Abstract
Catecholaminergic polymorphic ventricular tachycardia is a rhythm disorder that develops due to genetic reasons in the absence of structural cardiac abnormalities. Ventricular tachycardia, ventricular fibrillation, cardiac arrest, and death may occur. Two-year-old patient presented to the Emergency Department with sudden cardiac arrest. He had syncope attacks after playing with his brother and he was followed up by the pediatric neurology and cardiology clinics. Cardiopulmonary resuscitation was performed, and he was then transferred to the Intensive Care Unit because of hypotension; dobutamine and norepinephrine treatment was started. After treatment, ventricular tachycardia, ventricular fibrillation, and cardiac arrest developed. Dobutamine and noradrenaline was stopped immediately and amiodarone was started. A genetic test revealed heterozygote missense mutation (c.9110G>A(p.Gly3037Asp)) in exon 64 of the RYR2 gene, which is compatible with catecholaminergic polymorphic ventricular tachycardia. This mutation has been reported in the literature for the first time. This case is presented with the purpose of highlighting catecholaminergic polymorphic ventricular tachycardia.
Keywords: Catecholaminergic polymorphic ventricular tachycardia, RyR2, sudden cardiac arrest
Introduction
Catecholaminergic polymorphic ventricular tachycardia is a rhythm disorder that develops in relation with genetic causes in the absence of structural heart disease. Ventricular tachycardia, ventricular fibrillation, sudden cardiac arrest, and death, triggered by factors that increase sympathetic stimulation, may develop from young ages (1, 2). This disease is defined as the most fatal ion channel disorder (3). It is generally observed in children and young adults (1). Symptoms including drowsiness, dizziness, and fainting may be observed as a result of ventricular tachycardia in patients with catecholaminergic polymorphic ventricular tachycardia (1, 2). However, these patients are generally diagnosed late because the cause of the symptoms are associated with other morbidities.
It is mostly transmitted by an autosomal dominant pattern of inheritance over the RyR2 gene encoding the cardiac ryanodine receptor. Less frequently, it occurs sporadically or with mutations of the CASQ2 gene, which encodes the calsequestrin (1–4). These genes function in relation with intracellular calcium secretion and contraction. This case is presented to draw attention to catecholaminergic polymorphic ventricular tachycardia, which is rarely observed in patients who faint with exertion, and to report the gene mutation, which has been defined for the first time.
Case
A 2-year-old boy was brought to our Emergency Department by ambulance with a clinical picture of sudden cardiac arrest. The ambulance had reached his home approximately 15 minutes after the patient fainted and brought the patient to the Emergency Department in approximately 30 minutes. It was learned that he fainted while playing with his older brother at home and subsequently developed cardiac arrest. At presentation, the patient’s pulse could not be palpated and respiratory effort was absent. Cardiopulmonary resuscitation was performed and the patient was intubated. Following cardiopulmonary resuscitation, his pulse was felt weekly and his peripheral circulation was found to be impaired. He was internalized in the Pediatric Intensive Care Unit. In the history, it was learned that the patient was being followed up in Pediatric Neurology Outpatient Clinic in another center because of recurrent fainting spells triggered by exertion and his neuromotor development was compatible with his age. Phenobarbital treatment had been initiated because of fainting spells, considering epilepsy. However, the fainting spells continued and the patient was examined in the Pediatric Cardiology Outpatient Clinic. His electrocardiogram (ECG) and echocardiographic examination were found to be normal. The mother stated that the fainting spells generally developed immediately after playing with the older brother. The patient’s apical heart beat was found to be 120/min and blood pressure was found to be 80/40 mm Hg. He had a weak pulse and poor peripheral circulation. Normal saline was given twice at a dose of 20 cc/kg and treatment with positive inotropic drugs (dobutamine and noradrenaline) was initiated. Immediately after treatment with dobutamine and noradrenaline was initiated, ventricular tachycardia and subsequently ventricular fibrillation developed and sudden cardiac arrest occurred once again (Picture 1a, b). Treatment with dobutamine and noradrenaline was discontinued. Amiodarone treatment was given at a loading dosage of 5 mg/kg/day in 30 minutes. The patient’s arrhythmia was controlled with amiodarone. Amiodarone treatment was continued for 24 hours as infusion at a dosage of 10 mg/kg/day. The infusion dose was gradually tapered and oral maintenance treatment was initiated at a dosage of 5 mg/kg/day. An echocardiographic examination, which was performed in terms of structural cardiac problems, was found to be normal. The patient was closely monitored for the adverse effects of amiodarone. Severe neurologic injury and motor mental retardation developed in relation with hypoxia in the patient who had developed sudden cardiac arrest twice and undergone resuscitation. He was discharged on the 31st day of hospitalization with normal sinus rhythm and continued using amiodarone.
