Abstract
Aims
The pharmacokinetics (PK) of fluconazole and micafungin differ in neonates compared with children and adults. Dosing instructions in product labels appear to be inconsistent with the emerging scientific evidence. Limited information is available on the safety profile of these agents in neonates. Our objective was to study the population PK and safety of both drugs, randomly administered in neonates with suspected or confirmed systemic candidiasis.
Methods
Neonates were randomized 1:1 to fluconazole (loading dose 25 mg kg–1; maintenance dose 12 mg kg–1 day–1 or 20 mg kg–1 day–1, respectively, for infants <30 weeks or ≥30 weeks’ corrected gestational age) or micafungin (loading dose 15 mg kg–1 day–1; maintenance dose 10 mg kg–1 day–1). PK samples were taken on treatment days 1 and 5. Population parameters were determined using NONMEM and Monte Carlo simulations performed to reach predefined targets. Clinical and laboratory data, and adverse events were collected up to 36 weeks’ corrected gestational age or hospital discharge.
Results
Thirty‐six neonates were enrolled. The median (range) gestational age was 28.2 (24.1–40.1) and 26.8 (23.5–40.0) weeks for fluconazole and micafungin, respectively. Based on 163 PK samples, the median population clearance (l h–1 kg–1) and volume of distribution (l kg–1) for fluconazole were: 0.015 [95% confidence interval (CI) 0.008, 0.039] and 0.913, and for micafungin were: 0.020 (95% CI 0.010, 0.023) and 0.354 (95% CI 0.225, 0.482), respectively. The loading dose was well tolerated. No adverse events associated with micafungin or fluconazole were reported.
Conclusion
Based on Monte Carlo simulations, a loading dose for fluconazole and dosing higher than recommended for both drugs are required to increase the area under the plasma drug concentration–time curve target attainment rate in neonates.
Keywords: Candida spp, fluconazole, micafungin, neonate, pharmacokinetics, safety
What is Already Known about this Subject
Fluconazole and micafungin are used in suspected or proven invasive candidiasis in neonates.
Recommended dosing in the label for both drugs is lower than currently used, based on the current literature.
Evidence suggests that dosing instructions for patients with systemic candidiasis in the current label for fluconazole and micafungin are not adapted to neonates.
What this Study Adds
The maintenance of dosing schedules for fluconazole and micafungin that were higher than labelled (with a loading dose only for fluconazole), based on population pharmacokinetics, proved to be well tolerated, with no safety signals identified.
The dosage recommendations for both fluconazole and micafungin should be modified, to optimize the administration of both drugs in neonates.
Introduction
In neonates, fungal systemic infections are mainly caused by Candida spp. 1 and carry a high risk of spreading rapidly to the central nervous system 2, 3, 4. Effective and prompt treatment is essential as invasive candidiasis is often fatal (30–40% risk of death) and increases the risk of neurodevelopmental impairment (odds ratio 1.83), particularly in the youngest preterm neonates 2, 5, 6, 7.
Currently, fluconazole and micafungin are among the most frequently used antifungal agents for the treatment of neonatal invasive candidiasis 8, 9, 10, 11, 12. Fluconazole is a triazole that exerts antifungal activities by inhibiting the synthesis of ergosterol, with accumulation of toxic sterols in the cell membrane. Micafungin is an echinocandin which compromises fungal cell wall integrity, causing fungal cell death.
Despite their widespread use in neonatal clinical practice for the treatment of neonatal candidiasis, the optimal dosing regimens for both drugs are still questioned, resulting in huge differences between clinical practice, pharmacokinetic (PK) data and labelling information in neonatal dosing regimens. For fluconazole, the Summary of Product Characteristics (SPC) currently recommends a dose regimen of 6–12 mg kg–1 every 72 h and 48 h for the treatment of systemic candidiasis in term neonates aged 0–14 days and 15–27 days, respectively 13. Nonetheless, the most commonly used dosing regimen in clinical practice is 6 mg kg–1 or 12 mg kg–1 once daily 14. For micafungin, up to 2016, the SPC recommended a dose of 2–4 mg kg–1 once daily for the treatment of invasive candidiasis in all children (including neonates), whereas in practice the most commonly used dosing schemes ranged from 4 mg kg–1 to 10 mg kg–1 once daily 15. The recently updated SPC for micafungin now recommends a dose of 4–10 mg kg–1 once daily for the treatment of invasive candidiasis in children (including neonates) <4 months but underlines the need for further clinical studies in this age group 16. Indeed, limited micafungin PK and safety data are available in preterm neonates 17. Moreover, given the expected longer elimination half‐life of the two drugs in neonates and the emergency situation associated with critical sepsis, the loading dose strategy could be more appropriate in neonatal antifungal treatment. However, limited PK and safety data are currently available for this strategy in neonates 8, 18.
Given the high research priority of this issue, the Treat Infection in Neonates (TINN) Consortium, sponsored by the European Commission (EC) as part of the FP7 programme, conducted a randomized, open‐label phase II trial to evaluate the PK and safety of the administration of loading doses of fluconazole and micafungin, followed by high‐dose maintenance courses, in the treatment of neonatal candidiasis. The ultimate goal was to optimize antifungal treatment schemes in this vulnerable population.
Methods
Study design and participants
The TINN study was a prospective, randomized, open‐label, PK and safety study of fluconazole and micafungin in neonates with suspected or culture proven candidiasis. It was conducted to determine the PK and safety profile of the two drugs, administered as a loading dose followed by daily maintenance doses.
Five neonatal intensive care units (NICUs) in France and one in Spain participated after European Union (EU) regulatory validation was obtained in each country. The trial was conducted in accordance with the principles of Declaration of Helsinki, Good Clinical Practice Guidelines and European Clinical Trial Regulations 19, 20. The protocol and informed consent were approved by local ethics committees in France and Spain.
