Abstract
Aims
Voriconazole (VCZ) exhibits wide intrapatient pharmacokinetic variability, which is disadvantageous because of its narrow therapeutic range. A considerable part of this variation remains unexplainable, despite extensive knowledge of this drug. It is hypothesized that inflammation has an impact on VCZ pharmacokinetics. In the present study, we investigated the correlation between VCZ trough concentrations and various cytokines.
Methods
A prospective single‐centre analysis was performed in adult haematology patients receiving VCZ for possible, probable or proven invasive fungal disease. A linear mixed model was built to explore the contribution of each of the seven pro‐ and anti‐inflammatory cytokines to VCZ trough levels. The Akaike information criterion (AIC) was used to determine the model that fitted the best.
Results
Twenty‐two patients, with a total of 143 combined samples of VCZ trough levels and cytokines, were included. A significant correlation (P < 0.005) was found between VCZ trough concentrations and interleukin (IL) 6, IL‐8 and C‐reactive protein (CRP). IL‐6 showed the lowest AIC, although differences with the other mediators were marginal.
Conclusion
VCZ trough concentrations correlate with IL‐6, IL‐8 and CRP levels but only moderately explain the variability in VCZ pharmacokinetics. Future prospective studies should be undertaken to confirm these findings, and incorporate the data obtained into pharmacokinetic models, to refine the predictive behaviour.
Keywords: azoles, immune response, inflammation, pharmacokinetics, voriconazole
What is Already Known About this Subject
Inflammation, as measured by C‐reactive protein (CRP), has been associated with voriconazole (VCZ) exposure.
It is argued that CRP can be used to predict exposure to VCZ, and helps to explain the pharmacokinetic variability of this drug.
What this Study Adds
We confirmed the relationship between proinflammatory markers and VCZ trough concentrations but found large intersubject variability in correlations. We believe that more in‐depth mechanistic investigation into pro‐ and anti‐inflammatory markers is needed to resolve the impact on VCZ exposure.
We also provided a more in‐depth analysis incorporating other pro‐ and anti‐inflammatory markers, in addition to CRP.
We demonstrated that the correlation between inflammation and VCZ is weak and does not help to explain the large pharmacokinetic variability.
Introduction
Voriconazole (VCZ) is a broad‐spectrum triazole antifungal agent that is widely used in the treatment of invasive fungal disease (IFD), with pathogens of Aspergillus and Candida species 1. Treatment failure due to low VCZ trough concentrations is likely to occur, while hepatotoxicity has been associated with high VCZ trough concentrations 2, 3. Large interpatient and intrapatient variations in VCZ trough concentrations exist 2. The wide intrapatient variation in VCZ trough concentrations is particularly disadvantageous because of the known narrow therapeutic range of this agent 4. Concomitant factors, such as the route of administration, drug–drug interactions, liver dysfunction and genotypic variation in the genes of metabolic enzymes, are known to contribute to this variation 5, 6. However, a considerable part of the intrapatient variability still remains unexplained.
The metabolism of VCZ is regulated by liver enzymes, mainly by the drug‐metabolizing enzyme cytochrome P450 (CYP) 2C19, and to a lesser extent by http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1326 via oxidation 7, 8, 9. N‐oxide VCZ is the main circulating metabolite of VCZ in the blood and is 100‐fold less potent against fungal pathogens than VCZ. Next to N‐oxidation, hydroxylation is another important pathway of VCZ metabolism. Whereas http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=262#1328 is responsible for the main part of N‐oxidation, CYP3A4 appears to play a more important role in the hydroxylation pathway 9, 10. Oxidation via flavin‐containing mono‐oxygenase could be responsible for the remaining part of metabolism via N‐oxidation 11. CYP2C19 harbours many polymorphisms, of which the poor metabolizer phenotype has been investigated most extensively. High VCZ concentrations have been associated with the presence of the poor metabolizer phenotype 3. However, genotyping in predicting VCZ trough levels is not routinely used in clinical practice 2. Emerging evidence actually indicates that inflammation influences the metabolic phenotype of both CYP3A4 and CYP2C19 12, 13, 14.
Most patients with IFD are severely immunocompromised, and a considerably large subgroup suffers from a haematological malignancy. The disease‐intrinsic immunodeficiency, in combination with the treatment‐induced immunosuppression, severely increases the risk of IFDs in these patient 15. Intensive chemotherapy, used for the treatment of acute leukaemia and allogeneic stem cell transplantation (SCT), induces tissue damage, neutropenia and cellular immune dysfunction, resulting in a profound inflammatory response and the onset of fever.
C‐reactive protein (CRP) is commonly used as a marker reflecting the severity of inflammation, but is aspecific and in fact is a surrogate marker of the acute phase response. CRP is produced by hepatocytes, predominantly under the transcriptional control of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 16. Recently, a significant correlation between CRP and VCZ trough concentrations was reported 14, 17, 18. As in vitro studies have demonstrated that IL‐6 and other upstream proinflammatory cytokines downregulate CYP2C19 and CYP3A4 gene expression 19, 20, downregulation of these CYP enzymes by elevated concentrations of IL‐6 might cause an increase in VCZ trough concentrations.
