Abstract
Despite its good initial response and significant survival benefit in patients with castration-resistant prostate cancer (CRPC), taxane therapy inevitably encounters drug resistance in all patients. Deep understandings of taxane resistant mechanisms can significantly facilitate the development of new therapeutic strategies to overcome taxane resistance and improve CRPC patient survival. Multiple pathways of resistance have been identified as potentially crucial areas of intervention. First, taxane resistant tumor cells typically have mutated microtubule binding sites, varying tubulin isotype expression, and upregulation of efflux transporters. These mechanisms contribute to reducing binding affinity and availability of taxanes. Second, taxane resistant tumors have increased stem cell like characteristics, indicating higher potential for further mutation in response to therapy. Third, the androgen receptor pathway is instrumental in the proliferation of CRPC and multiple hypotheses leading to this pathway reactivation have been reported. The connection of this pathway to the AKT pathway has received significant attention due to the upregulation of phosphorylated AKT in CRPC. This review highlights recent advances in elucidating taxane resistant mechanisms and summarizes potential therapeutic strategies for improved treatment of CRPC.
KEY WORDS: Castration-resistant prostate cancer, Drug efflux transporters, Taxane resistance, Androgen receptor, PI3K/AKT pathway, Microtubules, Cancer stem cells, Efflux transporter
Graphical abstract
This review highlights recent advances in elucidating taxane resistant mechanisms and summarizes potential therapeutic strategies for improved treatment of castration-resistant prostate cancer (CRPC).
1. Introduction
Despite recent decreases in cancer diagnoses in the western world, prostate cancer still accounts for approximately 1 in 5 new cancer diagnoses in men and remains one of the leading causes of death. It is estimated that about 164,000 new cases will develop and 29,000 men will die in 2018 in the United States alone due to prostate cancer1. Worldwide, incidence rates continue to increase in the developing world, while rates in Asia remain the lowest of all major geographical regions2.
Typically, most patients diagnosed with prostate cancer are first treated through androgen deprivation therapy (ADT). While ADT has shown to be effective at first, the vast majority of patients develop resistance to this treatment, developing castration-resistant prostate cancer (CRPC) and other therapeutic options are required to treat the disease3, 4, 5, 6. Many times therapy involves the use of taxanes, microtubule stabilizing agents, which have shown to disrupt the G2/M-phase of cell cycle and induce apoptosis7, 8, 9. Paclitaxel (PXL), its nanoformulation Abraxane, and docetaxel (DXL) are the three 1st line taxanes approved to treat cancer in this manner. DXL is the approved choice in treating prostate cancer due to its effectiveness at prolonging the lifespan of prostate cancer patients when used in combination with prednisone10. Many problems still exist with these taxanes including development of resistance, high toxicity (especially neutropenia and peripheral neuropathy), and limited bioavailability11, 12, 13, 14.
As such, efforts to tackle any or all of these issues have increased in recent years. In 2010 the U.S. Food and Drug Administration (FDA) approved a new taxane, cabazitaxel (CXL), for treatment of prostate cancer for those who have already been treated with DXL and developed resistance15. Additionally, four other non taxane treatments have been approved in the last seven years. Abiraterone and enzalutamide inhibit androgen receptor (AR) function through biosynthesis inhibition and nuclear translocation respectively16, 17. Sipuleucel-T, the first vaccine approved for treatment of hormone refractory prostate cancer, has unknown exact mechanism of action but stimulates T-cell response against highly expressed antigen presenting cells18. Lastly, Radium-223, an alpha particle emitter, has been approved for patients whose cancer has spread to the bones19.
With all of these treatments approved, prostate cancer patients have an exceptional number of options for therapy. Yet, prostate cancer is still one of the leading causes of death in men and more work is needed to understand the mechanism behind resistance development and treatment failures, especially for the taxane class of drugs. This review will focus on taxanes and discuss their mechanisms of resistance and therapeutic strategies to overcome them.
2. Microtubule and taxane structures
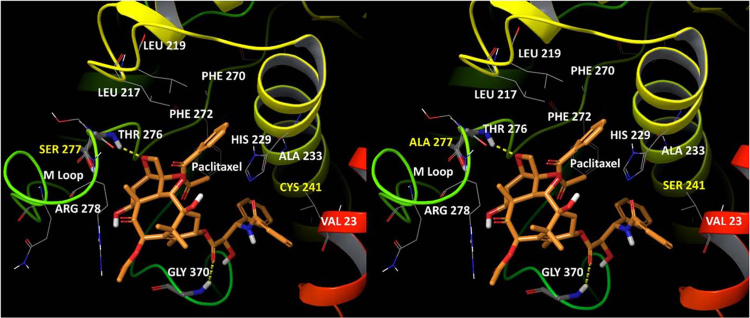
Microtubules are characterized as hollow tubes formed through the heterodimerization of alpha and beta tubulin, resulting in polar protofilaments. They assist in a variety of functions for the cell including structural integrity, transportation and migration, and mitosis as they are a main constituent of mitotic spindles. They are also dynamically active, constantly growing and shrinking in size20. The structure of microtubules has been well established. Their outer surface has high alpha helix expression as well as high expression of the C-terminal helices H11 and H12. On the other hand, the inner surface of the microtubules is characterized by long loops21. Cryoelectron microscopy has further shown that PXL binds behind the M-loop in the beta-tubulin and interacts with adjacent beta-tubulin H1-S2 loops (Fig. 1)22. More recently, taxanes have been implicated in promoting microtubule assembly through creating a short helix in the M-loop, possibly reducing strain and allowing for an easier transition from the curved unbound tubulin to the straight protofilament dimers23.
Figure 1.
Paclitaxel bound to the active site on βI-tubulin (left) and βIII-tubulin (right). The yellow lines indicate hydrogen bonding between PXL and β-tubulin (Gly370 and Thr276). Other key amino acids which potentially interact with PXL are included. Of note, His229 and Phe272 are residues of π–π interactions. Residues 241 and 277 are mutated in βIII-tubulin from cysteine and serine to serine and alanine, respectively.
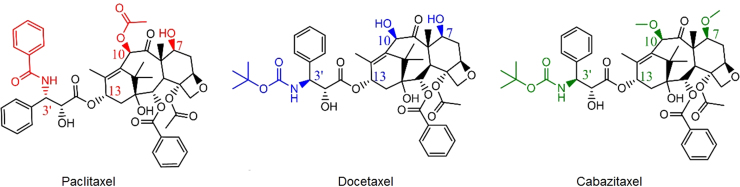
First identified in the early 1970s, PXL is a natural plant substance which binds to microtubules with high affinity (Kapp = 0.87 μmol/L)24, 25. Its structure (Fig. 2, left) contains a baccatin (the molecule which acts as PXL's precursor) core, a side chain at the C13 position, and three prominent functional groups: a benzyl amide at the C3′ position, a hydroxyl at C7, and an acetyloxy at C1026. As previously mentioned, two other taxanes, DXL and CXL, have been developed since PXL's discovery. While possessing the same backbone, the functional groups of these two molecules differ compared to PXL. DXL (Fig. 2, center) features a t-butyl carbamate functionality and a hydroxyl at C3′ and C10, respectively, while CXL (Fig. 2, right) contains methoxy groups at C7 and C10. The slight variation in structure affects not only binding to the microtubule active site, but efflux pumps as well. Most notably, CXL is not a strong substrate for P-glycoprotein (P-gp)7, 27.
Figure 2.
2D structures of paclitaxel, docetaxel, and cabazitaxel.
3. Mechanisms of taxane resistance and strategies to overcome this resistance
3.1. Microtubules
3.1.1. Microtubule mutations contribute to taxane resistance
The active binding site of taxanes to microtubules has been the subject of extensive research in the attempt to elucidate the cause of taxane resistance. Mutations in βI-tubulin have been associated with resistance to PXL in other forms of cancer including ovarian cancer28. Specifically, different amino acid residues appear to change in the mutated forms of resistance cell lines. The M40 isotype of βI-tubulin expresses valine and threonine at residue locations β270 and β364 instead of phenylalanine and alanine respectively28. A mutation of the β270 site to isoleucine has also been shown to occur in a prostate cancer DXL resistant cell line, further corroborating the importance of this amino acid site for taxane resistance29. Others have shown that a leucine cluster (β215, β217, β228) in the most common form of βI-tubulin may change to a variety of other amino acids resulting in PXL resistance30. It is not entirely clear whether these mutations result in weaker PXL binding to the active site, or reduction in the subsequent over stabilization of the microtubules, although structural analysis of PXL binding to microtubules indicates valine (β:Val23), leucine (β:Leu217 and 219), alanine (β:Ala233), and arginine (β:Arg278 and 282) all play important roles22, 31.
