Abstract
Aims
Some antipsychotics increase prolactin levels, which might increase the risk of breast cancer. Existing evidence is conflicting and based on sparse data, especially for the increasingly used second‐generation antipsychotics. We conducted a nationwide case–control study of the association between antipsychotic use and incident breast cancer.
Methods
From the Danish Cancer Registry, we identified women with a first‐time diagnosis of breast cancer 2000–2015 (n = 60 360). For each case, we age‐matched 10 female population controls. Using conditional logistic regression, we calculated odds ratios (ORs) for breast cancer associated with use of antipsychotics. We stratified antipsychotics by first‐ and second‐generation status and by ability to induce elevation of prolactin.
Results
In total, 4951 cases (8.1%) and 47 643 controls (7.9%) had ever used antipsychotics. Long‐term use (≥10 000 mg olanzapine equivalents) was associated with breast cancer, with an adjusted OR of 1.18 [95% confidence interval (CI), 1.06, 1.32]. A weak dose–response pattern was seen, with ORs increasing to 1.27 (95% CI 1.01, 1.59) for ≥50 000 mg olanzapine equivalents. Associations were similar for first‐ and second‐generation antipsychotics (ORs 1.17 vs. 1.11), but also for nonprolactin inducing‐antipsychotics (OR 1.17). Stratifying by oestrogen receptor status, positive associations were seen for oestrogen receptor‐positive cancers (long‐term use: OR 1.29; 95% CI 1.13, 1.47) while no associations were observed for oestrogen receptor‐negative cancers.
Conclusions
Overall, our results do not suggest a clinically important association between antipsychotic use and risk of breast cancer. The importance of drug‐induced prolactin elevation is unclear but may lead to a slightly increased risk of oestrogen receptor‐positive breast cancer.
Keywords: antipsychotics, breast cancer, pharmacoepidemiology
What is Already Known About this Subject
Some antipsychotics possess prolactin‐inducing properties, and their use might thereby infer an increased risk of breast cancer.
Existing evidence is conflicting and generally based on small studies.
Use of second‐generation antipsychotics are poorly studied in this context.
What this Study Adds
Use of antipsychotics does not seem to be associated with a meaningful increase in risk of breast cancer, although long‐term use may confer a slightly increased risk.
This risk pertains specifically to oestrogen receptor positive breast cancers.
For the prolactin‐inducing second‐generation antipsychotics risperidone and olanzapine, a slightly increased risk was observed.
Introduction
Breast cancer is one of the most commonly diagnosed malignant diseases worldwide 1. The aetiology of breast cancer is complicated with many contributing aspects, including a number of endocrine factors 2. Prolactin, a hormone essential to growth and differentiation of mammary gland cells 3, 4, 5, 6, 7, is likely to play a role in the aetiology of breast cancer, although this is somewhat controversial and incompletely understood 3, 7, 8, 9, 10. Upon binding of the ligand, the prolactin receptor initiates various downstream pathways that improve differentiation, proliferation and survival of epithelial cells of mammary glands 11, 12. Prolactin receptors are typically overexpressed in breast cancers, and their activation induces mammary carcinoma in animal models 13, 14.
By their antidopaminergic mode of action, antipsychotic drugs can increase serum prolactin levels to varying degrees. Most first‐generation antipsychotics (FGA) appear to induce substantial increases in prolactin levels, while the picture for second‐generation antipsychotics (SGA) is less clear. The most commonly prescribed SGAs, risperidone and olanzapine, are associated with increased levels of prolactin, while quetiapine and aripiprazole have little or no effect on prolactin levels 5, 15.
Longitudinal observational studies suggest that prolactin levels to some extent predict development of breast cancer 16, 17. Epidemiological association studies between exposure to antipsychotic drugs and risk of breast cancer have provided contradictory evidence, with most data suggesting a null‐association 7, 18, 19, 20. While some data suggest that the risk be greater among postmenopausal women, epidemiological data do not consistently demonstrate a risk differentiation be present with respect to menopausal status of the women 7, 16, 21. Further, some studies found a positive association between prolactin levels and tumours expressing oestrogen and progesterone receptors, and for tumours only expressing oestrogen receptors, with a null‐association for tumours not expressing oestrogen receptors 16, 22.
The most abundant data are for FGA, while studies on SGA are sparse, even in the last decade, during which SGAs have essentially replaced FGAs in clinical practice 7, 23, 24, 25. The largest study on the association between SGA and breast cancer is a case–control study including only 96 SGA‐exposed breast cancer cases, reporting no excess risk of SGA compared with FGA 26. The lack of data on SGA and risk of breast cancer has resulted in clinical ambiguity on how to counsel patients with indications for antipsychotic treatment about their risk for development of breast cancer 5, 18.
To investigate this association further, we used the nationwide Danish health registries to perform a population‐based case–control study on the risk of breast cancer following long‐term exposure to antipsychotic drugs.
Methods
We compared the use of antipsychotics among women diagnosed with breast cancer (cases) to that of cancer‐free women (controls) to obtain the odds ratio (OR) as an estimate of the incidence rate ratio associating antipsychotics use with breast cancer incidence.
Data sources
We used six Danish nationwide registries: the Danish Cancer Registry 27, 28, the National Prescription Registry 29, the National Patient Register 30, the Danish Pathology Register 31, the Danish Psychiatric Central Register 32, the Danish registers in Statistics Denmark on educational level and income 33, 34, and the Civil Registration System 35. The data sources are described in detail in Appendix S1, while codes for cancer diagnoses, drug exposures and covariates are provided in Appendix S2.
Data were linked by the personal identification number, a unique identifier assigned to all Danish residents since 1968 36. All linkages were performed within Statistics Denmark, a government institution that collects and processes information for a variety of statistical and scientific purposes. Virtually all medical care in Denmark is furnished by the national health authorities, allowing population‐based register linkage studies covering all legal residents of Denmark.
