Abstract
Aims
Several antihypertensive drugs are used in the treatment of severe hypertension in pregnancy. The present study is a network meta‐analysis comparing the efficacy and safety of these drugs.
Methods
Electronic databases were searched for randomized clinical trials comparing drugs used in the treatment of severe hypertension in pregnancy. The number of women achieving the target blood pressure (BP) was the primary outcome. Doses required and time taken for achieving the target BP, failure rate, and incidences of maternal tachycardia, palpitation, hypotension, headache, and neonatal death and stillbirth were the secondary outcomes. Mixed treatment comparison pooled estimates were generated using a random‐effects model. Odds ratios for the categorical and mean difference for the numerical outcomes were the effect estimates.
Results
Fifty‐one studies were included in the systematic review and 46 in the meta‐analysis. No significant differences in the number of patients achieving target BP was observed between any of the drugs. Diazoxide [−15 (−20.6, −9.4)], nicardipine [−11.8 (−22.3, −1.2)], nifedipine/celastrol [−19.3 (−27.4, −11.1)], nifedipine/vitamin D [−17.1 (−25.7, −9.7)], nifedipine/resveratrol [−13.9 (−22.6, −5.2)] and glyceryl trinitrate [−33.8 (−36.7, −31)] were observed to achieve the target BP (in minutes) more rapidly than hydralazine. Nifedipine required fewer doses than hydralazine for achieving the target BP. Glyceryl trinitrate and labetalol were associated with fewer incidences of tachycardia and palpitation respectively than hydralazine. Trial sequential analysis concluded adequate evidence for hydralazine and nifedipine compared with labetalol. Moderate quality of evidence was observed for direct comparison estimate between labetalol and hydralazine but was either low or very low for other comparisons.
Conclusion
The present evidence suggests similar efficacy between nifedipine, hydralazine and labetalol in the treatment of severe hypertension in pregnancy. Subtle differences may exist in their safety profile. The evidence is inadequate for other drugs.
Keywords: dihydralazine, hydralazine, ketanserin, labetalol, nifedipine, systematic review
What is Already Known about this Subject
There is no consensus on the relative efficacy and safety of anti‐hypertensive drugs used in the treatment of severe hypertension in pregnancy.
Currently, head‐to‐head clinical trials comparing several such drugs are lacking.
Several methodological flaws are noted with the existing systematic reviews of this topic.
What this Study Adds
A similar efficacy was observed between hydralazine and nifedipine compared to labetalol.
Adequacy of evidence for the above findings has been confirmed by trial sequential analysis.
No significant differences exist in the safety profile of these drugs.
Introduction
Hypertension in pregnancy is defined as systolic blood pressure (SBP) above 140 mmHg and/or diastolic blood pressure (DBP) above 90 mmHg; severe hypertension as SBP ≥ 160 mmHg with or without DBP ≥ 110 mmHg 1. Hypertension in pregnancy can be categorized as: chronic hypertension (before 20 weeks of gestation), gestational hypertension (later than 20 weeks) or pre‐eclampsia (associated with organ damage) 2. A prevalence of 3.6–9.1% has been reported for hypertension in pregnancy and 1.4–4% for pre‐eclampsia 3. Hypertensive women with pre‐eclampsia have an increased risk of renal failure, hepatic failure, stroke and perinatal mortality 4. Hypertensive crisis occurs in 1–2% of pregnant women 5.
Target blood pressure (BP) is recommended to be less than 140–150 mmHg for systolic and less than 90–100 mmHg for diastolic blood pressures in pregnant women with hypertension 6. A trial of oral anti‐hypertensives can be attempted for managing severe hypertension in pregnancy before initiating parenteral therapy 7. Labetalol, nifedipine and hydralazine are the commonly used drugs for treating severe hypertension in pregnancy 8. Despite being used for several decades, there is no consensus on the relative efficacy and safety of drugs used in treating severe hypertension in pregnancy, and a recent Cochrane review was inconclusive 9. This is mainly due to the lack of head‐to‐head clinical trials comparing these drugs. A network meta‐analysis offers advantage in comparing the interventions in the absence of head‐to‐head comparisons through a common comparator 10. Hence, we carried out the present network meta‐analysis to compare the drugs used for treating severe hypertension in pregnancy.
Methods
Search strategy
This review's protocol has been registered in PROSPERO (CRD42017076188). PubMed and Cochrane CENTRAL were searched with an appropriate search strategy (see supplementary appendix). We did not place any restrictions on the publication language or year. Additionally, we hand searched the cross‐references of the included studies.
Eligibility criteria
We included only randomized clinical trials carried out in patients with severe hypertension that had compared more than one drug. No strict criterion was placed for severe hypertension in the present review but we have carried out a sensitivity analysis by excluding trials with different blood pressure criteria in their study participants. We excluded trials comparing different formulations/doses/routes of the same drug and that had evaluated either intravenous magnesium sulphate/oral atenolol/oral alpha‐methyldopa as the standalone anti‐hypertensive drugs. Number of patients achieving the target BP was the primary outcome. Doses required and time taken for achieving the target BP, failure rate, incidences of maternal tachycardia, palpitation, maternal hypotension, headache, stillbirth, number of neonates with appearance, pulse, grimace, activity, respiration (APGAR) score <7, neonatal death and number of patients with new hypertensive crisis were the secondary outcomes.
Study procedure
Two authors performed an independent literature search and extracted the following details: trial site, year, trial design, participants, interventions and outcomes. Any disagreements between the authors were resolved through discussion. The present meta‐analysis complies with the preferred reporting items in systematic review and meta‐analysis (PRISMA) guidelines 11. The risk of bias of the included studies was assessed using the Cochrane risk of bias tool 12. We assessed publication bias only for those comparisons with at least five studies, using funnel plots and Egger's regression test 13. We used a random‐effects model for generating direct and mixed treatment comparison estimates. Direct estimates for any two interventions were obtained by pooling the data from head‐to‐head clinical trials comparing the same interventions. Mixed treatment comparison pooled estimates for the interventions were obtained by pooling the data both from the head‐to‐head clinical trials comparing the interventions and with the indirect estimates between the interventions through a common comparator. Odds ratio [95% confidence interval] was the effect estimate for categorical and weighted mean difference [95% confidence interval] for numerical outcomes. Inconsistency between direct and indirect pooled effect estimates was assessed by statistics, wherein a value of <3 was considered as minimal, 3–6 as modest and >6 as large 14. Sub‐group analyses were carried out for severe pre‐eclampsia, with different initial blood pressure thresholds, and for different definitions for target blood pressures. Sensitivity analysis was carried out by excluding trials that did not report the initial blood pressure criteria from the overall analysis and those trials that had recruited post‐partum women with severe hypertension. Trial sequential analysis (TSA, Copenhagen, DK) was conducted for comparisons with a minimum of five studies to assess the cumulative evidence according to the information size achieved to date 15. A relative risk reduction of 10% was considered as the clinically meaningful difference in the primary outcome. MetaXL was used for generating the pooled estimates 16. Grading of the evidence for key comparisons was carried out using the grades of recommendation, assessment, development and evaluation (GRADE) working group approach 12.