The purpose of the present study therefore was to determine whether concentrations of various pro‐ and anti‐inflammatory cytokines correlate with VCZ trough concentrations.
Methods
Patients and study design
The study was carried out in accordance with the applicable rules concerning biomedical research using patient data. Patient data were collected and analysed anonymously in a prospective single‐centre study of adult haematology patients (≥18 years of age) from the Radboud University Medical Center (Nijmegen, the Netherlands). Ethical approval for retrospective data collection was obtained. Patient data were eligible for analysis when patients were treated with VCZ dose according to the label for a possible, probable or proven IFD and underwent controlled sampling. IFD diagnosis was classified according to European Organization for Research and Treatment of Cancer (EORTC) criteria being possible, probable or proven 21. Furthermore, underlying haematological disease, the treatment received and basic characteristics such as age, gender, height and weight were recorded, as well as liver function tests [alanine transaminase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), gamma‐glutamyl transferase (γ‐GT), alkaline phosphatase (ALP) and bilirubin (conjugated and unconjugated)]. Liver function tests were only recorded if the interval between their sampling time and the corresponding VCZ trough concentration sampling time was <24 h. The hospital information system (HIS) was used to retrieve data on dosing regimens (dose, time of dose and route of administration) and to retrieve data on co‐medications that might interact with VCZ. VCZ samples were excluded if co‐medications that were known to influence VCZ metabolism were taken simultaneously. Differences in time within patient sampling and between patient sampling were taken into account using a specific statistical analysis that corrects for different values of time.
VCZ trough concentration determination
Only samples for VCZ trough concentrations determination were used. A VCZ concentration was confirmed to be a valid trough concentration when this was recorded in the HIS. VCZ trough concentrations were monitored twice weekly in the first weeks of treatment, followed by weekly concentrations from the third week, allowing dose modifications based on the results of therapeutic drug monitoring. VCZ trough concentration was determined by a validated routine ultra‐high performance liquid chromatography–ultraviolet plasma assay, as described in 22. The lower detection limit of this assay was 0.05 mg l–1 and the upper detection limit was 10 mg l–1. If VCZ trough concentrations were above 10 mg l–1, plasma samples were diluted with commercial blank blood plasma to reach a concentration lower than 10 mg l–1.
Cytokine concentration determination
Cytokine and CRP samples were stored at −80°C immediately after collection. Plasma concentrations of the proinflammatory cytokines tumour necrosis factor‐alpha (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=334), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974, IL‐6, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=822 and interferon‐gamma (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1725), as well as the anti‐inflammatory cytokines http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5878 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1900, were analysed batchwise in plasma obtained from lithium–heparin anticoagulated blood using a simultaneous Luminex assay according to the manufacturer's instructions (Milliplex; Millipore, Billerica, MA, USA). The lower detection limit of the assay was 6.4 pg ml–1 for all measured cytokines. The intraday coefficient of variation was between 1.6% and 2.6%, whereas the interday coefficient of variation ranged from 3.5% to 18.3%. Accuracy was above 94.9 for all inflammatory markers.
CRP was determined by means of the COBAS auto‐analyzer (Roche Diagnostics, Mannheim, Germany) using the C‐Reactive Protein Gen 3 test. The intraday coefficient of variation at 7 mg l–1 and 48 mg l–1 was 0% and 2%, respectively. Accuracy was >99.88% at low concentrations and total imprecision was 2.5%.
Statistical analysis
To explore the possible associations of interest, we calculated the Pearson correlation coefficient between the log‐transformed concentrations of each of the seven cytokines and the VCZ trough concentrations, also logarithmically transformed. These overall correlations do not adjust for the repeated character of the observations within patients over time. To account for correlations between repeated log‐VCZ trough concentrations within a patient, linear mixed models for repeated measurements were applied with Gaussian serial covariance structure in combination with a random intercept.
These models also accounted for missing data, provided that missing data were missing at random. For each cytokine and CRP, a linear mixed model was used to analyse its effect on VCZ. To correct for possible confounders, we incorporated fixed effects: dose per kg, route of administration (oral/intravenous), ALT, AST, LDH, γ‐GT, ALP and bilirubin (conjugated and unconjugated). These factors were chosen at the discretion of the researchers. To compare the predictive powers with respect to VCZ concentrations of the cytokines and CRP, we used the fit statistics Akaike information criterion (AIC).
Values lower than the detection limit were treated as missing values. Two‐sided P‐values <0.05, uncorrected for multiple testing, were considered to indicate statistical significance. The SAS software package (version 8.2, Huizen, The Netherlands) was used to fit the models.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org,thecommon portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 23, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 24, 25.
Results
Patient characteristics
A total of 22 haematology patients were included in our study. The baseline patient characteristics are depicted in Table 1. Sixteen out of 22 patients were classified as having probable invasive pulmonary aspergillosis (IA), and five with possible pulmonary IA. One patient was suspected to have an IA but did not match the criteria in the end.