Selection of breast cancer cases and population controls
From the Danish Cancer Registry, we identified cases as all women in Denmark with a first‐time diagnosis of invasive breast cancer between 2000 and 2015, defining the date of cancer diagnosis as the index date. To maximize validity of our case definition, we included only histologically verified breast cancer diagnoses. Exclusion criteria were age outside the range 18–85 years at index date and any residency outside Denmark within 10 years prior to index date, thus ensuring at least 10 years of follow‐up for all study subjects and a minimum of 5 years of prescription data (the prescription registry opened in 1995). We further excluded individuals with previous cancers (except nonmelanoma skin cancer) or previous mastectomy.
Controls were selected by risk set sampling. For each case, we sampled 10 controls from all eligible Danish women who were born in the same year. Controls were assigned the same index date as the case to whom they were matched. Cases were eligible for sampling as controls before their diagnosis date. Thereby, the calculated ORs are unbiased estimates of the incidence rate ratios that would have emerged from a cohort study in the same source population 37.
Main exposure variables and covariates
Exposure to different antipsychotics was standardized using olanzapine equivalents 38. For drugs not assigned a conversion factor, one defined daily dose (DDD), per WHO definitions, was considered equivalent to 10 mg olanzapine 39. We applied a pre‐specified main exposure measure corresponding to a cumulative exposure of 10 000 mg olanzapine, while restricting to antipsychotics with prolactin inducing properties (Appendix S2). We included all exposure from 1995 (the opening of the Prescription Registry) until 1 year before an individual's index date. The largely arbitrary cut‐off of 10 000 mg olanzapine equivalents was selected based on pharmacological consideration that if antipsychotic use inferred a risk of breast cancer, a substantial use was likely to be necessary to detect an increased risk. For dose–response analyses, we used the following prespecified categories: 0–4999 mg, 5000–9999 mg, 10 000–19 999 mg, 20 000–49 999 mg and ≥50 000 mg. These strata were selected to ensure that we did not overlook risk associated with either very short or very high use of antipsychotics. In all exposure calculations, we disregarded prescriptions redeemed within 1 year before the index date to reduce the possibility of reverse causation 40, and because such recent exposure is unlikely to affect cancer development.
The following potential confounders were identified and incorporated in the analyses: (i) use of drugs known or suspected to modify breast cancer risk, including low‐dose aspirin, nonaspirin nonsteroidal anti‐inflammatory drugs, digoxin, statins, spironolactone, oral steroids, metoclopramide, domperidone, loop diuretics, β‐blockers, vascular calcium‐channel blockers, oral contraceptives (gestagen, combination therapy and intravaginal handled separately), hormone replacement therapy (oestrogen, progesterone/gestagen and combination therapy; modelled to include both cumulative use and recency of use), and selective serotonin reuptake inhibitors; (ii) prior diagnoses of diabetes, chronic obstructive pulmonary disease, and alcohol‐related disease; (iii) prior psychiatric diagnoses of schizophrenia, schizotypical and delusional disorders, manic episode, bipolar affective disorder, mood (affective) disorders, phobic anxiety disorders, other anxiety disorders, and neurotic, stress‐related and somatoform disorders; (iv) Charlson comorbidity index scores (0, low; 1–2, medium; or ≥ 3, high) 41, 42; and (v) highest achieved education (as a crude measure of socioeconomic status). As in the assessment of drug exposure, we disregarded the period 1 year prior to the index date in the classification of confounder status.
Main analysis
The analysis followed a conventional matched case–control approach. We computed the frequency and proportion of cases and controls within categories of exposure and covariates. Using conditional logistic regression and adjusting for potential confounders, we estimated ORs for breast cancer associated with antipsychotics with either prolactin‐elevating or nonprolactin elevating effects. A formal dose–response analysis was performed by restricting to ever users and estimating the incremental OR for each 10 000 mg olanzapine equivalent, using ordinary logistic regression with adjustment for age as a continuous variable. Analyses were also carried out for specific exposure to FGA, SGA and to single antipsychotic drug products (risperidone/paliperidone, olanzapine, quetiapine and aripiprazole). In all analyses, use of antipsychotics was compared with nonuse.
Sensitivity and supplementary analyses
We performed several preplanned subanalyses and sensitivity analyses.
First, we assessed the risk of confounding by indication, i.e. that the clinical indication for antipsychotic therapy somehow affected breast cancer risk, either as a direct effect or mediated through, for example, higher or lower health care surveillance. To this end, we performed an analysis restricting exposure to antipsychotics with no or little prolactin‐inducing effect. An increased risk for these drugs would suggest an association confounded by psychiatric comorbidity and related risk factors, assuming that elevated prolactin is the sole biological mechanism by which exposure affects breast cancer risk.
Second, we examined the association between long‐term use of antipsychotics and breast cancer within subgroups defined by age, type of breast cancer, oestrogen receptor status and by clinical stage, i.e. localized or nonlocalized disease.
Third, to estimate the proportion of breast cancer cases that, during our study period, could be attributed to use of antipsychotics, we calculated the ‘population‐level attributable proportion’ (APpop) using the following equation: APpop = propcases × (OR – 1) / OR. Here, propcases denotes the proportion of all breast cancer cases classified as exposed to long‐term use of antipsychotics and the OR was that obtained for the main analysis.
Fourth, as two sensitivity analyses, we first removed the 1‐year lag‐time applied in the main analysis and then increased it to 2 years, respectively.
Last, we performed a quantitative bias assessment to evaluate the potential for unmeasured confounding to have influenced our results. In our models, we were unable to adjust for potential confounding by obesity, smoking, alcohol use or nulliparity. While there is no evidence that these factors are strongly related to use of antipsychotics, such a relation is reasonable to consider. We therefore conducted a probabilistic bias analysis 43 to evaluate whether confounding by these breast cancer risk factors may account for all or part of the observed association. A full account of the bias analysis methods and our chosen bias parameters is provided in Appendix S3.
Other
All analyses were performed using Stata Release 14.2 (StataCorp, College Station, TX, USA). The study was approved by the Danish Data Protection Agency. According to Danish law, studies based solely on register data do not require approval from an ethics review board 44.
Results
The final study population included 60 360 histologically verified breast cancer cases (Figure 1) matched with 603 600 controls. The median age was 62 years (interquartile range, 53–70); most cases were ductal adenocarcinomas (76%), followed by lobular adenocarcinomas (13%; Table 1). Differences in baseline characteristics between cases and controls were generally minor, except for a slightly higher use of hormone replacement therapy and slightly longer education among cases compared to controls (Table 1).
