Abstract
Aims
To examine the association between statin use before and after intracranial haemorrhage (ICH) and the risk of poststroke epilepsy (PSE).
Methods
Patients with new‐onset ICH between 2004 and 2012 were identified from the Taiwan National Health Insurance Research Database. The main outcome was the occurrence of epilepsy after stroke. Multivariable Cox regression modelling was used to estimate the association between statin use and the risk of PSE, with poststroke medication exposures being treated as time‐dependent variables.
Results
A total of 7435 patients with ICH were enrolled with a median follow‐up of 17.6 months. Within the study cohort, 709 patients developed PSE. Poststroke, but not prestroke, stain use was associated with a reduced risk of PSE (adjusted hazard ratio 0.62, 95% confidence interval 0.42–0.90, P = 0.01). In subanalyses, a trend of a dose–response relationship was observed. A significant PSE risk reduction was correlated with a higher cumulative statin dose. Moreover, the risk of PSE was lower in patients receiving moderate‐to‐high‐intensity statin therapy (adjusted hazard ratio 0.37, 95% confidence interval 0.18–0.75, P = 0.01). Lipophilic and hydrophilic statins were similar with regard to their associations with the reduced risk of PSE.
Conclusions
Statin therapy may reduce the risk of PSE after ICH, especially with moderate‐to‐high therapy intensity. Further research is needed to understand the mechanisms underlying the potential protective effects of statins against PSE in this patient population.
Keywords: epilepsy, pharmacoepidemiology, pharmacotherapy, statins, stroke
What is Already Known about this Subject
Poststroke epilepsy (PSE) is a common complication in patients with intracranial haemorrhage (ICH), and prophylactic use of antiepileptics is not recommended in clinical guidelines.
Statins have been found to possess neuroprotective effects and may reduce the risk of epilepsy in the elderly as well as seizure after ischaemic stroke. However, evidence of statins for epilepsy prevention in ICH is scarce.
What this Study Adds
Statin use after ICH was associated with a reduced risk of PSE.
The risk of PSE was lower in patients with moderate‐to‐high‐intensity statin therapy.
This study provides evidence on the potential protective effects of statins against post‐ICH epilepsy. Further research is warranted to verify our findings and to determine underlying mechanisms.
Introduction
Stroke is a major cause of epilepsy in adults and the elderly 1. The incidence of poststroke epilepsy (PSE) is higher with intracranial haemorrhage (ICH) than with ischaemic stroke. Approximately 4.1–11.1% of patients with ICH develop PSE 2, 3, 4, and PSE is often diagnosed within first 3 years after stroke 2. Patients with PSE have higher risks of disability and mortality 5, 6. Prophylactic use of antiepileptic drugs (AEDs), however, does not improve neurological outcomes or mortality and therefore is not recommended 6, 7, 8.
Activation of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4268 receptors plays an important role in epilepsy 9, and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=639 inhibitors (statins), a class of potent lipid‐lowering agent, have been shown to have neuroprotective and antiepileptic effects by protecting against NMDA‐induced excitotoxicity and neuronal damage in animal models and in vitro studies 10, 11, 12. Recent observational studies suggested that statin treatment may lower the risk of epilepsy in elderly patients and those with cardiovascular diseases, as well as reduce the risk of early‐onset seizure after ischaemic stroke 4, 13, 14. However, the pathophysiology and risk factors of epilepsy in ICH differs from that in ischaemic stroke 1, 15, and it remains unknown whether statin use alters the risk of PSE in patients with ICH. Therefore, we conducted a population‐based cohort study to investigate the association between statin use and the risk of PSE among patients with ICH. Further analyses were conducted to examine if the choice and cumulative dose of statin affect its effects on PSE.
Methods
Data source
The National Health Insurance (NHI) in Taiwan is a universal health insurance program with over 99.9% coverage of the total population (23 million). De‐identified administrative and claims data were derived from the NHI program to generate the National Health Insurance Research Database (NHIRD), which includes registration files and all medical claims for inpatient and outpatient services and pharmacy dispensing. In this study, we used three datasets of the Longitudinal Health Insurance Database, subsets of the NHIRD that contains all claims data from three million subjects who were randomly sampled from all beneficiaries in the years 2000, 2005 and 2010. Data from 2003 to 2013 were analysed. The study protocol was approved by the Research Ethics Committee of National Taiwan University Hospital. Owing to the anonymous nature of the data, informed consent was waived.
Study population
The study cohort consisted of all patients aged >20 years who were admitted to the hospital between January 2004 and December 2012 with a first‐ever diagnosis of an ICH (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] codes 430 for subarachnoid haemorrhage, 431 for ICH, and 432 for other and unspecified ICH). The date of hospital admission was defined as the index date in each patient, and 1 year before the index date served as the baseline observational period to determine prestroke medication exposure and comorbidities. Owing to the limited clinical information available in the NHIRD, the stroke severity was determined by the Stroke Severity Index (SSI) 16, which has been validated to estimate stroke severity in ICH patients using data from the NHIRD 17. Subjects were excluded if they: (i) had a history of any type of stroke, seizure/epilepsy, brain tumour or head trauma during or prior to the observational period; (ii) used AEDs prior to ICH; (iii) had missing registration data or inpatient claims; (iv) were not continuously enrolled in the NHI program during the baseline observational period (i.e. 1 year before the index date; to prevent mis‐recording of the baseline comorbidities and prestroke statin use); or (v) started statins and were newly diagnosed with seizure or epilepsy within the same hospital admission (because the temporal sequence of these events cannot be clarified). The Anatomical Therapeutic Chemical (ATC) codes for AEDs and ICD‐9‐CM codes used in the exclusion criteria are listed in the Supporting Information (Table S1).
