Abstract
Aim
To evaluate the association of the presence and extent of adenoid biofilms and the frequency of upper airway infections in children with upper airway obstruction.
Material and Methods
This cross-sectional study was conducted from October 2014 to December 2015 on pediatric patients who were candidates for adenoidectomy due to obstructive sleep apnea. After removal of the adenoid tissue and fixation in 2.5% glutaraldehyde, the samples were sent to the electron microscopy unit. The extent of biofilm formation was examined using environmental scanning electron microscopy. These results were then confirmed using image analysis software.
Results
Fifty-seven children with a mean age of 7.31 (±2.65) years were included in the study. Forty-three (75.4%) were male and 14 (24.6%) were female. The average number of upper airway infections during the last 12 months before adenoidectomy was 10.01 (±5.38). Biofilm structures were detected in all (100%) samples. As the main outcome, the extent of biofilm grading exhibited a statistically significant correlation with the frequency of upper airway infections (p<0.001). There was no significant correlation between sex and adenoid size with the biofilm extent.
Conclusion
The present study showed that the extent of adenoid biofilm had a significant relationship with the frequency of upper airway infection rate. It seems that the presence of a biofilm on the adenoid surface as a reservoir for microorganisms could cause chronic inflammation.
Keywords: Adenoidectomy, biofilms, infection, obstructive sleep apnea
Introduction
Biofilms are structured bacterial communities enclosed in a self-produced polymeric matrix that adheres to an inert or living surface (1). Biofilm bacteria are embedded in a substrate rich in polysaccharides, nucleic acids, and proteins known as extracellular polymeric substances (EPSs) (2). These complex structures provide a mechanism for bacteria to survive non-biologic hazards 10–1000 times more than genetically identical planktonic bacteria. Such a marked resistance is likely due to a decrease in metabolic rates, which makes the cells less susceptible to antibiotics acting on these target metabolic processes. Decreased metabolic activity, decreased growth rate, and transmission of resistance genes all contribute to the antibiotic-resistant nature of biofilms (3).
Biofilms are increasingly recognized as playing a role in Ear, Nose, and Throat (ENT) diseases. The role of biofilm in the persistence of chronic, mucosal-based ENT-related infections was first recognized in otitis media (4); however, definitive proof was lacking until the demonstration of bacterial biofilms on the middle ear mucosa of children with chronic otitis media with effusion (COME) and recurrent otitis media (ROM). Since then, biofilms have been shown to be involved in the etiology of otitis media, sinusitis, cholesteatoma, tonsillitis, adenoiditis, and device contaminations (5).
The upper airway seems to be at high risk for this type of colonization. Chronic and/or recurrent upper airway infections may be related to the complex structural and biochemical organization of the biofilm, which interferes with the activity of antibiotics, thus promoting the establishment of a chronic infection that can only be eradicated with surgical treatment (6). The presence of biofilms on the surface of adenoid tissue has been established and numerous studies have shown a correlation between the presence of biofilms on the adenoid and the occurrence of chronic upper respiratory tract infections. This was further supported by studies that showed a decrease in infection recurrence with the removal of adenoid tissue. Believing that chronic bacterial infections are biofilm-related is fundamental to developing rational strategies for the treatment and prevention based on tissue removal (7). The adenoid is a bacterial reservoir that contributes to chronic otolaryngologic infections. Removal of the adenoid can be effective in controlling pediatric sinusitis and otitis media (5). Biofilms have been reported on the adenoid surfaces of children with acute otitis media (AOM) and COME (8). Adenoid hypertrophy is a common finding in childhood and is probably associated with AOM, ROM with effusion, and obstructive sleep apnea (OSA), and adenoidectomy has been known as an effective treatment for COME, which involves removal of the physical obstruction of the Eustachian tube and the establishment of normal pressure and drainage of mucus in the middle ear (8).
Considering the studies about the role of biofilm formation in nasopharyngeal infections, we designed this cross-sectional study to evaluate the association between the extent of biofilm on the adenoid surface in children who had respiratory obstructive symptoms with the frequency of upper respiratory infections in the 12-month period before admission, using a scanning electron microscope (SEM).
