig. 3C). Following knockdown of Sox2, the colony number was also decreased. A longer incubation time was required to generate colonies of equivalent size to those generated by Cal27-shRNA control cells (P<0.05; Fig. 3D). In addition, knockdown of Sox2 significantly reduced the cell invasion and wound-healing ability (P<0.05; Fig. 3E and F). Furthermore, UM2 cells were stably transfected with Sox2 cDNA and the expression of Sox2 was higher in UM2-Sox2 than that in Sox2-control (Fig. 4A). UM2 cells transfected with Sox2 (UM2-Sox2) showed significantly higher cell proliferation (P<0.05; Fig. 4B). Overexpression of Sox2 in UM2 cells increased the colony numbers by 78.4% (P<0.05; Fig. 4C). Also, Sox2-overexpressing cells invaded faster than control cells (P<0.05; Fig. 4D). In addition, wound-healing assays confirmed these results (Fig. 4E), suggesting that Sox2 affects the motility in UM2 cells.
Figure 3.
Ability for cell proliferation, migration and invasion in Sox2-knockdown tongue squamous cell carcinoma cells. (A) Relative expression level of Sox2 in immortalized oral mucosal epithelial cells (hTERT+-OME) and 4 TSCC cells (UM1, UM2, SCC9 and Cal27). (B) Sox2 expression in Cal27-shRNA-NC and Cal27-shRNA-Sox2. (C) MTT assay. (D) Plate clone formation assay. (E) Transwell assay (magnification, ×200). (F) Wound-healing assay at different time courses. Data shown is from triplicate experiments. *P<0.05. OD, optical density; Sox2, Sry-box2; NC, negative control; shRNA, short hairpin RNA.
Figure 4.
Ability of cell proliferation, migration and invasion in Sox2-upregulated tongue squamous cell carcinoma cells. (A) Sox2 expression in UM2-control and UM2-Sox2. (B) MTT assay. (C) Plate clone formation assay. (D) Transwell assay (magnification, ×200). (E) Wound-healing assay at different time courses. Data shown is from triplicate experiments. *P<0.05. Sox2, Sry-box2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To determine if there is an assoicaation between Sox2 expression and the process of EMT, RT-qPCR and western blot analysis were performed. Compared with the cells transfected with shRNA-NC, the mesenchymal cell markers (Snail, Twist, vimentin and Slug) were downregulated, while the epithelial cell marker (E-cadherin) was upregulated in Cal27-shRNA-Sox2 cells. By contrast, upregulation of the mesenchymal cell markers and downregulation of the epithelial marker was found in Sox2-overexpressing cells (Fig. 5A and B).
Figure 5.
Expression of EMT markers and β-catenin in TSCC cells with alterations in Sox2 expression. (A) The results of reverse transcription-quantitative polymerase chain reactio and (B) western blot analyses showed the expression of EMT markers (E-cadherin, vimentin, Twist, Slug and Snail) and β-catenin in TSCC cells with alterations in Sox2 expression. Normalized mRNA and protein expression levels are from triplicate experiments. *P<0.05. EMT, epithelial-mesenchymal transition; Sox2, Sry-box2; shRNA, short hairpin RNA; NC, negative control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TSCC, tongue squamous cell carcinoma.
Sox2 modulates β-catenin expression
Since the Wnt/β-catenin signaling pathway is closely associated with cell tumorigenesis and migration, and it is also an important route via which to promote the process of EMT, experiments were designed to interrogate whether Sox2 expression was closely associated with the expression of β-catenin. As shown in Fig. 5, β-catenin expression increased significantly in UM2-Sox2 cells and decreased in Cal27-shRNA-Sox2 cells, at the mRNA and protein levels. These results suggest that β-catenin may associated with a higher malignant phenotype and EMT progression in TSCC cells with alterations in Sox2 expression.
Discussion
TSCC, as the most common tumor of the oral cavity, has a high frequency of local recurrence and distant metastatic characteristics (18). Recent studies indicated that EMT and the Wnt/β-catenin signaling pathway were associated with oncogenesis, invasion and metastasis in various tumors (19-22). Thus, understanding the underlying molecular mechanism of TSCC aggression and transformation processes could enhance the treatment outcome of patients with TSCC. The present study demonstrated that Sox2 can affect tumor aggressiveness and EMT in TSCC.