Results
Search results
A total of 320 articles were retrieved, of which 51 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 were included in the systematic review and 46 in the meta‐analysis. The PRISMA flow chart is depicted in Figure 1. Table S1 represents the key characteristics of the included studies. Overall assessment of risk of bias revealed low risk for reporting and attrition bias with some of studies associated with either unclear or high risk in other domains (Figure S1). The following interventions were included in the systematic review: direct‐acting vasodilators (hydralazine, dihydralazine, diazoxide, glyceryl trinitrate), sympatholytics (labetalol, ketanserin and urapidil), calcium channel blockers (nicardipine, nifedipine and isradipine), prostaglandins/prostaglandin analogues [prostaglandin A1 (PGA1) and epoprostenol], centrally acting antihypertensive drug (clonidine), angiotensin converting enzyme inhibitor (captopril) and drug combinations (nifedipine/celastrol, nifedipine/resveratrol and nifedipine/vitamin D). All the above drugs except clonidine and captopril were also evaluated in the meta‐analysis.
Figure 1.
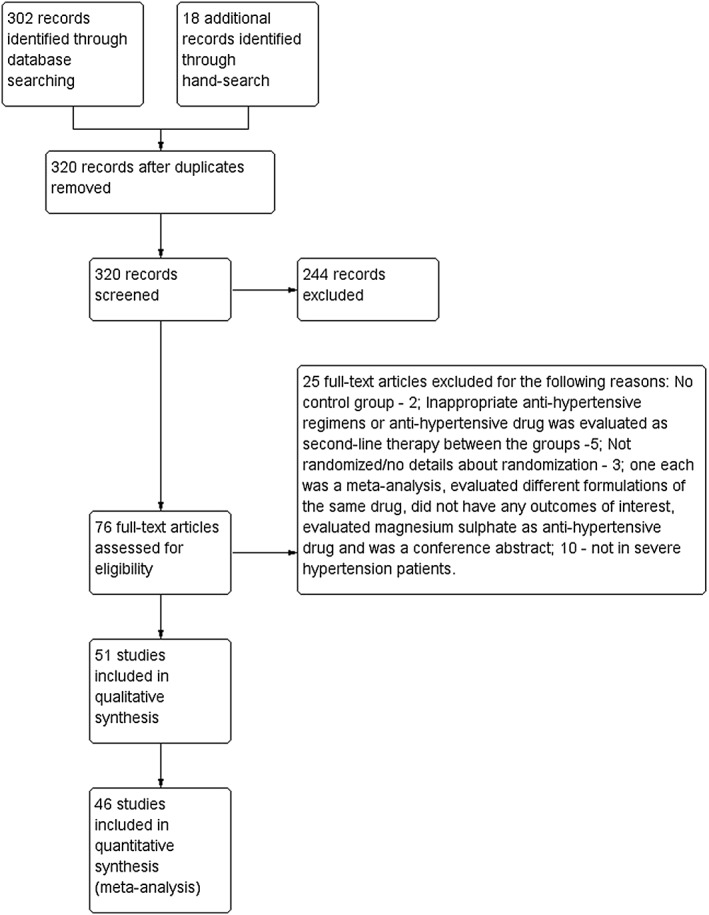
PRISMA flow diagram. Fifty‐one studies were included in this systematic review and 46 in this meta‐analysis
Overall analysis of the pooled estimates for the primary outcome
Thirty‐two studies with 3236 participants were included for the analysis of primary outcome. The network plot of the interventions assessed for the primary outcome is shown in Figure 2. No significant differences were observed in the proportion of patients achieving target blood pressure between the drugs. Surprisingly, nicardipine was observed with a better estimate compared to diazoxide (Table 1). However, for this comparison, there were no head‐to‐head clinical trials and the mixed treatment pooled estimate was based on the indirect comparison. Mild inconsistencies were observed between the direct and mixed treatment comparison estimates ( ranged between 1 and 1.47).
Figure 2.
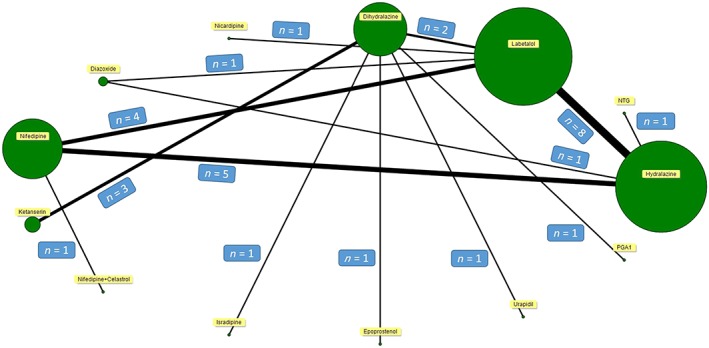
Network plot for primary outcome. The majority of the studies compared hydralazine with labetalol followed by nifedipine and labetalol
Table 1.