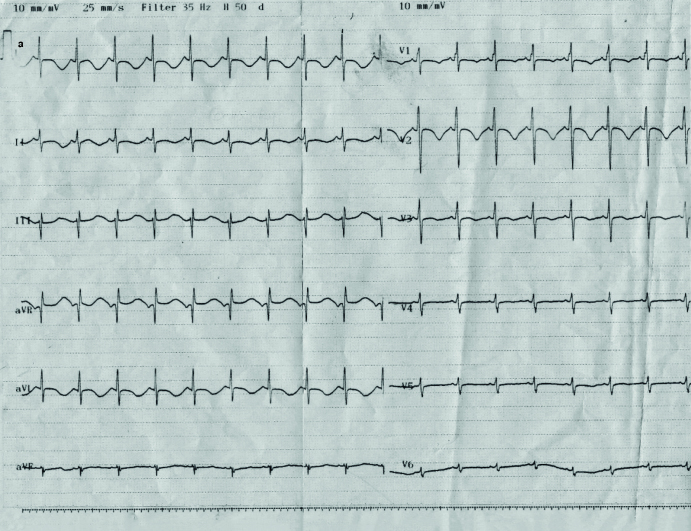
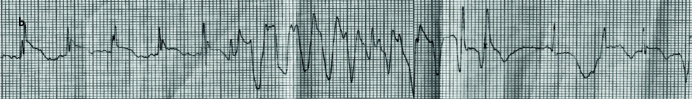
Picture 1.a, b.
Normal sinus rhythm recorded during Intensive Care Unit monitoring (a). Ventricular tachycardia recorded during Intensive Care Unit monitoring (b)
In light of this information, catecholaminergic polymorphic ventricular tachycardia was suspected primarily. The Pediatric Cardiology Division to which he was previously referred was contacted. It was learned that sinus rhythm and a normal corrected QT interval was found on the ECG performed at the time of presentation to the outpatient clinic. It was thought that the fainting spells triggered by exertion arose from possible cardiac arrhythmias that developed secondary to catecholamine release, and this diagnosis was supported by the fact that ventricular tachycardia, ventricular fibrillation, and sudden cardiac arrest developed after the use of catecholaminergic drugs and no structural cardiac problem was found. Genetic analysis of the patient revealed a heterozygous missense mutation in exon 64 in the RyR2 gene, which was compatible with the diagnosis of catecholaminergic polymorphic ventricular tachycardia. The mother was found to be a carrier. Amiodarone treatment was discontinued and propranolol (beta blocker) treatment was initiated because a diagnosis of catecholaminergic polymorphic ventricular tachycardia was made. The other therapeutic options were discussed with the family. Treatment with left cardiac sympathetic denervation and an implantable cardiac defibrillator were presented as a therapeutic option. However, the family did give consent for these treatments, because the patient had severe neurologic injury and motor mental retardation. The patient is still receiving propranolol with the diagnosis of catecholaminergic polymorphic ventricular tachycardia. He has been followed up in Pediatric Cardiology Outpatient Clinic for 20 months and no cardiac problem has been found. Verbal consent was obtained from the patient’s parents.
Discussion
Catecholaminergic polymorphic ventricular tachycardia is defined as ventricular tachycardia that develops in relation with genetic causes and is related with catecholamine release, which is triggered by exertion and emotional stress in the absence of structural cardiac disease (1, 2). Patients may present with sudden cardiac arrest. In the literature, it has been reported that approximately 30% of patients present with sudden cardiac arrest; sudden cardiac arrest develops in the first 30 years of life in more than half of all patients (1, 2). Another reason for physician referrals is fainting spells. Patients may be followed up with erroneous diagnoses including epilepsy or vasovagal syncope because of recurrent fainting spells and episodes of loss of consciousness. In the study by Priori et al. (5), it was reported that 16 of 26 patients presented with the symptom of fainting for the first time and the diagnosis was made only after 2±0.8 years because the fainting was associated with vasovagal syncope and neurologic causes. It is very difficult to make the diagnosis, especially in young children. In the absence of effort and stress, ECG and 24-hour rhythm recordings may be completely normal. Structural cardiac disorder is not found on echocardiography. The diagnosis can be made with stimulation of ventricular tachycardia using exercise tests or isoproterenol infusion (1). Inability to perform exercise tests in young children may render it difficult to make the diagnosis, like in our case.
A mutation in the RyR2 gene is present in approximately 60% of patients with catecholaminergic polymorphic ventricular tachycardia (1). Priori et al. (6) described the mutations in the cardiac ryanodine receptor gene in patients with catecholaminergic polymorphic ventricular tachycardia in 2001 for the first time. In the last 10 years, more than 100 mutations associated with catecholaminergic polymorphic ventricular tachycardia have been found (1, 2, 4, 5). The heterozygous c.9110G>A(p.Gly3037Asp) missense mutation in exon 64 found in our patient is reported for the first time in the literature. Mutations in the calsequestrin 2 (CASQ2) gene are responsible for 1–2% of cases of catecholaminergic polymorphic ventricular tachycardia (1). In addition, mutations in the calmodulin 1 (CALM1), triadin (TRDN), and SCN5A genes have also been reported (1). Catecholaminergic polymorphic ventricular tachycardia may be transmitted by autosomal dominant or autosomal recessive inheritance. It may also be sporadic. If patients with bi-directional ventricular tachycardia triggered by exercise or patients with sudden cardiac arrest display an autosomal dominant inheritance, sequence analysis in the RyR2 gene is recommended. If there is autosomal recessive inheritance, the CASQ2 gene should be examined (1). There is currently is no genetic treatment available for humans. However, successful animal studies have been published recently in which viral vectors carrying the CASQ2 gene were used (1).
