Abstract
Aims
The aim of this study was to investigate the putative link between dipeptidyl peptidase‐4 inhibitor (DPP‐4i) use and the risk of fracture in patients with type 2 diabetes.
Methods
This propensity‐score‐matched population‐based cohort study was performed between 2009 and 2013 on patients with type 2 diabetes who were stable metformin users. A total of 3996 patients with type 2 diabetes used DPP‐4i as a second‐line antidiabetic drug. The same number of matched non‐DPP‐4i users were followed up until fracture occurrence, health insurance policy termination, or the end of 2013. The incidence rates of overall and cause‐specific fractures were estimated based on the Poisson assumption. A multiple Cox proportional hazard model was used to estimate the covariate‐adjusted hazard ratio (HR) and 95% confidence interval (CI) to determine the association between DPP‐4i use and overall and cause‐specific fractures stratified by age and sex.
Results
Over a maximum follow‐up period of 5 years, 340 DPP‐4i users and 419 non‐DPP‐4i users were newly diagnosed with fractures, yielding incidence rates of 28.03 and 32.04 per 1000 people per year, respectively. The Cox proportional hazard model revealed that DPP‐4i use significantly reduced the risk of all‐cause fractures and upper extremity fractures, with adjusted HRs of 0.86 (95% CI: 0.74–0.99) and 0.75 (95% CI: 0.59–0.95), respectively. The aforementioned associations of DDP‐4i use with fracture were sustained across sex and age stratifications.
Conclusions
The results of this study supported the premise that DPP‐4i usage is associated with a reduced risk of all‐cause fractures and upper extremity fractures in patients with type 2 diabetes.
Keywords: type 2 diabetes, fracture, cohort study, dipeptidyl peptidase‐4 inhibitor
What is Already Known about this Subject
Patients with type 2 diabetes mellitus have increased risks of osteoporosis, bone fragility and even bone fractures.
Dipeptidyl peptidase‐4 inhibitors (DPP‐4is) are a relatively new antihyperglycaemic drug that has been on the market since 2006.
Previous randomized controlled trials and meta‐analyses revealed either positive or negative effects of the association between DPP‐4i use and fracture risks.
What this Study Adds
This five‐year population‐based cohort study demonstrated that DPP‐4i use is significantly associated with decreased risks of all fractures and lower extremity fractures in patients with type 2 diabetes.
Our study reveals a potential beneficial effect sustained across all age and sex stratifications, as well as the overall and cause‐specific fractures.
Although some potential mechanisms have been proposed for the putative link between DPP‐4i use and the reduced risk of fracture, additional pharmacoepidemiological studies are required to provide more concrete evidence for the seemingly beneficial effect of DPP‐4i use.
Introduction
Patients with type 2 diabetes mellitus have increased risks of osteoporosis, bone fragility, and even bone fractures 1, 2. Among such patients, bone fractures are usually triggered by recurrent falls 3 caused by diabetes‐related comorbidities such as retinopathy, loss of balance, neuropathy and hypoglycaemic events 1, 4, 5, as well as hyperglycaemia‐induced alterations in the tissue–matrix composition. Glucose‐lowering medications such as thiazolidinedione have been reported to reduce bone density 6, 7 and increase the risk of fracture 8, 9. A recent large‐scale study integrated data from two trials and proposed that canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, could increase the risk of fracture 10. In addition, despite its neutral effect on bone density 11, insulin therapy is associated with an increased fracture risk 12, 13, 14.
The pharmacological target of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1612 (DPP‐4) is part of the prolyl oligopeptidase family 15. DPP‐4 is well known for its role in glucose homeostasis and has become a validated therapeutic target for the treatment of type 2 diabetes 16. DPP‐4 inhibitors (DPP‐4is) reduce the rate of GLP‐1 inactivation and are a relatively new antihyperglycaemic drug that has been on the market since 2006 17. Since then, five DPP‐4i drugs have received coverage from Taiwan National Health Insurance: http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6286 on 1 March, 2009; http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6316 on 1 March, 2011; http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6310 on 1 August, 2011; http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6318 on 1 June, 2012; and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6319 on 1 November, 2015. Two other drugs belonging to this class, teneligliptin and anagliptin, have not receive marketing authorization in Taiwan. DPP‐4is might influence bone metabolism, potentially reducing fracture risk 18. A meta‐analysis of randomized controlled trials (RCTs) showed a reduced fracture risk in DPP‐4i users 19. Similarly, a previous study demonstrated that the serum levels of total alkaline phosphatase and urinary deoxypyridinoline considerably decreased in postmenopausal women with diabetes who received 12 weeks of sitagliptin therapy 20. By contrast, other meta‐analyses of RCTs revealed no significant associations between fracture events and DPP‐4i use compared with placebo or active comparator use 21, 22. Moreover, a series of observational studies found no associations between DPP‐4i use and fracture risk 23, 24, 25, 26.
In addition to inconsistency in the findings of previous studies, one major limitation of observational studies and RCTs is that the actual duration of DPP‐4i use might be too short to reveal a real association between DPP‐4i use and fracture risk 19, 23, 24. The longest recorded follow‐up period for DPP‐4i use is only 1.6 years 25. In some observational studies, fracture was not the primary endpoint and data on fractures were not routinely collected 23, 24, 27. Moreover, some meta‐analyses have combined heterogeneous comparison groups characterized by different ages, sexes and sites of fracture 19, 22. However, the incidence rates of fractures are different for men and women, and the disease burden of fractures increases with age 28. A previous meta‐analysis suggested that thiazolidinediones caused differing bone fracture incidence rates for men and women and that the fracture risk associated with thiazolidinediones increases with age 29. Additionally, another study also showed that the fracture risk associated with thiazolidinediones differed depending on the fracture site, especially in older patients 30. Despite this data, no studies had attempted to measure the potential effects of age and sex on DPP‐4i use and the risk of fracture.
Because of contradictory evidence and a lack of information concerning the incidence rates of fractures after long‐term DPP‐4i use 19, 23, 24 depending on age, sex and fracture site, we conducted the current five‐year population‐based cohort study to elucidate the relationship between DPP‐4i use and fracture risk. In addition to overall fracture risk, we investigated site‐specific fracture risk associated with DPP‐4i use.