Direct and mixed treatment comparison pooled estimates for primary outcome
| Drugs | PGA1 | GTN | Labetalol | Dihydralazine | Nifedipine | Diazoxide | Ketanserin | N/C | Nicardipine | Isradipine | Epoprostenol | Urapidil | Hydralazine | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | M | D | M | D | M | D | M | D | M | D | M | D | M | D | M | D | M | D | M | D | M | D | M | D | M | |
| PGA1 | – | 0.8 [0, 270] | – | 0.9 [0, 259] | 1 [0.1, 55.3] | 1 [0.1, 55.3] | – | 0.7 [0, 215] | – | 5.5 [0, 1851] | – | 1.6 [0, 106] | – | 0.7 [0, 407] | – | 0.6 [0, 206] | – | 0.2 [0, 21.4] | – | 0.2 [0, 32.1] | – | 1 [0, 287] | – | 1.2 [0, 359] | ||
| GTN | – | 1.1 [0.3, 4.9] | – | 1.3 [0, 93] | – | 0.4 [0.1, 3.3] | – | 0.5 [0.1, 2.4] | – | 2.1 [0, 169] | – | 0.4 [0, 13.1] | – | 0.8 [0.1, 5.2] | – | 0.3 [0, 34.8] | – | 0.3 [0, 52.8] | – | 1.3 [0, 449] | 1.5 [0.4, 5.6] | 1.5 [0.4, 5.6] | ||||
| Labetalol | 1.2 [0.6, 3.5] | 1.2 [0.6, 3.5] | 1.2 [0.6, 2.7] | 0.7 [0.3, 1.5] | 6.3 [1.7, 24] | 1.3 [0.1, 16.6] | – | 1.8 [0, 116.7] | – | 0.8 [0, 14.5] | 0.7 [0.3, 2.2] | 0.7 [0.3, 2.2] | – | 0.2 [0, 24.6] | – | 0.2 [0, 38] | – | 1.2 [0, 330] | 1.4 [0.7, 2.8] | 2.3 [0.3, 15.8] | ||||||
| Dihydralazine | – | 0.7 [0, 41.7] | – | 5.5 [0.1, 373] | 1.6 [0.5, 4.7] | 1.6 [0.5, 4.7] | – | 0.7 [0, 97.9] | – | 0.6 [0, 40.6] | 0.2 [0, 2.1] | 0.2 [0, 2.1] | 0.2 [0, 4.6] | 0.2 [0, 4.6] | 1 [0.1, 54.2] | 1 [0.1, 54.2] | – | 1.2 [0, 69.2] | ||||||||
| Nifedipine | – | 3.6 [0.6, 20.5] | – | 2.3 [0, 155] | 1 [0.1, 10] | 1 [0.1, 10] | – | 0.9 [0.2, 3.5] | – | 0.3 [0, 32.3] | – | 0.3 [0, 49.8] | – | 1.4 [0, 430] | 3.7 [0.7, 18.8] | 2.1 [0.9, 5.2] | ||||||||||
| Diazoxide | – | 0.3 [0, 22.7] | – | 0.8 [0, 20.8] | – | 0.1 [0, 0.6]* | – | 0.4 [0, 32.7] | – | 0.1 [0, 7.1] | – | 0.2 [0, 61] | – | 1.7 [0.1, 30.3] | ||||||||||||
| Ketanserin | – | 0.4 [0, 68.9] | – | 0.4 [0, 29.2] | – | 0.1 [0, 1.7] | – | 0.1 [0, 3.5] | – | 0.6 [0, 39.1] | – | 0.7 0, 49.7] | ||||||||||||||
| N/C | – | 0.9 [0, 19.9] | – | 0.3 [0, 69] | – | 0.3 [0, 100.8] | – | 1.0 [0, 815] | – | 3.7 [0.1, 93] | ||||||||||||||||
| Nicardipine | – | 0.3 [0, 37.6] | – | 0.3 [0, 57.6] | – | 1.6 [0, 495] | – | 1.8 [0.5, 6.7] | ||||||||||||||||||
| Isradipine | – | 1 [0, 46.5] | – | 4.8 [0, 474] | – | 5.6 [0.1, 600] | ||||||||||||||||||||
| Epoprostenol | – | 4.8 [0, 746] | – | 5.7 [0, 940] | ||||||||||||||||||||||
| Urapidil | – | 1.2 [0, 354] | ||||||||||||||||||||||||
| Hydralazine | ||||||||||||||||||||||||||
D, Direct pooled estimates; GTN, glyceryl trinitrate; M, Mixed treatment comparison estimates; N/C, nifedipine/celastrol; PGA1, prostaglandin A1.
P < 0.05 (statistically significant).
Sub‐group analyses for the primary outcome
Route of administration
Most of the drugs were administered intravenously (IV) either as bolus or infusion except for nifedipine. Details of the individual routes of the drugs used in the trials are given in Table S1. Twenty‐eight studies (2799 participants) were included for the sub‐group analysis compared to hydralazine IV bolus (Figure S2). Labetalol IV infusion [17.8 (3.9, 81)] and diazoxide IV bolus [2.8 (1.4, 5.8)] were observed with significantly increased proportion of patients achieving target BP. The direct comparison analysis revealed a better response with labetalol IV infusion compared to diazoxide IV bolus [6.3 (1.7, 24)]. No inconsistency was observed between the direct and mixed treatment comparison pooled estimates ( = 1).
Severe pre‐eclampsia
We carried out a sub‐group analysis of studies that had enrolled patients with severe pre‐eclampsia. We have also included those studies where more than two‐thirds of the patients were diagnosed with severe pre‐eclampsia. Twenty‐seven studies (2801 participants) were included and no significant differences were observed in the mixed treatment comparison pooled estimates between the drugs (Figure S3). However, the direct comparison pooled estimate for labetalol was significantly better than diazoxide [6.3 (1.7, 24)]. Mild inconsistency was observed between the direct and mixed treatment comparison estimates ( = 1.48).
Initial blood pressure threshold
Studies varied in their definition of severe hypertension (Table S1). Due to paucity of studies, the sub‐group analyses for this entity was restricted to only three categories (Table S2). No significant differences were observed with labetalol, nifedipine or glyceryl trinitrate with hydralazine, and labetalol, isradipine and ketanserin with dihydralazine. No inconsistency was observed between the direct and mixed treatment comparison pooled estimates ( = 1).
Target blood pressure
The studies also varied in the definitions of target blood pressure in their study participants (Table S1). Due to paucity of studies in most of the categories, sub‐group analyses were carried out for only two categories (Table S3). In the subset of studies with target blood pressure <160/100 mmHg, nifedipine was observed to outperform hydralazine [48.9 (5.6, 428.6)]. No inconsistency was observed between the direct and mixed treatment comparison pooled estimates ( = 1).
Sensitivity analysis and publication bias for the primary outcome
Four studies did not report the initial blood pressure criterion for recruiting their study participants, of which data from one was included in the analysis of primary outcome. Removal of data from this study did not significantly change the overall analysis (Figure S4).
Three studies recruited post‐natal women of which data from two were included for the analysis of primary outcome. No significant changes were observed in the pooled estimates of the drugs after removing the data from the above studies (Figure S5).
No publication bias was detected for the following comparisons: hydralazine with labetalol (P = 0.84), nifedipine with hydralazine (P = 0.69) and nifedipine and labetalol (P = 0.3) for the primary outcome (Figure S6).
Trial sequential analysis for primary outcome
Trial sequential analysis was carried out between hydralazine and labetalol; nifedipine and hydralazine; and nifedipine with labetalol for the primary outcome. The pooled estimates were similar for hydralazine and nifedipine compared to labetalol and the evidence is sufficient for concluding the same (Figures S7 and S8). However, the evidence is inconclusive for nifedipine compared to hydralazine (Figure S9).
Pooled estimates for secondary outcomes
A summary of pooled estimates for all the secondary outcomes is listed in Table 2. Compared to hydralazine, glyceryl trinitrate, nicardipine, diazoxide, nifedipine/celastrol, nifedipine/vitamin D and nifedipine/resveratrol were associated with significantly shorter time to achieve the target blood pressure. Fewer doses were required for nifedipine compared to hydralazine for achieving the target blood pressure, whereas nicardipine and isradipine required significantly more. Glyceryl trinitrate and labetalol were associated with lesser incidences of tachycardia and palpitation respectively than hydralazine.
Table 2.