Increased expression of βIII-tubulin has also been associated with taxane resistance8, 32. One study conducted by Terry et al.33 examined βIII-tubulin expression both in vivo and in vitro. Clinically, only seven samples out of 74 samples (4%) tested positive for βIII-tubulin if the patient had not been treated with any hormonal therapy. However, 24 out of 40 samples (60%) from patients chronically treated with hormonal therapy tested positive for βIII-tubulin. Similar results were observed in vitro as androgen insensitive cell lines (PC3 and DU145) expressed βIII-tubulin but androgen-sensitive cells (LNCaP) did not. It was also shown that βIII-tubulin is more highly expressed in a PXL resistant prostate cancer cell line compared to the parental sensitive cells34. Additionally, DXL resistance in multiple cancer types including breast cancer, lung cancer, and prostate cancer has been linked to βIII-tubulin expression35, 36, 37, 38. CXL has also shown to be less effective in high expressing βIII-tubulin cell lines, indicating βIII-tubulin is a key factor in all of taxane resistance39. Key amino acid changes in βIII-tubulin around the binding site of PXL are Ser241 and Ala277, compared with Cys241 and Ser277 in βI-tubulin40. Hypoxia has been identified as a potential cause of increased expression, but evidence as to why βIII-tubulin is so highly expressed in CRPC is still a mystery. Further studies to identify the underlying mechanisms of this change may be beneficial to patients. A potent inhibitor of this mechanism used in conjunction with taxane therapy may very well prevent development of resistance.
3.1.2. Cabazitaxel and other tubulin inhibitors can overcome resistance to PXL and DXL
Both DXL and CXL's initial improved efficacy in PXL resistant cell lines and CXLs improved efficacy in DXL resistant cell lines may very well be attributed to variation in structure between the three molecules. Dynamic simulations from Churchill and colleagues41 of the three drugs binding to microtubules have given insight into the slight variations which very well cause these differences. Both DXL and PXL exhibit relatively rigid structure binding, with emphasis on π–π interactions within the M-loop and hydrogen bonding. β:His229 and β:Phe272 seem to constituent the active sites contribution to the π–π interactions, while aspartate and arginine are essential for the hydrogen interactions. DXL did display slightly better interactions, especially for hydrogen bonding, most likely because of the hydroxyl additions. On the other hand, CXL seems to form a more collapsed form, completely unlike PXL and DXL. This indicates weaker interactions all around and thus most likely weaker binding to the active site.
Another method that has been popular in attempting to overcome the mutations to the taxane binding site is targeting one of two different sites to inhibit tubulin: the colchicine active site or the vinca alkaloid active site. Vinca alkaloids (VA), while successful in treating other forms of cancer, have exhibited mixed results in treating prostate cancer and currently no VA is FDA approved for this disease42, 43, 44. No molecule targeting the colchicine active site has been approved for treatment of any cancer, although two prodrug variations of combretastatin A-4, which disrupts angiogenesis, advanced to clinical trials for the treatment of solid tumors45, 46, 47. Both VA and colchicine binding agents are known to destabilize tubulin dynamics, although most of the exact mechanisms of action are still unknown.
3.2. Cancer stem cells
3.2.1. CSC development leads to taxane resistance
The development of cancer stem cells (CSC) has been associated with various forms of cancer for some time with CSCs being identified in prostate cancer in 200548, 49, 50, 51, 52, 53, 54, 55, 56. CD133, CD44, ALDH, and α1β2 integrin have all been associated biomarkers for stemness in prostate cancer and new evidence suggests resistance to taxane therapy in prostate cancer is at least partially derived from the formation of cells with some or all of these markers50, 57, 58, 59. While taxanes are effective at causing cell arrest, not every cell actually dies when its G2/M-phase is disrupted. One study showed that some survive and become a new subset of multinucleated polyploids (MP) which have recently been shown to express CD44 and express resistance to DXL60. CD133 has also been associated with taxane resistance in both melanoma and prostate cancer and is quite often highly expressed along with CD44 in prostate cancer61, 62, 63. As mentioned, ALDH and α1β2 integrin are also common markers. However, it remains to be seen whether they correspond to taxane resistance. Studies further examining the impact of taxane therapy on these crucial biomarkers could elucidate their importance. Current research has focused on both preventing CSC development and using CSC targeting molecules in conjunction with taxanes.
3.2.2. Preventing CSC development
A new taxoid, SBT-1214, has shown potential anti CSC effects, reducing expression of several stem cell related transcription factors including c-Myc and SOX2, as well as increasing expression of pro-apoptotic proteins p53 and p21 in prostate cancer64, 65. In a CSC enriched cell line, PPT2, SBT-1214 suppresses cell growth better than PXL in concentration ranging from 10 nmol/L to 10 μmol/L65. Additionally, a novel nanoemulsion formulation of this compound has displayed enhanced pharmacokinetic properties and improved tumor suppression (IC50 of ~ 6 nmol/L) in the same cell line compared to both SBT-1214 and Abraxane66.
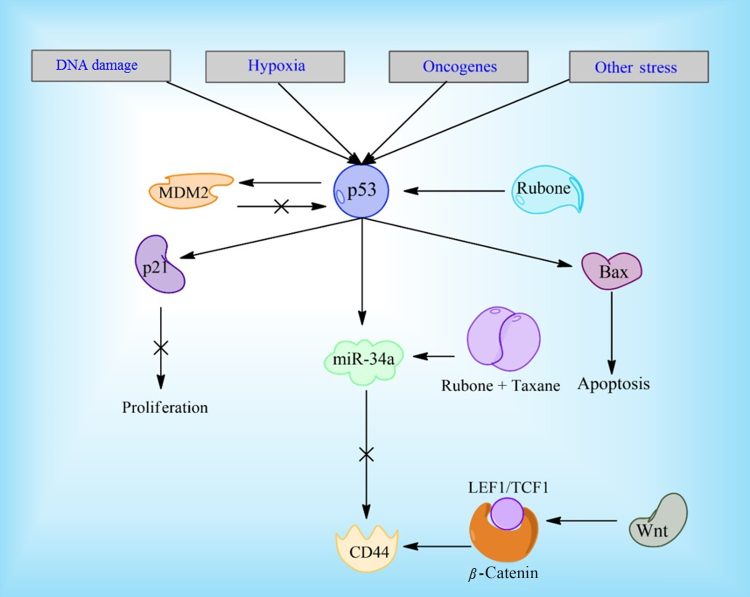
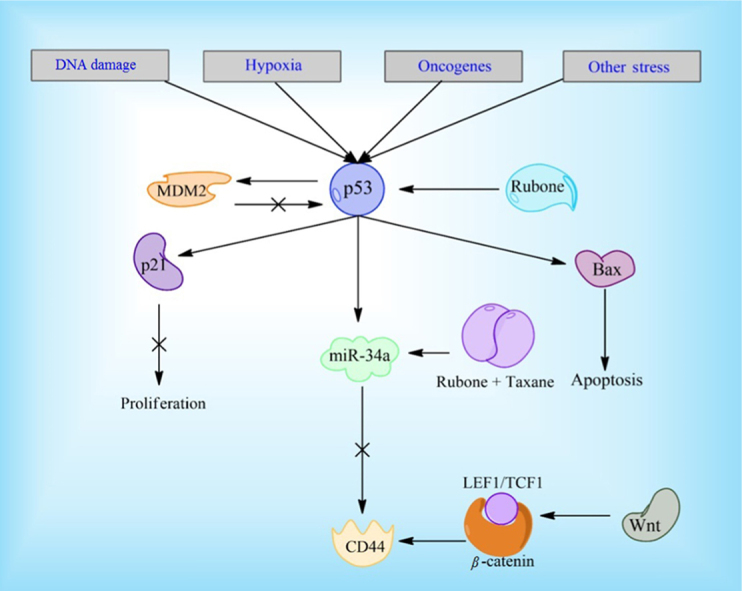
Reduction in microRNA miR-34a has also been associated with CSC development and its expression is reduced in CD44+ prostate cancer cells. Upregulation of miR-34a has displayed repressive qualities and both on its own and in combination with PXL67, 68. Studies have previously shown that p53 down regulates CD44 expression and the miR-34a pathway is a likely mechanism for this regulation69. A small molecule, rubone, has been especially effective at increasing iR-34a expression, although knockdown of p53 significantly reduces efficacy in hepatocellular carcinoma cells70, 71. However, a micellar delivery of PXL and rubone increases miR-34a regardless of p53 expression in prostate cancer cell lines68. This indicates multiple pathways are responsible for miR-34a up and down regulation in prostate cancer lines and rubone has an effect on at least two of these pathways (Fig. 3). It could be a potentially useful molecule clinically, since p53 is often absent in cancer cells. The Wnt signaling pathway has also been associated with abnormal CD44 expression through binding of β-catenin-TCF1/LEF1 to CD44's gene promoter72. LGK974, a Wnt inhibitor, is currently undergoing clinical testing and could possibly be tested further in combination with DXL to combat prostate cancer. Additionally, napabucasin, a STAT3 inhibitor currently undergoing clinical trials, has shown the ability to kill prostate cancer stem cells73. It is being tested in combination with Abraxane for a variety of other cancers and could potentially be used in combination with DXL for prostate cancer treatment.