Methods
Data source
In this study, we analysed the medical claims data of the Longitudinal Cohort of Diabetes Patients (LHDB) from 1999 to 2013. The data were retrieved from the Taiwan National Health Insurance Research Database (NHIRD). Details of the LHDB were reported in our earlier study 31. Briefly, the LHDB consists of a sample of 120 000 incident cases of diabetes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code 250.xx) between 1999 and 2013. These patients were randomly selected from all patients with diabetes who are covered by the National Health Insurance program (NHIP), a mandatory‐enrolment, single‐payment, state‐run system that covers more than 99% of Taiwan's population. In our study, in order to be considered a patient with diabetes, an individual must meet at least one of the following three criteria: (1) two or more ambulatory care visits with a diagnosis of diabetes within 1 year; (2) one ambulatory care visit with a diagnosis of diabetes and prescription of antidiabetic drugs within 1 year; and (3) one or more hospitalizations with a discharge diagnosis of diabetes or prescription of antidiabetic drugs within 1 year 32, 33. Because the NHIP was implemented in 1995, the LHDB excludes patients diagnosed with diabetes before 1999 to ensure that only incident cases of diabetes are included. In total, 1 197 371 patients with diabetes were initially identified.
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital, who granted a waiver from obtaining informed consent from individual patients because the patients in the database analysed, the NHIRD, were de‐identified (Approval No. A‐ER‐103‐298).
Study cohort and design
We established two inclusion criteria for the study cohort based on the LHDB. First, only patients who fit the ICD‐9‐CM diagnosis code of 250.x0 or 250.x2 (type 2 diabetes) were included in this study. Second, patients were required to have at least three consecutive refills of metformin with an interval between any two consecutive refills of <30 days during the follow‐up period. A total of 427 223 patients met these two criteria from 1999 to 2013 and were considered patients with type 2 diabetes who were stable metformin users.
Because DPP‐4i use was not approved by the NHIP until 2009 34, we further identified patients with any second‐line antidiabetic drug use during 2009–2012. In Taiwan, various DPP‐4 inhibitors have been used, including sitagliptin, vildagliptin, saxagliptin, alogliptin, linagliptin, gemigliptin, and evogliptin. The daily dose of DPP‐4i prescribed to patients with type 2 diabetes in clinical settings has ranged from 5 mg (saxagliptin and linagliptin) to 100 mg (sitagliptin). The starting date of second‐line antidiabetic drug use was defined as the index date. Patients using any second‐line antidiabetic drug or receiving insulin therapy within 180 days before the index date were excluded to ensure that all the study patients were new users of dual therapy (n = 53 347). To ensure the validity of the incidence rate of fracture, we further excluded patients with a history of fracture (ICD‐9‐CM codes 800–829) or cancer (ICD‐9‐CM codes 140–243) within 1 year before the index date (n = 8401). Finally, 44 946 patients were included in the study cohort.
In the study cohort, 3996 patients used a DPP‐4i as a second‐line antidiabetic drug, and the remaining 40 950 patients used other second‐line antidiabetic drugs including sulfonylurea, acarbose, meglitinide, and thiazolidinedione. Figure 1 presents the details of study cohort enrolment.
Figure 1.
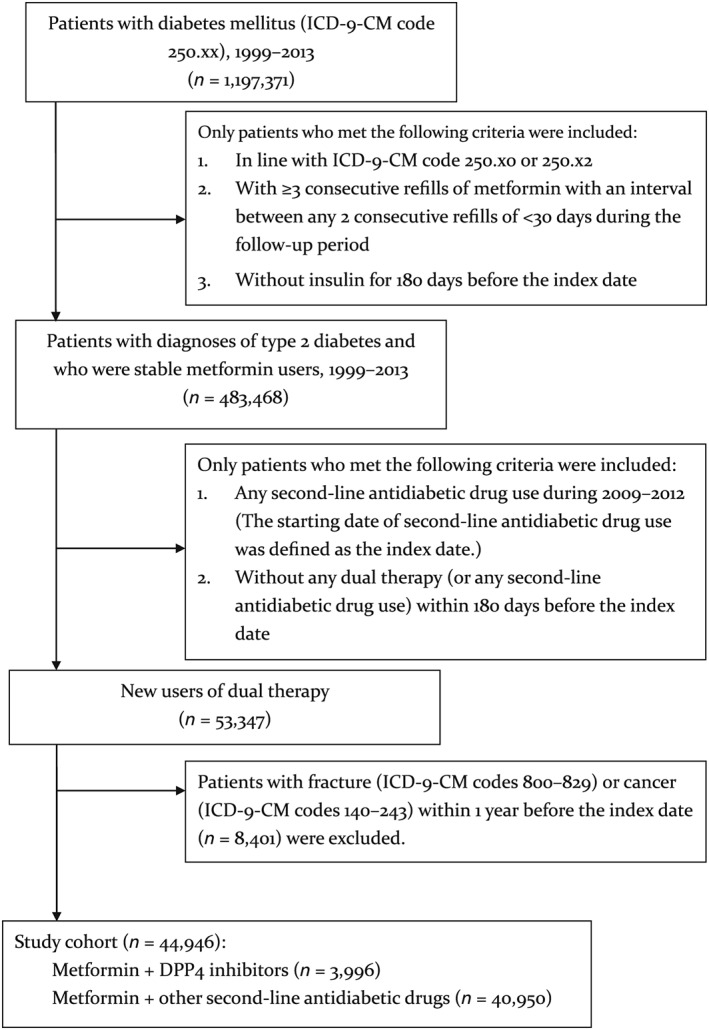
Flowchart of study cohort enrolment
To minimize the potential for confounding by indication, we performed one‐on‐one propensity score matching to select non‐DPP‐4 users. The matching algorithm determines first the ‘best’ matches and subsequently the ‘next‐best’ matches in a hierarchical sequence until no more matches can be determined (i.e., greedy matching technique) 35. For each study patient, the propensity score was calculated using all the variables listed in Table 1 to compare the baseline characteristics of DPP‐4i users and non‐DPP‐4i users before and after propensity score matching. The information in Table 1 indicates that after propensity score matching, the baseline characteristics of DPP‐4i users and non‐DPP‐4i users were fairly comparable.
Table 1.