Mixed treatment comparison pooled estimates for secondary outcomes in comparison with hydralazine
| Drugs | Outcomes (Total number of studies; Total number of patients) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal hypotension a (18; 2737) | Time for achieving target BP b (16; 2702) | Failure rate a (17; 1803) | Number of doses required to achieve target BP b (16; 1575) | Stillbirth a (12; 1055) | Neonatal death a (12; 800) | Maternal tachycardia a (17; 2884) | Headache a (29; 3828) | Palpitation a (8; 782) | Number of patients with new HT crisis a (4; 313) | Number of neonates with APGAR <7 a (13; 2647) | |
| Labetalol | 0.23 [0, 1.2] | −1 [−22.2, 23] | 0.6 [0.2, 1.6] | −0.1 [−0.3, 0.2] | 0.9 [0.4, 2.3] | 0.8 [0.2, 3.6] | 0.4 [0.1, 1.5] | 0.7 [0.3, 1.9] | 0.4 [0.2, 0.9]* | 0.4 [0.1, 1.9] | 1.4 [0.8, 2.4] |
| Nifedipine | 0.7 [0.1, 4] | −3.5 [−26.5, 19.7] | 0.7 [0.3, 1.5] | −0.4 [−0.7, −0.1]* | 1.4 [0.4, 5.1] | 0.4 [0.1, 2.5] | 0.6 [0.1, 2.1] | 0.8 [0.3, 2.3] | 1.5 [0.4, 5.9] | 0.5 [0.3, 1.1] | 1.1 [0.6, 2] |
| Glyceryl trinitrate | 0.9 [0.5, 1.7] | −33.8 [−36.7, −31]* | 0.7 [0.2, 2.4] | – | 0.7 [0.1, 4] | – | 0.5 [0.2, 0.9]* | 0.4 [0.1, 3.9] | 1.3 [0.1, 18.2] | – | 0.6 [1.1, 2.5] |
| PGA1 | – | – | – | – | – | – | – | – | – | – | – |
| Dihydralazine | 2.3 [0.1, 48] | – | 1.9 [0.1, 27.5] | −0.2 [−1.3, 1] | 0.1 [0, 4.4] | 0.2 [0, 10.1] | 6.4 [0.5, 91.6] | 3.1 [0.4, 3.4] | – | – | – |
| Nicardipine | – | −11.8 [−22.3, −1.2]* | 0.6 [0.2, 2.3] | 0.9 [0.5, 1.3]* | – | – | – | 0.6 [0.2, 2.3] | 3.5 [0.2, 82] | – | – |
| Diazoxide | 0.8 [0.1, 12.6] | −15 [−20.6, −9.4]* | 0.5 [0.1, 2.8] | – | 2.4 [0.1, 71] | 3.8 [0.1, 126.2] | 1 [0.3, 3.4] | 0.3 [0.1, 3.3] | – | – | 0.9 [0.2, 4] |
| Ketanserin | 0.3 [0.1, 8.3] | – | 20.5 [0.8, 496.6] | −0.1 [−1.2, 1.1] | 0.6 [0.1, 4.9] | 1 [0.1, 165.6] | 0.3 [0, 8.3] | 0.3 [0.1, 8.3] | – | – | – |
| Nifedipine/Celastrol | 0.6 [0.1, 15.4] | −19.3 [−27.4, −11.1]* | – | – | – | – | 0.4 [0.1, 4.2] | 1 [0.1, 8.2] | – | – | 1.1 [0.4, 2.7] |
| Isradipine | – | – | – | 1.7 [0.3, 2.1]* | 0.1 [0, 4.6] | 0.5 [0, 99] | – | – | – | – | – |
| Epoprostenol | – | – | – | – | – | 0.2 [0, 27.8] | – | 0.2 [0, 31.8] | – | – | – |
| Urapidil | 1.1 [0, 181] | – | – | – | – | 0.1 [0, 12.2] | – | – | – | – | – |
| Nifedipine/Vitamin D | 0.3 [0.1, 5.8] | −17.7 [−25.7, −9.7]* | – | – | – | – | 0.4 [0.1, 3.5] | 1.5 [0.3, 8.1] | – | – | 0.9 [0.4, 2.3] |
| Nifedipine/Resveratrol | 0.2 [0.1, 7.4] | −13.9 [−22.6, −5.2]* | – | −0.3 [−1.3, 0.8] | – | – | 0.7 [0.1, 10.6] | 1.5 [0.2, 10.7] | – | – | 0.8 [0.3, 2.2] |
P < 0.05 (statistically significant).
Pooled estimates expressed in odds ratio [95% confidence interval].
Pooled estimates expressed in mean difference [95% confidence interval].
BP, blood pressure; MTC, mixed treatment pooled estimates; PGA1, Prostaglandin A1.
Grading the evidence
Grading of the quality of evidence was carried out for key comparisons. For the primary outcome, moderate quality was observed for the direct comparison pooled estimates between labetalol and hydralazine. Grading of the quality of evidence for other comparisons revealed either low or very low quality (Table 3).
Table 3.
Grading the quality of evidence for key comparisons
| Comparisons | Illustrative comparative risks (95% confidence intervals) | Effect estimate and quality of evidence for direct comparisons | Effect estimate and quality of evidence for mixed treatment comparisons | |
|---|---|---|---|---|
| Assumed risk a | Corresponding risk b | |||
| Number of patients achieving target BP with labetalol compared to hydralazine | 881 per 1000 | 914 per 1000 (833 to 956) |
1.4 [0.7, 2.8] ⊕⊕⊕⊝ Moderate c |
2.3 [0.3, 15.8] ⊕⊕⊝⊝ Low c , e |
| Number of patients achieving target BP with nifedipine compared to hydralazine | 881 per 1000 | 964 per 1000 (833 to 992) |
3.7 [0.7, 18.8] ⊕⊕⊝⊝ Low c , e |
2.1 [0.9, 5.2] ⊕⊕⊝⊝ Low c , e |
| Number of doses required to achieve target BP with nifedipine compared to hydralazine | NA | NA |
−0.1 [−1.1, 1] ⊕⊝⊝⊝ Very low c , d , e |
−0.4 [−0.7, −0.1] ⊕⊝⊝⊝ Very low c , d , e |
| Time for achieving target BP with glyceryl trinitrate compared to hydralazine | NA | NA | −33.8 [−36.7, −31] ND | −33.8 [−36.7, −31] ND |
| Time for achieving target BP with nicardipine compared to hydralazine | NA | NA | −1.3 [−3.9, 1.3] ND | −11.8 [−22.3, −1.2] ND |
| Time for achieving target BP with diazoxide compared to hydralazine | NA | NA | −15 [−20.6, −9.4] ND | −15 [−20.6, −9.4] ND |
| Time for achieving target BP with nifedipine/celastrol compared to hydralazine | NA | NA | −21 [−24, −17.8] ND | −19.3 [−27.4, −11.1] ND |
| Time for achieving target BP with nifedipine/vitamin D compared to hydralazine | NA | NA | −19.3 [−22.2, − 16.6] ND | −17.7 [−25.7, −9.7] ND |
| Time for achieving target BP with nifedipine/resveratrol compared to hydralazine | NA | NA | −15.5 [−19.8, −11.1] ND | −13.9 [−22.6, −5.2] ND |
Assumed risk was the median control group risk across the studies for the categorical variables.