Figure 3.
Rubone and the p53 pathway. Rubone can upregulate p53 directly, leading to a decrease in stem cell qualities in cancer cells. The combination of PXL and rubone has shown to be effective in upregulating miR-34a regardless of p53 expression, through another unknown pathway.
These novel potential treatments back the hypothesis that CSCs are a key problem in the fight against prostate cancer progression. However, while CSCs may explain how prostate cells develop resistance to therapy, the exact mechanism of the resistance is still unknown. It is most likely the cause of a variety of mutations which vary in different cell lines in vitro and even within a single patient clinically, highlighting the heterogeneity of the disease74.
3.3. Efflux transporters
3.3.1. Efflux transporter upregulation precipitate taxane resistance
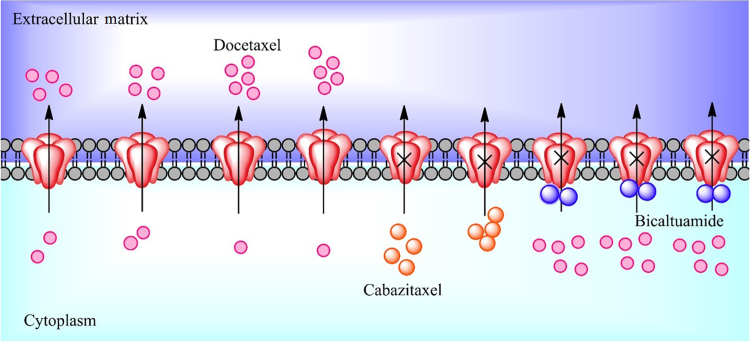
The Multidrug Resistance (MDR) family of efflux transporters is known to be heavily involved in resistance to various forms of chemotherapy. Increased P-gp expression, certain genetic variants of P-gp, as well as ABCC4 expression have been linked to increased DXL resistance in prostate cancer, although interestingly do not seem to affect the pharmacokinetics of the molecule75, 76. Additionally, it has been shown that ABCB5 transfected HEK293 cells exhibit higher resistance (2–3 folds) to PXL and DXL and ABCB5 ATPase activity increases in the presence of DXL77. This resistance and activity may be reduced via ABCB5 targeted siRNA. Some cell lines which either do not express or overexpress these transporters still develop resistance, indicating this cannot be the only mechanism for developing taxane resistance34. CXL has shown significantly decreased affinity for P-gp and can even cross the blood brain barrier which heavily expresses efflux transporters78. This mechanism likely contributes to its increased effectiveness in cell lines resistant to other taxanes, indicating avoiding major efflux transporters could be key in preventing resistance to taxanes78.
3.3.2. Resensitizing cells to taxane therapy via efflux inhibition
Recently, anti-androgens have been studied as possible inhibitors of the most important efflux transporters (Fig. 4). Both P-gp and ABCC4 respond to anti-androgen therapy. Bicalutamide and enzalutamide seem to reduce P-gp activity, leading to sensitivity to DXL once again4. Bicalutamide also reduces ABCC4 activity and this is associated with a decrease in ABCC4 mRNA expression as well79. However, the mechanism for how anti-androgen therapy may resensitize cells to taxanes is still unclear and may be a direction for future studies. Other inhibitors of P-gp have also been developed and studied in combination with taxane therapy. Verapamil, a known P-gp inhibitor has exhibited synergistic effects with PXL, resulting in a nearly 10-fold decrease in IC50 in a PXL resistant breast cancer cell line80. Quinine heterodimers and derivatives of coumarin have also shown to improve efficacy of PXL through P-gp inhibition, leading researchers to believe this could be a key to treating taxane resistant prostate cancer81, 82. Abraxane, an FDA approved nanoformulation of PXL, is known to escape elimination from P-gp83. This has sparked research into other formulations which could possibly do the same, especially those containing DXL. Cellax, a PEGylated carboxymethylcellulose conjugate of DXL, is one example of such a formulation84. Binding to albumin in plasma and being internalized via an albumin and SPARC complex, Cellax shows improved tumor distribution and sustained concentrations compared to DXL in vivo85. The formulation's slow release prevents cancer cells from upregulating P-gp expression, indicating maximum tumor concentration of taxanes may be an important factor in the development of resistance86. Variations of Cellax including those with CXL and podophyllotoxin, a microtubule destabilizer, have also been tested with success and the CXL conjugate showed potential to negate bone loss in treating bone metastatic prostate cancer27, 87. Other formulation attempts have used pH, lysosomes, and PLGA among others to achieve superior efficacy in vivo88, 89, 90.
Figure 4.
Efflux transporters and taxanes. DXL (pink) is a major substrate of P-gp, but bicaltuamide (blue) can reverse this sensitivity and sustain efficacy. CXL (orange) is not a P-gp substrate and is effective in many DXL resistant cancers.
3.4. Reactivation of the androgen receptor pathway can reduce taxane efficacy
Androgens, most importantly testosterone and its more active derivative dihydrotestosterone (DHT), are essential for the growth and proliferation of prostate cancer as they are known to stimulate proliferation as well as inhibit apoptosis of these cells91, 92. This is the reasoning behind using ADT as the frontline therapy for prostate cancer. However, all three currently approved taxanes have shown to inhibit AR nuclear translocation and/or AR expression too9, 93, 94. Evidence indicates that microtubules play an important role in AR translocation, so hyperstabilzation caused by the taxanes is the most likely cause of this inhibition95. Specifically, the androgen receptor, bound to both androgen and its microtubule binding site, is expressed in high concentrations in the cytoplasm post PXL administration95. Much like after ADT, however, resistance to this therapy develops, indicating the cells have found a way to foster androgen translocation and biosynthesis. This has been further supported by a recent study in which enzalutamide resistant cells also displayed cross resistance to DXL96. It is worth noting that, in the same study, CXL did not exhibit this cross resistance in terms of overall proliferation, although AR translocation was still restored to relatively normal levels.
Dehydoepiandrosterone (DHEA) is a native adrenal androgen and its sulfonated form (DHEA-SO4) has been implicated in an alternative pathway for prostate cancer proliferation in the presence of ADT5, 97. DHEA can be converted to DHT and it is often found in excess in circulation. This pathway depends on the presence of organic anion transporting polypeptides (OATPs) which uptake the molecules into the cell. The expression of these transporters is controlled by the gene expression of the solute carrier organic anion (SLCO) family and these have been shown to be markedly increased in prostate cancer cells98. However, this does not account for the impact of taxanes on the ability of DHT to translocate to the nucleus. Studies focusing on this in androgen independent cell lines could give insight into how prostate cancer may exhibit cross resistance to both DXL and enzalutamide, but not CXL.
Enzalutamide and abiraterone have been approved to treat CRPC with proposed mechanisms of inhibiting AR function and are used often in clinics either in conjunction with taxanes or pre/post taxane treatment. As previously mentioned, a recent study conducted by Zhu et al.4 has implicated enzalutamide in inhibiting P-gp efflux activity in a dose dependent manner, which in turn helped facilitate greater efficacy when used in combination with DXL. In the resistant cell lines, the IC50 of DXL decreased from 50 to 7 nmol/L when used in combination with 40 μmol/L of enzalutamide and recorded 60% inhibition of P-gp. However, resistance can develop to these treatments as well. It is also very difficult for doctors to decide the order and/or combination in which various chemotherapies should be given, as there is evidence to suggest both synergy and inhibition in various cases7, 99. It is clear that androgens are needed for prostate cancer to proliferate and continued research and development of drugs which hinder this pathway could prove vital for effective treatment.