The eligibility criteria were: (i) neonates and infants between 24 and 42 weeks’ corrected gestational age (CGA), with a postnatal age of 48 h of life up to day of life 120 at the time of culture acquisition; (ii) requiring antifungal therapy according to a medical decision by the attending physician for microbiologically documented or clinically suspected Candida infection independently of the availability of any positive culture for Candida spp.; (iii) written informed consent from the parents or the legally authorized representative obtained prior to entry; and (iv) neonates had to have sufficient venous access to permit the administration of study medication and monitoring of safety variables.
Infants were excluded if: (i) they had been exposed to fluconazole or micafungin prophylaxis prior to inclusion; (ii) they had received more than 48 h of systemic antifungal therapy (any product) prior to the first dose of the study drug for treatment of the current Candida infection; (iii) they had a concomitant medical condition, and their participation in the study might create an unacceptable additional risk, in the opinion of the investigator and/or medical adviser; (iv) they had previously been enrolled in this study; (v) they were coinfected with a non‐Candida fungal organism; (vi) they had a history of a hypersensitivity or severe vasomotor reaction to any echinocandin or fluconazole product; or (vii) they had a pre‐existing hepatic or renal disease.
Randomization and masking
Neonates were randomized 1:1 via the e‐Case Report Form (CRF) to the trial drug, either fluconazole (Fluconazole®, Kabi Fresenius, Paris, France, 2 mg ml–1) or micafungin (Mycamine®, Astellas, Levallois‐Perret, France, 10 mg ml–1 or 20 mg ml–1). The e‐CRF was developed by ClinInfo (the clinical studies partner, Lyon, France), which was responsible for the random allocation of the treatments. Trial drugs were bought and distributed by Théradis Pharma (Cagnes‐sur‐mer, France). Neither participants' families nor trial personnel were masked to treatment assignment.
Procedures
Patients received a loading dose followed by a maintenance dose, administered intravenously once a day over 2 h by using a syringe pump. For fluconazole, the loading dose was 25 mg kg–1 and the maintenance dose was adjusted according to CGA: 12 mg kg–1 day–1 or 20 mg kg–1 day–1 (for infants <30 weeks’ or ≥30 weeks’ CGA, respectively). For micafungin, the loading dose was 15 mg kg–1 day–1 and the maintenance dose was10 mg kg–1 day–1, irrespective of patient age. To determine the dosage and dosing scheme for fluconazole, modelling and simulation of data from juvenile mice, adults and in vitro/in silico data, as well as the existing literature, were used 21, 22. The dose and frequency of administration for micafungin were based on previously published data 5, 23, 24, 25.
The duration of treatment was planned to be at least 5 days at trial doses. On day 6, the physician decided whether or not to continue antifungal treatment, according to clinical conditions and microbiological results. From day 6 of treatment, the dose of fluconazole and micafungin could be maintained as per protocol or lowered, and crossover from fluconazole to micafungin or vice versa was permitted. The maximum treatment duration was 42 days.
During study drug treatment, a limited PK sampling procedure was implemented: PK blood samples of 200 μl each were collected according to a predefined 2–3 time point schedule, depending on CGA (Table 1).
Table 1.
Pharmacokinetic sampling schedules on days 1 and 5 of treatment
| Age group by corrected gestational agea | Sampling schedule | Sampling time(s) (h) after the start of study drug infusion | Number of patients sampled | |
|---|---|---|---|---|
| Fluconazole (N = 18) | Micafungin (N = 18) | |||
| <32 weeks | A | 2 h and 12 h | 4 | 7 |
| B | 2 h and 18 h | 8 | 4 | |
| ≥32 weeks | C | 2 h, 4 h and 16 h | 3 | 5 |
| D | 2.5 h, 4 h and 24 h | 3 | 2 | |
In each age group, the physician could choose between two sampling schedules
These samples were drawn on the first and fifth days of treatment. Plasma samples were frozen at −20°C prior to analysis. Plasma fluconazole and micafungin concentrations were determined using a validated high‐performance liquid chromatography–tandem mass spectrometry (HPLC‐MSMS) assay developed in the department of Pediatric Pharmacology and Pharmacogenetics (Hopital Robert Debré, Paris, France). The HPLC‐MSMS apparatus was a UPLC I class XevoTQD (Waters, Vélizy‐Villacoublay, France). Mass transitions were M/Z 306.93 >237.99 for fluconazole electrospray positive ionization (ESI+) and M/Z 1268.6 > 46.92 electrospray negative ionzation (ESI–). The mobile was a gradient of phase A (formic acid 0.1%) and phase B (acetonitrile‐formic acid 0.1%), composed of A+B starting at 95‐5%, 5‐95% at 2 min and 95‐5% at 2.5 min respectively. The validation ranges were from 0.2 mg l–1 to 30 mg l–1 and the lower limit of quantification for micafungin and fluconazole in plasma was set as 0.2 mg l–1. Intraday (n = 10) and interday (n = 15) coefficients of variation were less than 2% and 6%, respectively, for fluconazole, and less than 8% and 7%, respectively, for micafungin.
Baseline assessments at randomization included: (i) demographic data: age, gender, birth weight and current weight; (ii) clinical data: medical history, medication history, history of insertion/removal of central catheters, nutrition, physical examination data and vital signs; and (iii) biological data: serum creatinine concentration, liver function parameters, C‐reactive protein concentration and haematology parameters. Microbiology samples were collected for all neonates upon arrival at the unit. At least one sample from the ear canal or gastric aspirate was collected, in neonates were admitted to hospital in the first two days of life. Additional cultures from venous and arterial catheters were requested if clinically indicated.