Study outcome
The primary outcome was PSE after the index date. PSE was defined as having a diagnosis of epilepsy (ICD‐9‐CM code 345.x, except 345.6: infantile spasms), two diagnoses of seizure (780.39) on separate dates, or one diagnosis of seizure with continuous outpatient prescriptions of AEDs for at least 3 months after ICH 18, 19. The operational definition was suggested by the International League Against Epilepsy for using ICD‐coded data in health research. The supply of each prescription refill for chronic diseases was typically 28–30 days in Taiwan. Individuals were allowed a 50% grace period on the previous supply timeframe to refill the next prescription when measuring continuous medication use.
Follow‐up
Patients with ICH were followed from the index date until PSE development, three years after the index date, death, withdrawal from the health insurance program, or the end of data available period (31 December 2013), whichever occurred first. Since PSE tends to develop within 3 years after stroke 2, 20, 21, patients were followed for a maximum period of 3 years to minimize the likelihood of including epilepsy caused by other factors such as brain tumour and head injury 14.
Exposure definition
Different approaches were used to study pre‐ and poststroke statin exposure. Patients were divided into the following groups based on prestroke statin use: prestroke current users (duration of the last prescription covered the index date or ended within a month prior to the index date – considering carry‐over effect and gaps of refills), prestroke former users (the last prescription ended between 1 month and 1 year before the index date) and prestroke nonusers (no statin prescription within 1 year before the index date). By contrast, poststroke statin use was analysed as a time‐dependent variable – the presence or absence of statin use was recorded monthly after the index date and allowed to vary over time.
To evaluate the effects of different statin therapies, we classified statins by their lipophilicity and treatment intensity. Lipophilic statins include http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2949, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2951, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2739, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3035 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2955, whereas hydrophilic statins include http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2953 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2954 22. Statin therapy was categorized into low and moderate‐to‐high intensity according to the 2013 ACC/AHA Guideline 23. We further studied the dose–response effect of statin on PSE in prestroke nonusers, in consideration of avoiding potential carryover effects of prestroke therapy. The poststroke cumulative defined daily dose (cDDD) of statins was calculated monthly for each patient, with DDD used as the standardized unit of dose for measuring the total amount of statin exposure 24. The poststroke cDDD of statin was classified into four quartiles at each month and treated as a time‐dependent variable in the regression model 25.
Covariates
Age, sex, SSI, ICH subtypes, comorbidities and concurrent medications were included as covariates. The respective diagnosis codes and ATC codes for determining comorbidities and concurrent medications are listed in the Supporting Information (Tables S2 and S3). The comorbidities were identified based on the diagnoses given during the baseline observational period, including diabetes mellitus, metabolic disorders, dyslipidaemia, hypertension, heart diseases, kidney dysfunction, liver dysfunction, alcohol abuse, dementia and schizophrenia. Concurrent use of certain medications after stroke [including proconvulsant drugs (carbapenems, antidepressants, antipsychotics), potential neuroprotective drugs (metformin), and other lipid‐lowering agents (fibrates, ezetimibe)] was treated as time‐dependent variable in the model, allowing for the exposure status of each class of medications to change over time.
Hypertension is an important risk factor for ICH. In our analysis, however, patients with hypertension were associated with a lower risk of PSE. To control for potential residual confounding, we further included the average number of antihypertensive drug classes (as a proxy of the hypertension severity) and proportion of days covered (PDC; as a proxy of the hypertension severity and medication adherence) as covariates in the regression model. The equations for calculating the average number of antihypertensive drug classes and PDC are listed in the Supporting Information 26.
Statistical analysis
To compare the differences among patient groups based on the status of prestroke statin use, ANOVA and χ2 or Fisher's exact test were used for continuous and categorical variables, respectively. Extended Cox regression model was utilized to estimate the effect of statins on the risk of PSE, with poststroke statin and concurrent medications treated as time‐dependent variables. A backward elimination procedure was undertaken to keep the covariates of clinical and statistical significance; covariates (except for sex, age, SSI, ICH subtypes and prestroke statin use) that had a P‐value >0.2 and changed the effect estimates of statin use by ≤10% were removed from the final model 27. For subanalyses, additional Cox regression models were performed, using statin nonusers as the reference group, to study the effect of statin lipophilicity and therapy intensity on PSE. Hazard ratios for quartiles of cDDD were compared among the cohort of prestroke nonusers to explore the dose–response relationship of poststroke statin therapy.
Two additional analyses were conducted to ensure that the study findings were valid and specific to statin. A negative tracer analysis was performed with oral proton pump inhibitors (PPIs) selected as the negative exposure control. In addition, our study was repeated with acute gastroenteritis (ICD‐9‐CM: 558.9) appointed as the negative outcome control on which statins are not likely to cause an effect 28.
A series of sensitivity analyses were also conducted to test the robustness of our study findings. First, to exclude possible cases of acute symptomatic seizure and to assess the potential confounding effect by early poststroke seizures, patients with a diagnosis of seizure or epilepsy within 7 days and 14 days following the index date were excluded in the Sensitivity Analyses I‐1 and I‐2, respectively. Second, a different definition of PSE, i.e., having at least one diagnosis of epilepsy or two diagnoses of seizure on separate dates accompanied by at least one AED prescription, was used as the outcome endpoint in Sensitivity Analysis II. In Sensitivity Analysis III, more restrictive definitions of comorbidities were employed (Supporting Information Table S2). Fourth, the follow‐up period was reduced to 1 and 2 years to minimize the possibility of counting epilepsy from causes other than stroke (Sensitivity Analyses IV‐1 and IV‐2, respectively). Since patients may have received AEDs for seizure prophylaxis after ICH, which could confound the risk of PSE, we excluded those who were prescribed AEDs, regardless of the indication, without a diagnosis of PSE during the follow‐up period in Sensitivity Analysis V to assess the potential bias. Patients who had a diagnosis of ischaemic stroke (ICD‐9‐CM: 433–434) during the follow‐up period were excluded in Sensitivity Analysis VI, considering the increased risk of PSE associated with ischaemic stroke. In addition, we included only the patients with ICH, the major subtype of ICH, in Sensitivity Analysis VII. Finally, the time window for defining time‐dependent poststroke statin exposure was extended from 1 month to 2, 3, 6 and 12 months in Sensitivity Analysis VIII, and we expected that the magnitude of the effect estimate (risk of PSE associated with statin use) would reduce accordingly.