Material and Methods
Ethics
This study was ethically approved by the local Ethics Committee of the ENT-Head and Neck Surgery Research Center of Iran University of Medical Sciences. The study was also financially supported by this organization. The patients were enrolled in the study after a consent form was signed by their caregivers. The patients’ information remained confidential and was used only for research purposes.
Study design and population
In this cross-sectional study, we evaluated children who presented to the ENT and Pediatric Infectious Diseases Clinic of Ali-Asghar Children’s Hospital, from October 2014 to December 2015, with symptoms of upper airway obstruction and adenoid hypertrophy, who were candidates for adenoidectomy. The degree of the upper airway obstruction that compromised the quality of life was an indication for surgery. Patients with diseases such as cystic fibrosis, immune deficiency, allergic rhinitis, septal deviation, and respiratory disorders including asthma and those who had a history of antibiotic consumption during the two-week period prior to surgery were excluded from the study. The included patients were preoperatively evaluated using adenoid view X-ray for grading of adenoid size according to the nasopharyngeal size (adenoid nasopharynx ratio) and divided into four groups of less than 25% (grade 1), 25–50% (grade 2), 50–75% (grade 3) and more than 75% (grade 4). The rate of upper airway infection episodes, including common cold, pharyngitis, sinusitis, sinobronchitis, and rhinosinusitis was recorded according to the patient’s visit registry and/or their caregiver’s history during the 12-month period before surgery. Because all our patients had airway obstruction symptoms, otitis episodes were excluded from infections due to the probable mechanism for obstructive effects of adenoid hypertrophy in otitis etiology. The demographic information of the patients, including age, sex, duration of obstructive symptoms, the number of upper airway infections for the preceding 12 months, and adenoid size (according to adenoid view X-ray) were recorded in a pre-designed checklist, in addition to the clinical findings of prolonged upper airway obstructive symptoms, including OSA (recurrent episodes of apnea and hypopnea, secondary to collapse of the upper airways during sleep) (9), snoring, mouth breathing, adenoid facies, dental problems, speech disorders, enuresis, and restlessness during sleep (if accompanied by snoring at night). During the prospective data collection, patients were assigned to five categories according to the number of upper airway infections during the preceding 12 months: less than 5 infections, 5 to 8 infections, 9 to 12 infections, more than 12 infections, and more than 15 infections. All patients underwent surgery due to obstructive symptoms and there was no active infection during the admission for surgery.
The clinical definition of chronic rhinosinusitis (CRS) was considered as subjective symptoms for 12 continuous weeks, in addition to objective confirmation of sinonasal mucosal (10).
Samples preparation
Adenoidectomy was performed by the first author at an academic tertiary care pediatric hospital using an adenotome, and the specimens obtained from two different parts of the nasopharyngeal surface of adenoid tissue were cut with a knife by an otolaryngologist. Adenoid tissue specimens were washed in a sterile isotonic saline solution to remove blood and secretions, fixed in 2.5% glutaraldehyde for 24 hours, and sent to the electron microscope unit weekly. During this time the samples were kept in a dry and cold place of at 20–25°C. The samples were then dried in a vacuum vessel (10-3 Torr) and sputtered with a 30–50–nm-thick layer of gold (voltage 800 V, 100 mA) using physical vapor depositioning (Gold Coater: Yarnikan Saleh).