During the first 5 days of treatment, we collected: (i) PK data: time of start and completion of infusions, and precise PK sampling times; (ii) safety data: blood pressure and heart rate before, during and after study drug infusion; other clinical and biological parameters were monitored using routine clinical practice.
From the sixth day of treatment onwards (including in the case of drug discontinuation), safety monitoring was continued until discharge, transfer to another hospital, clinical trial closure (31 October 2015) or death, using local routine practices.
Outcomes
Our primary outcome was the number of patients achieving an area under the concentration–time curve from 0 to 24 h (AUC0–24) at steady state of ≥400 mg*h l–1 for fluconazole and ≥166.5 mg*h l–1 for micafungin 18, 23. Indeed, the AUC divided by the minimum inhibitory concentration (AUC/MIC ratio) is used as an efficacy criterion for both fluconazole and micafungin 18.
Secondary outcomes were the occurrence of adverse events (AEs) and abnormal laboratory findings during and after drug exposure. Descriptive safety analyses included all patients who received at least one dose of study drug. Investigators recorded AEs, including seriousness and causality 26. Medical history and AE data were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 19.0 27. Comedication was coded using the Anatomical Therapeutic Chemical (ATC) Classification 28.
General safety data analyses included AEs, reasons for discontinuation, deaths, laboratory data, vital signs and short‐term outcome. For vital signs and laboratory data, age‐corrected upper and lower limits of reference values were determined using the relevant literature 29.
Based on the undesirable effects described in the SPC for fluconazole and micafungin, additional data review included topics of interest such as hepatic disorders and serious AEs. The present review combined, where appropriate, AE, laboratory and vital sign data.
Safety data for patients crossing over between treatment groups were included in the respective treatment cohort. For example, patients originally randomized to micafungin having an AE after crossing over to fluconazole were counted in the fluconazole treatment cohort for the treatment duration for fluconazole. Thus, a patient crossing over could contribute to safety data in both treatment arms.
Patients could have more than one AE, abnormal laboratory result or vital sign. AE data were included regardless of reported causality, seriousness or outcome (i.e. including data from patients who died). Repeat AEs for the same patient were counted, if the prior AE episode had ended at least 24 h before the start of the repeat AE episode.
Statistical analysis
PK analysis was performed using the nonlinear mixed‐effects modelling software NONMEM v7.2 (Icon Development Solutions, San Antonio, TX, USA). First, population PK modelling was used to determine micafungin and fluconazole PK parameters in the study population (details about model building and validation are presented in Supporting information Data S1). The AUC0–24 at steady state was then calculated for each patient. Finally, a 500‐patient Monte Carlo simulation was performed for both drugs in order to compare the AUC0–24 obtained on the first and second days of treatment with the loading dose strategy vs. with the maintenance dose alone. These simulations used the parameter values of the validated population PK models.
Role of the funding source
The EU funding was used to organize and conduct the trial and support clinical and biological data collection and analysis. The sponsor had no role in the study design, the collection, analysis and interpretation of data, the writing of the report, or in the decision to submit the manuscript for publication.
Results
A total 36 patients were included in the present trial, 18 in each treatment arm (Figure 1). At baseline, the population characteristics were similar in both treatment arms (Table 2).
Figure 1.
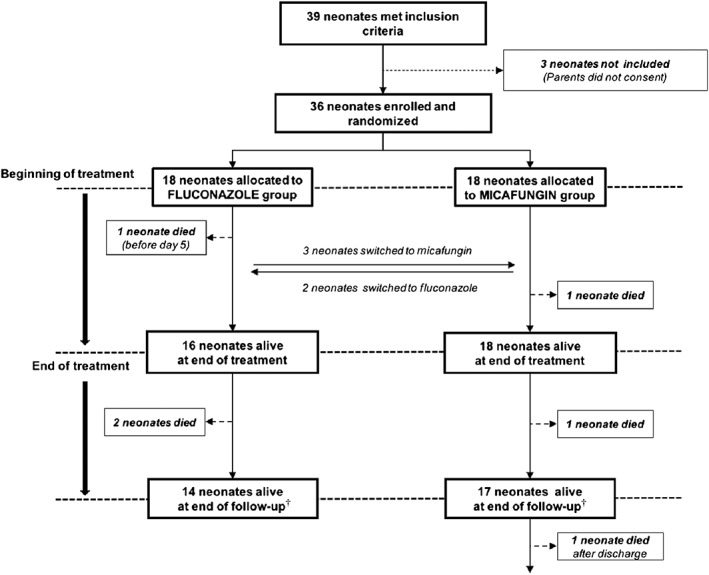
Trial flow chart. †The follow‐up ended at death, discharge, transfer to another hospital, clinical trial closure or 31 October 2015
Table 2.