Table 1.
Baseline characteristics
| Parameter | Results (n = 22) |
|---|---|
| Characteristics | |
| Female gender | 9 (41%) |
| Age (years), median (range) | 53 (47–61) |
| BMI, median (range) | 25.8 (21.1–27.9) |
| Underlying diseases | |
| Acute myeloid leukaemia | 10 (45%) |
| Myelodysplastic syndrome | 3 (14%) |
| Multiple myeloma | 2 (9%) |
| Other haematological malignancies | 7 (32%) |
| Diagnosis of invasive fungal disease | |
| Possible IA | 5 (32%) |
| Probable IA | 16 (73%) |
| Other (i.e. pulmonary Rhizomucor) | 1 (5%) |
| Treatment | |
| Chemotherapy | 22 (100%) |
| Allogeneic SCT | 13 (59%) |
Data are presented as median (interquartile range) or as n (%). BMI, body mass index; IA, invasive aspergillosis; SCT, stem cell transplantation
VCZ trough concentrations
In all, 268 VCZ concentrations were available from our patient cohort. Of these, 173 were confirmed to be valid VCZ trough concentrations. A total of 143 of the VCZ trough concentrations matched with samples that were available for cytokine and CRP determination. A median of seven samples per patient was available, with two patients accounting for only one determination per patient. The median VCZ trough concentration was 2.3 (1.5–3.7) mg l–1 and the median VCZ duration of treatment was 21 [3–61] days (see Table 2 ). Dose modification as a result of therapeutic drug monitoring was performed in 14 patients.
Table 2.
Laboratory parameters
| Laboratory parameters | ||
|---|---|---|
| Parameter | n | Value |
| VCZ trough (mg l –1 ) | 173 | 2.3 (1.5–3.7) |
| VCZ matches (pp) | 143 | 7 (3–10) |
| Duration of VCZ follow‐up (days) | 22 | 21 (3–61) |
| Cytokines | ||
| IFN‐γ (pg ml –1 ) | 48 | 15 (10–29) |
| IL‐10 (pg ml –1 ) | 92 | 35 (12–66) |
| IL1‐RA (pg ml –1 ) | 76 | 34 (18–106) |
| IL‐1β (pg ml –1 ) | 4 | 9.5 (8.5–11)‐ |
| Il‐6 (pg ml –1 ) | 101 | 32 (12–80) |
| IL‐8 (pg ml –1 ) | 139 | 54 (22–122) |
| TNF‐α (pg ml –1 ) | 130 | 26 (15–44) |
| Liver function tests | ||
| ALT (U l –1 ) | 136 | 35 (20–71) |
| AST (U l –1 ) | 135 | 33 (22–60) |
| LDH (U l –1 ) | 134 | 397 (259–666) |
| γ‐GT (U l –1 ) | 134 | 178 (62–443) |
| ALP (U l –1) | 135 | 123 (89–179) |
| Bilirubin (total) (μmol l –1 ) | 126 | 11 (8–20) |
| Bilirubin (unconjugated) (μmol l –1 ) | 37 | 15 (10–32) |
| CRP (mg l –1 ) | 134 | 37 (12–75) |
Data are presented as median (interquartile range). ALP, http://www.google.nl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwi94uH5yuzRAhWCuxQKHfmfAvgQFgghMAA&url=http%3A%2F%2Fwww.healthline.com%2Fhealth%2Falp&usg=AFQjCNFXA8stb8Y6n0ySDvIePcGeOONGDw&bvm=bv.145822982,d.d24 ALT, http://www.webmd.com/digestive-disorders/alanine-aminotransferase-alt
Determination of levels of cytokines and CRP, and liver function tests
In all 143 samples that were available for VCZ trough concentration determinations, at least one cytokine concentration was detectable. This resulted in different numbers of combinations between VCZ trough concentrations and specific cytokines. A total of 139 pairs of VCZ and IL‐8, 134 pairs of VCZ and CRP, and 101 pairs of VCZ were available. An overview of all measured laboratory parameters can be found in Table 2.
Correlations between inflammatory markers and VCZ trough concentrations
Of the seven cytokines, only IL‐6 and IL‐8 turned out to have a significant overall association with VCZ trough concentrations: IL‐6: r = 0.46, P < 0.0001, 100 observations; IL‐8: r = 0.42, P < 0.0001, 138 observations (Figure 1).
Figure 1.