Figure 1.
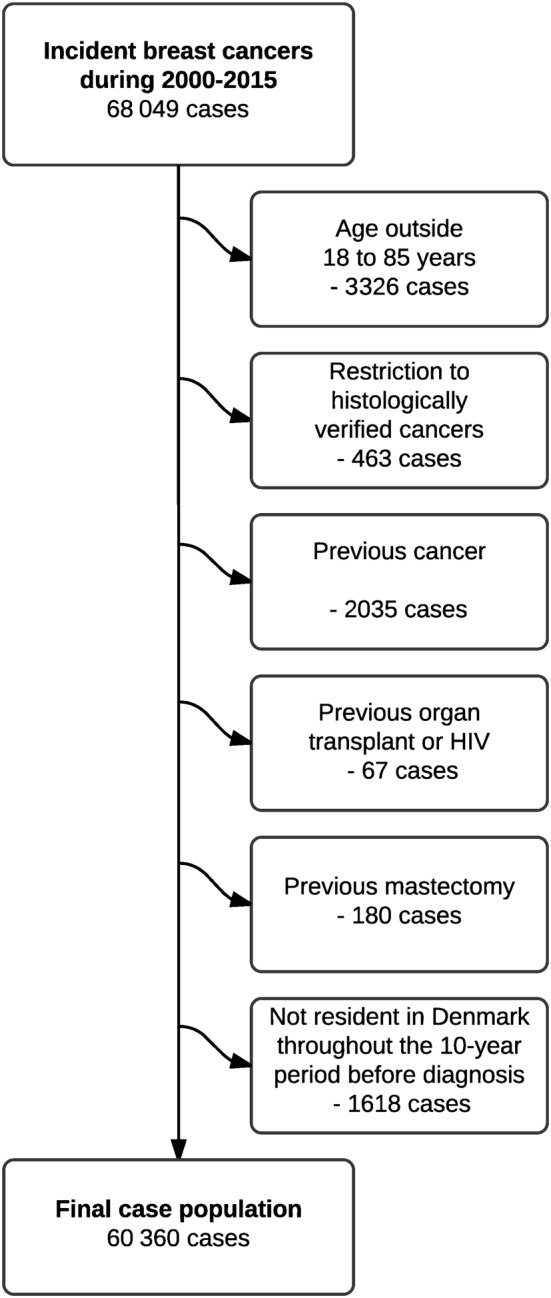
Flow‐chart of the selection of cases
Table 1.
Characteristics of breast cancer cases and their matched controls
| Cases | Controls | |
|---|---|---|
| (n = 60 360) | (n = 603 600) | |
| Age | ||
| Median (IQR, years) | 62 (53–70) | 62 (53–70) |
| < 50 years | 10 386 (17%) | 103 860 (17%) |
| 50–69 years | 34 259 (57%) | 342 590 (57%) |
| 70+ years | 15 715 (26%) | 157 150 (26%) |
| Cancer diagnosis | ||
| Ductal adenocarcinoma | 45 662 (76%) | NA |
| Lobular adenocarcinoma | 7546 (13%) | NA |
| Adenocarcinoma, not otherwise specified | 3631 (6.0%) | NA |
| Carcinoma, not otherwise specified | 3280 (5.4%) | NA |
| Other cancers | 241 (0.4%) | NA |
| Use of antipscyhotics | ||
| Never use (any antipsychotic) | 55 409 (92%) | 555 957 (92%) |
| Ever use (any antipsychotic) | 4951 (8.2%) | 47 643 (7.9%) |
| Ever use (prolactin‐inducing) | 4798 (7.9%) | 46 156 (7.6%) |
| Long‐term use * | 693 (1.1%) | 6323 (1.0%) |
| Charlson comorbidity index score | ||
| 0 | 45 917 (76%) | 463 552 (77%) |
| 1 | 8462 (14%) | 86 255 (14%) |
| 2 | 3543 (5.9%) | 31 675 (5.2%) |
| ≥3 | 2438 (4.0%) | 22 118 (3.7%) |
| Drugs | ||
| Low‐dose aspirin | 8067 (13%) | 80 180 (13%) |
| Nonaspirin NSAID | 32 801 (54%) | 318 551 (52%) |
| Digoxin | 1211 (2.0%) | 9377 (1.6%) |
| Statins | 8487 (14%) | 86 559 (14%) |
| Spironolactone | 1104 (1.8%) | 9778 (1.6%) |
| Oral steroids | 7742 (13%) | 76 471 (13%) |
| Metoclopramide | 3418 (5.7%) | 32 636 (5.4%) |
| Domperidone | 158 (0.3%) | 1588 (0.3%) |
| Loop diuretics | 5115 (8.5%) | 46 913 (7.8%) |
| Beta‐blockers | 2794 (4.6%) | 24 573 (4.1%) |
| Vascular calcium‐channel blockers | 7374 (12%) | 71 488 (12%) |
| Oral contraceptives | ||
| Gestagen | 9548 (16%) | 89 324 (15%) |
| Combination therapy | 1080 (1.8%) | 9642 (1.6%) |
| Intravaginal | 104 (0.2%) | 1036 (0.2%) |
| Hormone replacement therapy | ||
| Short term – distant | 2984 (4.9%) | 32 656 (5.4%) |
| Short term – recent | 1148 (1.9%) | 13 064 (2.2%) |
| Long term – distant | 6113 (10%) | 62 812 (10%) |
| Long term – recent | 13 204 (22%) | 100 683 (17%) |
| Selective serotonin reuptake inhibitors | 9541 (16%) | 90 837 (15%) |
| Diagnoses | ||
| Diabetes | 3325 (5.5%) | 32 387 (5.4%) |
| Chronic obstructive pulmonary disease | 2611 (4.3%) | 24 168 (4.0%) |
| Alcohol‐related disease | 1408 (2.3%) | 12 170 (2.0%) |
| Psychiatric diagnoses | ||
| Schizophrenia | 327 (0.5%) | 2671 (0.4%) |
| Schizotypical and delusional disorders | 491 (0.8%) | 4459 (0.7%) |
| Manic episode | 51 (0.1%) | 533 (0.1%) |
| Bipolar affective disorder | 336 (0.6%) | 2954 (0.5%) |
| Mood disorders | 1706 (2.8%) | 16 735 (2.8%) |
| Phobic anxiety disorders | 133 (0.2%) | 1360 (0.2%) |
| Other anxiety disorders | 518 (0.9%) | 5082 (0.8%) |
| Neurotic, stress‐related and somatoform disorders | 1509 (2.5%) | 14 329 (2.4%) |
| Highest achieved education | ||
| Short; Elementary school (7–10 years) | 21 421 (35%) | 230 371 (38%) |
| Medium; High school (11–13 years) | 21 370 (35%) | 204 124 (34%) |
| Long; College (>13 years) | 14 998 (25%) | 136 320 (23%) |
| Unknown | 2571 (4.3%) | 32 785 (5.4%) |
IQR = interquartile range; NSAID = nonsteroidal anti‐inflammatory drug
Exposure to different antipsychotics was standardized using olanzapine equivalents 38. Long‐term use was defined as a cumulative exposure of 10 000 mg olanzapine.