Patients' baseline characteristics
| Fluconazole group (n = 18) | Micafungin group (n = 18) | |
|---|---|---|
| Maternal fungal disorder at delivery (n) | 3 (17%) | 7 (39%) |
| Demographic data | ||
| Gender (M/F) | 11/7 | 11/7 |
| Gestational age (weeks + days) | 28 + 2 (24 + 1–40 + 1) | 26 + 6 (23 + 4–40 + 0) |
| Postnatal age (days) | 13.5 (2.0–101.0) | 12.5 (3.0–115.0) |
| Postmenstrual age (weeks) | 30.4 (25.6–49.1) | 29.9 (25.4–41.9) |
| Birth weight (g) | 995.0 (640.0–3960.0) | 885.0 (500.0–3630.0) |
| Current weight (g) | 1255.0 (750.0–4255.0) | 1090.0 (640.0–4615.0) |
| Patients with Candida isolates (n) | 10 (56%) | 7 (39%) |
| Positive peripheral cultures (n) | 19 | 9 |
| Positive blood/CSF cultures (n) | 1 | 0 |
| Biological data | ||
| Serum creatinine (μmol l–1) | 50.5 (12.0–138.0) | 43.0 (14.0–102.0) |
| Aspartate aminotransferase (IU l–1) | 21.0 (6.0–33.0) | 39.0 (21.0–1080.0) |
| Alanine aminotransferase (IU l–1) | 15.0 (6.0–43.0) | 13.0 (6.0–221.0) |
| Albumin (g l–1) | 30.0 (21.0–49.0) | 27.0 (14.0–36.0) |
| C‐reactive protein (mg l–1) | 15.0 (3.0–135.0) | 13.5 (3.0–225.0) |
| Haematocrit (%) | 42.8 (31.7–57.0) | 40.2 (25.2–50.8) |
| Clinical data | ||
| Invasive ventilation | 8 (44.4%) | 10 (55.6%) |
| Comedication | ||
| Aminoglycosides | 5 (27.8%) | 8 (44.4%) |
| Vancomycin | 9 (50.0%) | 12 (66.7%) |
| Inotropic agents | 6 (33.3%) | 7 (38.9%) |
| Diuretics | 2 (11.1%) | 4 (22.2%) |
| Caffeine | 9 (50.0%) | 8 (44.4%) |
Data represent median (range) or n (%). CSF, cerebrospinal fluid
At the time of giving birth, three (17%) and seven (39%) mothers in the fluconazole and micafungin arms, respectively, had a fungal infection and ten (55%) and seven (49%) of the neonates had a culture positive for Candida spp up to 7 days prior to randomization. Only one systemic culture was positive for Candida spp (fluconazole: one catheter). Most neonates were born at less than 28 weeks’ gestation and weighed less than 1000 g at birth. Over 80% of neonates received at least one concurrent antibacterial drug [(17 (85%) and 19 (90%) in the fluconazole and micafungin groups respectively)].
The median treatment duration was 6 days in fluconazole‐ and 4 days in micafungin‐treated patients. When antifungal treatment was maintained for more than 5 days, the drug dose was reduced by the treating physicians, in both treatment arms.
A total of 82 and 81 concentrations were available for fluconazole and micafungin PK modelling, respectively. The concentration–time plots are presented in Figure 2.
Figure 2.
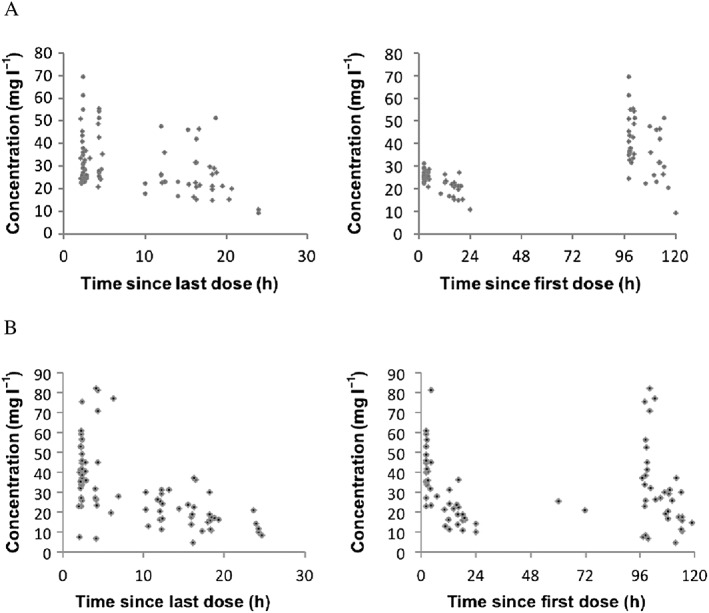
Concentrations vs. time profiles of fluconazole (A; 18 neonates, 82 concentrations) and micafungin (B; 18 neonates, 81 concentrations)
Two one‐compartment population PK models were developed and validated (see Figure S1). These models included the impact of current weight on fluconazole and micafungin clearance and the impact of CGA on micafungin clearance. The two models provided a good fit for the PK data. Table 3 presents estimates of the PK parameters for both drugs in the study population. Fluconazole and micafungin clearances were highly variable in the study population, with respective ranges of 0.008–0.042 l h–1 kg–1 and 0.010–0.024 l h–1 kg–1.
Table 3.
Fluconazole and micafungin treatment information and population pharmacokinetic results
| Fluconazole group (n = 18) | Micafungin group (n = 18) | |
|---|---|---|
| Treatment information a | ||
| Loading dose (mg kg–1 dose–1) | 25.0 (21.4–27.3) | 15.1 (12.1–18.8) |
| Maintenance dose up to day 5 (mg kg–1 day–1) | 18.6 (9.3–21.5) | 9.9 (8.1–11.4) |
| Maintenance dose from day 6 (mg kg–1 day–1) | 10.52 (4.78–20.43) | 4.76 (3.48 to 10.4) |
| Duration of treatment (days) | 6 (1–20) | 4 (1–35) |
| Pharmacokinetic parameters b | ||
| Clearance (l h–1 kg–1) | 0.015 (0.008–0.039) | 0.020 (0.010–0.023) |
| Volume of distribution (l kg–1) | 0.913 (0.913–0.913) | 0.354 (0.225–0.482) |
| t½ (h) | 40.9 (16.2–78.4) | 13.6 (9.9–21.7) |
| AUC0–24 (mg*h l–1) at day 1 | 490.9 (406.2–571.9) | 478.9 (400.6–734.4) |
| AUC0–24 (mg*h l–1) at steady state | 898.2 (503.4–1445.7) | 493.8 (437.5–1023.9) |
AUC0–24, area under the concentration–time curve from 0 to 24 h; t½, half‐life
Data represent median (range)
Data represent median (95% confidence interval)
At steady state, all patients reached the target systemic exposure of AUC0–24 ≥400 mg*h l–1 and ≥166.5 mg*h l–1 for fluconazole and micafungin, respectively. However, six patients (33%) did not achieve the 800 mg*h l–1 AUC0–24 target in fluconazole group.