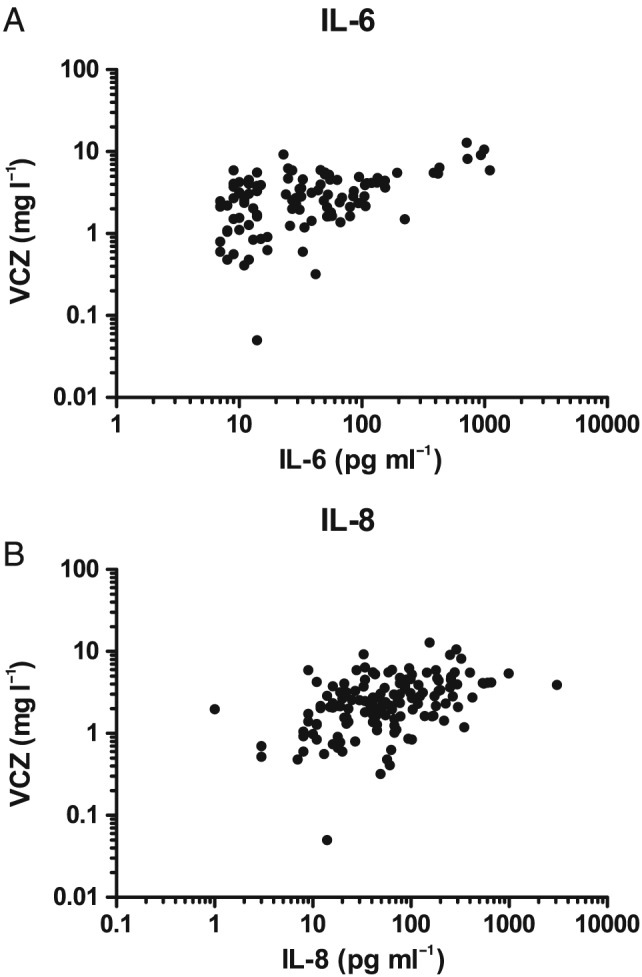
(A) Voriconazole (VCZ) concentrations (mg l–1) vs. interleukin (IL) 6 (pg ml–1) levels. (B) VCZ (mg l–1) vs. IL‐8 levels (pg ml –1)
Results on linear mixed‐model analyses pointed in the same direction: only IL‐6 (P = 0.0003) and IL‐8 (P = 0.0002) were significantly related to VCZ trough concentrations after correction for dose per kg and conjugated bilirubin. There was no need to correct for the potential confounders ALT, AST, LDH, γ‐GT, ALP and unconjugated bilirubin.
CRP levels showed an overall correlation with VCZ trough concentrations: r = 0.53, P < 0.0001, 133 observations (Figure 2).
Figure 2.
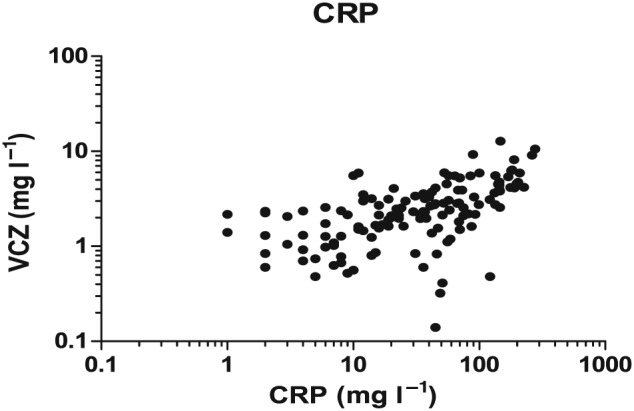
Voriconazole (VCZ) concentrations (mg l–1) vs. C‐reactive protein (CRP) levels (mg l–1)
Linear mixed‐model analysis showed that CRP levels were associated with VCZ trough concentrations after correction for dose per kg and conjugated bilirubin: P < 0.0001. The results for VCZ, CRP and IL‐6 concentrations per patient are shown in Figures 3 and 4, which show the differences between individuals in the correlation between VCZ trough concentration and IL‐6 and CRP.
Figure 3.
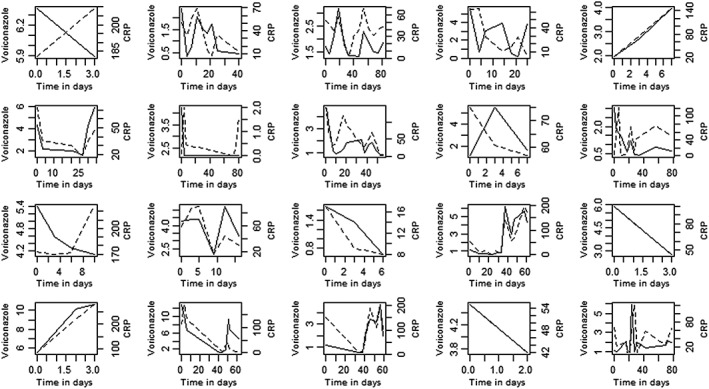
Voriconazole (VCZ) concentrations (mg l–1) (dotted line) and C‐reactive protein (CRP) levels (mg ml–1) (continuous line) vs. time (days) of VCZ treatment per individual patient (two patients were excluded because absence of repeated measurements over time)
Figure 4.