3.5. PI3K/AKT
3.5.1. Upregulation of PI3K/AKT signaling contributes to taxane resistance
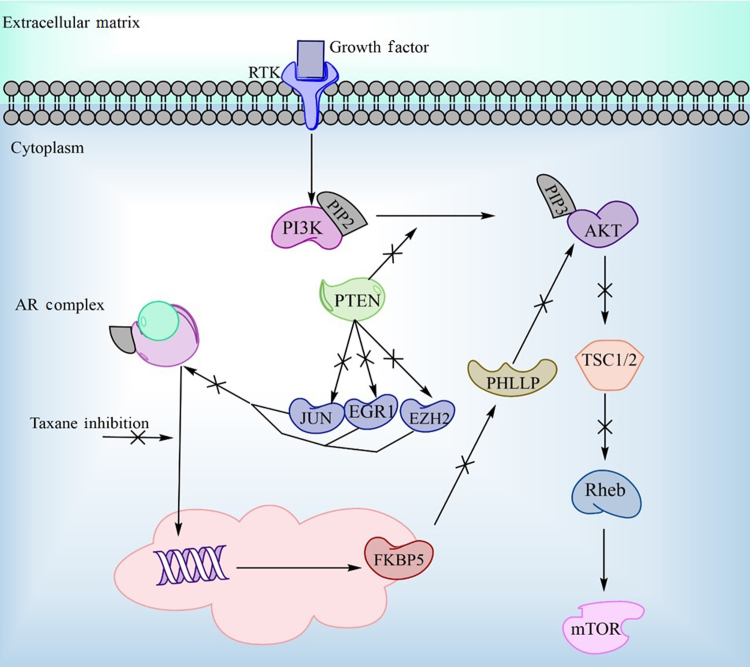
The phosphoinositide 3-kinase (PI3K)/AKT pathway regulates multiple cellular functions through important signaling inductions and increased activation of this pathway has been shown to be a key component in cancer proliferation. In more aggressive prostate cancers, phosphorylated AKT (pAKT) is upregulated, most likely due to the inactivation of the PTEN gene100. pAKT's involvement in the cell cycle as well as its relation to the AR pathway are of particular interest here. It promotes transition to the M-phase through inactivation of WEE1 (a known cdk1 inhibitor) which could promote sensitivity to taxane treatment101. Another study has correlated ADT and taxane therapy with upregulation of pAKT indicating this pathway may be an important mechanism for the cell to either reactivate the androgen pathway or reduce its dependence on it102. In support of this hypothesis, AKT has also been directly linked to AR signaling in multiple studies103, 104, 105 (Fig. 5).
Figure 5.
The relationship between AR translocation and the AKT signaling pathway. Taxanes can directly inhibit AR, but translocation can also be upregulated by increasing pAKT downstream signaling, possibly inducing resistance.
3.5.2. Targeting the PI3K/AKT pathway
Treatments targeting this pathway in cancer have been of extensive interest and a few have advanced to various stages of clinical trials. Generally, treatments aim to inhibit one of three important targets in the pathway: PI3K, AKT, or mTOR. PI3K inhibitors have shown limited clinical efficacy, although isoform specific inhibitors, which target only one type of p110 isoform (p110α), have shown effectiveness in other cancers while also reducing side effects106, 107. These specific inhibitors are yet to be tested in prostate cancer, and there is some concern that most prostate cancers exhibit the other p110 isoform (p110β) instead of the one targeted by these drugs108. Two different types of AKT inhibitors have shown various degrees of efficacy. Allosteric inhibitors displayed evidence of increased apoptosis and decreased cell proliferation in prostate cancers in vitro but no improved benefit from the current standard of care in clinical trials109, 110. On the other hand, some AKT ATP site inhibitors have shown greater antitumor effects in vivo compared to the allosteric inhibitors and a couple of these molecules are currently being tested in clinical trials in combination with AR inhibitors111. Similarly, allosteric mTOR inhibitors have been relatively ineffective in clinical trials112, 113, 114, but ATP site inhibitors of these compound types seem to prevent tumor growth115. One particularly interesting molecule, MLN0128, has shown good potency against prostate cancer mouse models, and is thought to act by targeting the 4EBP1/eIF4E axis and effecting translation of critical mRNA effect the invasive qualities of prostate cancer, PC-3 cells in particular115. Other mTOR inhibitors which do not target this axis and are not as effective in combination with taxane therapy. Last but not least, some molecules have been developed to target both the ATP active sites on p110 isoforms and on mTOR, which would inhibit most, if not all signaling from this pathway116, 117. Early clinical trials have demonstrated the effectiveness of these molecules in solid tumors and potency in metastatic CRPC is being tested in combination with abiraterone118.
Based on these data, blocking the ATP active binding sites seem to be a useful method for treating various forms of cancer, although clinical efficacy, side effects, and possible drug–drug interactions are still to be determined. In terms of prostate cancer, the data are much more unclear and treatment with these molecules would most likely need to be in combination with other targeted therapies as well. One AKT inhibitor (MK-2206) improved the efficacy of PXL in two ovarian cancer cell lines, SKOV3 (expresses active AKT) and ES2 (no active AKT), leading to increased apoptosis and IC50 reductions of approximately 80% and 55% in the two cell lines respectively119. This provides a basis for further research into the combination of AKT inhibitors in combination with taxane therapy for CRPC treatment.
3.6. Mitotic kinase mutations most likely do not contribute to taxane resistance
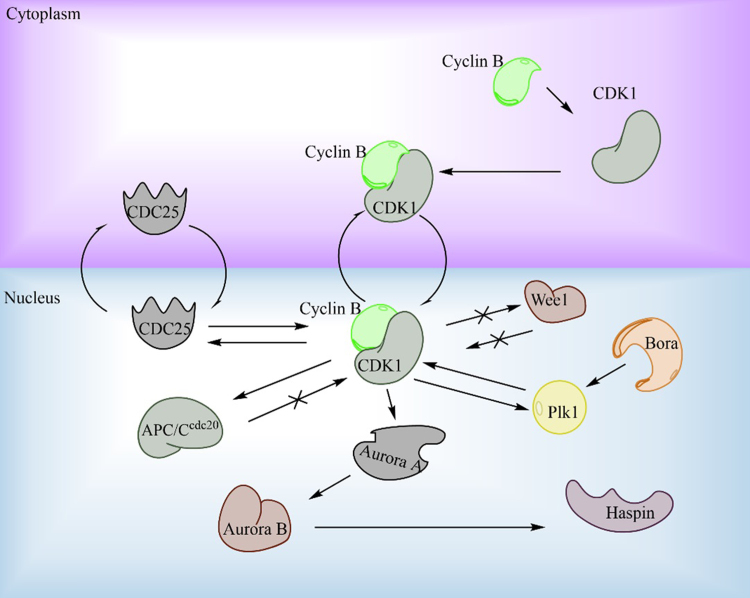
Based on the knowledge that taxanes are believed to interrupt the M-phase of the cell cycle, there have been efforts to directly target mitotic kinases, rather than microtubules in order to overcome the resistance to taxanes. It is hypothesized that similar efficacy could be produced with a reduction in side effects as well120, 121. The three major kinase targets have been Aurora A and B and Polo-like kinase (PLK1). Aurora A and B have principal roles in spindle formation and PLK1 is involved in cytokinesis, bipolar spindle formation, and a positive feedback loop of CDK1122, 123, 124, 125, 126 (Fig. 6). In vitro, where cells often double at very rapid paces, this strategy proved to be extremely effective. However, clinically these molecules did not exhibit this same effect, barely outperforming a placebo in a couple cases127, 128. The reasoning for this is believed to be that, in vivo, cells spend significantly less time in the M-phase129, 130, 131. Therefore directly targeting mitotic kinases will lead to a less effective therapeutic outcome. This is further supported by evidence which suggests taxanes inhibit cell function through multiple other mechanisms (such as inhibiting the AR pathway as discussed earlier)132. While showing initial promise, it seems that mitotic kinases are a dead-end for treating most types of cancer including prostate cancer and resistance is not likely to be related to mitotic kinase mutations. Future studies should focus on other areas of resistance.
Figure 6.
Critical protein pathways involved in cell cycle transition from G2 to M-phase. Cyclin B binds to CDK1, initiating the complexes translocation to the nucleus. Multiple positive and negative feedback loops namely, from CDC25, Wee1, and APC/Ccdc20 tightly regulate CDK1.