Comparison of baseline characteristics of DPP‐4i and non‐DPP‐4i users before and after propensity score matching
| Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|
| Non‐DPP‐4i users | DPP‐4i users | P‐value | Non‐DPP‐4i users | DPP‐4i users | P‐value | |
| n = 40 950 | n = 3996 | n = 3996 | n = 3996 | |||
| Men | 55.64% | 53.58% | 0.0123 | 54.48% | 53.58% | 0.4191 |
| Age (years) | 54.24 ± 12.42 | 54.00 ± 12.72 | 0.1787 | 53.86 ± 12.33 | 54.00 ± 12.72 | 0.7486 |
| CCI | 2.95 ± 1.37 | 2.90 ± 1.35 | 0.0535 | 2.90 ± 1.36 | 2.90 ± 1.35 | 0.7917 |
| DCSI | 0.80 ± 1.36 | 0.98 ± 1.50 | <0.0001 | 0.97 ± 1.58 | 0.98 ± 1.50 | 0.6851 |
| Duration of type 2 diabetes | 3.84 ± 3.24 | 4.15 ± 3.35 | <0.0001 | 4.17 ± 3.32 | 4.15 ± 3.35 | 0.8028 |
| Comorbidity | ||||||
| Fall | 0.04% | 0.03% | 0.7105 | 0.03% | 0.03% | 1.0000 |
| Osteoporosis | 2.16% | 2.65% | 0.434 | 2.40% | 2.65% | 0.4761 |
| Dizziness | 16.58% | 13.26% | <0.0001 | 12.56% | 13.26% | 0.3503 |
| Vertigo | 5.78% | 5.88% | 0.7850 | 5.71% | 5.88% | 0.7375 |
| Visual impairment | 36.39% | 40.92% | <0.0001 | 42.19% | 40.92% | 0.2470 |
| Hyperthyroidism | 1.27% | 1.90% | 0.0009 | 1.83% | 1.90% | 0.8041 |
| Parahypothyroidism | 0.05% | 0% | 0.1428 | 0% | 0% | – |
| Hypothyroidism | 0.56% | 1.48% | <0.0001 | 1.08% | 1.48% | 0.1108 |
| Arthropathy | 25.79% | 25.08% | 0.3221 | 25.78% | 25.08% | 0.4720 |
| Rheumatism | 30.46% | 30.21% | 0.7344 | 29.70% | 30.21% | 0.6253 |
| Dorsopathy | 26.06% | 24.60% | 0.0442 | 24.52% | 24.60% | 0.9379 |
| Osteopathy, chondropathy, and acquired musculoskeletal deformities | 1.50% | 1.40% | 0.6173 | 1.65% | 1.40% | 0.3616 |
| Joint pain | 24.69% | 23.87% | 0.2538 | 24.65% | 23.87% | 0.4186 |
| Pathological fracture | 0.17% | 0.25% | 0.2742 | 0.20% | 0.25% | 0.6370 |
| COPD | 11.13% | 10.56% | 0.2731 | 9.58% | 10.56% | 0.1472 |
| Alcohol‐related diseases | 1.24% | 0.73% | 0.0044 | 0.55% | 0.73% | 0.3254 |
| Anorexia nervosa and bulimia | 5.20% | 4.70% | 0.1794 | 4.23% | 4.70% | 0.3036 |
| Chronic kidney disease | 1.35% | 1.65% | 0.1189 | 1.33% | 1.65% | 0.2299 |
| Comedication | ||||||
| Steroid | 19.52% | 16.57% | <0.0001 | 15.02% | 16.57% | 0.0572 |
| Vitamin D | 0.06% | 0.10% | 0.3535 | 0.08% | 0.10% | 0.7053 |
| Calcium | 1.00% | 0.98% | 0.9015 | 0.73% | 0.98% | 0.2233 |
| Selective oestrogen receptor modulator | 0.07% | 0.18% | 0.0320 | 0.13% | 0.18% | 0.5634 |
| Parathyroid analogue | 0.01% | 0.05% | 0.1094 | 0.03% | 0.05% | 0.5636 |
| Biphosphate | 0.24% | 0.40% | 0.0488 | 0.23% | 0.40% | 0.1609 |
CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; DSCI, Diabetes Complications Severity Index
Follow‐up and study endpoint
For each study patient, the follow‐up period started from the index date and ended on the date of event (i.e., fracture) diagnosis or censoring, which was either the date of health insurance policy termination or the final day of 2013. The follow‐up length ranged from a minimum of 1 year (2012–2013) to a maximum of 5 years (2009–2013). The study endpoint was all‐cause fracture (ICD‐9‐CM codes 810–829). In addition, subgroup analyses were conducted to estimate the risks of hip fracture (ICD‐9‐CM codes 820–821), lower extremity fracture (ICD‐9‐CM codes 820–829) and upper extremity fracture (ICD‐9‐CM codes 810–819). The study endpoint was identified through the interlinking of the study cohort to both ambulatory and hospitalization medical claims.
Covariates
The covariates considered in this study included patient age on the index date, sex and various comorbidities identified within 1 year before the index date. The comorbidities considered were fall (ICD‐9‐CM E codes 880–888); osteoporosis (ICD‐9‐CM code 733); dizziness (ICD‐9‐CM code 780.4); vertigo (ICD‐9‐CM code 386); visual impairment (ICD‐9‐CM codes 360–379); hyperthyroidism (ICD‐9‐CM code 242); parahypothyroidism (ICD‐9‐CM code 252); hypothyroidism (ICD‐9‐CM code 244); arthropathy (ICD‐9‐CM codes 710–719); rheumatism (ICD‐9‐CM codes 714 and 725–729); dorsopathy (ICD‐9‐CM codes 720–724); osteopathy, chondropathy and acquired musculoskeletal deformities (ICD‐9‐CM codes 730–732 and 734–739, respectively); joint pain (ICD‐9‐CM codes 713, 715, 716, 718, and 719); cancer (ICD‐9‐CM codes 140–239); pathological fracture (ICD‐9‐CM code 733.1); smoking (chronic obstructive pulmonary disease; ICD‐9‐CM codes 490–496); alcohol‐related diseases (ICD‐9‐CM codes 291, 303, 305.0, and 571.0–571.3); anorexia nervosa and bulimia (ICD‐9‐CM codes 307 and 783); and chronic kidney disease (ICD‐9‐CM code 585). In addition, we calculated the Charlson comorbidity index 36 and Diabetes Complication Severity Index 37, 38 to represent each patient's comorbidity level and type 2 diabetes severity, respectively.