SEM Imaging
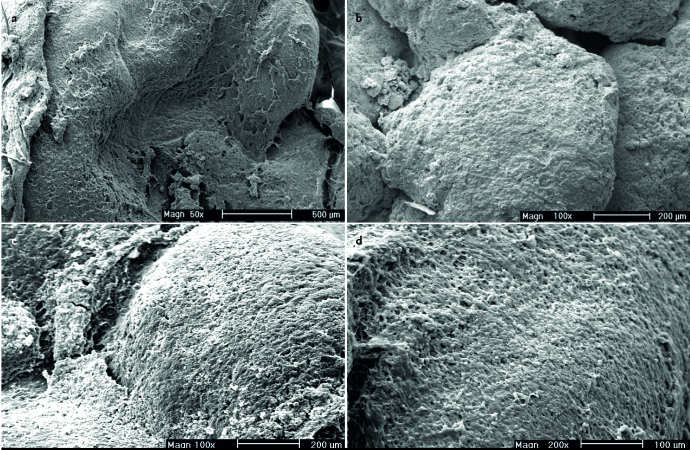
The images were examined under a Philips XL30 environmental scanning electron microscope (ESEM). We used the definition of Chole and Faddis for biofilm architecture as dense accumulations of bacteria within an amorphous matrix (11). To examine the areas of interest, the specimens were imaged with a voltage of 20–25 kVp and at a magnification range of ×15 to ×2500 (×15, ×50, ×100, ×200, ×500, ×1000 and ×2500). The surface layer of the adenoid tissue was examined for biofilm formation under an SEM in a single-blinded manner in the Electron Microscopy Laboratory (Kimiazi Analysis Research Group). The corresponding author was present at all imaging sessions and the facility staff performed imaging under his guidance. Biofilm extent grading, as the main outcome measure, was performed based on the adenoid surface area covered by the biofilm formation, in five categories of less than 20% (grade 1), 20–40% (grade 2), 40–60% (grade 3), 60–80% (grade 4), and more than 80% (grade 5). The results were then checked and confirmed using Clemex Vision digital image analysis software, Version 3.5 (Clemex Technologies Inc, Quebec, Canada).
Statistical analysis
Data were analyzed using Statistical Packages for the Social Sciences (SPSS) 18 (SPSS Inc, Chicago, IL, USA). Quantitative variables (including distances) are expressed as means and standard deviations (SD). Goodman and Kruskal’s gamma was used for correlation comparison between the adenoid size and biofilm extent with the upper airway infection groups. Fisher’s exact test was used to compare the relationship between sex and adenoid size and biofilm extent. P values less than 0.05 were considered significant.
Results
A total of 57 children with a mean age of 7.31 (±2.65) years (range, 28 months to 13 years) were included in the study, of whom 43 (75.4%) were male. The average number of episodes of upper airway infections during the last year before surgery was 10.01 (±5.38). Table 1 shows the frequencies of the associated symptoms. Snoring and mouth breathing were seen in all participants (100%). Adenoid facies (77.2%), dental problems (64.9%), OSA (63.2%), and speech disorders (54.4%) were the other most prevalent symptoms, respectively. The mean duration of symptoms was 2.5 years (30±21.4 months), ranging from 2 months to 7.5 years. Thirty-seven (64.9%) patients had used nasal corticosteroid sprays with a non-satisfactory response to treatment. None of the patients had a history of antibiotic therapy during the past two weeks prior to surgery. Based on the adenoid size, 33 (57.9%) patients were classified as grade 4, 22 (38.6%) as grade 3, and 2 (3.5%) as grade 2; no patients exhibited grade 1 (Table 2). Figure 1 shows the number of patients according to the number of upper airway infections. Biofilm formation was detected using SEM in all samples (100%) at different extents (Figure 2). As the main outcome of the study, there was a significant correlation between the grading of biofilm extent with upper airway infection prevalence (Goodman and Kruskal’s gamma, p<0.001). However, adenoid size had no correlation with the number of upper airway infections (Goodman and Kruskal’s gamma, p=0.109). There was no significant relationship between sex and the adenoid size (Fisher’s exact test, p=0.544) as well as the biofilm extent (Fisher’s exact test, p=0.737).
Table 1.
The frequencies of associated symptoms
| Associated symptoms | Number | Percent |
|---|---|---|
| Snoring | 57 | 100% |
| Oral breathing | 57 | 100% |
| Adenoid facies | 44 | 77.2% |
| Dental problems | 37 | 64.9% |
| Obstructive sleep apnea | 36 | 63.2% |
| Speech disorders | 31 | 54.4% |
| Restlessness during sleep | 26 | 45.6% |
| Enuresis | 13 | 22.8% |
| Sleepwalking | 10 | 17.5% |
Table 2.
Grading of adenoid size in terms of the percentage of nasopharyngeal obstruction
| Nasopharyngeal obstruction percentage | Grading | Number | Percent |
|---|---|---|---|
| 0–25% | I | 0 | 0% |
| 25–50% | II | 2 | 3.5% |
| 50–75% | III | 22 | 38.6% |
| 75–100% | IV | 33 | 57.9% |
Figure 1.