All P‐values were two‐sided, and the significance level was set at 0.05. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 29, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 30.
Results
Patient characteristics
The initial cohort consisted of 13 498 subjects who were admitted for ICH from 2004–2012. The median observation duration for exclusion screening was 5.4 years before the index date. After excluding patients who did not meet the study criteria, a total of 7435 patients were included in the final analyses (Figure 1). The mean age of the participants was 61.3 (standard deviation 15.0) years, 39.7% were female, and the mean SSI was 14.7 (standard deviation 6.1). About 74% of the patients were diagnosed with ICH. Hypertension was the most common comorbid disease. As shown in Table 1, > 90% of these patients did not use statin prior to the ICH event. Compared to prestroke former and current statin users, prestroke statin nonusers were younger, and less likely to be female or have comorbidities such as diabetes, dyslipidaemia, cardiovascular diseases and kidney dysfunction.
Figure 1.
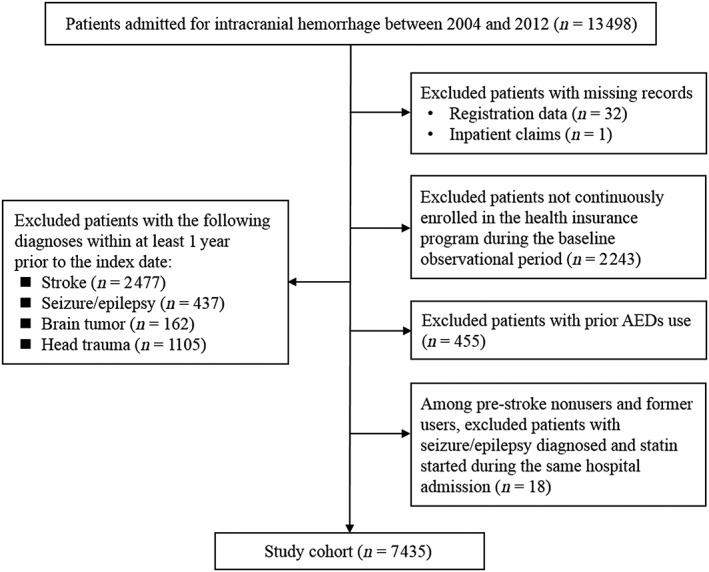
Flow diagram of the study
Table 1.
Patient baseline characteristics
| Variable | Overall n = 7435 | Prestroke statin use | P valuea | ||
|---|---|---|---|---|---|
| Nonuser n = 6838 | Former user n = 250 | Current user n = 347 | |||
| Age (mean ± SD) | 61.3 ± 15.0 | 61.0 ± 15.1 | 64.6 ± 12.4 | 65.6 ± 12.2 | <0.001b |
| Female, n (%) | 2954 (39.7) | 2654 (38.8) | 126 (50.4) | 174 (50.1) | <0.001 |
| SSI (mean ± SD) | 14.7 ± 6.1 | 14.7 ± 6.1 | 14.7 ± 6.5 | 14.4 ± 6.3 | 0.57b |
| ICH subtype, n (%) | |||||
| Subarachnoid haemorrhage | 1194 (16.1) | 1096 (16.0) | 39 (15.6) | 59 (17.0) | 0.12 |
| Intracerebral haemorrhage | 5511 (74.1) | 5084 (74.3) | 187 (74.8) | 240 (69.2) | |
| Other and unspecified intracranial haemorrhage | 730 (9.8) | 658 (9.6) | 24 (9.6) | 48 (13.8) | |
| Measures of stroke severity, n (%) | |||||
| Airway suctioning | 2269 (30.5) | 2082 (30.4) | 77 (30.8) | 110 (31.7) | 0.88 |
| Bacterial sensitivity test | 2251 (30.3) | 2065 (30.2) | 81 (32.4) | 105 (30.3) | 0.76 |
| General ward stay | 6166 (82.9) | 5666 (82.9) | 205 (82.0) | 295 (85.0) | 0.54 |
| Intensive care unit stay | 5921 (79.6) | 5460 (79.8) | 192 (76.8) | 269 (77.5) | 0.30 |
| Nasogastric intubation | 4019 (54.1) | 3697 (54.1) | 136 (54.4) | 186 (53.6) | 0.98 |
| Osmotherapy | 5124 (68.9) | 4752 (69.5) | 167 (66.8) | 205 (59.1) | <0.001 |
| Urinary catheterization | 3996 (53.7) | 3659 (53.5) | 138 (55.2) | 199 (57.3) | 0.34 |
| Comorbidities, n (%) | |||||
| Diabetes mellitus | 1490 (20.0) | 1186 (17.3) | 110 (44.0) | 194 (55.9) | <0.001 |
| Metabolic disorders | 485 (6.5) | 442 (6.5) | 25 (10.0) | 18 (5.2) | 0.05 |
| Dyslipidaemia | 803 (10.8) | 411 (6.0) | 158 (63.2) | 234 (67.4) | <0.001 |
| Hypertension | 4984 (67.0) | 4461 (65.2) | 218 (87.2) | 305 (87.9) | <0.001 |
| Heart failure | 374 (5.0) | 302 (4.4) | 25 (10.0) | 47 (13.5) | <0.001 |
| Coronary artery disease | 804 (10.8) | 622 (9.1) | 61 (24.4) | 121 (34.9) | <0.001 |
| Peripheral artery disease | 70 (0.9) | 57 (0.8) | 1 (0.4) | 12 (3.5) | <0.001c |
| Atrial fibrillation | 202 (2.7) | 173 (2.5) | 9 (3.6) | 20 (5.8) | 0.001 |
| Kidney dysfunction | 512 (6.9) | 412 (6.0) | 50 (20.0) | 50 (14.4) | <0.001 |
| Liver dysfunction | 78 (1.0) | 75 (1.1) | 2 (0.8) | 1 (0.3) | 0.43c |
| Alcohol abuse | 196 (2.6) | 188 (2.7) | 3 (1.2) | 5 (1.4) | 0.12 |
| Dementia | 169 (2.3) | 151 (2.2) | 8 (3.2) | 10 (2.9) | 0.43 |
| Schizophrenia | 37 (0.5) | 37 (0.5) | 0 (0.0) | 0 (0.0) | 0.33c |
P value for comparing the difference among three groups of prestroke statin use.