Treatment of catecholaminergic polymorphic ventricular tachycardia consists of limitation of moderate and high-intensity effort and administration of high-dose beta blocker drugs (propranolol, nadolol). Recently, nadolol was recommended for treatment instead of propranolol (7). Flecainide is an effective drug in arrhythmias developing under beta blockers. Flecainide is an antiarrhythmic agent that acts by various mechanisms. Direct RyR2 blockade is one such mechanism. There is currently no adequate information related to the use of amiodarone in the treatment of catecholaminergic polymorphic ventricular tachycardia. For the first time, Sioros et al. (8) reported that arrhythmia did not develop with 28-month amiodarone treatment in a patient who was diagnosed as having catecholaminergic polymorphic ventricular tachycardia. Amiodarone treatment was also used in our patient until the genetic test result was obtained. Arrhythmia was not found in our patient during this period. Beta-blocker treatment was initiated after the genetic test result was obtained. Left cardiac sympathetic denervation may provide long-term rhythm control in patients in whom arrhythmia cannot be controlled despite use of beta blocker drugs. In the study by De Ferrari et al. (9) with 63 patients, it was recommended that sympathetic denervation should be tried before implantable cardiac defibrillators as second-line treatment in patients who had fainting episodes despite appropriate medical treatment. In the guideline related to diagnosis and treatment of congenital arrhythmia syndromes published in 2013, use of implantable cardiac defibrillators was emphasized in arrhythmias that continue despite medical treatment and in patients who have experienced an event necessitating cardiorespiratory resuscitation (10). However, implantable cardiac defibrillators have some disadvantages. The most important problem is severe arrhythmia and death due to adrenergic discharge, which develops with inappropriate shocks.
In conclusion, rhythm disorders should be considered in patients with recurrent fainting spells triggered by exertion. In the history, it should definitely be interrogated as to whether fainting spells initiate with exertion. Patients followed up with a misdiagnosis of epilepsy or vasovagal syncope may present with sudden cardiac arrest as a result of adrenergic stimuli, like in our patient. Therefore, catecholaminergic polymorphic ventricular tachycardia should be considered in the differential diagnosis in cases of recurrent fainting spells triggered by exertion, though it is observed rarely.
Footnotes
Informed Consent: Verbal informed consent was obtained from patients’ parents who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.U.A.; Design - F.E.A.; Supervision - A.G.E.; Resources - R.D.; Materials - F.Ö.; Data Collection and/or Processing - F.E.A.; Analysis and/or Interpretation - F.Ö., Literature Search - A.K.; Writing Manuscript - F.E.A., S.U.A.; Critical Review - A.G.E.; Other - A.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Spears DA, Gollob MH. Genetics of inherited primary arrhythmia disorders. Appl Clin Genet. 2015;8:215–33. doi: 10.2147/TACG.S55762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kron J, Ellebogen K, Abbate A. Recurrent ventricular fibrillation in a young female carrying a previously unidentified RyR2 gene mutation. Int J Cardiol. 2015;201:222–4. doi: 10.1016/j.ijcard.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 3.Napolitano C, Priori SG. Diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:675–8. doi: 10.1016/j.hrthm.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 4.Lahat H, Pras E, Olender T, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–84. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.CIR.0000020013.73106.D8. [DOI] [PubMed] [Google Scholar]
- 6.Priori SG, Napolitano C, Schwartz PJ, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–4. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 7.Leren IS, Saberniak J, Majid E, Haland TF, Edvardsen T, Haugaa KH. Nadolol decreases the incidence and severity of ventricular arrhythmias during exercise stress testing compared with β1-selective β-blockers in patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2016;13:433–40. doi: 10.1016/j.hrthm.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Sioros L, Baltogiannis GG, Lysitsas DN, Kolettis TM. Treatment of catecholaminergic polymorphic ventricular tachycardia: lessons from one case. Hosp Chron. 2014;9:27–32. [Google Scholar]
- 9.De Ferrari GM, Dusi V, Spazzolini C, et al. Clinical management of catecholaminergic polymorphic ventricular tachycardia: the role of left cardiac sympathetic denervation. Circulation. 2015;131:2185–93. doi: 10.1161/CIRCULATIONAHA.115.015731. [DOI] [PubMed] [Google Scholar]
- 10.Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–63. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]