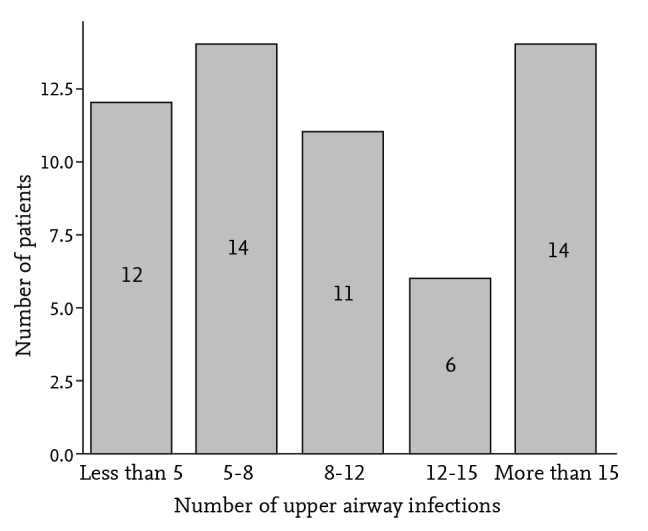
The number of patients according to the number of upper airway infections
Figure 2.
Organized communities of bacteria in a rich matrix of extracellular polymeric substances called bacterial biofilm in different magnifications
Discussion
In this cross-sectional study, we aimed to evaluate the correlation of biofilm extension in children undergoing adenoidectomy and the number of acute upper respiratory episodes in the prior 12-month period. We included a significant number of children undergoing adenoidectomy (n=57) and our results suggest that biofilm extension in the adenoid might contribute to the upper airway infection rate. In other words, an increase in the grading of biofilm formation resulted in an increase in the frequency of infections. This finding is somehow consistent with previous studies that introduced the presence of biofilm formations on the adenoid in the pediatric population as a cause of chronic upper respiratory infections (12, 13). Safaan et al. (1) studied biofilm formation in patients with adenoid hypertrophy, with and without COME, and introduced adenoid tissue as a cause of chronic infection and reported higher grades of biofilm formation in the group of patients with COME compared with those without COME.
Most studies have emphasized higher grading of biofilm formation in patients with chronic otolaryngologic infections such as COME, CRS, recurrent AOM, and chronic adenotonsilitis than in patients with pure upper respiratory tract obstruction, mostly OSA. To the best of our knowledge, our study is the first to show a correlation between the frequency of upper respiratory tract infections and biofilm grade. This may confirm adenoidectomy as an effective treatment in children with recurrent upper airway infections due to the presence of biofilm over the surface of adenoids as an infectious reservoir.
Our study showed no relationship between adenoid size and sex with the extent of biofilm. These findings are also consistent with the results of previous studies. For example, Saylam et al. (8) in 2010 reported no significant relationship between the adenoid size and age, sex, and duration of symptoms in patients with CRS comparison with patients with OSA. Naturally, in the analysis of adenoid size and other variables, sampling bias should be considered due to the inclusion criteria because the majority of children had hypertrophic adenoids.
In the current study, number of patients who had no nasopharyngeal obstruction was less than 25% (grade 1) and only two patients exhibited an obstruction between 25% and 50% (grade 2). The remaining 55 patients (96.5%) had nasopharyngeal obstruction of more than 50%, indicating that most of the patients became adenoidectomy candidates due to nasal obstruction, which is an acceptable finding because the main indication for adenoidectomy is upper airway obstruction and subsequent symptoms such as obstructive sleep apnea, snoring, and oral breathing, similar every participant in this project (100%). Saylam et al. (8) also divided patients according to airway obstruction caused by adenoid hypertrophy into three groups; less than 50%, 50% to 75%, and more than 75%; only two patients out of 34 were in the first group.