ANOVA F‐test
Fisher's exact test
SD, standard deviation
The median follow‐up duration was 17.6 months for total patients included in the study (a median of 5 months for patients with PSE and a median of 22 months for those without PSE). A total of 709 patients (9.5%) developed PSE, and more than half (57.2%) of the PSE occurred within 6 months after ICH. Among the 6838 prestroke statin nonusers, 868 initiated statin at different time points during the follow‐up period, and 461 (53.1%) had a diagnosis of dyslipidaemia. In prestroke statin nonusers who developed PSE, only 5.5% (36/659) had initiated statin prescriptions during their follow‐up period; in those who did not develop PSE, 13.4% (832/5347) had initiated statin. Atorvastatin and rosuvastatin were the most commonly used statins, and lovastatin and pitavastatin were used the least (Supporting Information Table S4).
Multivariable analysis and model selection
The multivariable Cox regression model showed that younger age, higher SSI, alcohol abuse, and dementia were associated with higher risk of PSE (Table 2), whereas hypertension was linked to a lower risk of PSE, even after adjusting for proxies of the hypertension severity and medication adherence. After the backward elimination procedure, age, gender, SSI, ICH subtypes, hypertension (along with the number of medication classes and PDC), coronary artery disease, atrial fibrillation, kidney dysfunction, liver dysfunction, alcohol abuse, dementia, schizophrenia, use of serotonin–norepinephrine reuptake inhibitors and antipsychotics were kept in the final model. All covariates in the final model had a variance inflation factor <10 and tolerance >0.1, which indicated that multicollinearity among the independent variables was less likely to exist.
Table 2.
Multivariable analysis of variables associated with poststroke epilepsy among intracranial haemorrhage patients (full model)†
| Covariate | HR (95% CI) | P value |
|---|---|---|
| Age (years) ‡ | 0.99 (0.98–0.99) | <0.001 |
| Female ‡ | 0.90 (0.77–1.05) | 0.19 |
| Stroke Severity Index ‡ | 1.10 (1.08–1.11) | <0.001 |
| Intracranial haemorrhage subtypes | ||
| Intracerebral haemorrhage | Reference | |
| Subarachnoid haemorrhage | 0.82 (0.66–1.02) | 0.07 |
| Other and unspecified intracranial haemorrhage | 1.07 (0.81–1.40) | 0.65 |
| Average number of types of antihypertensive agents ‡ | 0.95 (0.83–1.09) | 0.47 |
| PDC of antihypertensive therapy (for every 10% change) ‡ | 0.92 (0.64–1.34) | 0.68 |
| Comorbidities | ||
| Diabetes mellitus | 0.99 (0.78–1.25) | 0.94 |
| Metabolic disorders | 1.11 (0.83–1.47) | 0.47 |
| Dyslipidaemia | 0.86 (0.62–1.20) | 0.36 |
| Hypertension ‡ | 0.82 (0.69–0.98) | 0.03 |
| Heart failure | 0.76 (0.49–1.17) | 0.22 |
| Coronary artery disease ‡ | 1.27 (0.97–1.66) | 0.08 |
| Peripheral artery disease | 1.10 (0.49–2.46) | 0.83 |
| Atrial fibrillation ‡ | 0.58 (0.30–1.09) | 0.09 |
| Kidney dysfunction ‡ | 1.31 (0.96–1.79) | 0.09 |
| Liver dysfunction ‡ | 1.79 (0.95–3.39) | 0.07 |
| Alcohol abuse ‡ | 1.48 (1.00–2.18) | 0.05 |
| Dementia ‡ | 2.37 (1.57–3.60) | <0.001 |
| Schizophrenia ‡ | 1.72 (0.81–3.65) | 0.16 |
| Poststroke comedications | ||
| Potential epileptogenics | ||
| Carbapenems | 0.95 (0.63–1.43) | 0.81 |
| TCAs | −§ | 0.94 |
| SSRIs | 0.99 (0.63–1.55) | 0.96 |
| SNRIs ‡ | 1.43 (0.92–2.22) | 0.11 |
| Antipsychotics ‡ | 0.83 (0.64–1.08) | 0.16 |
| Metformin | 0.90 (0.66–1.24) | 0.52 |
| Lipid‐lowering agents other than statins | ||
| Fibrates | 0.73 (0.37–1.41) | 0.35 |
| Ezetimibe | 0.94 (0.13–6.81) | 0.95 |
CI, confidence interval; HR, hazard ratio; HTN, hypertension; PDC, proportion of days covered; SNRIs, serotonin–norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants.
The hazard ratios for prestroke and poststroke statin use are presented in Table 3.
Covariates included in the final model.