Monte Carlo simulations showed that the fluconazole target attainment rate at 24 h of treatment increases from 30% to 96% with the use of a 25 mg kg–1 loading dose. When using the same maintenance dose without the 25 mg kg–1 loading dose, the target attainment is delayed to 48 h of treatment. In this case, 30% and 93% of the patients would achieve the therapeutic target on the first and second day of treatment, respectively. For micafungin, 100% of simulated patients reached the AUC0–24 target of 166.5 mg*h l–1 with and without the 15 mg kg–1 loading dose (Figure 3).
Figure 3.
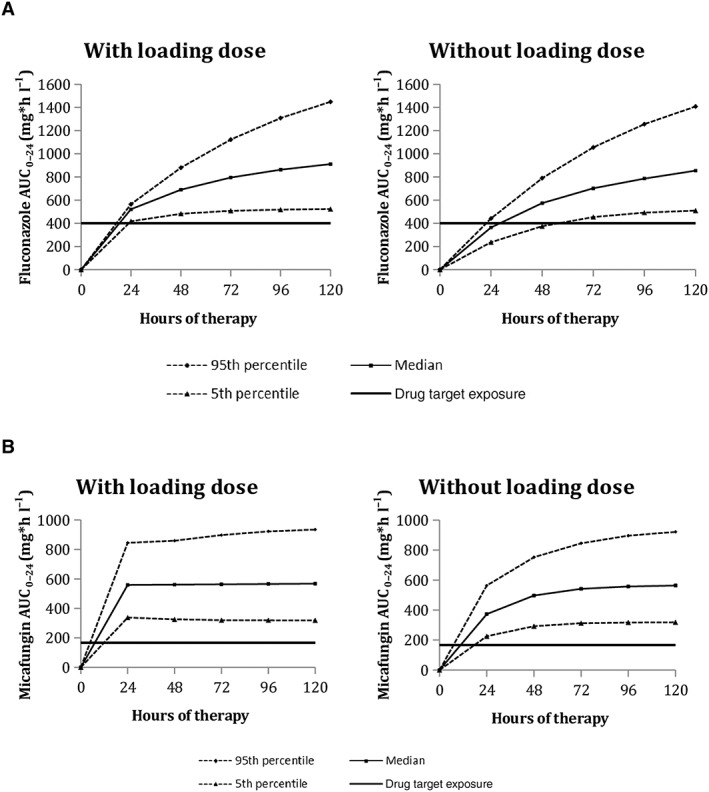
Target attainment rates for fluconazole and micafungin at days 1 and 2, administered with and without loading dose (100 simulated trials)
Safety data analyses included 20 and 21 patients for fluconazole and micafungin, respectively (five patients switched from one drug to the other and were part of both arms); no difference, in terms of safety, was detected between the two arms.
Over 60% of patients experienced at least one AE during treatment in both groups [fluconazole 13/20 (65%) and micafungin 14/21 (67%)], but few were reported as serious [fluconazole 6/20 (30%), micafungin 4/21 (19%)], none led to treatment discontinuation and none was reported as related to the antifungal drug administered.
During treatment, the most frequent AEs in both treatment arms were anaemia [fluconazole 6/20 (30%) and micafungin 8/21 (38%)], followed by staphylococcal sepsis [fluconazole 5/20 (25%) and micafungin 2/21 (10%)] and thrombocytopenia [fluconazole 3/20 (15%) and micafungin 3/21 (14%)]. No trend for a change in heart rate or blood pressure was observed during infusion in the first 5 days of treatment.
Data review by safety topic of interest did not identify any new safety signals. AEs reported during treatment were considered to be due to sepsis and/or population‐specific comorbidities.
In total, six neonates born prematurely between 23 weeks' and 32 weeks' gestation died but none of the deaths was attributed to the study drugs. Two of these (one per treatment arm) died of bacterial sepsis during study drug treatment. The remaining four neonates (two per treatment arm) died after discontinuation of the study drug: two died of bacterial sepsis, one due to Shone syndrome and one due to a homozygous ABCA3 mutation.
Overall, 15 patients in each treatment arm were alive at the type of censoring (discharge, transfert to another hospital, death ortrial closure). Weight gain since birth were similar in both treatment arms [fluconazole 18.19 (0.79–31.54) g day–1; micafungin 20.55 (4.19–26.54) g day–1]. Discharge diagnoses were characteristic of the study population and were similar overall, regardless of randomized treatment. The most frequent medical conditions reported in the discharge summary were bacterial infectious disorders [fluconazole 12/15 (80%) and micafungin 9/15 (60%)].
Discussion
We performed a randomized, controlled trial with the aim of determining the PK of fluconazole and micafungin when used at doses (both loading and maintenance) that were higher than those currently used in the treatment of suspected or proven neonatal candidiasis. Our results showed that, under the tested dosage schedule and in order that ≥90% of patients reached the target AUC at 24 h and 48 h after initiation of treatment, a loading dose is required for fluconazole but not for micafungin, and that an increased maintenance dose is needed for both drugs when continuing the therapeutic courses. Of note, these higher doses were well tolerated and there were no unexpected AEs for either drug. In addition, our data confirmed that weight (for both antifungal agents) and postmenstrual age (only for fluconazole) influence PK in preterm neonates.