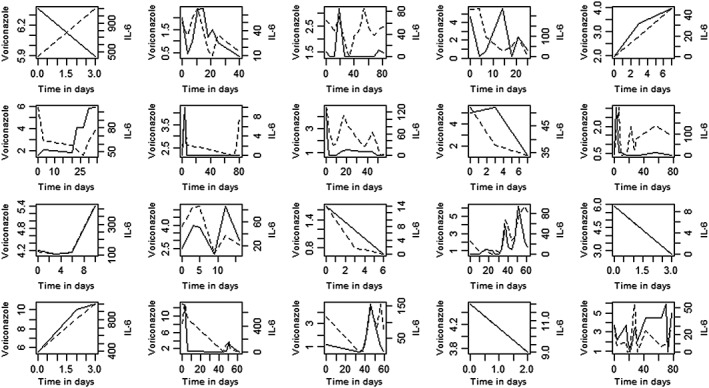
Voriconazole (VCZ) concentrations (mg l–1) (dotted line) and interleukin (IL) 6 levels (pg ml–1) (continuous line) vs. time (days) of VCZ treatment per individual patient (two patients were excluded because absence of repeated measurements over time)
Comparing the ability of cytokines to predict VCZ levels, in comparison with CRP
To compare the associations of Il‐6 and IL‐8, respectively, with VCZ trough concentrations on the one hand, and that of CRP with VCZ trough concentrations on the other, we fitted the above‐mentioned models on the same subset, consisting of all those observations with nonmissing values for VCZ, Il‐6, IL‐8, CRP, conjugated bilirubin and dose per kg at the same time points (Table 3). This resulted in a total of 82 observations containing no missing values.
Table 3.
Statistical comparison between the log‐IL‐6, log‐IL‐8 and log‐CRP models
| Estimated regression coefficient | Intercept | Dose per kg | Log‐bilirubin level | Log‐IL‐6, log‐IL‐8 or log‐CRP levels | Akaike Information Criteriona | Number of observations |
|---|---|---|---|---|---|---|
| Model with log‐IL‐6 | −2.1990 P = 0.0010 | 0.4162 P = 0.0002 | 0.3393 P = 0.0283 | 0.2254 P = 0.0004 | 169.6 | 82 |
| Model with log‐IL‐8 | −2.8777 P = 0.003 | 0.4728 P < 0.0001 | 0.3766 P = 0.0199 | 0.2881 P = 0.0014 | 170.6 | 82 |
| Model with log‐CRP | −2.2546 P = 0.0012 | 0.3754 P = 0.0011 | 0.3685 P = 0.0157 | 0.2419 P = 0.0005 | 172.5 | 82 |
CRP, C‐reactive protein; IL, interleukin
Smaller values are better
Inspection of the AICs of these three models, fitted on the same dataset of 82 observations, showed that the fit of the model with CRP (AIC = 172.5) was poorer than that of the models containing IL‐6 (AIC = 169.6) or IL‐8 (AIC = 170.6), although the differences were marginal.
Discussion
In the present study, we examined a panel of seven cytokines, both pro‐ and anti‐inflammatory, in specific models, for their correlation with VCZ trough concentrations. The levels of two of these proinflammatory cytokines, IL‐6 and IL‐8, correlated significantly with VCZ trough concentrations. We corrected for other known concomitant factors. The correlations between VCZ and IL‐6 levels, and between VCZ and IL‐8 levels were modest.
Our results highlight that proinflammatory markers only partly explain the intrapatient variability in VCZ. A relationship levels of pro‐inflammatoiry cytokines and decreased expression of CYP enzymes, and thereby higher concentrations of several drugs, has previously been suggested 26. Furthermore, a correlation between VCZ and inflammation, reflected by CRP levels, has been reported 18, 27. The possible relationship between upregulation of VCZ and specific proinflammatory markers had not yet been described. Recently, a study with a comparable design and patient group was published, using another linear mixed model to determine the association of CRP with VCZ concentrations 17. We used a different type of linear mixed model because of the skewness of our data, which made log‐transformation essential. In our opinion, our model ensured a better fit for showing a correlation between VCZ trough and cytokine/CRP concentrations. The Gaussian serial covariance structure in combination with a random intercept takes into account the fact that observations within a patient decrease if the time between observations increases. This resulted in lower values of AIC compared with using other approaches 17. The lower values of the AIC indicate a better fit of our data in this model. To account for correlations between repeated log‐VCZ trough concentrations within a patient, linear mixed models for repeated measurements were applied with Gaussian serial covariance structure in combination with a random intercept. In addition to the Gaussian serial covariance structure, we also explored the use of first‐order autoregressive and exponential serial covariance structures. As these structures resulted in higher values of AIC, indicating a poorer fit, we decided not to use them in the present study.
The confirmation of the correlation between IL‐6 and VCZ levels is interesting because it might imply that a higher immune response to IFD enhances the toxic effects of VCZ treatment. Patients suffering from IFDs produce many proinflammatory cytokines as a result of the infection and, in addition, mucosal barrier injury and graft‐vs.‐host disease result in increased levels of proinflammatory cytokines, which subsequently can result in the downregulation of CYP enzymes. This results in higher VCZ concentrations, with the likelihood of greater effectiveness in killing the mould, but at the expense of toxicity for the patient. The inflammatory response in our population was fairly modest, with an average CRP level of 37 mg l–1 (Table 2). It remains to be investigated whether the levels of pro‐inflammatoiry cytokines on the downregulation of VCZ metabolism.