The confidence interval was too wide to provide a precise effect estimate
Statin use and risk of PSE
The hazard ratios for statin use on PSE risk were estimated based on the final model after backward elimination (Table 3). After adjusting for the covariates, poststroke statin use was associated with a reduced risk of PSE; patients with recent statin use (within a month) had a lower risk of PSE than statin nonusers [adjusted hazard ratio (aHR) 0.62, 95% confidence interval (CI) 0.42–0.90, P = 0.01]. However, no significant effect was found with prestroke, either current or former, statin use. In the subanalysis in which poststroke statin therapy was classified by the intensity of lipid‐lowering therapy (Table 4), only moderate‐to‐high intensity was associated with a significantly decreased risk of PSE compared to nonusers (aHR 0.37, 95% CI 0.18–0.75, P = 0.01). In the subanalysis of the lipophilicity, the lipophilic statins were significantly associated with reduced risk of PSE (aHR 0.63, 95% CI 0.40–0.98, P = 0.04), while a similar trend was found in hydrophilic statins (aHR 0.59, 95% CI 0.30–1.16, P = 0.13). The dose–response relationship of poststroke statin was further explored using the cohort of prestroke statin nonusers. In general, the risk of PSE was lower for patients with higher quartiles of cDDD, although the effect was only statistically significant for the third quartile of cDDD (Table 4).
Table 3.
Associations between statin treatment and risk of poststroke epilepsy in patients with intracranial haemorrhage
| Crude HR (95% CI) | P value | Adjusted HR† (95% CI) in the full model | P value | Adjusted HR§ (95% CI) in the final model | P value | |
|---|---|---|---|---|---|---|
| Prestroke statin use | ||||||
| Nonuser | Reference | Reference | Reference | |||
| Former user | 0.82 (0.52–1.30) | 0.41 | 1.12 (0.68–1.85) | 0.66 | 1.00 (0.63–1.60) | 0.99 |
| Current user | 0.91 (0.63–1.30) | 0.60 | 1.50 (0.97–2.34) | 0.07 | 1.32 (0.89–1.97) | 0.17 |
| Poststroke statin use (time‐dependent variable) | ||||||
| 0.52 (0.36–0.74) | <0.001 | 0.64 (0.43–0.94) | 0.02 | 0.62 (0.42–0.90) | 0.01 | |
CI, confidence interval; HR, hazard ratio.
Table 4.
Associations between different statins and poststroke epilepsy and the dose–response relationship among patients with intracranial haemorrhage
| Poststroke use of statins | Crude HR (95% CI) | P value | Adjusted HR† (95% CI) | P value |
|---|---|---|---|---|
| By lipophilicity ‡ | ||||
| No statin use | Reference | Reference | ||
| Lipophilic statins | 0.52 (0.34–0.79) | 0.002 | 0.63 (0.40–0.98) | 0.04 |
| Hydrophilic statins | 0.53 (0.27–1.02) | 0.06 | 0.59 (0.30–1.16) | 0.13 |
| By intensity of statin therapy | ||||
| No statin use | Reference | Reference | ||
| Low | 0.75 (0.47–1.18) | 0.21 | 0.93 (0.58–1.49) | 0.76 |
| Moderate to high | 0.30 (0.15–0.60) | <0.001 | 0.37 (0.18–0.75) | 0.01 |
| By cumulative defined daily dose (cDDD) § | ||||
| 0 | Reference | Reference | ||
| Lowest quartile | 0.96 (0.58–1.61) | 0.89 | 1.10 (0.66–1.85) | 0.70 |
| Second quartile | 0.65 (0.35–1.21) | 0.17 | 0.73 (0.39–1.37) | 0.33 |
| Third quartile | 0.32 (0.13–0.78) | 0.01 | 0.38 (0.16–0.92) | 0.03 |
| Highest quartile | 0.39 (0.17–0.87) | 0.02 | 0.50 (0.22–1.12) | 0.09 |
CI, confidence interval: HR, hazard ratio.
HR adjusted for the covariates in the final model.
Lipophilic statins include atorvastatin, fluvastatin, lovastatin, pitavastatin, and simvastatin. Hydrophilic statins include pravastatin and rosuvastatin.
This analysis was conducted in prestroke statin nonusers.
Additional analysis and sensitivity analysis
The analysis with negative exposure control showed no association between PPI use and PSE risk (Supporting Information Table S5). In addition, post‐ICH statin use was not associated with either an increased or decreased risk of acute gastroenteritis, the negative control of study outcome. The results of the sensitivity analyses are given in Supporting Information Tables S6 and S7. Using different definitions of PSE and comorbidities (Sensitivity Analyses I to III) or restricting the maximum length of follow‐up period to 1 or 2 years (Sensitivity Analysis IV) did not significantly alter the magnitude of the association between statin use and the risk of PSE. Moreover, after excluding individuals taking AEDs without a diagnosis of PSE (43.0% of non‐PSE cases; Sensitivity Analysis V) or patients who developed ischaemic stroke after the index date (6.4%; Sensitivity Analysis VI), or when only patients with ICH were enrolled (Sensitivity Analysis VII), the trend of negative association between poststroke statin use and PSE remained. In Sensitivity Analysis VIII, the adjusted HR for poststroke statin use increased toward the null, as expected, while extending the time window for defining the exposure.
Discussion
The study showed that poststroke statin exposure was associated with a reduced risk of PSE in patients with ICH, whereas statin use prior to the incidence of ICH was not associated with such beneficial effects. While exploring the dose–response relationship using the cohort of prestroke statin nonusers, there was a trend toward reduced PSE risk with a higher cumulative dose of poststroke statin therapy. A similar trend of dose‐dependent protective effects of statins against seizure or epilepsy was observed previously in an animal model and in patients with cardiovascular diseases 13, 31.
Although the risk of PSE may be reduced by statin therapy in both ischaemic stroke and ICH 4, the aetiology of PSE differs in these two stroke types 1. The pathogenesis of PSE in ICH has not yet been clearly elucidated, but the deposition of haemosiderin, a product of blood metabolism, has been proposed to be accountable 1. The deposition of haemosiderin in brain parenchyma could generate free radicals and lipid peroxides, resulting in excitotoxicity of the adjacent neurons and proliferation of glial tissues through disrupting receptor activity, calcium homeostasis and neurotransmitter levels (e.g. glutamate, aspartate and phosphorylethanolamine) 32. Dysregulation of calcium homeostasis, mediated by the activation of NMDA receptors and a subsequent influx of calcium ions into the neurons, can lead to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2351‐dependent activation of neuronal nitric oxide synthetase, which is associated with seizure attack 33, 34. It has been shown that statins can reduce the association between subunit‐1 of NMDA receptors and lipid rafts, thereby protecting against NMDA‐induced excitotoxicity and neuronal damage 12. Moreover, statins can lower the levels of proinflammatory/proconvulsant cytokines such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 while increasing the synthesis of anti‐inflammatory/neuroprotective cytokine http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975 10, 35.