Ever use of prolactin‐elevating antipsychotics was seen among 7.9% of cases and 7.6% of controls, while long‐term use, i.e. ≥10 000 mg olanzapine equivalents, was found among 1.1% and 1.0%, respectively. This corresponded to an adjusted OR associating ever use of prolactin‐elevating antipsychotics with risk of breast cancer of 1.00 [95% confidence interval (CI), 0.97, 1.04]. The corresponding OR for long‐term use was 1.18 (95%CI, 1.06, 1.32). Adjustment for measured potential confounders had limited influence on the magnitude of the estimates.
The dose–response analysis (Table 2) revealed no excess for short‐term/sporadic use (<5000 mg olanzapine equivalents), with an adjusted OR of 0.97 (95%CI, 0.94, 1.01). Four in five antipsychotic users fell within this exposure category. For the remaining exposure levels, a dose response pattern was seen (P < 0.001), with slightly stronger associations with higher cumulative exposure, increasing from an OR of 1.19 (95%CI 1.05, 1.34) for limited use (5000–9999 mg) to an OR of 1.27 (95%CI, 1.01, 1.59) for use of more than 50 000 mg olanzapine equivalents (Table 2). Similar patterns of associations to that of the main analysis were seen when separating exposure into use of first‐ and second‐generation prolactin‐inducing antipsychotics (Table 3). Use of nonprolactin‐inducing antipsychotics also showed an association with breast cancer incidence (Table 3), although with a less apparent dose–response pattern (P = 0.58). In analyses of single antipsychotic drugs, more pronounced dose–response patterns were seen specifically for use of risperidone/paliperidone and olanzapine (Table S1).
Table 2.
Association between exposure to prolactin‐inducing antipsychotics and risk of breast cancer, specified by exposure pattern within the entire follow‐up‐period, excluding the last year prior to the index date
| Exposure group | Cases | Controls | Adjusted ORa | Adjusted ORb |
|---|---|---|---|---|
| Nonuse | 55 409 | 555 957 | 1.0 (ref.) | 1.0 (ref.) |
| Ever use | 4798 | 46 156 | 1.04 (1.01–1.08) | 1.00 (0.97–1.04) |
| Long‐term use c | 693 | 5659 | 1.23 (1.14–1.33) | 1.18 (1.06–1.32) |
| Cumulative use c | ||||
| 0–4999 mg | 3756 | 37 619 | 1.00 (0.97–1.04) | 0.97 (0.94–1.01) |
| 5000–9999 mg | 349 | 2878 | 1.21 (1.09–1.36) | 1.19 (1.05–1.34) |
| 10 000–19 999 mg | 243 | 2131 | 1.15 (1.01–1.32) | 1.11 (0.95–1.29) |
| 20 000–49 999 mg | 246 | 1993 | 1.24 (1.09–1.42) | 1.27 (1.07–1.50) |
| ≥50 000 mg | 204 | 1535 | 1.33 (1.15–1.54) | 1.27 (1.01–1.59) |
OR, odds ratio
Adjusted for age and calendar‐time (by design; risk‐set matching).
Fully adjusted model, see section ‘Analytical variables’.
Exposure to different antipsychotics was standardized using olanzapine equivalents 38. Long‐term use was defined as a cumulative exposure of 10 000 mg olanzapine.
Table 3.