Bacteria have historically been thought to be isolated organisms; however, it is now clear that the vast majority of bacteria exist in complex communities, attached to surfaces known as biofilms (2). The original finding of biofilms in tonsils by Chole and Faddis (11) was confirmed in a study in which 17 out of 24 tonsils (70.8%) removed for chronic or recurrent tonsillitis contained biofilms (14). Biofilms have been demonstrated in 65.6% of surgical specimens consisting of tonsils, adenoids, and ethmoid or maxillary sinus mucosa removed from patients with refractory upper airway infections (6). There is strong anatomic evidence for the presence of bacterial biofilms in chronically diseased adenoids. Bacterial biofilms within adenoids may explain the chronicity and recurrent nature of some forms of tonsillitis because sessile bacteria within biofilms are resistant to host defenses and antibiotics (11). In a similar study, Galli et al. (13) tried to document the presence of biofilms in surgical tissue specimens from patients with recurrent upper airway infections and identify their possible role in the chronicity of these infectious processes. They examined 32 surgical specimens from the upper respiratory tract of 28 patients with upper airway infections that had persisted despite repeated treatment with anti-inflammatory agents and antibiotics with in vitro efficacy. Over 80% of the tissue specimens in this study were culture-positive and bacterial biofilms were observed in 65.6% of the tissue samples (5).
Galli et al. (13) also reported biofilm positivity on the adenoid tissue in all patients examined with a diagnosis of recurrent upper airway infection and resistant OME. Lin et al. (7) compared adenoid hyperplasia and biofilm formation in children with S. aureus adenoiditis in Taiwan. The patients were divided into methicillin-resistant and methicillin-sensitive S. aureus-infected groups. In that study, the severity of adenoid hyperplasia and extensive biofilm formation were more prominent in patients infected with methicillin-resistant S. aureus than methicillin-sensitive S. aureus (MSSA).
It seems that biofilm formation in the nasopharynx of children might serve as a chronic reservoir for bacterial pathogens resistant to standard antibiotics. In addition, the mechanical debridement of nasopharyngeal biofilms might explain the observed clinical benefit associated with adenoidectomy in this subset of pediatric patients. Adenoidectomy is known to be effective in children with CRS and COME in preventing recurrent infection, and recent work identifying biofilms in adenoids may help explain this clinical observation. For example, adenoids removed from children with chronic rhinosinusitis have, on average, 94.9% of the mucosal surface area covered with biofilms, compared with 1.9% of the surface area removed from children with OSA (12).
As shown in other studies, the best way to estimate the percentage of adenoid surface covered by biofilm structure is to use an experienced SEM operator in the electron microscopy (EM) unit and image analysis software programs such as Clemex or Carnoy, which were used in this study for the confirmation of biofilm extent. While in the EM unit one might see different parts of the sample under different magnifications thereby giving the best estimation of the total adenoid surface area covered by biofilm structure. These software programs are usually suitable and accurate tools to quantify the exact percentage of adenoidal surface covered by biofilm; however, a cropped image cannot necessarily be extended to the whole sample surface. In other words, these software programs are suitable tools for engineering or specific biologic samples such as peripheral blood smears (PBS), in which cropped capture image analysis can be extended to the whole sample rather than for biofilm extent estimation. One of the advantages of our study was the use of ESEM, which is an electron microscope designed especially for wet biologic samples, in comparison with SEM usually used in other studies, which is basically designed for engineering and material analysis. Therefore, the quality of the imaging in our study is significantly better than that in previous studies.
Limitations of the study
Some limitations of this study should be taken into consideration. The history taking of the patients in the study was very detailed. Lack of upper airway infections registry caused us to completely rely on the patients’ or their caregivers’ history. Although it was an infrastructural problem and was not directly related to the design of study, we tried to lessen this shortcoming by categorizing the number of infections into subgroups and spending more time for an accurate history taking. The small number of patients and the lack of a control group could also be considered as limitations.
In conclusion, it seems that the frequency of upper airway infections is probably related to the presence and extension of biofilm on the adenoid surface as a reservoir of microorganisms. This finding may suggest adenoidectomy as an effective treatment in children with recurrent upper airway infections. Further studies are needed to show the important role of biofilm formation in the pathophysiology of otolaryngologic diseases.
Acknowledgement
The authors wish to thank Dr Mohammad Mahdi Ahadian for his kind assistance in the establishment of this project. We are also grateful to Kimiazi research analysis group staff, especially Mr. Babak Akbari.