In the present study, lipophilic and hydrophilic statins showed a similar trend in the association between their use and the risk of PSE. The nonsignificance in hydrophilic statins group might be due to the limited sample size while most statin users in the study were under lipophilic statins (about 70% of the person–time with statin use, Supporting Information Table S4). Because cholesterol metabolism in the central and peripheral nervous systems are regulated independently, there is a weak correlation between serum cholesterol and cerebral cholesterol 36, 37. If the use of hydrophilic statins was truly linked to a decreased risk of epilepsy, this would imply that the neuroprotection mediated by statins could be operated through mechanisms other than reducing the cholesterol levels in neurons, the effect of which should correlate with the drug ability to penetrate blood–brain barrier after the acute phase of ICH. There was also a trend that a moderate‐to‐high intensity of statin therapy was associated with a lower risk of PSE; however, the correlation between the intensity of statin therapy and cholesterol level in the brain remains unclear. Due to the limited sample size in individual statin groups, we were unable to determine the effect of individual statin on PSE prevention. Further studies are needed to elucidate the biological mechanisms underlying these observations and to investigate how the neuroprotective activity and anticonvulsant effect differ among the individual statins.
It is well established that hypertension is a major risk factor for ICH; unexpectedly, however, the present study revealed that hypertension was associated with a lower risk of PSE in the multivariable analysis after adjusting for antihypertensive medication use. A prior study by Hundozi et al. had similar findings 38. In their study, patients with hypertension when admitted for stroke had a lower risk of poststroke seizure. Although no definite pathophysiological mechanism could explain the results 38, we propose several possibilities herein. First, brain haemorrhages are often associated with uncontrolled high blood pressure 39, but patients might not be diagnosed with hypertension before stroke because of unawareness of their elevated blood pressure. Second, hypertensive cerebral haemorrhage is frequently located in deep brain structures such as basal ganglia, putamen, thalamus, cerebellum and pons, which are less likely to cause seizures 40, 41. The bleeding sites in nonhypertensive ICH (such as cerebral amyloid angiopathy or arteriovenous malformations), on the contrary, tend to involve the superficial cortical areas of the brain and might lead to a higher rate of stroke recurrence, worse prognosis, and elicit seizure 40, 41. Despite the possible explanations, this finding should be carefully interpreted in case there are unmeasured confounders that were not controlled in the model.
We found that dyslipidaemia was the main reason for statin‐naïve patients to initiate statin after ICH. Many other patients have initiated statin for secondary prevention of atherosclerotic cardiovascular disease or primary prevention for major cardiovascular events (if having major risk factors) since statins could be reimbursed for these patients based on the criteria by the National Health Insurance Administration in Taiwan. It is noteworthy that despite the positive findings of the current study, the overall benefit of statins for epilepsy prevention in this patient population could be partially offset by an increased risk of ICH. The post hoc analysis of Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial showed an increased risk of ICH in patients with recent stroke or transient ischaemic attack who were receiving high‐dose atorvastatin treatment 42. However, a more recent study has failed to find an association between statins and the risk of ICH in Asians 25. It remains controversial to use statins in patients with a history of ICH; therefore, routine use for secondary prevention in all patients is not recommended. Physicians should carefully weigh the balance of risks and benefits of statin therapy for individuals with ICH in their clinical practice.
To the best of our knowledge, this study was the first to focus on the association between statin use and post‐ICH epilepsy. We used a nationwide claims database with a representative sample that had a sufficient size. The extended Cox regression with time‐dependent variables was used to model the effect of statins and concurrent drugs to prevent exposure misclassification or immortal time bias, especially considering the exposure of these medications could vary over time during the follow‐up period 43. Our results showed that more than half of the PSE occurred within 6 months after ICH, and the follow‐up duration was appropriate to detect most PSE cases. A series of sensitivity analyses were also conducted to validate the results of our study. Of note, we found that although prophylactic AED use is not recommended in the guideline, approximately half of the patients without PSE have used AEDs during the follow‐up period. The negative relationship between statin use and PSE occurrence remained significant after excluding patients with prophylactic AEDs (Sensitivity Analysis V). Moreover, the negative findings of analyses with negative exposure and outcome controls confirm that the current results are specific to statin and PSE, respectively.
This study has several limitations. First, as in most administrative claims databases, detailed clinical information is lacking in the NHIRD, and some data, such as the lipid profile, stroke location, cortical involvement, haemorrhage volume, blood pressure, type of epilepsy, and laboratory results, are not available. Although we have controlled for the stroke severity (using SSI as a proxy), ICH subtypes, prestroke statin use, comorbidities and other covariates in the regression model to minimize selection bias, residual confounding remains possible. Second, no validation study on the accuracy of epilepsy diagnosis in the NHIRD was available, and there was no gold standard algorithm to identify epilepsy using administrative claims data 44. Therefore, we adopted the definition of epilepsy suggested by the International League Against Epilepsy and conducted several sensitivity analyses including using different definitions of PSE or excluding possible cases of acute symptomatic seizure. Third, due to the ICD‐9‐CM coding structure and insufficient details in the diagnosis codes, we could not distinguish the type of seizure for most of the PSE cases to understand the role of statins on different types of seizures. Fourth, as detailed data on the exact time of diagnoses and prescriptions during hospital admissions are not available in the NHIRD, we were unable to distinguish between early‐ and late‐onset PSE or to study the effect of statins during the acute phase of stroke. However, only 18 PSE cases were excluded because they were diagnosed with seizure or epilepsy and started on statins during the same hospitalization. Moreover, using the claims database as the data source, we only have pharmacy records for medications that have been prescribed and filled; therefore, the data may not fully represent patients' actual medication adherence.