Association between exposure to first‐generation and second‐generation prolactin‐inducing antipsychotics and nonprolactin‐inducing antipsychotics and risk of breast cancer, specified by exposure pattern within the entire follow‐up‐period, excluding the last year prior to the index date
| Exposure group | Cases | Controls | Adjusted ORa | Adjusted ORb |
|---|---|---|---|---|
| First‐generation prolactin‐inducing antipsychotics | ||||
| Nonuse | 55 409 | 555 957 | 1.0 (ref.) | 1.0 (ref.) |
| Ever use | 4538 | 43 132 | 1.06 (1.02–1.09) | 1.01 (0.97–1.05) |
| Long‐term use c | 516 | 4249 | 1.22 (1.11–1.34) | 1.17 (1.04–1.32) |
| Cumulative use c | ||||
| 0–4999 mg | 3704 | 36 434 | 1.02 (0.99–1.06) | 0.99 (0.95–1.02) |
| 5000–9999 mg | 318 | 2449 | 1.30 (1.16–1.46) | 1.25 (1.10–1.42) |
| 10 000–19 999 mg | 213 | 1778 | 1.22 (1.05–1.40) | 1.14 (0.97–1.34) |
| 20 000–49 999 mg | 171 | 1522 | 1.12 (0.96–1.31) | 1.15 (0.94–1.40) |
| ≥50 000 mg | 132 | 949 | 1.39 (1.15–1.66) | 1.35 (1.05–1.75) |
| Second‐generation prolactin‐inducing antipsychotics | ||||
| Nonuse | 55 409 | 555 957 | 1.0 (ref.) | 1.0 (ref.) |
| Ever use | 934 | 8888 | 1.06 (0.99–1.13) | 0.98 (0.90–1.07) |
| Long‐term use c | 257 | 2086 | 1.24 (1.09–1.41) | 1.11 (0.92–1.35) |
| Cumulative use c | ||||
| 0–4999 mg | 562 | 5852 | 0.97 (0.89–1.06) | 0.93 (0.84–1.03) |
| 5000–9999 mg | 115 | 950 | 1.21 (0.99–1.47) | 1.27 (1.01–1.59) |
| 10 000–19 999 mg | 84 | 832 | 1.01 (0.80–1.26) | 0.86 (0.65–1.13) |
| 20 000–49 999 mg | 113 | 860 | 1.34 (1.10–1.63) | 1.39 (1.06–1.83) |
| ≥50 000 mg | 60 | 394 | 1.52 (1.16–2.00) | 1.42 (0.96–2.10) |
| Nonprolactin‐inducing antipsychotics | ||||
| Nonuse | 55 409 | 555 957 | 1.0 (ref.) | 1.0 (ref.) |
| Ever use | 517 | 4810 | 1.08 (0.98–1.18) | 1.04 (0.93–1.17) |
| Long‐term use c | 111 | 894 | 1.23 (1.01–1.50) | 1.17 (0.88–1.55) |
| Cumulative use c | ||||
| 0–4999 mg | 364 | 3548 | 1.03 (0.93–1.15) | 1.01 (0.89–1.14) |
| 5000–9999 mg | 42 | 368 | 1.16 (0.84–1.60) | 1.17 (0.82–1.67) |
| 10 000–19 999 mg | 51 | 361 | 1.37 (1.02–1.83) | 1.22 (0.85–1.75) |
| 20 000–49 999 mg | 37 | 339 | 1.10 (0.78–1.54) | 1.02 (0.67–1.55) |
| ≥50 000 mg | 23 | 194 | 1.20 (0.78–1.86) | 0.98 (0.56–1.72) |
OR = odds ratio
Adjusted for age and calendar‐time (by design; risk‐set matching).
Fully adjusted model, see section ‘Analytical variables’.
Exposure to different antipsychotics was standardized using olanzapine equivalents 38. Long‐term use was defined as a cumulative exposure of 10 000 mg olanzapine.
Subgroup analyses (Table 4) revealed limited variation according to age. There was a tendency towards a stronger association with nonlocalized cancer (OR 1.37; 95%CI, 1.15, 1.65) compared to localized cancer (OR 1.01; 95%CI 0.85, 1.20). Associations were positive for oestrogen receptor‐positive cancers and null (with wider CIs) for oestrogen receptor‐negative cancers (OR for long‐term use of 1.29 vs. 0.92) and also showed a statistically significant dose–response pattern (P < 0.001), while no pattern was observed for oestrogen receptor negative cancers (Table S2). Further, an increased risk was seen specifically for cancers classified as carcinoma, not otherwise specified, with an OR of 1.90 (95%CI, 1.31, 2.76) compared to point estimates ranging from 1.03 to 1.20 for other histologies. A posthoc analysis confirmed that this histological designation was more common among the 693 cancer cases classified as long‐term users compared to the 55 409 never users (10.7% vs. 5.2%). Subgroup analyses specified by use of first‐ and second‐generation prolactin‐inducing and nonprolactin‐inducing antipsychotics generally showed patterns similar to the overall analyses, including an increased risk specific to nonspecified carcinomas (Table S3).
Table 4.
Associations between long‐term exposure to antipsychotics with prolactin‐inducing properties (equivalent to ≥10 000 mg of olanzapine) and risk of breast cancer, specified by patient subgroups or cancer subtype or stage
| Subgroup | Cases Exposed/unexposed | Controls Exposed/unexposed | Adjusted ORa | Adjusted ORb |
|---|---|---|---|---|
| Age | ||||
| <50 years | 66/9845 | 648/97 906 | 1.02 (0.79–1.31) | 1.02 (0.68–1.54) |
| 50–69 years | 441/31 424 | 3474/316 605 | 1.28 (1.16–1.42) | 1.20 (1.04–1.38) |
| 70+ years | 186/14 140 | 1537/141 446 | 1.21 (1.03–1.41) | 1.16 (0.96–1.41) |
| Subtype | ||||
| Ductal adenocarcinoma | 496/41 958 | 4259/421 053 | 1.17 (1.07–1.29) | 1.14 (1.01–1.30) |
| Lobular adenocarcinoma | 80/6984 | 728/69 373 | 1.09 (0.86–1.38) | 1.03 (0.75–1.43) |
| Adenocarcinoma NOS | 40/3342 | 324/33 402 | 1.23 (0.89–1.72) | 1.20 (0.77–1.87) |
| Carcinoma NOS | 74/2906 | 329/29 914 | 2.32 (1.79–3.00) | 1.90 (1.31–2.76) |
| Estrogen receptor status | ||||
| Positive | 483/34 712 | 3699/348 626 | 1.31 (1.19–1.45) | 1.29 (1.13–1.47) |
| Negative | 64/7540 | 742/75 453 | 0.87 (0.67–1.12) | 0.92 (0.65–1.29) |
| Unknown | 146/13 157 | 1218/131 878 | 1.20 (1.01–1.43) | 1.01 (0.80–1.28) |
| Stage | ||||
| Localized | 270/22 464 | 2471/224 821 | 1.09 (0.96–1.24) | 1.01 (0.85–1.20) |
| Nonlocalized | 263/18 798 | 2077/188 181 | 1.28 (1.13–1.46) | 1.37 (1.15–1.65) |
| Unknown | 160/14 147 | 1111/142 955 | 1.45 (1.23–1.72) | 1.24 (0.99–1.55) |
OR = odds ratio; NOS = tot otherwise specified
Adjusted for age and calendar‐time (by design).
Fully adjusted model, see section ‘Analytical variables’.
Changing the lag‐time to 2 or 0 years had no discernible effects on the estimates (data not shown) compared with the original 1‐year lag.
We applied the probabilistic bias adjustment to the main result associating antipsychotic use with breast cancer (OR 1.18; 95%CI 1.06, 1.32). The bias adjustment taking into account smoking, obesity, alcohol use and nulliparity had little effect on the result (OR 1.17; 95% simulation interval, 1.04, 1.31).