3.7. Other novel treatments to overcome resistance
Statins have traditionally been used to lower cholesterol for treating cardiovascular disease. However, there is growing evidence that some forms of CRPC use cholesterol to biosynthesize androgen for use, just as with the DHEA pathway discussed earlier133, 134, 135. Thus, statins could possess a useful mechanism to prevent the reuptake of androgen post initial ADT. In vitro experiments have displayed tumor suppression through both inhibition of androgen synthesize as well as AKT inhibition, with simvastatin exhibiting the strongest antitumor effects136. The potential for AKT inhibition means these compounds or possible future derivatives could be useful in combination with DXL or CXL to reduce resistance. While toxicity and is not a major concern considering statins are already FDA approved, more experiments need to be conducted to determine efficacy and possible drug–drug interactions in vivo in combination with taxanes.
Novel drug delivery mechanisms have also been subject to extensive research in hopes of improving the bioavailability and reducing systemic toxicity. The nanoemulsion formulation of the taxane SBT-1214 has already been discussed and this is just one example of these efforts. A recent publication from Souchek et al.137 showed the synergy between DXL and nanoparticles containing the weight loss drug orlistat which inhibits fatty acid synthase, an enzyme over expressed in many cancer types. Orlistat prevents the synthesis of phosphatidylcholine from C-choline, therefore reducing its incorporation in lipids. It is not known how orlistat works in synergy with taxanes, but one hypothesis is that it also improves microtubule stabilization, leading to further hyper stabilization and the nanoparticle delivery system improves bioavailability137. Corroborating this theory, Yang and colleagues138 provided evidence of orlistat binding to β-tubulin, although at what site the binding occurs and how this may affect taxane binding is still unknown. One other novel nano-formulation gaining attention is the use of polymeric micelles. These are spherical structures which contain a hydrophobic core surrounded by hydrophilic polymers, commonly polyethylene glycol (PEG). The hydrophobic core is excellent for maintaining the stability of hydrophobic drugs such as taxanes, while the outer hydrophilic molecules enhance the aqueous solubility139. One last novel delivery system worth mentioning is a surgically implantable device, which uses magnets to deliver drugs at a specific rate in vivo. In a mouse model, DXL was efficiently delivered to the tumor and provided similar efficacy to IV administered DXL, with a mice losing significantly less weight140. While a device of this type would probably prove difficult to use clinically, the study once again highlights the importance of increasing the bioavailability of a drug around the tumor site in order to reduce resistance development.
4. Conclusions
Taxanes are still currently the 1st line treatment for CRPC, but they may not solely behave in the manner initially thought, as mounting evidence would suggest. Thus development of resistance is most likely not a function of just one or two mechanisms (Table 1). For years, efflux pumps, P-gp in particular, have been associated with taxane resistance, along with resistance to other chemotherapeutic molecules. While upregulation of these proteins certainly play an integral role in resistance in most cell types, it cannot be the only mechanism. The new evidence supporting the common stem-cell like qualities of prostate cancer cells corroborate this hypothesis. The taxane binding site on microtubules is consistently mutated in resistant cell lines, probably reducing taxane affinity for the active site. The AR pathway seems to be the central link between many forms of inhibition, with both taxanes and AKT inhibitors showing effects on this pathway, in addition to the already approved abiraterone and enzalutamide. However, as previously mentioned, CXL still exhibits significant tumor suppression which is independent of the AR pathway, indicating yet another possible mechanism of resistance. Direct inhibition of the AKT pathway has shown potential in clinical trials and may be effective contributors to combination therapy. The tight regulation of the cell cycle has so far shown to be impervious to specific protein inhibitors such as mitotic kinases, possibly due to the minimal time cells in vivo spend in M-phase. Novel formulations and delivery methods of drugs which increase bioavailability may circumvent taxane resistance. Patients may benefit from experiments conducted on the pathways outlined in this paper.
Table 1.
Overview of the major resistance mechanisms to taxane therapy and the strategies to improve therapy.
| Resistance mechanism | Potential strategy to overcome resistance |
|---|---|
| Mutations to the microtubule binding site and increased expression of βIII-tubulin8, 28, 30, 32 | Development of other microtubule binding agents, which do not bind the same active site or bind to the mutated forms with high affinity42, 43, 44, 45, 46, 47 |
| Development of cancer stem cells50, 60 | Targeting miR-34a and known stem cell transcription factors such as SOX2 and c-Myc64, 65, 66, 67, 68, 70, 71 |
| Efflux transporter upregulation75, 76, 77 | Inhibiting efflux transporter activity or development of molecules which do not bind to highly expressed transporters4, 79, 83 |
| Androgen receptor pathway reactivation5, 96, 97 | Anti-androgen therapy given in combination with taxanes4, 7 |
| PI3K/AKT signal upregulation100, 102, 103, 104, 105 | Direct inhibition of pathway signaling through PI3K, AKT and mTOR inhibitors106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117 |
5. Future directions
Further investigation of all novel microtubule binding molecules, especially vinca alkaloids and colchicine binding agents, may help elucidate the most likely mechanism of action for these compounds. Understanding whether these molecules deliver similar effects to taxanes may help determine their usefulness. It is possible that binding to different active sites on microtubules may induce different downstream effects, besides that of G2/M-phase disruption. If so this may open new therapeutic opportunities for these compounds. Furthermore, preventing the development of stem cells is also an interesting point of study. This inhibition would possibly correlate to slower mutations times for cancer cells, leaving them more vulnerable to taxane therapy. Studies conducted on this topic may want to focus on the signaling pathways or mRNA involved and potential targets of inhibition. MicroRNA miR-34a, SOX2, and c-Myc have been identified as potential targets for further study. While AKT inhibitors have already been studied extensively in cancer treatment, the AKT pathway has become increasingly more important in prostate cancer growth and future studies should focus on the magnitude of its impact on cell cycle and androgen translocation. Finding a targetable pathway which disrupts cell cycle regulation in addition to taxanes could prove to be an extremely efficient combination therapy. Additionally, any inhibition of the androgen receptor and its subsequent translocation is crucial for halting the progression of the vast majority of prostate cancers. There may very likely be multiple pathways for AR upregulation. Lastly, novel delivery methods have gained popularity in drug development and continued research into this field could provide a unique method for reducing resistance. Studies testing all these agents on a variety of different prostate cancer cell lines, notably PC3, DU145, LNCaP, and their respective taxane resistant forms, may also give better indication to the possible biomarkers for the various subpopulations which have been known to develop in patients. It is imperative to understand the difference between these cell lines. This will provide crucial knowledge about how prostate cancer cells adapt when confronted with various perturbations allowing for better prediction of future resistance development and ensuing treatment.