Both fluconazole and micafungin have a marketing authorization in Europe for paediatric patients. However, SPC dose recommendations for administration in neonates affected with suspected or proven candidiasis have been questioned and higher doses of both drugs are currently administered. The results of a recent survey from our group showed that, currently, the most frequently used maintenance doses in European NICUs range from 6 mg kg–1 and 12 mg kg–1 for fluconazole, and is 10 mg kg–1 for micafungin 30. These findings clearly confirmed the need to harmonize clinical practice based on pharmacological data for these two drugs, an issue that we tried to address with our work.
In order to determine the dosage schedule for antimicrobials, the PK/pharmacodynamic relationship is fitted optimally by the AUC0–24/minimal inhibitory concentration (MIC) ratio. In adult settings, a single loading dose of 800 mg fluconazole on the first day of treatment is recommended for patients with candidemia by the Infectious Disease Society of America as a way of reducing the time needed to reach the target AUC 31, 32. No loading dose is required for micafungin. In adults, the daily maintenance dose for fluconazole is 400 mg, and for micafungin is 100 mg. In neonatal settings, limited PK data are available in this area, particularly for premature neonates, although scattered experiences with loading doses of antifungal agents have been reported 33.
For fluconazole, a minimum AUC of 400 mg*h l–1 is required to ensure that the PK/pharmacodynamic index of AUC/MIC stays >50 for Candida spp, with an MIC breakpoint ≤8 μg ml–1. When fluconazole is administered without a loading dose, it takes several days for therapeutic levels to be reached 21, 33, 34, 35, 36.
This issue can be critical in preterm neonates, a category of patients for whom high and rapidly effective amounts of antifungal drugs are urgently required owing to a very high severity of systemic candidiasis. In line with this need, a 25 mg kg–1 loading dose strategy was evaluated preliminarily in a small group (n = 8) near‐term infants, five of whom (63%) achieved a therapeutic target (AUC >400 mg*h–1l) on the first day of dosing 21, 33, 34. The data from the present study confirmed the above‐mentioned findings, in showing that a fluconazole loading dose of 25 mg kg−1 followed by a maintenance dose of 12 mg kg−1 day−1 and 20 mg kg−1 day−1 for neonates <30 weeks' CGA and >30 weeks' CGA, respectively, reduced the time needed to reach the target AUC/MIC, thus providing an important therapeutic benefit in such vulnerable patients.
For micafungin, it is known that an exposure ensuring AUC0–24 values exceeding 166.5 mg*h l–1 is desirable 23, 24, 25, 37. It has also been reported that, in neonates, a 15 mg kg–1 daily dose yields an average AUC0–24 value of 437.5 mg*h l−1, with the highest observed AUC0–24 level being 555.6 mg*h l–1 and, thus, well within the appropriate range 25. Our results showed that the loading dose yields no additional benefit as the target AUC of 166.5 mg*h l–1 will already have been attained at 24 h if a daily dose of 10 mg kg–1 is administered.
Overall, both drugs were well tolerated. Assessment of the tolerability of the loading dose considered liquid volume and sodium input; there was no impact on cardiac rhythm, blood pressure or natraemia. This aspect had to be considered carefully as fluid intake is a matter for extensive debate between restricted and liberal approaches 38, 39. According to available data, fluconazole administered as prophylaxis or for treatment of candidiasis is considered to be safe in paediatric patients, including neonates 40, 41, although hepatotoxicity is a recognized adverse drug reaction for both fluconazole and micafungin 17, 42. In summary, safety profile in our study were consistent with the recognized comorbidities of the patient population.
We acknowledge a number of limitations in our study. First, it was not designed or powered to demonstrate efficacy of the investigated drug regimens. Clinical trials in term and preterm neonates are difficult to conduct for well‐identified practical and ethical issues, including small sample sizes and the rarity of the studied conditions. Specially, the increased use of prophylactic fluconazole in NICUs over the past few years, together with a refinement of the management strategies of newborn infants, have led to a decreased incidence of systemic candida infection in preterm neonates. Therefore, it was challenging to recruit and enrol patients for the present study, similarly to what concomitantly happened in an international multicentre, randomized control trial of micafungin vs. amphotericin B treatment in preterm neonates, that was discontinued due to insufficient recruitment (Clinicaltrial.gov : NCT00815516). In addition, as an FP7 project, there were some difficulties in initiating and conducting the trial, primarily relating to regulation and sponsorship. Secondly, we assessed the PK of these two antifungal drugs in infants who were given this treatment for both suspected and microbiologically confirmed candida sepsis. We could not exclude the possibility that the relationship between the drug and the host might be different in neonates with confirmed infection compared with those who have been allocated to treatment on an empirical basis, without conformation of the infection.
Conclusions
To our knowledge, this was the first study in neonates that compared the PK and safety of two antifungal drugs. As discussed, the small number of patients included may have limited the generalizability of our data, although randomization and central analysis of drug concentrations were strengths of the study. We demonstrated that the dose regimen, with a loading dose of fluconazole and daily doses higher than those recommended for both fluconazole and micafungin, enabled the target AUC to be reached earlier.
The micafungin and fluconazole dosing schemes adopted in the present study led to safe achievement of therapeutic targets in most neonates. No safety signals of concern were detected.
Competing Interests
This work was part of the TINN (Treat Infections in NeoNates) network, supported by the European Commission under the Health Cooperation Work Programme of the 7th Framework Programme (Grant agreement No. 223 614). The authors have no conflict of interest to report related to the present study. B.A. was an employee of Novartis between 2009 and 2015, and GlaxoSmithKline between 2006 and 2009. She currently holds pension funds at GlaxoSmithKline.