In a posthoc analysis, we restricted exposure to that obtained within the five years before sampling, while still employing a 1‐year lag period, which did not lead to materially different results (Table S4). When incorporating recency into the exposure criteria, positive associations were seen with recent long‐term use (requiring both long‐term use and that the most recent antipsychotic prescription to be filled <2 years from index date), which yielded an OR of 1.20 (95% CI 1.07, 1.34) while distant long‐term use (long‐term use and last prescription filled >2 years before index date) yielded neutral associations (OR 0.94, 95% CI 0.70, 1.27).
Discussion
In this large study of >50 000 breast cancer cases, we found evidence of a weak association between use of prolactin‐inducing antipsychotics and the risk of breast cancer, oestrogen receptor positive cancers in particular. Four in five antipsychotic users fell in the lowest exposure category, and for such exposure no increased risk was observed. Among the more highly exposed users, the association displayed a weak dose‐dependent pattern. Similar results were found for FGA and SGA as well as nonprolactin‐inducing antipsychotics. The subgroup analyses had, however, lower precision than the overall estimates, and are therefore more susceptible to chance variations.
The primary strength of our study is its nationwide approach, with complete coverage of an entire nation and their use of antipsychotics for up to 20 years, with limited risk of selection bias. Further, the size of our study—4951 events among antipsychotic ever users and 693 events among long‐term users—is much larger than previous studies, and allowed meaningful assessment of the risks associated with a wide range of exposure levels, including very long‐term use of up to 100 000 mg olanzapine equivalents. Last, the databases used, mainly the Prescription Registry and the Cancer Registry, are of high validity 28, 29.
Some limitations of our study need to be acknowledged, mainly the concern that our findings could be at least partly explained by confounding from unmeasured patient characteristics. The a priori defined supplementary analysis of nonprolactin‐inducing antipsychotics returned estimates comparable to that of the main analysis, which goes against our biological hypothesis. Whether the diagnosis of schizophrenia itself confers an increased risk of breast cancer is unclear 45. Further, we had no data on some specific risk factors for breast cancer, including obesity, smoking, alcohol consumption and parity. As these are not only risk factors for breast cancer, but might also be associated with use of antipsychotics (either positively or inversely), uncontrolled confounding from these factors might bias our findings. However, the results of the probabilistic bias analysis showed that these were unlikely to account for the observed association, conditional on the accuracy of the bias model. This lack of substantial bias is a function of the relatively low prevalence of these confounders and the relatively low strength of association between these risk factors and breast cancer risk, both of which diminish the potential for these factors to confound the association.
Despite these limitations, our results suggest a small excess risk with long‐term use of prolactin‐inducing antipsychotics. While dose–response patterns were generally weak, they did suggest a dose–response effect, especially for risperidone and olanzapine. Such a dose–response pattern is less likely to be explained by unmeasured confounding than is the overall result. These two drugs are known to elevate prolactin levels, so an increased breast cancer risk associated with their long‐term use is biologically plausible. The positive overall association to long‐term use is substantiated by our posthoc analysis stratifying long‐term use by recent and nonrecent use, which confirmed a positive association for the former but not the latter group. Importantly, however, the increased risk seems to achieve a clinically relevant magnitude only with long‐term use, which was only observed in about 1% of the population. As illustrated by the estimated population‐level attributable proportion of 0.2%, the increased risks, even if assuming causality, does not constitute a public health issue. Also, this is the first study to provide meaningful risk estimates of very long‐term exposure (up to 100 000 mg olanzapine equivalents); it is reassuring that we did not identify substantially increased risks associated with such exposure. All the same, it is worth noting that our reported association is of approximately the same magnitude as some other established—but much more prevalent—breast cancer risk factors.
Analysis stratified by oestrogen receptor status showed that the observed associations were specific to oestrogen receptor positive breast cancers. This finding in accordance with results from studies on prolactin levels and risk of breast cancer 16, 20. The possible underlying mechanisms are not well substantiated. It has been demonstrated that activation of nuclear prolactin receptor induces the expression of oestrogen receptors in breast cancer cells 46, 47. While contradictory results exist, some studies also suggest that oestrogen receptor and prolactin receptor may be co‐expressed in breast cancer cells and it has been suggested that prolactin may act as a local growth promoter 10, 48. While stratified analyses consistently showed null‐associations for oestrogen receptor negative cancers, the accompanying CIs reflect the significantly lower number of cases.
The only other study 26 that specifically addressed the use of FGA nested their analysis within antipsychotic ever users and found no increased risk when comparing SGA to FGA, which is in line with our findings of a comparable risk between the two. Further, they found no evidence of a dose–response pattern. Here, however, comparison with our study is less straight forward, as the small number of events and limited follow‐up precluded detailed dose–response analyses, and their highest exposure level (≥3000 mg) overlaps the lowest exposure level in our analysis (<5000 mg, Table 2). Other studies hold no or indiscriminate information on exposure to SGA. Wang et al. studied the relationship of breast cancer and dopamine antagonists including FGA and, specifically, aripiprazole among 52 819 exposed and 55 289 nonexposed women from psychiatric wards, nursing homes, and general medical patients 49. A weak dose‐dependent association was found for any dopamine antagonist with a specific hazard ratio of 1.19 (95% CI 1.08, 1.32) for the SGA group of phenothiazines comprising 508 exposed cases. Results were internally inconsistent with the underlying biological rationale though, as no association was found for butyphenones (comprising 240 exposed cases), which include the prolactin‐elevating FGA haloperidol. From the paper, it is not possible to identify cases exposed specifically to risperidone. A previous regional Danish study did not find an association between FGA and breast cancer in a cohort of 25 264 users of FGA (neuroleptics, ATC N05A) 50, with 258 women developing breast cancer corresponding to an adjusted incidence rate‐ratio of 1.06 (95% CI 0.93, 1.21). Last, a case–control study of 5814 women diagnosed with primary invasive breast cancer within the preceding year found no association with prior phenothiazine use (174 exposed) 51.
The increased risk specifically for cancers, classified as unclassified carcinoma, was a surprising finding for which we have no plausible explanation. One could consider whether diagnostic workup might be different in the face of severe psychiatric disorders. However, as we only included histologically verified cancers, this would require that the pathological evaluation differed according to psychiatric comorbidity, which is unlikely.