Acknowledgements
This work was partially supported by NIH grants 1R01CA148706 and 1R01CA193609 to Wei Li. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Ms. Shanshan Deng for assistance with the molecular modeling.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA: A Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C.K., Check D.P., Lortet-Tieulent J., Laversanne M., Jemal A., Ferlay J. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer. 2016;138:1388–1400. doi: 10.1002/ijc.29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edlind M.P., Hsieh A.C. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl. 2014;16:378–386. doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y., Liu C., Armstrong C., Lou W., Sandher A., Gao A.C. Anti-androgens inhibit ABCB1 efflux and ATPase activity and reverse docetaxel resistance in advanced prostate cancer. Clin Cancer Res. 2015;21:4133–4142. doi: 10.1158/1078-0432.CCR-15-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penning T.M. Mechanisms of drug resistance that target the androgen axis in castration resistant prostate cancer (CRPC) J Steroid Biochem Mol Biol. 2015;153:105–113. doi: 10.1016/j.jsbmb.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluemn E.G., Coleman I.M., Lucas J.M., Coleman R.T., Hernandez-Lopez S., Tharakan R. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017;32:474–489. doi: 10.1016/j.ccell.2017.09.003. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzpatrick J.M., de Wit R. Taxane mechanisms of action: potential implications for treatment sequencing in metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:1198–1204. doi: 10.1016/j.eururo.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Lee K.M., Cao D., Itami A., Pour P.M., Hruban R.H., Maitra A. Class III β-tubulin, a marker of resistance to paclitaxel, is overexpressed in pancreatic ductal adenocarcinoma and intraepithelial neoplasia. Histopathology. 2007;51:539–546. doi: 10.1111/j.1365-2559.2007.02792.x. [DOI] [PubMed] [Google Scholar]
- 9.Gan L., Chen S., Wang Y., Watahiki A., Bohrer L., Sun Z. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 2009;69:8386–8394. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 11.Kellokumpu-Lehtinen P., Tuunanen T., Asola R., Elomaa L., Heikkinen M., Kokko R. Weekly paclitaxel---an effective treatment for advanced breast cancer. Anticancer Res. 2013;33:2623–2627. [PubMed] [Google Scholar]
- 12.Benbow J.H., Mann T., Keeler C., Fan C., Hodsdon M.E., Lolis E. Inhibition of paclitaxel-induced decreases in calcium signaling. J Biol Chem. 2012;287:37907–37916. doi: 10.1074/jbc.M112.385070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Docetaxel and adjuvant treatment of breast cancer. Prescrire Int. 2011;20:149. [PubMed] [Google Scholar]
- 14.Cella D., Peterman A., Hudgens S., Webster K., Socinski M.A. Measuring the side effects of taxane therapy in oncology. Cancer. 2003;98:822–831. doi: 10.1002/cncr.11578. [DOI] [PubMed] [Google Scholar]
- 15.de Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.P., Kocak I. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 16.de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scher H.I., Fizazi K., Saad F., Taplin M.E., Sternberg C.N., Miller K. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 18.Anassi E., Ndefo U.A. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. Pharm Ther. 2011;36:197–202. [PMC free article] [PubMed] [Google Scholar]
- 19.Parker C., Nilsson S., Heinrich D., Helle S.I., O'Sullivan J.M., Fosså S.D. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 20.Akhmanova A., Steinmetz M.O. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- 21.Nogales E., Whittaker M., Milligan R.A., Downing K.H. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- 22.Löwe J., Li H., Downing K.H., Nogales E. Refined structure of αβ-tubulin at 3.5 å resolution. J Mol Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 23.Prota A.E., Bargsten K., Zurwerra D., Field J.J., Díaz J.F., Altmann K.H. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science. 2013;339:587–590. doi: 10.1126/science.1230582. [DOI] [PubMed] [Google Scholar]
- 24.Wani M.C., Taylor H.L., Wall M.E., Coggon P., McPhail A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 25.Parness J., Horwitz S.B. Taxol binds to polymerized tubulin in vitro. J Cell Biol. 1981;91:479–487. doi: 10.1083/jcb.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastropaolo D., Camerman A., Luo Y., Brayer G.D., Camerman N. Crystal and molecular structure of paclitaxel (taxol) Proc Natl Acad Sci U S A. 1995;92:6920–6924. doi: 10.1073/pnas.92.15.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoang B., Ernsting M.J., Tang W.H., Bteich J., Undzys E., Kiyota T. Cabazitaxel-conjugated nanoparticles for docetaxel-resistant and bone metastatic prostate cancer. Cancer Lett. 2017;410:169–179. doi: 10.1016/j.canlet.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 28.Giannakakou P., Sackett D.L., Kang Y.K., Zhan Z., Buters J.T., Fojo T. Paclitaxel-resistant human ovarian cancer cells have mutant β-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 29.Hara T., Ushio K., Nishiwaki M., Kouno J., Araki H., Hikichi Y. A mutation in β-tubulin and a sustained dependence on androgen receptor signalling in a newly established docetaxel-resistant prostate cancer cell line. Cell Biol Int. 2010;34:177–184. doi: 10.1042/CBI20090030. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Garay M.L., Chang L., Blade K., Menick D.R., Cabral F.A. β-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274:23875–23882. doi: 10.1074/jbc.274.34.23875. [DOI] [PubMed] [Google Scholar]
- 31.Rao S., He L., Chakravarty S., Ojima I., Orr G.A., Horwitz S.B. Characterization of the taxol binding site on the microtubule. Identification of Arg282 in β-tubulin as the site of photoincorporation of a 7-benzophenone analogue of taxol. J Biol Chem. 1999;274:37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- 32.Katsetos C.D., Herman M.M., Mörk S.J. Class III β-tubulin in human development and cancer. Cell Motil Cytoskelet. 2003;55:77–96. doi: 10.1002/cm.10116. [DOI] [PubMed] [Google Scholar]
- 33.Terry S., Ploussard G., Allory Y., Nicolaiew N., Boissière-Michot F., Maillé P. Increased expression of class III β-tubulin in castration-resistant human prostate cancer. Br J Cancer. 2009;101:951–956. doi: 10.1038/sj.bjc.6605245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobue S., Mizutani N., Aoyama Y., Kawamoto Y., Suzuki M., Nozawa Y. Mechanism of paclitaxel resistance in a human prostate cancer cell line, PC3-PR, and its sensitization by cabazitaxel. Biochem Biophys Res Commun. 2016;479:808–813. doi: 10.1016/j.bbrc.2016.09.128. [DOI] [PubMed] [Google Scholar]
- 35.Shalli K., Brown I., Heys S.D., Schofield A.C. Alterations of β-tubulin isotypes in breast cancer cells resistant to docetaxel. FASEB J. 2005;19:1299–1301. doi: 10.1096/fj.04-3178fje. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi Y., Kuriyama H., Umezu H., Tanaka J., Yoshimasu T., Furukawa T. Class III β-tubulin expression in tumor cells is correlated with resistance to docetaxel in patients with completely resected non-small-cell lung cancer. Intern Med. 2009;48:203–208. doi: 10.2169/internalmedicine.48.1659. [DOI] [PubMed] [Google Scholar]
- 37.Ploussard G., Terry S., Maillé P., Allory Y., Sirab N., Kheuang L. Class III β-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res. 2010;70:9253–9264. doi: 10.1158/0008-5472.CAN-10-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan S.F., Zhu L.J., Zheng W.E., Chen H., Wu L.L., Zhang W. Expression of β-tubulin III and survivin in advance stage breast cancer correlates with chemotherapeutic effects of docetaxel. Asian Pac J Cancer Prev. 2012;13:361–365. doi: 10.7314/apjcp.2012.13.1.361. [DOI] [PubMed] [Google Scholar]
- 39.Duran G.E., Wang Y.C., Francisco E.B., Rose J.C., Martinez F.J., Coller J. Mechanisms of resistance to cabazitaxel. Mol Cancer Ther. 2015;14:193–201. doi: 10.1158/1535-7163.MCT-14-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magnani M., Ortuso F., Soro S., Alcaro S., Tramontano A., Botta M. The β I/β III-tubulin isoforms and their complexes with antimitotic agents. FEBS J. 2006;273:3301–3310. doi: 10.1111/j.1742-4658.2006.05340.x. [DOI] [PubMed] [Google Scholar]
- 41.Churchill C.D., Klobukowski M., Tuszynski J.A. Elucidating the mechanism of action of the clinically approved taxanes: a comprehensive comparison of local and allosteric effects. Chem Biol Drug Des. 2015;86:1253–1266. doi: 10.1111/cbdd.12595. [DOI] [PubMed] [Google Scholar]