Statistical analysis
Patient characteristics were analysed using descriptive statistics, which included the mean, standard deviation, frequency and proportion. Baseline covariates were compared using the independent t‐test or the Pearson chi‐square (χ2) test. The incidence rate of fractures was calculated under the Poisson assumption as the total number of people who developed fractures during the follow‐up period divided by people at risk per year. The people at risk per year were calculated from the index date to the occurrence of the endpoint, NHIP termination, or the end of 2013.
A Cox proportional hazard model was used to estimate crude and covariate‐adjusted hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs). We assessed the potential problem of collinearity among the covariates by examining the estimated slope coefficients and SEM and found no indication of collinearity. The proportional hazard assumption was verified using plots of log(−log(survival function)) vs. log(time) and Schoenfeld residuals vs. time. We found no evidence to contradict the proportionality assumption for the Cox proportional model. The significance level of all hypothesis testing was set at 0.05. SAS 9.4 software (SAS Institute, Inc., Cary, NC) was utilized for the aforementioned analyses.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 39, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 15.
Results
The characteristics of the study population are shown in Table 1. After propensity score matching, the distributions of age, sex, comorbidity level, severity and duration of diabetes, and selected comorbidities that predispose patients to fracture risk were comparable between DPP‐4i users and non‐DPP‐4i users.
Over a maximum follow‐up period of 5 years, 340 (8.5%) DPP‐4i users and 419 (10.5%) non‐DPP‐4i users developed fractures, corresponding to the incidence rates of 28.03 and 32.04 per 1000 people per year, respectively. The incidence rates of fracture for male DPP‐4i users and non‐DPP‐4i users were 21.51 and 25.01 per 1000 patients per year, respectively, and the corresponding rates for females were much higher at 35.57 and 40.18 per 1000 people per year, respectively. The Cox proportional hazard model indicated that DPP‐4i use was significantly associated with a reduced risk of all fractures (adjusted HR: 0.86 [95% CI: 0.74–0.99]). A similar reduction in HR was also noted for men (adjusted HR: 0.87 [95% CI: 0.69–1.08]) and women (adjusted HR: 0.88 [95% CI: 0.73–1.07]). However, this sex‐specific reduction did not reach statistical significance (P = 0.75). Additional sex‐ and age‐stratified analyses revealed that women aged 45–54 years were the only age group of either sex who exhibited a significantly reduced risk of all fractures (adjusted HR: 0.48 [95% CI: 0.32–0.73]; Table 2). Figure 2 compares the Nelson–Aalen cumulative hazard (event) rates of the onset of all‐cause and site‐specific fractures for patients receiving DPP‐4i and those not. The patients receiving DPP‐4i had lower event rates over the study period.
Table 2.
Overall, age‐specific, and sex‐specific incidence rates and hazard ratios of all fractures (ICD‐9‐CM codes 800–820) in association with DPP‐4i use in patients with type 2 diabetesa
| Variable | Non‐DPP‐4i users | DPP‐4i users | Crude HR (95% CI) | Adjusted HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | Number of events | Incidence rate b | Number of patients | Number of events | Incidence rate b | |||
| Men | ||||||||
| ≤44 | 619 | 52 | 25.43 | 605 | 40 | 21.50 | 0.87 (0.57–1.32) | 0.87 (0.57–1.31) |
| 45–54 | 752 | 55 | 22.07 | 689 | 39 | 18.34 | 0.82 (0.54–1.23) | 0.78 (0.51–1.19) |
| 55–65 | 445 | 35 | 24.20 | 486 | 30 | 20.30 | 0.83 (0.51–1.35) | 0.88 (0.54–1.45) |
| ≥65 | 361 | 36 | 31.80 | 361 | 31 | 29.67 | 0.96 (0.59–1.55) | 0.90 (0.54–1.50) |
| Total | 2177 | 178 | 25.01 | 2141 | 140 | 21.51 | 0.87 (0.69–1.08) | 0.84 (0.66–1.05) |
| Women | ||||||||
| ≤44 | 364 | 23 | 18.11 | 384 | 25 | 20.76 | 1.18 (0.66–2.08) | 1.23 (0.69–2.19) |
| 45–54 | 560 | 72 | 39.50 | 572 | 34 | 19.08 | 0.48 (0.32–0.73) | 0.43 (0.28–0.65) |
| 55–64 | 520 | 60 | 36.70 | 496 | 63 | 41.14 | 1.11(0.78–1.58) | 1.02 (0.70–1.46) |
| ≥65 | 375 | 79 | 72.02 | 403 | 78 | 70.65 | 0.98 (0.72–1.34) | 0.97 (0.69–1.33) |
| Total | 1819 | 234 | 40.18 | 1855 | 200 | 35.57 | 0.88 (0.73–1.07) | 0.86 (0.71–1.04) |
| Overall | 3996 | 419 | 32.04 | 3996 | 340 | 28.03 | 0.86 (0.74–0.99) | 0.86 (0.74–0.99) |
The P‐values for the interactions of DPP‐4i use with sex, age in men and age in women were 0.75, 0.94 and 0.93, respectively.
Per 1000 people per year.
CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; HR, hazard ratio; ICD‐9‐CM, International Classification of Diseases, Ninth Reversion, Clinical Modification. Bold numbers were referred to significance.
Figure 2.