In conclusion, our results, from what is the most extensive set of data reported to date, do not suggest an overall clinically important association between exposure to SGA – or any antipsychotic drugs – and risk of breast cancer. Long‐term use of antipsychotic drugs may confer a slightly increased risk of oestrogen receptor‐positive breast cancer.
Competing Interests
There are no competing interests to declare.
This work was supported by the Danish Council for Independent Research (4004‐00234B).
Martin T. Ernst (University of Southern Denmark) is acknowledged for valuable help with data management. Søren Friis (the Danish Cancer Society) is acknowledged for valuable help with classification of morphology codes for subtypes of breast cancer.
Supporting information
Appendix S1 Data sources
Appendix S2 Codes and definitions
Appendix S3 Probabilistic bias analysis
Table S1 Association between the second‐generation antipsychotics risperidone, olanzapine, quetiapine and aripriprazole and risk of breast cancer, specified by exposure pattern within the entire follow‐up‐period, excluding the last year prior to the index date
Table S2 Association between exposure to prolactin‐inducing antipsychotics and risk of breast cancer, specified by exposure pattern within the entire follow‐up‐period, excluding the last year prior to the index date and stratified by oestrogen receptor status
Table S3 Associations between long‐term exposure (equivalent to ≥10 000 mg of olanzapine) to first‐generation antipsychotics and second‐generation antipsychotics with prolactin‐inducing properties as well as nonprolactin‐inducing antipsychotics and the risk of breast cancer, specified by patient subgroups or cancer subtype or stage
Table S4 Association between exposure to prolactin‐inducing antipsychotics and risk of breast cancer, specified by exposure pattern within the last 5 years prior to index date, excluding the last year prior to the index date
Pottegård, A. , Lash, T. L. , Cronin‐Fenton, D. , Ahern, T. P. , and Damkier, P. (2018) Use of antipsychotics and risk of breast cancer: a Danish nationwide case–control study. Br J Clin Pharmacol, 84: 2152–2161. 10.1111/bcp.13661.
References
- 1. Anderson BO, Lipscomb J, Murillo RH, Thomas DB. Breast cancer In: Cancer Dis Control Priorities, Third edn, Vol. 3 [Internet], eds Gelband H, Jha P, Sankaranarayanan R, Horton S. Washington (DC): The International Bank for Reconstruction and Development/The World Bank, 2015. [cited 2016 May 13]. Available at http://www.ncbi.nlm.nih.gov/books/NBK343636/. [Google Scholar]
- 2. Anothaisintawee T, Wiratkapun C, Lerdsitthichai P, Kasamesup V, Wongwaisayawan S, Srinakarin J, et al Risk factors of breast cancer: a systematic review and meta‐analysis. Asia‐Pac J Public Health 2013; 25: 368–387. [DOI] [PubMed] [Google Scholar]
- 3. Ben‐Jonathan N, Liby K, McFarland M, Zinger M. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol Metab 2002; 13: 245–250. [DOI] [PubMed] [Google Scholar]
- 4. Oakes SR, Rogers RL, Naylor MJ, Ormandy CJ. Prolactin regulation of mammary gland development. J Mammary Gland Biol Neoplasia 2008; 13: 13–28. [DOI] [PubMed] [Google Scholar]
- 5. Rahman T, Clevenger CV, Kaklamani V, Lauriello J, Campbell A, Malwitz K, et al Antipsychotic treatment in breast cancer patients. Am J Psychiatry 2014; 171: 616–621. [DOI] [PubMed] [Google Scholar]
- 6. Vanneste M, Naderi A. Prolactin‐induced protein regulates cell adhesion in breast cancer. Biochem Biophys Res Commun 2015; 468: 850–856. [DOI] [PubMed] [Google Scholar]
- 7. De Hert M, Peuskens J, Sabbe T, Mitchell AJ, Stubbs B, Neven P, et al Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: a critical review. Acta Psychiatr Scand 2016; 133: 5–22. [DOI] [PubMed] [Google Scholar]
- 8. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin 2014; 64: 52–62. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez I, Touraine P, Goffin V. Prolactin and human tumourogenesis. J Neuroendocrinol 2010; 22: 771–777. [DOI] [PubMed] [Google Scholar]
- 10. Clevenger CV. Role of prolactin/prolactin receptor signaling in human breast cancer. Breast Dis 2003; 18: 75–86. [DOI] [PubMed] [Google Scholar]
- 11. Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol 2012; 1: 533–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LaPensee EW, Ben‐Jonathan N. Novel roles of prolactin and estrogens in breast cancer: resistance to chemotherapy. Endocr Relat Cancer 2010; 17: R91–R107. [DOI] [PubMed] [Google Scholar]
- 13. Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV. Expression of prolactin and its receptor in human breast carcinoma. Endocrinology 1997; 138: 5555–5560. [DOI] [PubMed] [Google Scholar]
- 14. Wennbo H, Gebre‐Medhin M, Gritli‐Linde A, Ohlsson C, Isaksson OG, Törnell J. Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest 1997; 100: 2744–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 2014; 28: 421–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tworoger SS, Eliassen AH, Zhang X, Qian J, Sluss PM, Rosner BA, et al A 20‐year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res 2013; 73: 4810–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tworoger SS, Rice MS, Rosner BA, Feeney YB, Clevenger CV, Hankinson SE. Bioactive prolactin levels and risk of breast cancer: a nested case‐control study. Cancer Epidemiol Biomark Prev 2015; 24: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Froes Brandao D, Strasser‐Weippl K, Goss PE. Prolactin and breast cancer: the need to avoid undertreatment of serious psychiatric illnesses in breast cancer patients: a review. Cancer 2016; 122: 184–188. [DOI] [PubMed] [Google Scholar]
- 19. Goodman G, Bercovich D. Prolactin does not cause breast cancer and may prevent it or be therapeutic in some conditions. Med Hypotheses 2008; 70: 244–251. [DOI] [PubMed] [Google Scholar]
- 20. Tworoger SS, Hankinson SE. Prolactin and breast cancer etiology: an epidemiologic perspective. J Mammary Gland Biol Neoplasia 2008; 13: 41–53. [DOI] [PubMed] [Google Scholar]
- 21. De Hert M, Vancampfort D, Stubbs B, Sabbe T, Wildiers H, Detraux J. Antipsychotic treatment, prolactin, and breast tumorigenesis. Psychiatr Danub 2016; 28: 243–254. [PubMed] [Google Scholar]