Overexpression of Sox2 is often associated with increased cancer aggressiveness, resistance to chemo-radiation therapy and decreased survival rate, which have been reported in various cancer types, including breast, prostate, lung, ovarian and colon cancer (9,10,23-25). The present study revealed that Sox2 is vital in the regulation of TSCC motility, invasion and tumorigenicity. The results indicated that upregulated Sox2 expression was significantly associated with the progression of TSCC, as we previously described (26). Sox2 expression was significant higher in primary TSCC tissues, the para-tumoral tissues and oral epithelial dysplastic (pre-invasive) tissues compared with the oral normal mucosa (26). Sox2 was detected in oral pre-invasive lesions, suggesting that Sox2 upregulation may be an early event in TSCC carcinogenesis.
The present data also revealed that Sox2 expression was higher in UM1 cells than in UM2 cells. UM1 and UM2 cells were established from the same tongue carcinoma tissue from patients without any adjuvant treatment. The growth pattern of UM1 was scattered, while UM2 cells formed colonies with firm adhesion. Compared with UM2 cells, UM1 cells exhibit a spontaneous EMT pattern, with higher motility, invasive and metastatic activities in viv and in vitro (27). From the present results, we speculate that Sox2 may serve an important role in the aggressive behavior of TSCC. To confirm this hypothesis, two TSCC cell lines were constructed to evaluate the role of Sox2 through lentiviral-mediated overexpression and lentiviral-mediated knockdown. From this investigation, it was noted that Sox2 could modulate cell aggression and motility by affecting the capability of migration, invasion and proliferation in TSCC cells.
EMT is well known for its major role in embryogenesis and development, which involves growth and differentiation (28). The regulatory changes that enable EMT to drive as a normal process of increased growth and differentiation in developing populations of cells within an organism. Nevertheless, when the same modifications occur in cancer cells, metastasis can happen. As an important factor associated with cancer aggression, EMT is often involved in invasion and metastasis during tumor progression (29). It has been confirmed that numerous classical molecular pathways are associated with EMT, including the Wnt/β-catenin, Snail/Slug, transforming growth factor-β (TGF-β), Twist and Cripto pathways (30). Recent studies showed that cytokines, including TGF-β, epidermal growth factor (EGF) and insulin-like growth factor, mediated the occurrence of EMT in normal epithelial cells and cancer cells by autocrine or paracrine signaling (31-33). Nuclear transcription factors, which include Twist, Snail and Slug, also served important roles in the process of tumor metastasis by promoting EMT (34-36). In the present study, Sox2 was shown to possess the capacity to promote the characteristics of EMT in TSCC cells. Upregulation of Sox2 in UM2 led to the enhanced expression of Snail, Slug, Twist and vimentin, and the reduction in the protein level of E-cadherin. In addition, silencing of Sox2 could attenuate the expression of mesenchymal markers, and could elevate the epithelial marker expression in Cal27 tongue cancer cells.
Sox2 has been described in certain cases as an oncogene, and it can control cancer physiology via promoting oncogenic signaling pathways, such as that of Wnt/β-catenin and phosphoinositide 3-kinase/protein kinase B (37,38). Meanwhile, Sox2 is mediated by certain upstream signals to regulate the cell survival, tumorigenesis, invasion and migration reported in recent literature. These signals include EGF receptor signals, the Hedgehog/glioma-associated oncogene homolog zinc finger protein signaling pathway, and transcription factor mammalian target of rapamycin and signal transducer and activator of transcription signals (39-42). Sox2 acts as an important factor in these signaling pathways.
The Wnt/β-catenin signaling pathway is associated with malignant cancer progression, and is also an important way to promote the process of EMT. Abundant evidence has shown that Sox2 promotes tumor metastasis by stimulating EMT via the Wnt/β-catenin pathway in breast and laryngeal cancer (12,43). β-catenin itself is one of the vital downstream molecules that mediate EMT induced by Sox2 (12). In the present study, the results revealed that β-catenin expression was closely associated with Sox2 expression, which suggests that β-catenin may be associated with a higher malignant phenotype and EMT progression in TSCC cells with alteration of Sox2 expression.
In conclusion, the present study illustrated the pivotal role of Sox2 in the progression of TSCC. The results provide an explanation for TSCC aggression and metastasis by linking them with the overexpression of Sox2, and the possible mechanism by which Sox2 may modulate EMT by targeting β-catenin. These data can be extended in in viv and in viv models to further elucidate the role of Sox2 in the carcinogenesis and metastasis of TSCC. Hence, the study indicates that Sox2 can be a predictive and therapeutic target for TSCC with aggressive clinical behavior.