Contributors
Contributing members of the FP7 TINN project: Danny Benjamin (USA), Maurizio Bonati (Italy), Imti Choonara (UK), John van Den Anker (Nederlands), Pierre Henri Jarreau (France), Rene Kornelisse (Nederland's), Jean‐Paul Langhendries (Belgium), Chantal Le Guellec (France), Paolo Manzoni (Italy), Anders Rane (Sweden). NICUs neonatal investigators involved in the fluconazole–micafungin trial: In France: S. Goudjil, G. Kongolo (Amiens); O. Baud, P. H. Jarreau (Paris); B. Dusang (La Réunion); S. Decoopman (Lille); O. Claris (Lyon); In Belgium: C. Debauche (Brussels); M. Kalenga (Liège); E. Henrion (Namur); J. P. Langhendries, P. Maton (Rocourt); In the Netherlands: R. Kornelisse (Rotterdam); In Spain: R. Garcia Sanchez (Salamanca), M. G. Lopez (Malaga). The eCRF was housed and managed by M. Drogue, P. Foerster and P. Chevarier (ClinInfo, Lyon, France).
E.J.‐A. was the principal investigator of the present trial. She submitted the FP7 project and ran the Work Package on fluconazole. She was responsible for the conception of the trial, and drug measurement, and contributed to pharmacokinetic analysis and the writing of the scientific report. S.L. contributed to the trial organization and pharmacokinetic analysis, and drafted the first version of the manuscript. V.E. and F.L. managed the trial, including writing the protocol, standard operating procedures and regulatory submissions, and managing the data. W.Z. and C.B.‐Le‐G. conceived the pharmacokinetic plan and contributed to the pharmacokinetic study design and analysis. B.A. carried out the drug safety data and outcome analysis. V.B., B.D. S.G., S.D. and R.G.S. recruited patients and conducted the trial in their respective centres. P.M. was responsible of the Work Package of the trial in the FP7 project, participated in the conception of the trial and reviewed the manuscript. All authors contributed to writing the manuscript, approved the final version as submitted and agree to be accountable for all aspects of the work.
Supporting information
Data S1 Pharmacokinetic modelling: method, final population pharmacokinetic models and model validation
Figure S1 Model validation: goodness‐of‐fit plots for fluconazole and micafungine
Leroux, S. , Jacqz‐Aigrain, E. , Elie, V. , Legrand, F. , Barin‐Le Guellec, C. , Aurich, B. , Biran, V. , Dusang, B. , Goudjil, S. , Coopman, S. , Garcia Sanchez, R. , Zhao, W. , Manzoni, P. , and on behalf of the FP7 TINN (Treat Infections in NeoNates) consortium (2018) Pharmacokinetics and safety of fluconazole and micafungin in neonates with systemic candidiasis: a randomized, open‐label clinical trial. Br J Clin Pharmacol, 84: 1989–1999. 10.1111/bcp.13628.
Clinical Trial Registration: EudraCT registration number: 2012‐001916‐41; http://clinicaltrials.gov registration number: NCT02145832; Community and Research Information Service of the European Commission (CORDIS): https://www.cordis.europa.eu/project/rcn/89361_en.html
References
- 1. Oeser C, Vergnano S, Naidoo R, Anthony M, Chang J, Chow P, et al Neonatal invasive fungal infection in England 2004–2010. Clin Microbiol Infect 2014; 20: 936–941. [DOI] [PubMed] [Google Scholar]
- 2. Adams‐Chapman I, Bann CM, Das A, Goldberg RN, Stoll BJ, Walsh MC, et al Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J Pediatr 2013; 163: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin DK, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end‐organ damage: a critical appraisal of the literature using meta‐analytic techniques. Pediatrics 2003; 112: 634–640. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin DK, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006; 117: 84–92. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin DK, Stoll BJ, Gantz MG, Walsh MC, Sanchez PJ, Das A, et al Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics 2010; 126: e865–e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Invasive fungal infection in very low birthweight infants: national prospective surveillance study. Arch Dis Child Fetal Neonatal Ed 2006; 91: F188–F192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puig‐Asensio M, Padilla B, Garnacho‐Montero J, Zaragoza O, Aguado JM, Zaragoza R, et al on behalf of the CANDIPOP Project and GEIH‐GEMICOMED (SEIMC) and REIPI. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population‐based surveillance in Spain. Clin Microbiol Infect 2014; 20: 245–254. [DOI] [PubMed] [Google Scholar]
- 8. Botero‐Calderon L, Benjamin DK, Cohen‐Wolkowiez M. Advances in the treatment of invasive neonatal candidiasis. Expert Opin Pharmacother 2015; 16: 1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, et al ESCMID Fungal Infection Study Group. ESCMID guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 2012; 18 (Suppl. 7)): 38–52. [DOI] [PubMed] [Google Scholar]
- 10. Almirante B, Rodríguez D. Antifungal agents in neonates: issues and recommendations. Paediatr Drugs 2007; 9: 311–321. [DOI] [PubMed] [Google Scholar]
- 11. Manzoni P, Mostert M, Jacqz‐Aigrain E, Farina D. The use of fluconazole in neonatal intensive care units. Arch Dis Child 2009; 94: 983–987. [DOI] [PubMed] [Google Scholar]
- 12. Diflucan . Summary of Product Characteristics. European Medicines Agency [online]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Diflucan_30/WC500121908.pdf (last accessed 15 September 2017).