- 22. Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE. Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res 2004; 64: 6814–6819. [DOI] [PubMed] [Google Scholar]
- 23. Morrens M, Destoop M, Cleymans S, van der Spek S, Dom G. Evolution of first‐generation and second‐generation antipsychotic prescribing patterns in Belgium between 1997 and 2012: a population‐based study. J Psychiatr Pract 2015; 21: 248–258. [DOI] [PubMed] [Google Scholar]
- 24. Pringsheim T, Gardner DM. Dispensed prescriptions for quetiapine and other second‐generation antipsychotics in Canada from 2005 to 2012: a descriptive study. CMAJ Open 2014; 2: E225–E232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huskamp HA, O'Malley AJ, Horvitz‐Lennon M, Taub AL, Berndt ER, Donohue JM. How quickly do physicians adopt new drugs? The case of second‐generation antipsychotics. Psychiatr Serv 2013; 64: 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azoulay L, Yin H, Renoux C, Suissa S. The use of atypical antipsychotics and the risk of breast cancer. Breast Cancer Res Treat 2011; 129: 541–548. [DOI] [PubMed] [Google Scholar]
- 27. Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry – history, content, quality and use. Dan Med Bull 1997; 44: 535–539. [PubMed] [Google Scholar]
- 28. Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health 2011; 39: 42–45. [DOI] [PubMed] [Google Scholar]
- 29. Pottegård A, Schmidt SAJ, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data Resource Profile: The Danish National Prescription Registry. Int J Epidemiol 2017; 46: 798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7: 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health 2011; 39: 72–74. [DOI] [PubMed] [Google Scholar]
- 32. Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health 2011; 39: 54–57. [DOI] [PubMed] [Google Scholar]
- 33. Jensen VM, Rasmussen AW. Danish Education Registers. Scand J Public Health 2011; 39: 91–94. [DOI] [PubMed] [Google Scholar]
- 34. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health 2011; 39: 103–105. [DOI] [PubMed] [Google Scholar]
- 35. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014; 29: 541–549. [DOI] [PubMed] [Google Scholar]
- 36. Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011; 39: 22–25. [DOI] [PubMed] [Google Scholar]
- 37. Rothman KJ, Greenland S, Lash TL. Modern epidemiology, 3rd edn. Philadelphia: Wolters Kluwer Health, Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 38. Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry 2010; 167: 686–693. [DOI] [PubMed] [Google Scholar]
- 39. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2016. Oslo, 2016.
- 40. Pottegård A, Hallas J. New use of prescription drugs prior to a cancer diagnosis. Pharmacoepidemiol Drug Saf 2016; 26: 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 42. Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD‐10 diagnostic coding used to assess Charlson comorbidity index conditions in the population‐based Danish National Registry of Patients. BMC Med Res Methodol 2011; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lash TL, Fox MP, Fink AK. Applying quantitative bias analysis to epidemiological data. New York: Springer, 2009. [Google Scholar]
- 44. Thygesen LC, Daasnes C, Thaulow I, Brønnum‐Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health 2011; 39: 12–16. [DOI] [PubMed] [Google Scholar]
- 45. Catalá‐López F, Suárez‐Pinilla M, Suárez‐Pinilla P, Valderas JM, Gómez‐Beneyto M, Martinez S, et al Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta‐analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother Psychosom 2014; 83: 89–105. [DOI] [PubMed] [Google Scholar]
- 46. Gutzman JH, Miller KK, Schuler LA. Endogenous human prolactin and not exogenous human prolactin induces estrogen receptor alpha and prolactin receptor expression and increases estrogen responsiveness in breast cancer cells. J Steroid Biochem Mol Biol 2004; 88: 69–77. [DOI] [PubMed] [Google Scholar]
- 47. Rasmussen LM, Frederiksen KS, Din N, Galsgaard E, Christensen L, Berchtold MW, et al Prolactin and oestrogen synergistically regulate gene expression and proliferation of breast cancer cells. Endocr Relat Cancer 2010; 17: 809–822. [DOI] [PubMed] [Google Scholar]
- 48. Bhatavdekar JM, Patel DD, Shah NG, Vora HH, Suthar TP, Ghosh N, et al Prolactin as a local growth promoter in patients with breast cancer: GCRI experience. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2000; 26: 540–547. [DOI] [PubMed] [Google Scholar]
- 49. Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R, et al Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 2002; 59: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 50. Dalton SO, Johansen C, Poulsen AH, Nørgaard M, Sørensen HT, McLaughlin JK, et al Cancer risk among users of neuroleptic medication: a population‐based cohort study. Br J Cancer 2006; 95: 934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kelly JP, Rosenberg L, Palmer JR, Rao RS, Strom BL, Stolley PD, et al Risk of breast cancer according to use of antidepressants, phenothiazines, and antihistamines. Am J Epidemiol 1999; 150: 861–868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Data sources
Appendix S2 Codes and definitions
Appendix S3 Probabilistic bias analysis
Table S1 Association between the second‐generation antipsychotics risperidone, olanzapine, quetiapine and aripriprazole and risk of breast cancer, specified by exposure pattern within the entire follow‐up‐period, excluding the last year prior to the index date
Table S2 Association between exposure to prolactin‐inducing antipsychotics and risk of breast cancer, specified by exposure pattern within the entire follow‐up‐period, excluding the last year prior to the index date and stratified by oestrogen receptor status
Table S3 Associations between long‐term exposure (equivalent to ≥10 000 mg of olanzapine) to first‐generation antipsychotics and second‐generation antipsychotics with prolactin‐inducing properties as well as nonprolactin‐inducing antipsychotics and the risk of breast cancer, specified by patient subgroups or cancer subtype or stage
Table S4 Association between exposure to prolactin‐inducing antipsychotics and risk of breast cancer, specified by exposure pattern within the last 5 years prior to index date, excluding the last year prior to the index date