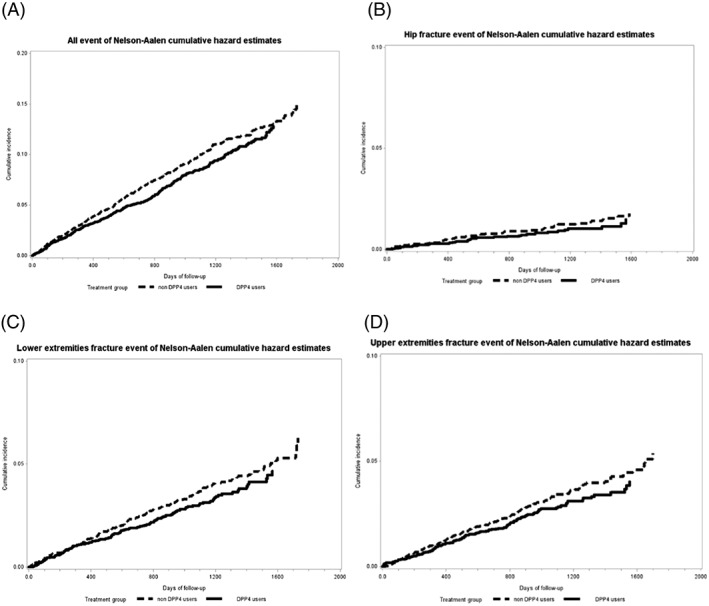
Comparison of Nelson–Aalen cumulative hazard estimates of the onset of (A) all fractures (ICD‐9‐CM codes 800–820), (B) hip fracture (ICD‐9‐CM codes 820–821), (C) lower extremity fracture (ICD‐9‐CM codes 820–829), and (D) upper extremity fracture (ICD‐9‐CM codes 810–819) between the DPP‐4i and non‐DPP‐4i groups
Supporting Information Tables S1–S3 show the HRs of fractures at specific sites in relation to DPP‐4i use. DPP‐4i use did not significantly reduce the risk of hip fracture (adjusted HR: 0.89 [95% CI: 0.57–1.39]). Such null results were observed across all age and sex stratifications (Supporting Information Table S1). Table S4 also revealed that DPP‐4i use did not reduce the overall or sex‐specific risk of lower extremity fracture. However, sex‐ and age‐stratified analyses indicated that DPP‐4i use significantly reduced the risk of lower extremity fractures among men and women aged 46–55 years (adjusted HR: 0.40 [95% CI: 0.19–0.81] and 0.48 [95% CI: 0.25–0.91], respectively; Supporting Information Table S2). DPP‐4i use was found to significantly reduce the risk of upper extremity fracture among all study patients (adjusted HR: 0.75 [95% CI: 0.59–0.95]), all female patients (adjusted HR: 0.68 [95% CI: 0.49–0.95]), and women aged 55–64 years (0.52 [95% CI: 0.28–0.97]; Supporting Information Table S3). Nonetheless, the aforementioned sex and age differences in relation to the risks of lower and upper extremity fractures did not reach statistical significance in the multiple testing of subgroup analyses. Additional analyses were performed for head‐to‐head comparisons of fracture risks between patients receiving DPP‐4i and those receiving other oral antidiabetes drugs individually. These data, which are presented in Supporting Information Table S4, demonstrated that patients treated with DPP‐4i tended to experience consistently lower risk of all fractures and site‐specific fracture compared with patients treated with other drugs except acarbose.
Discussion
In this population‐based cohort study, we discovered that DPP‐4i use was significantly associated with reduced risks of all‐cause and upper extremity fractures in patients with type 2 diabetes. No significant interaction was observed between DPP‐4i use and age or sex, but the data did indicate the presence of a significant association between DPP‐4i and a reduced risk of all fractures only in women with diabetes aged 45–54 years. However, we should conservatively interpret the results of subgroup analyses because multiple comparisons could have yielded false positive findings. Furthermore, site‐specific analysis suggested that a significantly lower risk was noted only for upper extremity fractures in women, particularly those aged 55–64 years. The aforementioned results are unlikely to have been confounded by demographic characteristics, diabetes severity or fracture‐related comorbidities. To the best of our knowledge, the current study is the first age‐ and sex‐stratified analysis to evaluate the relationship of DPP4i usage according to different fracture sites.
An earlier meta‐analysis of 28 randomized clinical trials involving 11 880 and 9175 patients with DPP‐4i and comparator use, respectively, found that compared with placebo or other treatments, DPP‐4i use was associated with a reduced risk of fractures (odds ratio: 0.60, 95% CI: 0.37–0.99, P = 0.045) 19. However, such a protective effect was not observed in a subsequent updated meta‐analysis, which incorporated more study participants (62 206 including 33 452 patients treated with DPP‐4i) 22. In addition to the inconsistent study findings, the durations of most trials included in the aforementioned meta‐analyses were relatively short (mostly 24 months), which does not facilitate the drawing of inferences on the long‐term effects of DPP‐4i use on fractures. Furthermore, bone fractures were not the primary endpoints in any of the aforementioned trials and have been reported only as adverse events, which constitute only a fraction of all fractures 19, 21.
Driessen et al. 23 conducted a retrospective population‐based cohort study by using data from the Clinical Practice Research Datalink (CPRD) database from 2007 to 2012. The researchers found that DPP4‐i use was not associated with fracture risk compared with controls and other noninsulin antidiabetic drugs. However, the actual duration of DPP4‐i use was only 1.3 years. In a subsequent case–control study, Driessen et al. 24 analysed the relationships between DPP4i use and various site‐specific risks of fracture including hip, radius or ulna, and vertebral fractures. The researchers showed that DPP4‐i use (current, recent, past or distant past) was not associated with the risk of hip fracture or radius or ulna fracture. However, although current DPP4‐i use was not associated with the risk of vertebral fracture, recent DPP4‐i use significantly decreased the risk of vertebral fracture 24. Using data from the CPRD database, Driessen et al. 25 recently conducted a retrospective population‐based cohort study covering a longer period (2007–2015). The study reported that continuous long‐term DPP‐4i use (defined as >4.0–8.5 years of DPP‐4i use for any fracture, >3.0–8.5 years for osteoporotic fracture, and >2.0–8.5 years for hip fracture) was not associated with the risk of any, osteoporotic or hip fracture. By contrast, a recent nationwide study in South Korea showed that DPP‐4i use might exert a protective effect on composite bone metabolism 40. However, meta‐analyses of RCTs 21, 22 and observational studies 23, 24, 25 have observed no significant protective effects of DPP‐4i on fracture. This discrepancy may be attributed to the potential heterogeneity in the study methodologies included in the meta‐analyses or to inadequate duration of DPP4i use. Moreover, in contrast to the aforementioned real‐life observational studies that have used a multivariate regression model to remove potential confounding factors 23, 24, 25, the current study used the propensity score matching technique to select controls, which is considered relatively effective for controlling for confounding factors by indication 41. This may have also contributed to the dissimilarity in findings between previous studies and the current study.
Because DPP‐4 is an enzyme involved in the degradation of glucagon‐like peptide‐1, which plays a role in promoting bone formation and inhibiting bone resorption, DPP‐4i may exert a beneficial effect on bone metabolism 42, 43, 44. In an animal study 45, the use of sitagliptin, a DPP‐4i, was found to increase trabecular bone volume, cortical bone volume and bone mineral density in diabetic male rats. Sitagliptin use was also associated with the attenuation of bone strength loss and reduction of bone resorption. These findings were supported by those of another animal study 46, where high‐fat‐diet‐fed mice treated with sitagliptin showed an increase in vertebral bone mineral density. Moreover, a recent in vivo study showed that MK‐0626, another DPP‐4i, exerted neutral effects on cortical and trabecular bone in a type 2 diabetes mouse model, and MK‐0626 did not alter osteoblast differentiation 47. Thus, bone quality may be more crucial than bone density in predicting the increased risk of fracture in patients with type 2 diabetes 5.