Conclusions
Our study showed that the use of statins, especially statins of moderate‐to‐high intensity, was associated with a lower risk of PSE after ICH. Although routine use of statins is not recommended at this point and the decision for statin therapy should be based on individualized assessment of the risks and benefits, this study provided more evidence on the anticonvulsant effect of statins. Further research is required to prove causation and understand the biological mechanisms for the neuroprotective and anticonvulsant effects of statins in ICH.
Competing Interests
There are no competing interests to declare.
|This study is based, in part, on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Department of Health or National Health Research Institutes.
Contributors
H.‐W.L., study concept and design, analysis and interpretation of data and drafting of the manuscript. Y.‐F.H., study concept and design, interpretation of data, critical revision of the manuscript for intellectual content and study supervision. F.‐J.L., study concept and design, interpretation of data, critical revision of the manuscript for intellectual content and study supervision.
Supporting information
Table S1 List of ICD‐9‐CM codes for excluding conditions and Anatomical Therapeutic Chemical codes used in the study for antiepileptic drugs
Table S2 Definition of comorbidities (ICD‐9‐CM codes)
Table S3 Definition of concurrent drug use (Anatomical Therapeutic Chemical codes)
Table S4 Summary of statin use during the follow‐up period among the patients with intracranial haemorrhage
Table S5 Additional analyses with negative exposure and outcome control
Table S6 Sensitivity analyses on the study definitions and patient inclusion criteria
Table S7 Sensitivity Analysis VIII on the time window for defining time‐dependent poststroke statin use
Lin, H.‐W. , Ho, Y.‐F. , and Lin, F.‐J. (2018) Statin use associated with lower risk of epilepsy after intracranial haemorrhage: A population‐based cohort study. Br J Clin Pharmacol, 84: 1970–1979. 10.1111/bcp.13626.
References
- 1. Silverman IE, Restrepo L, Mathews GC. Poststroke seizures. Arch Neurol 2002; 59: 195–201. [DOI] [PubMed] [Google Scholar]
- 2. Chen TC, Chen YY, Cheng PY, Lai CH. The incidence rate of post‐stroke epilepsy: a 5‐year follow‐up study in Taiwan. Epilepsy Res 2012; 102: 188–194. [DOI] [PubMed] [Google Scholar]
- 3. Graham NS, Crichton S, Koutroumanidis M, Wolfe CD, Rudd AG. Incidence and associations of poststroke epilepsy: the prospective South London Stroke Register. Stroke 2013; 44: 605–611. [DOI] [PubMed] [Google Scholar]
- 4. Guo J, Guo J, Li J, Zhou M, Qin F, Zhang S, et al Statin treatment reduces the risk of poststroke seizures. Neurology 2015; 85: 701–707. [DOI] [PubMed] [Google Scholar]
- 5. Berger AR, Lipton RB, Lesser ML, Lantos G, Portenoy RK. Early seizures following intracerebral hemorrhage: implications for therapy. Neurology 1988; 38: 1363–1365. [DOI] [PubMed] [Google Scholar]
- 6. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 2032–2060. [DOI] [PubMed] [Google Scholar]
- 7. Messe SR, Sansing LH, Cucchiara BL, Herman ST, Lyden PD, Kasner SE. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care 2009; 11: 38–44. [DOI] [PubMed] [Google Scholar]
- 8. Naidech AM, Garg RK, Liebling S, Levasseur K, Macken MP, Schuele SU, et al Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke 2009; 40: 3810–3815. [DOI] [PubMed] [Google Scholar]
- 9. Meldrum BS. The role of glutamate in epilepsy and other CNS disorders. Neurology 1994; 44 (11 Suppl 8): S14–S23. [PubMed] [Google Scholar]
- 10. De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, et al Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci 2000; 12: 2623–2633. [DOI] [PubMed] [Google Scholar]
- 11. Gouveia TL, Scorza FA, Iha HA, Frangiotti MI, Perosa SR, Cavalheiro EA, et al Lovastatin decreases the synthesis of inflammatory mediators during epileptogenesis in the hippocampus of rats submitted to pilocarpine‐induced epilepsy. Epilepsy Behav 2014; 36: 68–73. [DOI] [PubMed] [Google Scholar]
- 12. Ponce J, de la Ossa NP, Hurtado O, Millan M, Arenillas JF, Davalos A, et al Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol‐mediated effect in neuroprotection. Stroke 2008; 39: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 13. Etminan M, Samii A, Brophy JM. Statin use and risk of epilepsy: a nested case‐control study. Neurology 2010; 75: 1496–1500. [DOI] [PubMed] [Google Scholar]
- 14. Pugh MJ, Knoefel JE, Mortensen EM, Amuan ME, Berlowitz DR, Van Cott AC. New‐onset epilepsy risk factors in older veterans. J Am Geriatr Soc 2009; 57: 237–242. [DOI] [PubMed] [Google Scholar]
- 15. Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Cote R, et al Seizures after stroke: a prospective multicenter study. Arch Neurol 2000; 57: 1617–1622. [DOI] [PubMed] [Google Scholar]
- 16. Sung SF, Hsieh CY, Kao Yang YH, Lin HJ, Chen CH, Chen YW, et al Developing a stroke severity index based on administrative data was feasible using data mining techniques. J Clin Epidemiol 2015; 68: 1292–1300. [DOI] [PubMed] [Google Scholar]
- 17. Hung LC, Sung SF, Hsieh CY, Hu YH, Lin HJ, Chen YW, et al Validation of a novel claims‐based stroke severity index in patients with intracerebral hemorrhage. J Epidemiol 2017; 27: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jette N, Beghi E, Hesdorffer D, Moshe SL, Zuberi SM, Medina MT, et al ICD coding for epilepsy: past, present, and future‐‐a report by the International League Against Epilepsy Task Force on ICD codes in epilepsy. Epilepsia 2015; 56: 348–355. [DOI] [PubMed] [Google Scholar]