IL‐6 is a cytokine that has effects not only on immune cells, but also on liver cells 28. It firstly binds to its receptor (IL‐6R), then recruits glycoprotein 130 (gp130) and forms a complex that subsequently activates signal transducer and activator of transcription 3 (STAT3), and leads to transcription 29. IL‐6R is expressed in only a limited number of cells – mainly hepatocytes, macrophages and neutrophils 29. Other cells in the host can also respond to IL‐6 but will activate gp130 because IL‐6 and soluble IL‐6R form a complex that circulates and binds to gp130, which is called trans‐signalling 30. Hepatocytes express abundant gp130 and therefore can be activated directly and via trans‐signalling, underscoring the significant impact that IL‐6 can have on hepatocytes 31. In the setting of patients with IFD that are treated with VCZ, IL‐6 could still be beneficial for the host by enhancing the treatment of IFD through its effects on hepatocytes, despite being ineffective in optimizing host responses against the infection. As the effects of IL‐6 are dependent on STAT3, it is tempting to speculate that other signalling cascades that modulate STAT3 activity might also play a significant role in regulating VCZ metabolism in hepatocytes.
As suggested, in vitro studies have clearly shown a direct influence of IL‐6 on CYP2C19 and CYP3A4 transcription and expression. Studies that investigated the influence of IL‐6 on different CYP enzymes demonstrated that IL‐6‐mediated downregulation is more pronounced for CYP3A4 than for CYP2C19. This could play an important role in poor metabolizers because the expected main responsible metabolic pathway in these metabolizers is the hydroxylation route via CYP3A4. According to this theory, poor metabolizers of CYP2C19 with high IL‐6 concentrations should have even higher VCZ trough concentrations than poor metabolizers without high IL‐6 concentrations. The impact of inflammation in patients who use VCZ could thereby be even bigger in poor metabolizers. Unfortunately, we were not able to determine the genotype of our patients, so we could not investigate this hypothesis.
To confirm the impact of the immune response on the clearance pathways of VCZ, the metabolic ratio of VCZ and N‐oxide VCZ might have provided more information. Knowledge on shifts in this ratio would have helped to determine the downregulation of metabolic processes involved in VCZ turnover and further substantiate the hypothesis of a reduction in clearance caused by the inflammatory response. Unfortunately, this information was not available from our cohort.
We also found that IL‐8 concentrations were associated with VCZ trough concentrations. There are several possible explanations for the similar relationships between IL‐8 and VCZ, and between IL‐6 and VCZ. First, similar to IL‐6, IL‐8 might have direct effects on CYP enzymes. However, in contrast to the in vitro studies on IL‐6 and CYP enzymes, there are no reports on the effects of IL‐8 on these enzymes. Secondly, the production of both IL‐6 and IL‐8 is induced by recognition of pathogen‐associated molecular patterns and under the transcriptional control of, among others, the master inflammatory transcription factor, nuclear factor κB 32, 33, 34. Therefore, the relationship between IL‐8 and VCZ might be an epiphenomenon, caused by the fact that IL‐8 is induced alongside IL‐6 in this patient group, but has no direct effects on CYP enzymes. This is in line with an inter‐correlation we found between IL‐6 and IL‐8, but which we did not show in our results. Regarding the known direct effect of IL‐6 on CYP enzymes, we hypothesize that IL‐6 might play a more important role in VCZ metabolism by downregulating CYP enzymes, although the AIC for IL‐6 was only marginally better than that for IL‐8, which cannot serve as a confirmation of this hypothesis.
The correlation between CRP and VCZ trough concentrations will be more useful in clinical practice compared with the use of IL‐6 or IL‐8 levels. The benefit of using these cytokine levels is that they provide more downstream‐selective information on inflammation. CRP levels are measured more frequently than IL‐6 or IL‐8 in everyday healthcare settings, and at a lower cost than for these cytokines. As such, CRP might be easier to use in clinical practice, keeping in mind that the correlation between CRP and VCZ alone is not enough to explain the specific contribution of the high intrapatient variability in VCZ trough concentrations.
A possible limitation to the study was the time difference between the actual determination of VCZ trough concentrations and measurement of cytokine/CRP levels. Sampling and determination of VCZ trough concentrations took place on the same day, but measurement of cytokine levels took place after a period of 2–5 years’ storage at −80°C. The stability of different cytokines has not been investigated for a period longer than 2 years 35. However, even if the cytokine concentrations were to be lower than expected, they would still allow us to determine whether VCZ concentrations can be predicted by these cytokine levels owing to an equally relevant decay over time. By using our linear mixed model, the specific value of the cytokine concentrations is less important than the relative comparison with increased or decreased VCZ trough concentrations.
Finally, as can be seen in Figure 3, VCZ and proinflammatory markers mimic each other well in some cases but poorly in other cases. We could not establish a plausible cause for this, such as dose adaptations. No significant difference was found between patients undergoing therapeutic drug monitoring and those who did not.