This study has the following strengths. First, this study adopted a large, nationwide, population‐based design with high representativeness of all patients with type 2 diabetes in Taiwan during 2009–2013. The study sample size was sufficiently large for performing age‐ and sex‐stratified analyses to investigate the potential modifiers of the effect of DPP‐4i use on fracture risk without comprising statistical power. Moreover, additional analyses were performed for head‐to‐head comparisons of fracture risks between patients receiving DPP‐4i and those receiving other oral antidiabetes drugs individually. We used the propensity score matching technique to select and pair the patients not receiving DPP‐4i to each group of patients receiving oral antidiabetes drugs other than DPP‐4i. The results indicated that patients receiving DPP‐4i tended to experience a consistently lower risk of all fractures and site‐specific fractures compared with patients receiving other drugs.
Second, in contrast to previous meta‐analyses of RCTs or observational studies of which the average study duration was relatively short (mostly 24 months), the present study adopted a longer follow‐up period (maximum of 5 years) to observe the occurrence of fracture events. Moreover, in contrast to many previous meta‐analyses 19, 21, 22 that did not use fracture as the primary endpoint, our study was designed to specifically investigate the risk of fracture in patients with type 2 diabetes, thereby also minimizing the likelihood of type 1 error inflation. Furthermore, we calculated in years the average time period from patient enrolment to the occurrence of an all‐cause fracture for patients who experienced a fracture. The average time‐to‐event periods for patients experiencing a fracture in the DPP‐4i and non‐DPP‐4i groups were 1.69 and 1.87 years, respectively. Such short drug exposure periods were less likely to change the bone metabolism. Further studies of patients after a longer period of drug exposure may provide evidence concerning the long‐term effect of DPP‐4i on fracture. Although this analysis was limited to patients who had already developed a fracture, our results did not indicate a significant difference in the risk of or time period for a fracture occurrence between patients experiencing fractures because of falls consequent to hypoglycaemia (e.g., sulfonylureas and meglitinides) and fractures directly associated with bone metabolism (e.g., thiazolidinediones).
Third, in contrast to many previous observational studies that used clinical data from several hospitals 23, 24, 25, 26, the present study is the first to use the insurance claims data of the entire population of a nation, thereby ensuring complete coverage of patients with type 2 diabetes or fractures. In addition to the overall fracture risk presented in the aforementioned South Korean study 40, we further confirmed the effect of DPP‐4i use on fractures by conducting subgroup analyses of fractures at various sites including hip, lower extremity and upper extremity fractures to ensure the robustness of our findings.
Fourth, the type 2 diabetes cohort in this study was recruited from the LHDB and all research information was retrieved from the medical claims data in the NHIRD, thereby minimizing the likelihood of erroneous recall of drug use, nonresponse and loss to follow‐up.
Fifth, to accurately estimate the incidence of fracture, we identified patients with fracture from inpatient and outpatient settings. Finally, we used propensity score matching to select controls, thereby considerably minimizing the potential for confounding by indication.
Despite the aforementioned methodological strengths, our study has several limitations. First, diagnoses of type 2 diabetes and fractures were completely dependent on ICD‐9‐CM codes, which are subject to disease misclassification. This constitutes a major limitation of this study compared with studies based on information obtained from standardized clinical examinations of patients. However, the National Health Insurance Administration of the Ministry of Health and Welfare conducts quarterly expert reviews of any hospital with outlier charges or outlier practice patterns, which could effectively minimize the potential for disease misclassification.
Second, although we adjusted for certain potential confounders in the analysis, other known risk factors for fracture were not adjusted for in the analysis. These risk factors included patients' socioeconomic background, family history of fracture 48, 49, and health behaviours (e.g., glucose control, physical activity and body mass index 50, 51, 52. Failure to comprehensively adjust for these potential confounders may result in residual confounding at least to a certain degree. However, we further conducted falsification analyses using upper gastrointestinal (UGI) haemorrhage as a negative study endpoint. UGI was not expected to be related to DPP‐4i usage. The analysis demonstrated that patients who used DPP‐4i had a slightly but insignificantly lower hazard ratio (covariate‐adjusted HR: 0.93 [95% CI: 0.80–1.09]) for all UGI haemorrhage. This additional analysis provided reassurance that the inverse relationship between DPP‐4i use and the risk of fracture found in our study was unlikely to be entirely caused by unmeasured confounders.
Third, we did not account for the fracture risk associated with a specific DPP‐4i in our study. Determining whether a dose–gradient relationship exists between DPP‐4i use and fracture risk in patients with type 2 diabetes may help to elucidate the mechanism underlying the effect of DPP‐4i use on fracture risk. Additionally, renal function adjustment was lacking in our study because no laboratory data were available in the NHIRD. However, we compared the numbers of DPP‐4i and non‐DPP‐4i users before and after propensity score matching and no significant difference was observed. Subgroup analyses of drugs belonging to this class (e.g., sitagliptin, linagliptin, saxagliptin and alogliptin) would also be needed in further studies. However, we should also interpret our study results with caution because multiple comparisons with several subgroup analyses might have increased the chance of false positive findings.
Finally, the majority of our cohort was Asian; therefore, whether the results may be extrapolated to other populations (e.g., Caucasians) must be answered through further studies.
Conclusions
This population‐based cohort study demonstrated that DPP‐4i use is significantly associated with decreased risks of all fractures and lower extremity fractures in patients with type 2 diabetes. Such a negative association was seemingly sustained across all age and sex stratifications. Although some potential mechanisms have been proposed for the putative link between DPP‐4i use and the reduced risk of fracture, additional pharmacoepidemiological studies are required to provide more concrete evidence for the seemingly beneficial effect of DPP‐4i use. Biological studies exploring the actual mechanisms underlying the association between DPP‐4i use and the risk of fracture are also warranted.
Contributors
W.H.H. designed the study, performed statistical analyses, drafted the initial manuscript, and revised the manuscript for critical content. C.K.C. analysed the data and drafted the statistical sections of the manuscript. C.Y.L. participated in the study design, interpretation of results, and revising the submitted work. H.T.O. is the guarantor of this work, has full access to all the data used in the study, and takes responsibility for the integrity of the data and accuracy of the data analysis.