Computed only for the categorical outcomes based on assumed risk.
Downgraded one level for including studies with high risk of bias.
Downgraded one level as publication bias could not be ruled out.
Downgraded one level for serious limitations in the precision of the estimates.
Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality: We are very uncertain about the estimate.
BP, blood pressure; NA, not assessed as the risk assessment was performed only for the categorical variable; ND, not determined due to very serious limitations in the precision of the estimates, publication bias could not be assessed and high risk of bias and the estimate was derived from only one study.
Discussion
The present network meta‐analysis was carried out to compare the efficacy and safety of drugs for treating severe hypertension in pregnancy. We have included 51 studies in this systematic review and 46 in the meta‐analysis. No significant differences in the number of patients achieving target BP was noted between the drugs. Glyceryl trinitrate, nicardipine, diazoxide, nifedipine/celastrol, nifedipine/vitamin D and nifedipine/resveratrol were observed to require a significantly shorter time to achieve the target BP than hydralazine. Nifedipine requires fewer doses than hydralazine to achieve the target BP. Glyceryl trinitrate and labetalol were associated with fewer incidences of tachycardia and palpitation respectively than hydralazine. Sub‐group analyses revealed that labetalol IV infusion and diazoxide IV bolus could outperform hydralazine IV bolus. Similarly, labetalol IV infusion may perform better than diazoxide IV bolus, including in patients with severe pre‐eclampsia. Trial sequential analysis concluded the presence of adequate evidence for hydralazine and nifedipine compared to labetalol. Moderate quality of evidence was observed for nifedipine and hydralazine but was either low or very low for others.
Network meta‐analysis can estimate the relative effect even in the absence of head‐to‐head clinical trials through a common comparator; for example, if there are three interventions, namely A, B and C, and we assume that head‐to‐head clinical trials compared either A with B or B with C. With the aid of network meta‐analysis modelling, through the common comparator (B), we can compute the relative effect estimate between A and C 10. In the present network meta‐analysis, we observed a similar efficacy profile between the several anti‐hypertensive drugs used in pregnancy despite the absence of head‐to‐head comparisons for many drugs. The only other robust quantitative synthesis was from Duley et al. 9, where the authors included 35 studies but the results were inconclusive. Further, the authors of the study did not take into account the route of administration of the anti‐hypertensive drugs, various initial and target blood pressure values and pre‐eclampsia status; we have addressed all the above issues in the present study. Additionally, they did not validate their results by adjusting the pooled estimates to type 1 error for which we have carried out the trial sequential analysis. Trial sequential analysis can be considered as an interim analysis that accounts for the statistical diversity relating the accumulated evidence to the total sample size required 68. We had observed that the evidence is sufficient to conclude a similar efficacy (in terms of number of patients achieving the target BP) between hydralazine and nifedipine compared to labetalol. Future investigators should be aware that conducting clinical trials with a similar comparison is futile. Also, we have noted that some of the investigators in recent times have started combining drugs such as vitamin D and resveratrol along with conventional antihypertensive drugs (nifedipine). Though few such trials were included in the present meta‐analysis, no advantages have been observed with such drug combinations.
Our results are in line with the recommendations from Royal College of Obstetricians and Gynaecologists and American College of Obstetrics and Gynaecology where labetalol, nifedipine and hydralazine are listed as first‐line drugs 69, 70. Interestingly, the World Health Organization (WHO) has listed only intravenous hydralazine in its latest essential drugs list (EDL) for treating severe hypertension in pregnancy 71. Although cost‐effectiveness data for hydralazine and nifedipine is not available, because nifedipine can be administered orally, available as the generic preparation with a similar efficacy and safety profile confirmed in the present review and in previous reviews 72, the WHO may consider including nifedipine in the EDL for primary health care. Moreover, we observed that nifedipine performs better than hydralazine when the target BP is less than 160/110 mmHg.
We have observed that labetalol IV infusion performs better than hydralazine IV bolus. However, there were only two studies (with 51 patients) where labetalol was administered as IV infusion. Reports indicate the risk of severe hypotension with labetalol IV infusion 73. Hence, considering the very low quality of evidence for this comparison and the potential risk involved, we do not recommend labetalol IV infusion. Further studies should explore the therapeutic utility of labetalol IV infusion for treating either severe or refractory hypertension in pregnancy.
We did not observe any significant differences in terms of either maternal or fetal safety profile between the evaluated drugs. In addition, maternal mortality is reported rarely in the included studies. We have excluded studies that have assessed the role of oral alphamethyl dopa/atenolol, as their role for treating severe hypertension in contemporary practice is not favoured due to weak anti‐hypertensive effects 74. Similarly, guidelines from the multidisciplinary working group of the National Partnership for Maternal Safety has advised not using magnesium sulphate for the sole anti‐hypertensive purpose, and so we excluded it in this review. 75
This is the first network meta‐analysis in this field. The estimates generated from the present model will be useful to practitioners as it may take several years to conduct head‐to‐head clinical trials comparing several drugs for treating severe hypertension in pregnancy. Additionally, we have also confirmed the presence of adequate evidence at least for key comparisons. Various sub‐group analyses based on route of administration, comorbidity with severe pre‐eclampsia, pre‐treatment and target blood pressure values were carried out. However, the following are the limitations of the study: the differences in the pre‐treatment blood pressure values varied widely between the studies; differences in the therapeutic response between primigravida and multigravida could not be explored as the authors rarely reported this variable in their studies; definition of failure rate differed across the studies; effects on uterine artery/umbilical artery/placental blood flow could not be assessed; effects on fetus such as fetal distress/congenital anomalies/small for gestational age was not assessed; and no strict definition of pre‐eclampsia was assumed in this review.
In conclusion, the present network meta‐analysis suggests similar efficacy between nifedipine, hydralazine and labetalol in the treatment of severe hypertension in pregnancy. The above drugs may also be useful in treating hypertension in severe pre‐eclampsia. Moderate quality of evidence was observed for direct comparison pooled estimate between labetalol and hydralazine but was either low or very low for other comparisons. Negligible differences were observed in the individual safety profile. The cumulative evidence is inadequate for any meaningful conclusion for other drugs.
Competing Interests
There are no competing interests to declare.
We thank Cochrane Review Group for the Revman ® software for assessing the risk of bias. We are grateful to PROSPERO for registering the protocol of this review and EpiGear for using MetaXL in generating mixed treatment comparison estimates.