We realize that the study might have benefited from a larger sample size, to determine the specific contribution of IL‐6 or IL‐8 to VCZ trough concentrations. Despite the prospective design of our study, samples had to be excluded from analysis. Ideally, samples of VCZ trough and cytokines are available more frequent (for instance at least three times weekly) and with fully identical timing of both cytokines as well as voriconazole trough concentration. A study with a larger patient cohort and focusing on altered clearance patterns (including metabolite information) is required to predict the influence of specific cytokines more precisely. A prospective study should be performed in two groups of comparable healthy volunteers, one group receiving intravenous VCZ without stimulation of proinflammatory cytokine production, and the other receiving intravenous VCZ with stimulation of proinflammatory cytokine production – for example, with lipopolysaccharide. Both groups should have similar patient characteristics, including a confirmed extensive metabolizer phenotype of CYP2C19.
By measuring IL‐6, IL‐8, CRP and VCZ trough concentrations, we were able to determine the potential contribution of IL‐6 and IL‐8 in the upregulation of VCZ trough concentrations and compare this to the contribution of CRP levels. This outcome should be tested in future pharmacokinetic modelling exercises as a relevant covariate on clearance. This might help us to retrieve more information on the currently unexplained differences within a patient over time. If this result proves valid, it will help us to make valid recommendations for dose adaptation, taking this finding into account. In conclusion, VCZ trough concentrations show a significant but modest correlation with IL‐6, IL‐8 and CRP levels. Future studies should investigate this prospectively, and incorporate the data obtained into pharmacokinetic models to refine this predictive behaviour.
Competing Interests
The current study was funded by the Radboud University Medical Center. R.J.B. has served as a consultant to, and received unrestricted research and educational grants from Astellas Pharma, Inc., F2G, Gilead Sciences, Merck Sharp & Dohme Corp. and Pfizer, Inc. All contracts were through Radboudumc and payments were invoiced by Radboudumc. None of the work is related to this manuscript. None of the other authors have any competing interests to declare.
The authors would like to thank Jelle Gerretsen for performing the cytokine analysis, Khalid Asouit for performing the VCZ analysis, Lisa Martial for designing Figures 3 and 4 , Maikel Couwenberg for collecting dosing data from old patient records, and haematologist Caroline Mandigers, from Canisius‐Wilhelmina Hospital, Nijmegen, for looking after some dosing regimens. We thank Mr A. F. J. de Haan for his assistance in the statistical analysis.
Vreugdenhil, B. , van der Velden, W. J. F. M. , Feuth, T. , Kox, M. , Pickkers, P. , van de Veerdonk, F. L. , Blijlevens, N. M. A. , and Brüggemann, R. J. M. (2018) Moderate correlation between systemic IL‐6 responses and CRP with trough concentrations of voriconazole. Br J Clin Pharmacol, 84: 1980–1988. 10.1111/bcp.13627.
References
- 1. Herbrecht R, Patterson TF, Slavin MA, Marchetti O, Maertens J, Johnson EM, et al Application of the 2008 definitions for invasive fungal diseases to the trial comparing voriconazole versus amphotericin B for therapy of invasive aspergillosis: a collaborative study of the Mycoses Study Group (MSG 05) and the European Organization for Research and Treatment of Cancer Infectious Diseases Group. Clin Infect Dis 2015; 60: 713–720. [DOI] [PubMed] [Google Scholar]
- 2. Dolton MJ, Mikus G, Weiss J, Ray JE, McLachlan AJ. Understanding variability with voriconazole using a population pharmacokinetic approach: implications for optimal dosing. J Antimicrob Chemother 2014; 69: 1633–1641. [DOI] [PubMed] [Google Scholar]
- 3. Dolton MJ, McLachlan AJ. Voriconazole pharmacokinetics and exposure‐response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents 2014; 44: 183–193. [DOI] [PubMed] [Google Scholar]
- 4. Bruggemann RJ, Donnelly JP, Aarnoutse RE, Warris A, Blijlevens NM, Mouton JW, et al Therapeutic drug monitoring of voriconazole. Ther Drug Monit 2008; 30: 403–411. [DOI] [PubMed] [Google Scholar]
- 5. Gautier‐Veyret E, Fonrose X, Tonini J, Thiebaut‐Bertrand A, Bartoli M, Quesada JL, et al Variability of voriconazole plasma concentrations after allogeneic hematopoietic stem cell transplantation: impact of cytochrome p450 polymorphisms and comedications on initial and subsequent trough levels. Antimicrob Agents Chemother 2015; 59: 2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol 2006; 46: 235–243. [DOI] [PubMed] [Google Scholar]
- 7. Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N‐oxidation of voriconazole. Drug Metab Dispos 2003; 31: 540–547. [DOI] [PubMed] [Google Scholar]
- 8. Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, et al The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab Dispos 2003; 31: 731–741. [DOI] [PubMed] [Google Scholar]