Competing Interests
There are no competing interests to declare.
This study was supported by two grants from the Ministry of Science and Technology, Taiwan (MOST 104‐2314‐B‐006‐020‐MY2) and (MOST 104‐2320‐B‐006‐008‐MY3). The funders have no role in conducting and submitting this work. The authors gratefully thank Taipei Medical University Hospital, National Cheng Kung University, and their affiliated hospitals for all their support.
Supporting information
Table S1 Overall, age‐specific and sex‐specific incidence rates and hazard ratios of hip fracture (ICD‐9‐CM codes 820–821) in association with DPP‐4i use in patients with type 2 diabetes
Table S2 Overall, age‐specific and sex‐specific incidence rates and hazard ratios of lower extremity fracture (ICD‐9‐CM codes 820–829) in association with DPP‐4i use in patients with type 2 diabetes
Table S3 Overall, age‐specific and sex‐specific incidence rates and hazard ratios of upper extremity fracture (ICD‐9‐CM codes 810–819) in association with DPP‐4i use in patients with type 2 diabetes
Table S4 Comparison of covariate adjusted hazard ratio for fracture between patients receiving DPP‐4i and those receiving other oral antidiabetic agents
Hou, W.‐H. , Chang, K.‐C. , Li, C.‐Y. , and Ou, H.‐T. (2018) Dipeptidyl peptidase‐4 inhibitor use is associated with decreased risk of fracture in patients with type 2 diabetes: a population‐based cohort study. Br J Clin Pharmacol, 84: 2029–2039. 10.1111/bcp.13636.
This study was based partially on data from the National Health Insurance Research Database provided by the National Health Insurance Administration and Department of Health and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Department of Health, or National Health Research Institutes.
References
- 1. Vestergaard P. Bone metabolism in type 2 diabetes and role of thiazolidinediones. Curr Opin Endocrinol Diabetes Obes 2009; 16: 125–131. [DOI] [PubMed] [Google Scholar]
- 2. Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 1999; 22: 1196–1200. [DOI] [PubMed] [Google Scholar]
- 3. Wallace C, Reiber GE, LeMaster J, Smith DG, Sullivan K, Hayes S, et al Incidence of falls, risk factors for falls, and fall‐related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care 2002; 25: 1983–1986. [DOI] [PubMed] [Google Scholar]
- 4. Carnevale V, Romagnoli E, D'Erasmo E. Skeletal involvement in patients with diabetes mellitus. Diabetes Metab Res Rev 2004; 20: 196–204. [DOI] [PubMed] [Google Scholar]
- 5. Montagnani A, Gonnelli S. Antidiabetic therapy effects on bone metabolism and fracture risk. Diabetes Obes Metab 2013; 15: 784–791. [DOI] [PubMed] [Google Scholar]
- 6. Grey A. Thiazolidinedione‐induced skeletal fragility – mechanisms and implications. Diabetes Obes Metab 2009; 11: 275–284. [DOI] [PubMed] [Google Scholar]
- 7. Yaturu S, Bryant B, Jain SK. Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care 2007; 30: 1574–1576. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–2443. [DOI] [PubMed] [Google Scholar]
- 9. Home PD, Pocock SJ, Beck‐Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open‐label trial. Lancet 2009; 373: 2125–2135. [DOI] [PubMed] [Google Scholar]
- 10. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 11. Stolk RP, Van Daele PL, Pols HA, Burger H, Hofman A, Birkenhager JC, et al Hyperinsulinemia and bone mineral density in an elderly population: The Rotterdam Study. Bone 1996; 18: 545–549. [DOI] [PubMed] [Google Scholar]
- 12. Monami M, Cresci B, Colombini A, Pala L, Balzi D, Gori F, et al Bone fractures and hypoglycemic treatment in type 2 diabetic patients: a case‐control study. Diabetes Care 2008; 31: 199–203. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE, et al Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25: 1749–1754. [DOI] [PubMed] [Google Scholar]
- 14. Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Diabetes and risk of fracture: the Blue Mountains Eye Study. Diabetes Care 2001; 24: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 15. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 17. Dicker D. DPP‐4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care 2011; 34 (Suppl 2): S276–S278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mannucci E, Dicembrini I. Drugs for type 2 diabetes: role in the regulation of bone metabolism. Clin Cases Miner Bone Metab 2015; 12: 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monami M, Dicembrini I, Antenore A, Mannucci E. Dipeptidyl peptidase‐4 inhibitors and bone fractures: a meta‐analysis of randomized clinical trials. Diabetes Care 2011; 34: 2474–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hegazy SK. Evaluation of the anti‐osteoporotic effects of metformin and sitagliptin in postmenopausal diabetic women. J Bone Miner Metab 2015; 33: 207–212. [DOI] [PubMed] [Google Scholar]
- 21. Mamza J, Marlin C, Wang C, Chokkalingam K, Idris I. DPP‐4 inhibitor therapy and bone fractures in people with type 2 diabetes – a systematic review and meta‐analysis. Diabetes Res Clin Pract 2016; 116: 288–298. [DOI] [PubMed] [Google Scholar]
- 22. Fu J, Zhu J, Hao Y, Guo C, Zhou Z. Dipeptidyl peptidase‐4 inhibitors and fracture risk: an updated meta‐analysis of randomized clinical trials. Sci Rep 2016; 6: 29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Driessen JH, van Onzenoort HA, Henry RM, Lalmohamed A, van den Bergh JP, Neef C, et al Use of dipeptidyl peptidase‐4 inhibitors for type 2 diabetes mellitus and risk of fracture. Bone 2014; 68: 124–130. [DOI] [PubMed] [Google Scholar]
- 24. Driessen JH, van Onzenoort HA, Starup‐Linde J, Henry R, Neef C, van den Bergh J, et al Use of dipeptidyl peptidase 4 inhibitors and fracture risk compared to use of other anti‐hyperglycemic drugs. Pharmacoepidemiol Drug Saf 2015; 24: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 25. Driessen JH, van den Bergh JP, van Onzenoort HA, Henry RM, Leufkens HG, de Vries F. Long‐term use of dipeptidyl peptidase‐4 inhibitors and risk of fracture: a retrospective population‐based cohort study. Diabetes Obes Metab 2017; 19: 421–428. [DOI] [PubMed] [Google Scholar]