- 13. Pandolfini C, Kaguelidou F, Sequi M, Jacqz‐Aigrain E, Choonara I, Turner MA, et al Wide intra‐ and inter‐country variability in drug use and dosage in very‐low‐birth‐weight newborns with severe infections. Eur J Clin Pharmacol 2013; 69: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manzoni P, Mostert M, Castagnola E. Update on the management of Candida infections in preterm neonates. Arch Dis Child Fetal Neonatal Ed 2015; 100: F454–F459. [DOI] [PubMed] [Google Scholar]
- 15. Mycafungin . Summary of Product Characteristics. European Medicines Agency [online]. Available at https://www.medicines.org.uk/emc/product/6315/smpc (last accessed 9 June 2018).
- 16. European Medicines Agency . Mycamine: EPAR‐Summary of product characteristics [online]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-Public_assessment_report/human/000734/WC500031079.pdf (last accessed 9 June 2018).
- 17. Manzoni P, Wu C, Tweddle L, Roilides E. Micafungin in premature and non‐premature infants: a systematic review of 9 clinical trials. Pediatr Infect Dis J 2014; 33: e291–e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Autmizguine J, Guptill JT, Cohen‐Wolkowiez M, Cohen‐Wolkowiez M, Benjamin DK, Capparelli EV. Pharmacokinetics and pharmacodynamics of antifungals in children: clinical implications. Drugs 2014; 74: 891–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. International Conference on Harmonization of technical requirements for registration of pharmaceutical for human use. Current Step 4 ‐ Version dated 10 June 1996. Available at https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (last accessed 10 June 2018).
- 20. International Conference of Harmonisation for Better Health . E11 Clinical Trials in Pediatric Population [online]. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/ethical_considerations_en.pdf (last accessed 9 June 2018).
- 21. Wade KC, Wu D, Kaufman DA, Benjamin DK Jr, Sullivan JE, Ramey N, et al National Institute of Child Health and Development Pediatric Pharmacology Research Unit Network. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother 2008; 52: 4043–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao W, Le Guellec C, Benjamin DK Jr, Hope WW, Bourgeois T, Watt KM, et al First dose in neonates: are juvenile mice, adults and in vitro‐in silico data predictive of neonatal pharmacokinetics of fluconazole? Clin Pharmacokinet 2014; 53: 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hope WW, Mickiene D, Petraitis V, Petraitiene R, Kelaher AM, Hughes JE, et al The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis 2008; 197: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ascher S, Smith PB, Benjamin DK. Safety of micafungin in infants: insights into optimal dosing. Expert Opin Drug Saf 2011; 10: 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hope WW, Smith PB, Arrieta A, Buell DN, Roy M, Kaibara A, et al Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob Agents Chemother 2010; 54: 2633–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use . Harmonised tripartite guideline. Clinical safety data management: Definitions and standards for expedited reporting E2A [online]. 1994. Available at https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf (last accessed 15 September 2017)
- 27. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . MedDRA Term Selection: Points to consider. ICH‐Endorsed Guide for MedDRA Users. Release 4.11, Based on MedDRA Version 19.0 [online]. Available at https://www.meddra.org/sites/default/files/guidance/file/9491-1900_termselptc_r4_11_mar2016_0.pdf (last accessed 5 September 2017)
- 28. World Health Organization . Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment [online]. Available at: https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines (last accessed 10 September 2017)
- 29. Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics 2009; 123: e333–e337. [DOI] [PubMed] [Google Scholar]
- 30. Kaguelidou F, Pandolfini C, Manzoni P, Choonara I, Bonati M, Jacqz‐Aigrain E. European survey on the use of prophylactic fluconazole in neonatal intensive care units. Eur J Pediatr 2012; 171: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, et al Practice guidelines for the treatment of candidiasis. Clin Infect Dis 2000; 30: 662–678. [DOI] [PubMed] [Google Scholar]
- 32. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky‐Zeichner L, et al Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 15: e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Piper L, Smith PB, Hornik CP, Cheifetz IM, Barrett JS, Moorthy G, et al Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J 2011; 30: 375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wade KC, Benjamin DK, Kaufman DA, Ward RM, Smith PB, Jayaraman B, et al Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr Infect Dis J 2009; 28: 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clancy CJ, Yu VL, Morris AJ, Snydman DR, Nguyen MH. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob Agents Chemother 2005; 49: 3171–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clancy CJ, Staley B, Nguyen MH. In vitro susceptibility of breakthrough Candida bloodstream isolates correlates with daily and cumulative doses of fluconazole. Antimicrob Agents Chemother 2006; 50: 3496–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benjamin DK Jr, Smith PB, Arrieta A, Castro L, Sánchez PJ, Kaufman D, et al Safety and pharmacokinetics of repeat‐dose micafungin in young infants. Clin Pharmacol Ther 2010; 87: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2001; 3: CD000503. [DOI] [PubMed] [Google Scholar]
- 39. Oh W, Poindexter BB, Perritt R, Lemons JA, Bauer CR, Ehrenkranz RA, et al Neonatal Research Network. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr 2005; 147: 786–790. [DOI] [PubMed] [Google Scholar]
- 40. Egunsola O, Adefurin A, Fakis A, Jacqz‐Aigrain E, Choonara I, Sammons H. Safety of fluconazole in paediatrics: a systematic review. Eur J Clin Pharmacol 2013; 69: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee J, Kim HS, Shin SH, Choi CW, Kim EK, Choi EH, et al Efficacy and safety of fluconazole prophylaxis in extremely low birth weight infants: multicenter pre‐post cohort study. BMC Pediatr 2016; 16: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Grossman LB. Twice weekly fluconazole prophylaxis for prevention of invasive Candida infection in high‐risk infants of <1000 grams birth weight. J Pediatr 2005; 147: 172–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Pharmacokinetic modelling: method, final population pharmacokinetic models and model validation
Figure S1 Model validation: goodness‐of‐fit plots for fluconazole and micafungine