- 9. Scholz I, Oberwittler H, Riedel KD, Burhenne J, Weiss J, Haefeli WE, et al Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br J Clin Pharmacol 2009; 68: 906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murayama N, Imai N, Nakane T, Shimizu M, Yamazaki H. Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N‐oxidized metabolite formation from voriconazole, a new anti‐fungal agent, in human liver microsomes. Biochem Pharmacol 2007; 73: 2020–2026. [DOI] [PubMed] [Google Scholar]
- 11. Yanni SB, Annaert PP, Augustijns P, Bridges A, Gao Y, Benjamin DK Jr, et al Role of flavin‐containing monooxygenase in oxidative metabolism of voriconazole by human liver microsomes. Drug Metab Dispos 2008; 36: 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aitken AE, Richardson TA, Morgan ET. Regulation of drug‐metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol 2006; 46: 123–149. [DOI] [PubMed] [Google Scholar]
- 13. Morgan ET. Impact of infectious and inflammatory disease on cytochrome P450‐mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther 2009; 85: 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Veringa A, Ter Avest M, Span LF, van den Heuvel ER, Touw DJ, Zijlstra JG, et al Voriconazole metabolism is influenced by severe inflammation: a prospective study. J Antimicrob Chemother 2017; 72: 261–267. [DOI] [PubMed] [Google Scholar]
- 15. Antachopoulos C, Roilides E. Cytokines and fungal infections. Br J Haematol 2005; 129: 583–596. [DOI] [PubMed] [Google Scholar]
- 16. Pepys MB, Hirschfield GM. C‐reactive protein: a critical update. J Clin Invest 2003; 111: 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Encalada Ventura MA, van Wanrooy MJ, Span LF, Rodgers MG, van den Heuvel ER, Uges DR, et al Longitudinal analysis of the effect of inflammation on voriconazole trough concentrations. Antimicrob Agents Chemother 2016; 60: 2727–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Wanrooy MJ, Span LF, Rodgers MG, van den Heuvel ER, Uges DR, van der Werf TS, et al Inflammation is associated with voriconazole trough concentrations. Antimicrob Agents Chemother 2014; 58: 7098–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein M, Thomas M, Hofmann U, Seehofer D, Damm G, Zanger UMA. systematic comparison of the impact of inflammatory signaling on absorption, distribution, metabolism, and excretion gene expression and activity in primary human hepatocytes and HepaRG cells. Drug Metab Dispos 2015; 43: 273–283. [DOI] [PubMed] [Google Scholar]
- 20. Li AP, Yang Q, Vermet H, Raoust N, Klieber S, Fabre G. Evaluation of human hepatocytes under prolonged culture in a novel medium for the maintenance of hepatic differentiation: results with the model pro‐inflammatory cytokine interleukin 6. Drug Metab Lett 2014; 8: 12–18. [DOI] [PubMed] [Google Scholar]
- 21. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wissen CP, Burger DM, Verweij PE, Aarnoutse RE, Bruggemann RJ. Simultaneous determination of the azoles voriconazole, posaconazole, isavuconazole, itraconazole and its metabolite hydroxy‐itraconazole in human plasma by reversed phase ultra‐performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 2012; 887–888: 79–84. [DOI] [PubMed] [Google Scholar]
- 23. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al THE Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 2017; 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Renton KW. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin Drug Metab Toxicol 2005; 1: 629–640. [DOI] [PubMed] [Google Scholar]
- 27. Encalada Ventura MA, Span LF, van den Heuvel ER, Groothuis GM, Alffenaar JW. Influence of inflammation on voriconazole metabolism. Antimicrob Agents Chemother 2015; 59: 2942–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997; 276: 60–66. [DOI] [PubMed] [Google Scholar]
- 29. Taga T, Kishimoto T. Gp130 and the interleukin‐6 family of cytokines. Annu Rev Immunol 1997; 15: 797–819. [DOI] [PubMed] [Google Scholar]
- 30. Peters M, Muller AM, Rose‐John S. Interleukin‐6 and soluble interleukin‐6 receptor: direct stimulation of gp130 and hematopoiesis. Blood 1998; 92: 3495–3504. [PubMed] [Google Scholar]
- 31. Peters M, Jacobs S, Ehlers M, Vollmer P, Mullberg J, Wolf E, et al The function of the soluble interleukin 6 (IL‐6) receptor in vivo: sensitization of human soluble IL‐6 receptor transgenic mice towards IL‐6 and prolongation of the plasma half‐life of IL‐6. J Exp Med 1996; 183: 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffmann E, Dittrich‐Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin‐8 gene expression. J Leukoc Biol 2002; 72: 847–855. [PubMed] [Google Scholar]
- 33. Kishimoto T. IL‐6: from its discovery to clinical applications. Int Immunol 2010; 22: 347–352. [DOI] [PubMed] [Google Scholar]
- 34. Diepold M, Noellke P, Duffner U, Kontny U, Berner R. Performance of interleukin‐6 and interleukin‐8 serum levels in pediatric oncology patients with neutropenia and fever for the assessment of low‐risk. BMC Infect Dis 2008; 8: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert‐Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol 2009; 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]