Supporting information
Figure S1 Summary of risk of bias of the included studies
Figure S2 Forest plot of the pooled estimates of mixed treatment comparisons of drugs with their route of administration and hydralazine IV bolus
Figure S3 Forest plot of the pooled estimates of mixed treatment comparisons in severe pre‐eclampsia
Figure S4 Forest plot of pooled estimates of mixed treatment comparisons in the sensitivity analysis by removing studies that did not report the initial blood pressure values
Figure S5 Forest plot of pooled estimates of mixed treatment comparisons in the sensitivity analysis by excluding studies carried out in post‐partum women
Figure S6 Funnel plots for assessment of publication bias for the primary outcome
Figure S7 Trial sequential analysis graph for hydralazine compared to labetalol for the primary outcome
Figure S8 Trial sequential analysis graph for nifedipine compared to labetalol for the primary outcome
Figure S9 Trial sequential analysis graph for nifedipine compared to hydralazine for the primary outcome
Table S1 Key characteristics of the included studies
Table S2 Direct and mixed treatment comparison pooled estimates for primary outcome with various blood pressure threshold values
Table S3 Direct and mixed treatment comparison pooled estimates for primary outcome with various target blood pressure values compared to hydralazine
Appendix S1 Search strategy used in PubMed
Sridharan, K. , and Sequeira, R. P. (2018) Drugs for treating severe hypertension in pregnancy: a network meta‐analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol, 84: 1906–1916. 10.1111/bcp.13649.
References
- 1. Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, SOGC Hypertension Guideline Committee . Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can 2014; 36: 575–576. [DOI] [PubMed] [Google Scholar]
- 2. Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM, et al Hypertensive disorders of pregnancy. J Prenat Med 2009; 3: 1–5. [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al Population‐based trends in pregnancy hypertension and pre‐eclampsia: an international comparative study. BMJ Open 2011; 1: e000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tuffnell D, Shennan AH, Waugh JJS, Walker JJ. The management of severe pre‐eclampsia/eclampsia. RCOG guideline number 10(A) In: Royal College of Obstetricians and Gynaecologists, 2006. [Google Scholar]
- 5. Too GT, Hill JB. Hypertensive crisis during pregnancy and postpartum period. Semin Perinatol 2013; 37: 280–287. [DOI] [PubMed] [Google Scholar]
- 6. Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving mothers' lives: reviewing maternal deaths to make motherhood safer – 2003–2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London: CEMACH; 2007.
- 7. Arulkumaran N, Lightstone L. Severe pre‐eclampsia and hypertensive crises. Best Pract Res Clin Obstet Gynaecol 2013; 27: 877–884. [DOI] [PubMed] [Google Scholar]
- 8. Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs 2014; 74: 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev 2013, Issue 7 Art. No. CD001449; 10.1002/14651858.CD001449.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al Interpreting indirect treatment comparisons and network meta‐analysis for health‐care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011; 14: 417–428. [DOI] [PubMed] [Google Scholar]
- 11. Hutton B, Salanti G, Caldwell DM, Schmid CH, Cameron C, Ioannidis JP, et al The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Green S. (editors). Cochrane handbook for systematic reviews of interventions. 5.1.0 edition Available from http://www.cochrane-handbook.org (last accessed 1 February 2018). [Google Scholar]
- 13. Sedgwick P, Marston L. How to read a funnel plot in a meta‐analysis. BMJ 2015; 16 (351): h4718. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 15. Thorlund K, Engstrom J, Wetterslev J, Brok J, Imberger G, Gluud C. Trial sequential analysis. Copenhagen trial unit. Available at: http://www.ctu.dk/tools-and-links/trial-sequential-analysis.aspx (last accessed on 2 February 2018).
- 16. Barendregt JJ, Doi SA. MetaXL user guide. Available at: http://www.epigear.com/index_files/MetaXL%20User%20Guide.pdf (last accessed on 2 February 2018).
- 17. Aali BS, Nejad SS. Nifedipine or hydralazine as a first‐line agent to control hypertension in severe preeclampsia. Acta Obstet Gynecol Scand 2002; 81: 25–30. [DOI] [PubMed] [Google Scholar]
- 18. Ali RM, Salah D, Mansour DY. The effect of nitroglycerin infusion versus hydralazine infusion as antihypertensive therapy in acute management of patients with severe pre‐eclampsia. Ain Shams J Anesthesiol 2015; 8: 499–504. [Google Scholar]
- 19. Ashe RG, Moodley J, Richards AM, Philpott RH. Comparison of labetalol and dihydralazine in hypertensive emergencies of pregnancy. S Afr Med J 1987; 71: 354–356. [PubMed] [Google Scholar]
- 20. Baggio MR, Martins WP, Calderon AC, Berezowski AT, Marcolin AC, Duarte G, et al Changes in fetal and maternal Doppler parameters observed during acute severe hypertension treatment with hydralazine or labetalol: a randomized controlled trial. Ultrasound Med Biol 2011; 37: 53–58. [DOI] [PubMed] [Google Scholar]
- 21. Delgado De Pasquale S, Velarde R, Reyes O, De La Ossa K. Hydralazine vs labetalol for the treatment of severe hypertensive disorders of pregnancy: a randomized, controlled trial. Pregnancy Hypertens 2014; 4: 19–22. [DOI] [PubMed] [Google Scholar]
- 22. Dhananjaya BS, Jamuna R. Oral nifedipine versus intravenous labetalol in hypertensive emergencies of pregnancy: a randomised trial. Res J Pharm Biol Chem Sci 2015; 6: 1673–1682. [Google Scholar]
- 23. Elatrous S, Nouira S, Ouanes Besbes L, Marghli S, Boussarssar M, Sakkouhi M, et al Short‐term treatment of severe hypertension of pregnancy: prospective comparison of nicardipine and labetalol. Intensive Care Med 2002; 28: 1281–1286. [DOI] [PubMed] [Google Scholar]
- 24. Garden A, Davey DA, Dommisse J. Intravenous labetalol and intravenous dihydralazine in severe hypertension in pregnancy. Clin Exp Hypertens B 1982; 1: 371–383. [DOI] [PubMed] [Google Scholar]
- 25. Vigil‐De Gracia P, Lasso M, Ruiz E, Vega‐Malek JC, de Mena FT, López JC, et al Severe hypertension in pregnancy: hydralazine or labetalol: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol 2006; 128: 157–162. [DOI] [PubMed] [Google Scholar]