- 42.Hainsworth J.D., Meluch A.A., Lane C.M., Spigel D.R., Burris H.A., III, Gandhi J.G. Single agent vinflunine in the salvage treatment of patients with castration-resistant prostate cancer: a phase II trial of the sarah cannon research consortium. Cancer Investig. 2010;28:275–279. doi: 10.3109/07357900902918460. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C., Jing T., Wang F., Gao X., Xu C., Sun Y. Chemotherapy plus estramustine for management of castration-resistant prostate cancer: meta-analysis of randomized controlled trials. Actas Urol Esp. 2014;38:184–191. doi: 10.1016/j.acuro.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Grenader T., Plotkin Y., Rosengarten O. Intravenous vinorelbine as first line chemotherapy in patients with castration-resistant prostate cancer. Harefuah. 2014;153:731–734. 752. [PubMed] [Google Scholar]
- 45.Delmonte A., Sessa C. AVE8062: a new combretastatin derivative vascular disrupting agent. Expert Opin Investig Drugs. 2009;18:1541–1548. doi: 10.1517/13543780903213697. [DOI] [PubMed] [Google Scholar]
- 46.Del Conte G., Bahleda R., Moreno V., Damian S., Perotti A., Lassau N. A phase I study of ombrabulin (O) combined with bevacizumab (B) in patients with advanced solid tumors ( NCT01193595) J Clin Oncol. 2012;30:3080. [Google Scholar]
- 47.Salmon H.W., Siemann D.W. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin Cancer Res. 2006;12:4090–4094. doi: 10.1158/1078-0432.CCR-06-0163. [DOI] [PubMed] [Google Scholar]
- 48.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 50.Collins A.T., Berry P.A., Hyde C., Stower M.J., Maitland N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 51.O'Brien C.A., Pollett A., Gallinger S., Dick J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 52.Li C., Heidt D.G., Dalerba P., Burant C.F., Zhang L., Adsay V. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 53.Eramo A., Lotti F., Sette G., Pilozzi E., Biffoni M., Di Virgilio A. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 54.Schatton T., Murphy G.F., Frank N.Y., Yamaura K., Waaga-Gasser A.M., Gasser M. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang S., Balch C., Chan M.W., Lai H.C., Matei D., Schilder J.M. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Z.F., Ho D.W., Ng M.N., Lau C.K., Yu W.C., Ngai P. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Burger P.E., Gupta R., Xiong X., Ontiveros C.S., Salm S.N., Moscatelli D. High aldehyde dehydrogenase activity: a novel functional marker of murine prostate stem/progenitor cells. Stem Cells. 2009;27:2220–2228. doi: 10.1002/stem.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Hoogen C., van der Horst G., Cheung H., Buijs J.T., Lippitt J.M., Guzmán-Ramírez N. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70:5163–5173. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 59.Castellón E.A., Valenzuela R., Lillo J., Castillo V., Contreras H.R., Gallegos I. Molecular signature of cancer stem cells isolated from prostate carcinoma and expression of stem markers in different gleason grades and metastasis. Biol Res. 2012;45:297–305. doi: 10.4067/S0716-97602012000300011. [DOI] [PubMed] [Google Scholar]
- 60.Mittal K., Donthamsetty S., Kaur R., Yang C., Gupta M.V., Reid M.D. Multinucleated polyploidy drives resistance to docetaxel chemotherapy in prostate cancer. Br J Cancer. 2017;116:1186–1194. doi: 10.1038/bjc.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Khattouti A., Selimovic D., Haïkel Y., Megahed M., Gomez C.R., Hassan M. Identification and analysis of CD133+ melanoma stem-like cells conferring resistance to taxol: an insight into the mechanisms of their resistance and response. Cancer Lett. 2014;343:123–133. doi: 10.1016/j.canlet.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 62.Castillo V., Valenzuela R., Huidobro C., Contreras H.R., Castellon E.A. Functional characteristics of cancer stem cells and their role in drug resistance of prostate cancer. Int J Oncol. 2014;45:985–994. doi: 10.3892/ijo.2014.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mimeault M., Johansson S.L., Henichart J.P., Depreux P., Batra S.K. Cytotoxic effects induced by docetaxel, gefitinib, and cyclopamine on side population and nonside population cell fractions from human invasive prostate cancer cells. Mol Cancer Ther. 2010;9:617–630. doi: 10.1158/1535-7163.MCT-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Botchkina G.I., Zuniga E.S., Das M., Wang Y., Wang H., Zhu S. New-generation taxoid SB-T-1214 inhibits stem cell-related gene expression in 3D cancer spheroids induced by purified colon tumor-initiating cells. Mol Cancer. 2010;9:192. doi: 10.1186/1476-4598-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Botchkina G.I., Zuniga E.S., Rowehl R.H., Park R., Bhalla R., Bialkowska A.B. Prostate cancer stem cell-targeted efficacy of a new-generation taxoid, SBT-1214 and novel polyenolic zinc-binding curcuminoid, CMC2.24. PLoS One. 2013;8:e69884. doi: 10.1371/journal.pone.0069884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmad G., El Sadda R., Botchkina G., Ojima I., Egan J., Amiji M. Nanoemulsion formulation of a novel taxoid DHA-SBT-1214 inhibits prostate cancer stem cell-induced tumor growth. Cancer Lett. 2017;406:71–80. doi: 10.1016/j.canlet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C., Kelnar K., Liu B., Chen X., Calhoun-Davis T., Li H. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen D., Peng Y., Lin F., Singh R.K., Mahato R.I. Micellar delivery of miR-34a modulator rubone and paclitaxel in resistant prostate cancer. Cancer Res. 2017;77:3244–3254. doi: 10.1158/0008-5472.CAN-16-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Godar S., Ince T.A., Bell G.W., Feldser D., Donaher J.L., Bergh J. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao Z., Li C.H., Chan S.L., Xu F., Feng L., Wang Y. A small-molecule modulator of the tumor-suppressor miR34a inhibits the growth of hepatocellular carcinoma. Cancer Res. 2014;74:6236–6247. doi: 10.1158/0008-5472.CAN-14-0855. [DOI] [PubMed] [Google Scholar]
- 71.Xiao Z., Chen Y. Small molecule targeting miR-34a for cancer therapy. Mol Cell Oncol. 2015;2:e977160. doi: 10.4161/23723556.2014.977160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yun E.J., Zhou J., Lin C.J., Hernandez E., Fazli L., Gleave M. Targeting cancer stem cells in castration-resistant prostate cancer. Clin Cancer Res. 2016;22:670–679. doi: 10.1158/1078-0432.CCR-15-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y., Jin Z., Zhou H., Ou X., Xu Y., Li H. Suppression of prostate cancer progression by cancer cell stemness inhibitor napabucasin. Cancer Med. 2016;5:1251–1258. doi: 10.1002/cam4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boyd L.K., Mao X., Lu Y.J. The complexity of prostate cancer: genomic alterations and heterogeneity. Nat Rev Urol. 2012;9:652–664. doi: 10.1038/nrurol.2012.185. [DOI] [PubMed] [Google Scholar]
- 75.Sissung T.M., Baum C.E., Deeken J., Price D.K., Aragon-Ching J., Steinberg S.M. ABCB1 genetic variation influences the toxicity and clinical outcome of patients with androgen-independent prostate cancer treated with docetaxel. Clin Cancer Res. 2008;14:4543–4549. doi: 10.1158/1078-0432.CCR-07-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oprea-Lager D.E., Bijnsdorp I.V., van Moorselaar R.J., van den Eertwegh A.J., Hoekstra O.S., Geldof A.A. ABCC4 decreases docetaxel and not cabazitaxel efficacy in prostate cancer cells in vitro. Anticancer Res. 2013;33:387–391. [PubMed] [Google Scholar]
- 77.Kawanobe T., Kogure S., Nakamura S., Sato M., Katayama K., Mitsuhashi J. Expression of human ABCB5 confers resistance to taxanes and anthracyclines. Biochem Biophys Res Commun. 2012;418:736–741. doi: 10.1016/j.bbrc.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 78.Fumoleau P., Trigo J.M., Isambert N., Sémiond D., Gupta S., Campone M. Phase I dose-finding study of cabazitaxel administered weekly in patients with advanced solid tumours. BMC Cancer. 2013;13:460. doi: 10.1186/1471-2407-13-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y.F., Ji H.H., Zhang Z.L., Zhang T.T., Gan W., Zhang S.F. Targeting MRP4 expression by anti-androgen treatment reverses MRP4-mediated docetaxel resistance in castration-resistant prostate cancer. Oncol Lett. 2017;14:1748–1756. doi: 10.3892/ol.2017.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang F., Zhang D., Zhang Q., Chen Y., Zheng D., Hao L. Synergistic effect of folate-mediated targeting and verapamil-mediated P-gp inhibition with paclitaxel–polymer micelles to overcome multi-drug resistance. Biomaterials. 2011;32:9444–9456. doi: 10.1016/j.biomaterials.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 81.Pires M.M., Emmert D., Hrycyna C.A., Chmielewski J. Inhibition of P-glycoprotein-mediated paclitaxel resistance by reversibly linked quinine homodimers. Mol Pharmacol. 2009;75:92–100. doi: 10.1124/mol.108.050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee K., Chae S.W., Xia Y., Kim N.H., Kim H.J., Rhie S. Effect of coumarin derivative-mediated inhibition of P-glycoprotein on oral bioavailability and therapeutic efficacy of paclitaxel. Eur J Pharmacol. 2014;723:381–388. doi: 10.1016/j.ejphar.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 83.Nehate C., Jain S., Saneja A., Khare V., Alam N., Dubey R.D. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11:666–686. doi: 10.2174/1567201811666140609154949. [DOI] [PubMed] [Google Scholar]