Acknowledgments
Not applicable.
Funding
This study was supported by Shandong Provincial Natural Science Foundation (grant no. ZR2018PH027) the Natural Science Foundation of Guangdong, China (grant no. 2014A030313153), the Science and Technology Plan Projects of Guangzhou (grant no. 201510010268) and the National Natural Science Foundation of China, with funding from the Youth Foundation of The First Affiliated Hospital of Zhengzhou University (grant no. 81200796).
Availability of data and materials
The data and materials are available upon request.
Authors' contributions
XL and QT conceived and designed the study. XL, BQ, TZ and FH performed the experiments. XL and AKYL wrote the paper. AKYL and QT reviewed and edited the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
Informed consent was obtained on the use of surgically resected specimens for research purposes, according to the guidelines for research for human tissues and samples set by the Institution Review Board (IRB) of Sun Yat-sen University, who also approved the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–316. doi: 10.1016/S1535-6108(04)00090-X. [DOI] [PubMed] [Google Scholar]
- 3.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 5.Zha L, Zhang J, Tang W, Zhang N, He M, Guo Y, Wang Z. HMGA2 elicits EMT by activating the Wnt/β-catenin pathway in gastric cancer. Dig Dis Sci. 2013;58:724–733. doi: 10.1007/s10620-012-2399-6. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Ginther C, Kim J, Mosher N, Chung S, Slamon D, Vadgama JV. Expression of Wnt3 activates Wnt/β-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol Cancer Res. 2012;10:1597–1606. doi: 10.1158/1541-7786.MCR-12-0155-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin LC, Hsu SL, Wu CL, Hsueh CM. TGFβ can stimulate the p(38)/β-catenin/PPARγ signaling pathway to promote the EMT, invasion and migration of non-small cell lung cancer (H460 cells) Clin Exp Metastasis. 2014;31:881–895. doi: 10.1007/s10585-014-9677-y. [DOI] [PubMed] [Google Scholar]
- 8.Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16:182–187. doi: 10.1016/S0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, Sun L, Yang X, Wang Y, Zhang Y, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem. 2008;283:17969–17978. doi: 10.1074/jbc.M802917200. [DOI] [PubMed] [Google Scholar]
- 10.Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, Tom W, Paner GP, Szmulewitz RZ, Vander Griend DJ. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS One. 2013;8:e53701. doi: 10.1371/journal.pone.0053701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A, Corte G. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T, Liu Y, Li X, Xiang R, Li N. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/β-catenin signal network. Cancer Lett. 2013;336:379–389. doi: 10.1016/j.canlet.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Kokalj Vokač N, Cizmarević B, Zagorac A, Zagradišnik B, Lanišnik B. An evaluation of SOX2 and hTERC gene amplifications as screening markers in oral and oropharyngeal squamous cell carcinomas. Mol Cytogenet. 2014;7:5. doi: 10.1186/1755-8166-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michifuri Y, Hirohashi Y, Torigoe T, Miyazaki A, Kobayashi J, Sasaki T, Fujino J, Asanuma H, Tamura Y, Nakamori K, et al. High expression of ALDH1 and SOX2 diffuse staining pattern of oral squamous cell carcinomas correlates to lymph node metastasis. Pathol Int. 2012;62:684–689. doi: 10.1111/j.1440-1827.2012.02851.x. [DOI] [PubMed] [Google Scholar]
- 15.Sobin LH, Wittekind C. TNM classification of malignant tumours. 6th edition. New York: Wiley-Liss; 2002. [Google Scholar]
- 16.Qiao B, Gopalan V, Chen Z, Smith RA, Tao Q, Lam AK. Epithelial-mesenchymal transition and mesenchymal-epithelial transition are essential for the acquisition of stem cell properties in hTERT-immortalised oral epithelial cells. Biol Cell. 2012;104:476–489. doi: 10.1111/boc.201100077. [DOI] [PubMed] [Google Scholar]