- 26. Vigil‐De Gracia P, Ruiz E, López JC, de Jaramillo IA, Vega‐Maleck JC, Pinzón J. Management of severe hypertension in the postpartum period with intravenous hydralazine or labetalol: a randomized clinical trial. Hypertens Pregnancy 2007; 26: 163–171. [DOI] [PubMed] [Google Scholar]
- 27. Hennessy A, Thornton CE, Makris A, Ogle RF, Henderson‐Smart DJ, Gillin AG, et al A randomised comparison of hydralazine and mini‐bolus diazoxide for hypertensive emergencies in pregnancy: the PIVOT trial. Aust N Z J Obstet Gynaecol 2007; 47: 279–285. [DOI] [PubMed] [Google Scholar]
- 28. Jegasothy R, Paranthaman S. Sublingual nifedipine compared with intravenous hydrallazine in the acute treatment of severe hypertension in pregnancy: potential for use in rural practice. J Obstet Gynaecol Res 1996; 22: 21–24. [DOI] [PubMed] [Google Scholar]
- 29. Khan A, Hafeez S, Nasrullah FD. Comparison of hydralazine and labetalol to lower severe hypertension in pregnancy. Pak J Med Sci 2017; 33: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lakshmi BS, Dasari P. Oral nifedipine versus intravenous labetalol in hypertensive urgencies and emergencies of pregnancy: a randomized clinical trial. Obstet Med 2012; 5: 171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol 1987; 70: 328–333. [PubMed] [Google Scholar]
- 32. Martins‐Costa S, Ramos JG, Barros E, Bruno RM, Costa CA, Goldin JR. Randomized, controlled trial of hydralazine versus nifedipine in preeclamptic women with acute hypertension. Clin Exp Hypertens B 1992; 11: 25–44. [Google Scholar]
- 33. Michael CA. Intravenous labetalol and intravenous diazoxide in severe hypertension complicating pregnancy. Aust N Z J Obstet Gynaecol 1986; 26: 26–29. [DOI] [PubMed] [Google Scholar]
- 34. Morris R, Sunesara I, Darby M, Novotny S, Kiprono L, Bautista L, et al Impedance cardiography assessed treatment of acute severe pregnancy hypertension: a randomized trial. J Matern Fetal Neonatal Med 2016; 29: 171–176. [DOI] [PubMed] [Google Scholar]
- 35. Noronha Neto CC, Maia SS, Katz L, Coutinho IC, Souza AR, Amorim MM. Clonidine versus captopril for severe postpartum hypertension: a randomized controlled trial. PLoS One 2017; 12: e0168124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bijvank SW, Visser W, Duvekot JJ, Steegers EA, Edens MA, Roofthooft DW, et al Ketanserin versus dihydralazine for the treatment of severe hypertension in early‐onset preeclampsia: a double blind randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2015; 189: 106–111. [DOI] [PubMed] [Google Scholar]
- 37. Patel P, Koli D, Maitra N, Sheth T, Vaishnav P. Comparison of efficacy and safety of intravenous labetalol versus hydralazine for management of severe hypertension in pregnancy. J Obstet Gynecol India 2017. 10.1007/s13224-017-1053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rezaei Z, Sharbaf FR, Pourmojieb M, Youefzadeh‐Fard Y, Motevalian M, Khazaeipour Z, et al Comparison of the efficacy of nifedipine and hydralazine in hypertensive crisis in pregnancy. Acta Med Iran 2011; 49: 701–706. [PubMed] [Google Scholar]
- 39. Raheem IA, Saaid R, Omar SZ, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomised trial. BJOG 2012; 119: 78–85. [DOI] [PubMed] [Google Scholar]
- 40. Sabir S, Yasmin S, Abbas G. Comparison of oral nifedipine with intravenous hydralazine for acute hypertensive emergencies of pregnancy. J Postgrad Med Inst 2016; 30: 328–330. [Google Scholar]
- 41. Scardo JA, Vermillion ST, Newman RB, Chauhan SP, Hogg BB. A randomized, double‐blind, hemodynamic evaluation of nifedipine and labetalol in preeclamptic hypertensive emergencies. Am J Obstet Gynecol 1999; 181: 862–866. [DOI] [PubMed] [Google Scholar]
- 42. Seabe SJ, Moodley J, Becker P. Nifedipine in acute hypertensive emergencies in pregnancy. S Afr Med J 1989; 76: 248–250. [PubMed] [Google Scholar]
- 43. Sharma C, Soni A, Gupta A, Verma A, Verma S. Hydralazine vs nifedipine for acute hypertensive emergency in pregnancy: a randomized controlled trial. Am J Obstet Gynecol 2017; 217: 687.e1–687.e6. [DOI] [PubMed] [Google Scholar]
- 44. Shekhar S, Sharma C, Thakur S, Verma S. Oral nifedipine or intravenous labetalol for hypertensive emergency in pregnancy: a randomized controlled trial. Obstet Gynecol 2013; 122: 1057–1063. [DOI] [PubMed] [Google Scholar]
- 45. Shi DD, Yang FZ, Zhou L, Wang N. Oral nifedipine vs. intravenous labetalol for treatment of pregnancy‐induced severe pre‐eclampsia. J Clin Pharm Ther 2016; 41: 657–661. [DOI] [PubMed] [Google Scholar]
- 46. Shi DD, Wang Y, Guo JJ, Zhou L, Wang N. Vitamin D enhances efficacy of oral nifedipine in treating preeclampsia with severe features: a double blinded, placebo‐controlled and randomized clinical trial. Front Pharmacol 2017; 8: 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tariq S, Shahid A, Yousof T. Comparison of maternal hypotension after administration of labetalol versus hydralazine in treating patients having severe pregnancy induced hypertension. Pak J Med Health Sci 2017; 11: 541–543. [Google Scholar]
- 48. Vermillion ST, Scardo JA, Newman RB, Chauhan SP. A randomized, double‐blind trial of oral nifedipine and intravenous labetalol in hypertensive emergencies of pregnancy. Am J Obstet Gynecol 1999; 181: 858–861. [DOI] [PubMed] [Google Scholar]
- 49. Xiao S, Zhang M, Liang Y, Wang D. Celastrol synergizes with oral nifedipine to attenuate hypertension in preeclampsia: a randomized, placebo‐controlled, and double blinded trial. J Am Soc Hypertens 2017; 11: 598–603. [DOI] [PubMed] [Google Scholar]
- 50. Bolte AC, van Eyck J, Strack van Schijndel RJ, van Geijn HP, Dekker GA. The haemodynamic effects of ketanserin versus dihydralazine in severe early‐onset hypertension in pregnancy. Br J Obstet Gynaecol 1998; 105: 723–731. [DOI] [PubMed] [Google Scholar]
- 51. Ding J, Kang Y, Fan Y, Chen Q. Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy‐induced preeclampsia. Endocr Connect 2017; 6: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Das S, Biswas S, Das P, Mahapatra B. Comparative study of intravenous labetalol and oral nifedipine for control of blood pressure in severe preeclampsia. J Dent Med Sci 2015; 14: 22–27. [Google Scholar]
- 53. Duggan PM, McCowan LM, Stewart AW. Antihypertensive drug effects on placental flow velocity waveforms in pregnant women with severe hypertension. Aust N Z J Obstet Gynaecol 1992; 32: 335–338. [DOI] [PubMed] [Google Scholar]