- 19. Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, et al Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011; 52 (Suppl 7): 2–26. [DOI] [PubMed] [Google Scholar]
- 20. Faught E, Peters D, Bartolucci A, Moore L, Miller PC. Seizures after primary intracerebral hemorrhage. Neurology 1989; 39: 1089–1093. [DOI] [PubMed] [Google Scholar]
- 21. Biffi A, Rattani A, Anderson CD, Ayres AM, Gurol EM, Greenberg SM, et al Delayed seizures after intracerebral haemorrhage. Brain 2016; 139: 2694–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 2005; 19: 117–125. [DOI] [PubMed] [Google Scholar]
- 23. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129 (25 Suppl 2): S1–S45. [DOI] [PubMed] [Google Scholar]
- 24. Flint AC, Kamel H, Navi BB, Rao VA, Faigeles BS, Conell C, et al Statin use during ischemic stroke hospitalization is strongly associated with improved poststroke survival. Stroke 2012; 43: 147–154. [DOI] [PubMed] [Google Scholar]
- 25. Chang CH, Lin CH, Caffrey JL, Lee YC, Liu YC, Lin JW, et al Risk of intracranial hemorrhage from statin use in Asians: a nationwide cohort study. Circulation 2015; 131: 2070–2078. [DOI] [PubMed] [Google Scholar]
- 26. Lai SW, Su LT, Lin CH, Tsai CH, Sung FC, Hsieh DP. Polypharmacy increases the risk of Parkinson's disease in older people in Taiwan: a population‐based study. Psychogeriatrics 2011; 11: 150–156. [DOI] [PubMed] [Google Scholar]
- 27. Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989; 79: 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipsitch M, Tchetgen ET, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010; 21: 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alexander SP, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to Pharmacology 2017/18: ligand‐gated ion channels. Br J Pharmacol 2017; 174 (Suppl 1): S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russo E, Donato di Paola E, Gareri P, Siniscalchi A, Labate A, Gallelli L, et al Pharmacodynamic potentiation of antiepileptic drugs' effects by some HMG‐CoA reductase inhibitors against audiogenic seizures in DBA/2 mice. Pharmacol Res 2013; 70: 1–12. [DOI] [PubMed] [Google Scholar]
- 32. Ruan D, Yu XB, Shrestha S, Wang L, Chen G. The role of hemosiderin excision in seizure outcome in cerebral cavernous malformation surgery: a systematic review and meta‐analysis. PLoS One 2015; 10: e0136619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamida T, Takeda Y, Fujiki M, Abe T, Abe E, Kobayashi H. Nitric oxide synthase and NMDA receptor expressions in cavernoma tissues with epileptogenesis. Acta Neurol Scand 2007; 116: 368–373. [DOI] [PubMed] [Google Scholar]
- 34. Murashima YL, Yoshii M, Suzuki J. Role of nitric oxide in the epileptogenesis of EL mice. Epilepsia 2000; 41 (Suppl 6): S195–S199. [DOI] [PubMed] [Google Scholar]
- 35. Vezzani A. Brain inflammation and seizures. Epilepsy Curr 2004; 4: 73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sierra S, Ramos MC, Molina P, Esteo C, Vazquez JA, Burgos JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood‐brain‐barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis 2011; 23: 307–318. [DOI] [PubMed] [Google Scholar]
- 37. Wolozin B, Brown J 3rd, Theisler C, Silberman S. The cellular biochemistry of cholesterol and statins: insights into the pathophysiology and therapy of Alzheimer's disease. CNS Drug Rev 2004; 10: 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hundozi Z, Shala A, Boshnjaku D, Bytyqi S, Rrustemi J, Rama M, et al Hypertension on admission is associated with a lower risk of early seizures after stroke. Seizure 2016; 36: 40–43. [DOI] [PubMed] [Google Scholar]
- 39. Woo D, Haverbusch M, Sekar P, Kissela B, Khoury J, Schneider A, et al Effect of untreated hypertension on hemorrhagic stroke. Stroke 2004; 35: 1703–1708. [DOI] [PubMed] [Google Scholar]
- 40. Kase CS. Intracerebral hemorrhage: non‐hypertensive causes. Stroke 1986; 17: 590–595. [DOI] [PubMed] [Google Scholar]
- 41. Mehndiratta MM, Dwivedi RN. Intracerebral hemorrhage (ICH) therapy: current status and future directions. Med Update 2011; 229–235. [Google Scholar]
- 42. Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators , Karam JG, Loney‐Hutchinson L, McFarlane SI. High‐dose atorvastatin after stroke or transient ischemic attack: The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. J Cardiometab Syndr 2008; 3: 68–69. [DOI] [PubMed] [Google Scholar]
- 43. Jones M, Fowler R. Immortal time bias in observational studies of time‐to‐event outcomes. J Crit Care 2016; 36: 195–199. [DOI] [PubMed] [Google Scholar]
- 44. Kee VR, Gilchrist B, Granner MA, Sarrazin NR, Carnahan RM. A systematic review of validated methods for identifying seizures, convulsions, or epilepsy using administrative and claims data. Pharmacoepidemiol Drug Saf 2012; 21 (Suppl 1): 183–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 List of ICD‐9‐CM codes for excluding conditions and Anatomical Therapeutic Chemical codes used in the study for antiepileptic drugs
Table S2 Definition of comorbidities (ICD‐9‐CM codes)
Table S3 Definition of concurrent drug use (Anatomical Therapeutic Chemical codes)
Table S4 Summary of statin use during the follow‐up period among the patients with intracranial haemorrhage
Table S5 Additional analyses with negative exposure and outcome control
Table S6 Sensitivity analyses on the study definitions and patient inclusion criteria
Table S7 Sensitivity Analysis VIII on the time window for defining time‐dependent poststroke statin use