- 26. Driessen JH, de Vries F, van Onzenoort H, Harvey NC, Neef C, van den Bergh JP, et al The use of incretins and fractures – a meta‐analysis on population‐based real life data. Br J Clin Pharmacol 2017; 83: 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 28. Geusens P, Dinant G. Integrating a gender dimension into osteoporosis and fracture risk research. Gend Med 2007; 4 (Suppl B): S147–S161. [DOI] [PubMed] [Google Scholar]
- 29. Loke YK, Singh S, Furberg CD. Long‐term use of thiazolidinediones and fractures in type 2 diabetes: a meta‐analysis. CMAJ 2009; 180: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Betteridge DJ. Thiazolidinediones and fracture risk in patients with type 2 diabetes. Diabet Med 2011; 28: 759–771. [DOI] [PubMed] [Google Scholar]
- 31. Hou WH, Chang KC, Li CY, Ou HT. Dipeptidyl peptidase‐4 inhibitor use is not associated with elevated risk of severe joint pain in patients with type 2 diabetes: a population‐based cohort study. Pain 2016; 157: 1954–1959. [DOI] [PubMed] [Google Scholar]
- 32. Chen HF, Ho CA, Li CY. Age and sex may significantly interact with diabetes on the risks of lower‐extremity amputation and peripheral revascularization procedures: evidence from a cohort of a half‐million diabetic patients. Diabetes Care 2006; 29: 2409–2414. [DOI] [PubMed] [Google Scholar]
- 33. Chang CH, Shau WY, Jiang YD, Li HY, Chang TJ, Sheu WH, et al Type 2 diabetes prevalence and incidence among adults in Taiwan during 1999–2004: a national health insurance data set study. Diabet Med 2010; 27: 636–643. [DOI] [PubMed] [Google Scholar]
- 34. Ou SM, Shih CJ, Chao PW, Chu H, Kuo SC, Lee YJ, et al Effects on clinical outcomes of adding dipeptidyl peptidase‐4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med 2015; 163: 663–672. [DOI] [PubMed] [Google Scholar]
- 35. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127: 757–763. [DOI] [PubMed] [Google Scholar]
- 36. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 37. Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care 2012; 18: 721–726. [PubMed] [Google Scholar]
- 38. Chen HL, Hsiao FY. Risk of hospitalization and healthcare cost associated with Diabetes Complication Severity Index in Taiwan's National Health Insurance Research Database. J Diabetes Complications 2014; 28: 612–616. [DOI] [PubMed] [Google Scholar]
- 39. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi HJ, Park C, Lee YK, Ha YC, Jang S, Shin CS. Risk of fractures and diabetes medications: a nationwide cohort study. Osteoporos Int 2016; 27: 2709–2715. [DOI] [PubMed] [Google Scholar]
- 41. Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity‐based weighting under conditions of nonuniform effect. Am J Epidemiol 2006; 163: 262–270. [DOI] [PubMed] [Google Scholar]
- 42. Adamson J, Ebrahim S, Dieppe P, Hunt K. Prevalence and risk factors for joint pain among men and women in the West of Scotland Twenty‐07 study. Ann Rheum Dis 2006; 65: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanz C, Vazquez P, Blazquez C, Barrio PA, Alvarez Mdel M, Blazquez E. Signaling and biological effects of glucagon‐like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am J Physiol Endocrinol Metab 2010; 298: E634–E643. [DOI] [PubMed] [Google Scholar]
- 44. Nuche‐Berenguer B, Moreno P, Portal‐Nunez S, Dapia S, Esbrit P, Villanueva‐Penacarrillo ML. Exendin‐4 exerts osteogenic actions in insulin‐resistant and type 2 diabetic states. Regul Pept 2010; 159: 61–66. [DOI] [PubMed] [Google Scholar]
- 45. Glorie L, Behets GJ, Baerts L, De Meester I, D'Haese PC, Verhulst A. DPP IV inhibitor treatment attenuates bone loss and improves mechanical bone strength in male diabetic rats. Am J Physiol Endocrinol Metab 2014; 307: E447–E455. [DOI] [PubMed] [Google Scholar]
- 46. Kyle KA, Willett TL, Baggio LL, Drucker DJ, Grynpas MD. Differential effects of PPAR‐γ activation versus chemical or genetic reduction of DPP‐4 activity on bone quality in mice. Endocrinology 2011; 152: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gallagher EJ, Sun H, Kornhauser C, Tobin‐Hess A, Epstein S, Yakar S, et al The effect of dipeptidyl peptidase‐IV inhibition on bone in a mouse model of type 2 diabetes. Diabetes Metab Res Rev 2014; 30: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kidwai SS, Wahid L, Siddiqi SA, Khan RM, Ghauri I, Sheikh I. Upper limb musculoskeletal abnormalities in type 2 diabetic patients in low socioeconomic strata in Pakistan. BMC Res Notes 2013; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Eisman JA, et al A family history of fracture and fracture risk: a meta‐analysis. Bone 2004; 35: 1029–1037. [DOI] [PubMed] [Google Scholar]
- 50. Ramchurn N, Mashamba C, Leitch E, Arutchelvam V, Narayanan K, Weaver J, et al Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med 2009; 20: 718–721. [DOI] [PubMed] [Google Scholar]
- 51. Feskanich D, Flint AJ, Willett WC. Physical activity and inactivity and risk of hip fractures in men. Am J Public Health 2014; 104: e75–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johansson H, Kanis JA, Oden A, McCloskey E, Chapurlat RD, Christiansen C, et al A meta‐analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014; 29: 223–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Overall, age‐specific and sex‐specific incidence rates and hazard ratios of hip fracture (ICD‐9‐CM codes 820–821) in association with DPP‐4i use in patients with type 2 diabetes
Table S2 Overall, age‐specific and sex‐specific incidence rates and hazard ratios of lower extremity fracture (ICD‐9‐CM codes 820–829) in association with DPP‐4i use in patients with type 2 diabetes
Table S3 Overall, age‐specific and sex‐specific incidence rates and hazard ratios of upper extremity fracture (ICD‐9‐CM codes 810–819) in association with DPP‐4i use in patients with type 2 diabetes
Table S4 Comparison of covariate adjusted hazard ratio for fracture between patients receiving DPP‐4i and those receiving other oral antidiabetic agents