- 17.Vermeire J, Naessens E, Vanderstraeten H, Landi A, Iannucci V, Van Nuffel A, Taghon T, Pizzato M, Verhasselt B. Quantification of reverse transcriptase activity by real-time PCR as a fast and accurate method for titration of HIV, lentiand retroviral vectors. PLoS One. 2012;7:e50859. doi: 10.1371/journal.pone.0050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma an update. CA Cancer J Clin. 2015;65:401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 19.Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, Willbanks A, Sarkar S. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Zhu X, Hu J, He G, Li X, Wu P, Ren X, Wang F, Liao W, Liang L, et al. The positive feedback between Snail and DAB2IP regulates EMT, invasion and metastasis in colorectal cancer. Oncotarget. 2015;6:27427–27439. doi: 10.18632/oncotarget.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demirkan B. The roles of epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET) in breast cancer bone metastasis: potential targets for prevention and treatment. J Clin Med. 2013;2:264–282. doi: 10.3390/jcm2040264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. Pte loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belotte J, Fletcher NM, Alexis M, Morris RT, Munkarah AR, Diamond MP, Saed GM. Sox2 gene amplification significantly impacts overall survival in serous epithelial ovarian cancer. Reprod Sci. 2015;22:38–46. doi: 10.1177/1933719114542021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann J, Bahr F, Horst D, Kriegl L, Engel J, Luque RM, Gerhard M, Kirchner T, Jung A. SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer. 2011;11:518. doi: 10.1186/1471-2407-11-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, Gao Y, Geng J, Qu D, Han Q, Qi J, Chen G. Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2846–2854. [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao B, He B, Cai J, Yang W. The expression profile of Oct4 and Sox2 in the carcinogenesis of oral mucosa. Int J Clin Exp Pathol. 2013;7:28–37. [PMC free article] [PubMed] [Google Scholar]
- 27.Nakayama S, Sasaki A, Mese H, Alcalde RE, Matsumura T. Establishment of high and low metastasis cell lines derived from a human tongue squamous cell carcinoma. Invasion Metastasis. 1999;18:219–228. doi: 10.1159/000024515. [DOI] [PubMed] [Google Scholar]
- 28.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Zhang H, Tang L, Chen H, Wu C, Zhao M, Yang Y, Chen X, Liu G. Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology. 2013;303:139–146. doi: 10.1016/j.tox.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, Wang XJ. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest. 2005;115:1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O'Regan RM. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 33.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 34.Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367:235–241. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 37.Ye X, Wu F, Wu C, Wang P, Jung K, Gopal K, Ma Y, Li L, Lai R. β-catenin, a Sox2 binding partner, regulates the DNA binding and transcriptional activity of Sox2 in breast cancer cells. Cell Signal. 2014;26:492–501. doi: 10.1016/j.cellsig.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Gen Y, Yasui K, Nishikawa T, Yoshikawa T. SOX2 promotes tumor growth of esophageal squamous cell carcinoma through the AKT/mammalian target of rapamycin complex 1 signaling pathway. Cancer Sci. 2013;104:810–816. doi: 10.1111/cas.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bora-Singhal N, Perumal D, Nguyen J, Chellappan S. Gli1-mediated regulation of Sox2 facilitates self-renewal of stem-like cells and confers resistance to EGFR inhibitors in non-small cell lung cancer. Neoplasia. 2015;17:538–551. doi: 10.1016/j.neo.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh S, Trevino J, Bora-Singhal N, Coppola D, Haura E, Altiok S, Chellappan SP. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol Cancer. 2012;11:73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corominas-Faja B, Cufí S, Oliveras-Ferraros C, Cuyàs E, López-Bonet E, Lupu R, Alarcón T, Vellon L, Iglesias JM, Leis O, et al. Nuclear reprogramming of luminal-like breast cancer cells generates Sox2-overexpressing cancer stem-like cellular states harboring transcriptional activation of the mTOR pathway. Cell Cycle. 2013;12:3109–3124. doi: 10.4161/cc.26173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bass AJ, Wang TC. An inflammatory situation: SOX2 and STAT3 cooperate in squamous cell carcinoma initiation. Cell Stem Cell. 2013;12:266–268. doi: 10.1016/j.stem.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Yang N, Hui L, Wang Y, Yang H, Jiang X. Overexpression of SOX2 promotes migration, invasion, and epithelial-mesenchymal transition through the Wnt/β-catenin pathway in laryngeal cancer Hep-2 cells. Tumour Biol. 2014;35:7965–7973. doi: 10.1007/s13277-014-2045-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are available upon request.