Footnotes
Informed Consent: Written informed consent was obtained from the parents of the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.B., S.S.; Design - G.B., S.S.; Supervision - G.B.; Data Collection and/or Processing - M.A.; Analysis and/or Interpretation - M.S.; Literature Review - M.S., S.S., F.K.; Writing - G.B., M.S., F.K.; Critical Review - G.B., M.S., S.S., F.K.; Other - G.B., S.S., M.S., F.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study was based on the M.D. thesis of Mahdi Safdarian under the supervision of Dr Gholamreza Bayazian and was approved and financially supported by the ENT and Head & Neck Research center and Department, Hazrat Rasoul Akram Hospital, Iran University of Medical Sciences (IUMS), Tehran, Iran.
References
- 1.Saafan ME, Ibrahim WS, Tomoum MO. Role of adenoid biofilm in chronic otitis media with effusion in children. Eur Arch Otorhinolaryngol. 2013;270:2417–25. doi: 10.1007/s00405-012-2259-1. [DOI] [PubMed] [Google Scholar]
- 2.Psaltis AJ, Ha KR, Beule AG, Tan LW, Wormald PJ. Confocal scanning laser microscopy evidence of biofilms in patients with chronic rhinosinusitis. Laryngoscope. 2007;117:1302–6. doi: 10.1097/MLG.0b013e31806009b0. [DOI] [PubMed] [Google Scholar]
- 3.Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother. 2004;48:2659–64. doi: 10.1128/AAC.48.7.2659-2664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–11. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuliani G, Carlisle M, Duberstein A, et al. Biofilm density in the pediatric nasopharynx: recurrent acute otitis media versus obstructive sleep apnea. Ann Otol Rhinol Laryngol. 2009;118:519–24. doi: 10.1177/000348940911800711. [DOI] [PubMed] [Google Scholar]
- 6.Galli J, Ardito F, Calo L, et al. Recurrent upper airway infections and bacterial biofilms. J Laryngol Otol. 2007;121:341–4. doi: 10.1017/S0022215106003896. [DOI] [PubMed] [Google Scholar]
- 7.Lin CD, Tsai MH, Lin CW, et al. Association of adenoid hyperplasia and bacterial biofilm formation in children with adenoiditis in Taiwan. Eur Arch Otorhinolaryngol. 2012;269:503–11. doi: 10.1007/s00405-011-1704-x. [DOI] [PubMed] [Google Scholar]
- 8.Saylam G, Tatar EC, Tatar I, Ozdek A, Korkmaz H. Association of adenoid surface biofilm formation and chronic otitis media with effusion. Arch Otolaryngol Head Neck Surg. 2010;136:550–5. doi: 10.1001/archoto.2010.70. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues AP, Pinto P, Nunes B, Bárbara C. Obstructive sleep apnea: epidemiology and portuguese patients profile. Rev Port Pneumol. 2017;23:57–61. doi: 10.1016/j.rppnen.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:3. 1–298. preceding table of contents. [PubMed] [Google Scholar]
- 11.Chole RA, Faddis BT. Anatomical evidence of microbial biofilms in tonsillar tissues: a possible mechanism to explain chronicity. Arch Otolaryngol Head Neck Surg. 2003;129:634–6. doi: 10.1001/archotol.129.6.634. [DOI] [PubMed] [Google Scholar]
- 12.Coticchia J, Zuliani G, Coleman C, et al. Biofilm surface area in the pediatric nasopharynx: Chronic rhinosinusitis vs obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2007;133:110–4. doi: 10.1001/archotol.133.2.110. [DOI] [PubMed] [Google Scholar]
- 13.Galli J, Calo L, Ardito F, et al. Bacterial biofilm identification in the rhinopharingeal mucosa of children with recurrent infection of the upper respiratory tract and otitis media. Pediatr Med Chir. 2008;30:31–4. [PubMed] [Google Scholar]
- 14.Kania RE, Lamers GE, Vonk MJ, et al. Demonstration of bacterial cells and glycocalyx in biofilms on human tonsils. Arch Otolaryngol Head Neck Surg. 2007;133:115–21. doi: 10.1001/archotol.133.2.115. [DOI] [PubMed] [Google Scholar]