- 54. Howarth GR, Seris A, Venter C, Pattinson RC. A randomized controlled pilot study comparing urapidil to dihydralazine in the management of severe hypertension in pregnancy. Hypertens Pregnancy 1997; 16: 213–221. [Google Scholar]
- 55. Maharaj B, Khedun SM, Moodley J, van der Byl K, Rapiti N. A comparative study of intravenous isradipine and dihydralazine in the treatment of severe hypertension of pregnancy in black patients. Hypertens Pregnancy 1997; 16: 1–9. [Google Scholar]
- 56. Manzur‐Verástegui S, Mandeville PB, Gordillo‐Moscoso A, Hernández‐Sierra JF, Rodríguez‐Martínez M. Efficacy of nitroglycerine infusion versus sublingual nifedipine in severe pre‐eclampsia: a randomized, triple‐blind, controlled trial. Clin Exp Pharmacol Physiol 2008; 35: 580–585. [DOI] [PubMed] [Google Scholar]
- 57. Moodley J, Gouws E. A comparative study of the use of epoprostenol and dihydralazine in severe hypertension in pregnancy. Br J Obstet Gynaecol 1992; 99: 727–730. [DOI] [PubMed] [Google Scholar]
- 58. Nabanita D, Chandra DG, Swagata B, Sangeeta Y. A comparative study of hydralazine versus labetalol in the management of pregnancy induced hypertension (PIH). Sch J App Med Sci 2016; 4: 3996–3999. [Google Scholar]
- 59. Nombur LI, Agida ET, Isah AY, Ekele BA. A comparison of hydralazine and labetalol in the management of severe preeclampsia. J Women's Health Care 2014; 3 10.4172/2167-0420.1000200. [DOI] [Google Scholar]
- 60. Rossouw HJ, Howarth G, Odendaal HJ. Ketanserin and hydralazine in hypertension in pregnancy – a randomised double‐blind trial. S Afr Med J 1995; 85: 525–528. [PubMed] [Google Scholar]
- 61. Steyn DW, Odendaal HJ. Dihydralazine or ketanserin for severe hypertension in pregnancy? Preliminary results. Eur J Obstet Gynecol Reprod Biol 1997; 75: 155–159. [DOI] [PubMed] [Google Scholar]
- 62. Swati T, Lila V, Lata R, Prachi G, Pratibha A, Tushar P. Comparative study of IV labetalol and IV hydralazine on mean arterial blood pressure changes in pregnant women with hypertensive emergency. Sch J App Med Sci 2016; 4: 2256–2259. [Google Scholar]
- 63. Wacker J, Werner P, Walter‐Sack I, Bastert G. Treatment of hypertension in patients with pre‐eclampsia: a prospective parallel‐group study comparing dihydralazine with urapidil. Nephrol Dial Transplant 1998; 13: 318–325. [DOI] [PubMed] [Google Scholar]
- 64. Wacker JR, Wagner BK, Briese V, Schauf B, Heilmann L, Bartz C, et al Antihypertensive therapy in patients with pre‐eclampsia: a prospective randomised multicentre study comparing dihydralazine with urapidil. Eur J Obstet Gynecol Reprod Biol 2006; 127: 160–165. [DOI] [PubMed] [Google Scholar]
- 65. Fenakel K, Fenakel G, Appelman Z, Lurie S, Katz Z, Shoham Z. Nifedipine in the treatment of severe preeclampsia. Obstet Gynecol 1991; 77: 331–337. [PubMed] [Google Scholar]
- 66. Toppozada MK, Darwish E, Barakat AA. Management of severe preeclampsia detected in early labor by prostaglandin A1 or dihydralazine infusions. Am J Obstet Gynecol 1991; 164: 1229–1232. [DOI] [PubMed] [Google Scholar]
- 67. Walss Rodriguez RJ, Flores Padilla LM. Management of severe pre‐eclampsia/eclampsia. Comparison between nifedipine and hydralazine as antihypertensive agents. Ginecol Obstet Mex 1993; 61: 76–79. [PubMed] [Google Scholar]
- 68. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta‐analysis. BMC Med Res Methodol 2017; 17: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. National Collaborating Centre for Women's and Children's Health (UK) . Medical management of severe hypertension or severe pre‐eclampsia in a critical care setting In: Hypertension in Pregnancy: the Management of Hypertensive Disorders during Pregnancy. (NICE Clinical Guidelines, No. 107.). London: RCOG Press, 2010. [PubMed] [Google Scholar]
- 70. Committee on Obstetric Practice . Committee Opinion No. 692: Emergent therapy for acute‐onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol 2017; 129: e90–e95. [DOI] [PubMed] [Google Scholar]
- 71. WHO model list of essential medicines. Available at: http://www.who.int/medicines/publications/essentialmedicines/20th_EML2017.pdf?ua=1 (last accessed 12 March 2018).
- 72. Shekhar S, Gupta N, Kirubakaran R, Pareek P. Oral nifedipine versus intravenous labetalol for severe hypertension during pregnancy: a systematic review and meta‐analysis. BJOG 2016; 123: 40–47. [DOI] [PubMed] [Google Scholar]
- 73. Fahed S, Grum DF, Papadimos TJ. Labetalol infusion for refractory hypertension causing severe hypotension and bradycardia: an issue of patient safety. Patient Saf Surg 2008; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ghanem FA, Movahed A. Use of antihypertensive drugs during pregnancy and lactation. Cardiovasc Ther 2008; 26: 38–49. [DOI] [PubMed] [Google Scholar]
- 75. Bernstein PS, Martin JN Jr, Barton JR, Shields LE, Druzin ML, Scavone BM, et al Consensus bundle on severe hypertension during pregnancy and the postpartum period. J Midwifery Womens Health 2017; 62: 493–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Summary of risk of bias of the included studies
Figure S2 Forest plot of the pooled estimates of mixed treatment comparisons of drugs with their route of administration and hydralazine IV bolus
Figure S3 Forest plot of the pooled estimates of mixed treatment comparisons in severe pre‐eclampsia
Figure S4 Forest plot of pooled estimates of mixed treatment comparisons in the sensitivity analysis by removing studies that did not report the initial blood pressure values
Figure S5 Forest plot of pooled estimates of mixed treatment comparisons in the sensitivity analysis by excluding studies carried out in post‐partum women
Figure S6 Funnel plots for assessment of publication bias for the primary outcome
Figure S7 Trial sequential analysis graph for hydralazine compared to labetalol for the primary outcome
Figure S8 Trial sequential analysis graph for nifedipine compared to labetalol for the primary outcome
Figure S9 Trial sequential analysis graph for nifedipine compared to hydralazine for the primary outcome
Table S1 Key characteristics of the included studies
Table S2 Direct and mixed treatment comparison pooled estimates for primary outcome with various blood pressure threshold values
Table S3 Direct and mixed treatment comparison pooled estimates for primary outcome with various target blood pressure values compared to hydralazine
Appendix S1 Search strategy used in PubMed