- 84.Ernsting M.J., Murakami M., Undzys E., Aman A., Press B., Li S.D. A docetaxel-carboxymethylcellulose nanoparticle outperforms the approved taxane nanoformulation, abraxane, in mouse tumor models with significant control of metastases. J Control Release. 2012;162:575–581. doi: 10.1016/j.jconrel.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 85.Hoang B., Ernsting M.J., Roy A., Murakami M., Undzys E., Li S.D. Docetaxel-carboxymethylcellulose nanoparticles target cells via a sparc and albumin dependent mechanism. Biomaterials. 2015;59:66–76. doi: 10.1016/j.biomaterials.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roy A., Murakami M., Ernsting M.J., Hoang B., Undzys E., Li S.D. Carboxymethylcellulose-based and docetaxel-loaded nanoparticles circumvent P-glycoprotein-mediated multidrug resistance. Mol Pharm. 2014;11:2592–2599. doi: 10.1021/mp400643p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roy A., Ernsting M.J., Undzys E., Li S.D. A highly tumor-targeted nanoparticle of podophyllotoxin penetrated tumor core and regressed multidrug resistant tumors. Biomaterials. 2015;52:335–346. doi: 10.1016/j.biomaterials.2015.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao S., Tan S., Guo Y., Huang J., Chu M., Liu H. pH-sensitive docetaxel-loaded D-α-tocopheryl polyethylene glycol succinate–poly(β-amino ester) copolymer nanoparticles for overcoming multidrug resistance. Biomacromolecules. 2013;14:2636–2646. doi: 10.1021/bm4005113. [DOI] [PubMed] [Google Scholar]
- 89.Tran T.H., Ramasamy T., Choi J.Y., Nguyen H.T., Pham T.T., Jeong J.H. Tumor-targeting, pH-sensitive nanoparticles for docetaxel delivery to drug-resistant cancer cells. Int J Nanomed. 2015;10:5249–5262. doi: 10.2147/IJN.S89584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hrkach J., Von Hoff D., Mukkaram Ali M., Andrianova E., Auer J., Campbell T. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 91.Feldman B.J., Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 92.Denmeade S.R., Lin X.S., Isaacs J.T. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 93.Kuroda K., Liu H., Kim S., Guo M., Navarro V., Bander N.H. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: implications for PSA surrogacy. Prostate. 2009;69:1579–1585. doi: 10.1002/pros.21004. [DOI] [PubMed] [Google Scholar]
- 94.Zhu M.L., Horbinski C.M., Garzotto M., Qian D.Z., Beer T.M., Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Darshan M.S., Loftus M.S., Thadani-Mulero M., Levy B.P., Escuin D., Zhou X.K. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–6029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Soest R.J., de Morrée E.S., Kweldam C.F., de Ridder C.M.A., Wiemer E.A.C., Mathijssen R.H.J. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol. 2015;67:981–985. doi: 10.1016/j.eururo.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 97.Cho E., Montgomery R.B., Mostaghel E.A. Minireview: SLCO and ABC transporters: a role for steroid transport in prostate cancer progression. Endocrinology. 2014;155:4124–4132. doi: 10.1210/en.2014-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamada A., Sissung T., Price D.K., Danesi R., Chau C.H., Sharifi N. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14:3312–3318. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Omlin A., Pezaro C., Gillessen Sommer S. Sequential use of novel therapeutics in advanced prostate cancer following docetaxel chemotherapy. Ther Adv Urol. 2014;6:3–14. doi: 10.1177/1756287213509677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malik S.N., Brattain M., Ghosh P.M., Troyer D.A., Prihoda T., Bedolla R. Immunohistochemical demonstration of phospho-Akt in high gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 101.Yang S.X., Costantino J.P., Kim C., Mamounas E.P., Nguyen D., Jeong J.H. Akt phosphorylation at ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer. J Clin Oncol. 2010;28:2974–2981. doi: 10.1200/JCO.2009.26.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kosaka T., Miyajima A., Shirotake S., Suzuki E., Kikuchi E., Oya M. Long-term androgen ablation and docetaxel up-regulate phosphorylated Akt in castration resistant prostate cancer. J Urol. 2011;185:2376–2381. doi: 10.1016/j.juro.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 103.Mulholland D.J., Tran L.M., Li Y., Cai H., Morim A., Wang S. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carver B.S., Chapinski C., Wongvipat J., Hieronymus H., Chen Y., Chandarlapaty S. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xin L., Teitell M.A., Lawson D.A., Kwon A., Mellinghoff I.K., Witte O.N. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci U S A. 2006;103:7789–7794. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonzalez-Angulo A.M., Juric D., Argilés G., Schellens J.H., Burris H.A., Berlin J. Safety, pharmacokinetics, and preliminary activity of the α-specific PI3K inhibitor BYL719: results from the first-in-human study. J Clin Oncol. 2013;31:2531. [Google Scholar]
- 107.Jessen K., Kessler L., Kucharski J., Guo X., Staunton J., Janes M. Abstract A171: a potent and selective PI3K inhibitor, INK1117, targets human cancers harboring oncogenic PIK3CA mutations. Mol Cancer Ther. 2011;10:A171. [Google Scholar]
- 108.Jia S., Liu Z., Zhang S., Liu P., Zhang L., Lee S.H. Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Posadas E.M., Gulley J.L., Arlen P.M., Trout A., Parnes H.L., Wright J. A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol Ther. 2005;4:1133–1137. doi: 10.4161/cbt.4.10.2064. [DOI] [PubMed] [Google Scholar]
- 110.Chee K.G., Longmate J., Quinn D.I., Chatta G., Pinski J., Twardowski P. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007;5:433–437. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 111.Thomas C., Lamoureux F., Crafter C., Davies B.R., Beraldi E., Fazli L. Synergistic targeting of PI3K/AKT pathway and androgen receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Mol Cancer Ther. 2013;12:2342–2355. doi: 10.1158/1535-7163.MCT-13-0032. [DOI] [PubMed] [Google Scholar]
- 112.Nakabayashi M., Werner L., Courtney K.D., Buckle G., Oh W.K., Bubley G.J. Phase II trial of RAD001 and bicalutamide for castration-resistant prostate cancer. BJU Int. 2012;110:1729–1735. doi: 10.1111/j.1464-410X.2012.11456.x. [DOI] [PubMed] [Google Scholar]
- 113.Armstrong A.J., Netto G.J., Rudek M.A., Halabi S., Wood D.P., Creel P.A. A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res. 2010;16:3057–3066. doi: 10.1158/1078-0432.CCR-10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Templeton A.J., Dutoit V., Cathomas R., Rothermundt C., Bärtschi D., Dröge C. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08) Eur Urol. 2013;64:150–158. doi: 10.1016/j.eururo.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 115.Hsieh A.C., Liu Y., Edlind M.P., Ingolia N.T., Janes M.R., Sher A. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maira S.M., Stauffer F., Brueggen J., Furet P., Schnell C., Fritsch C. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 117.Wallin J.J., Edgar K.A., Guan J., Berry M., Prior W.W., Lee L. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–2436. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 118.Wagner A.J., Bendell J.C., Dolly S., Morgan J.A., Ware J.A., Fredrickson J. A first-in-human phase I study to evaluate GDC-0980, an oral PI3K/mTOR inhibitor, administered QD in patients with advanced solid tumors. J Clin Oncol. 2011;29:3020. [Google Scholar]
- 119.Lin Y.H., Chen B.Y., Lai W.T., Wu S.F., Guh J.H., Cheng A.L. The akt inhibitor MK-2206 enhances the cytotoxicity of paclitaxel (Taxol) and cisplatin in ovarian cancer cells. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:19–31. doi: 10.1007/s00210-014-1032-y. [DOI] [PubMed] [Google Scholar]
- 120.Nakai R., Iida S., Takahashi T., Tsujita T., Okamoto S., Takada C. K858, a novel inhibitor of mitotic kinesin Eg5 and antitumor agent, induces cell death in cancer cells. Cancer Res. 2009;69:3901–3909. doi: 10.1158/0008-5472.CAN-08-4373. [DOI] [PubMed] [Google Scholar]
- 121.Jackson J.R., Patrick D.R., Dar M.M., Huang P.S. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 122.Hauf S., Cole R.W., LaTerra S., Zimmer C., Schnapp G., Walter R. The small molecule hesperadin reveals a role for aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J.J., Hoffmann M. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of Polo-like kinase 1. Curr Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 124.Hoar K., Chakravarty A., Rabino C., Wysong D., Bowman D., Roy N. MLN8054, a small-molecule inhibitor of Aurora A, causes spindle Pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol. 2007;27:4513–4525. doi: 10.1128/MCB.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burkard M.E., Randall C.L., Larochelle S., Zhang C., Shokat K.M., Fisher R.P. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci U S A. 2007;104:4383–4388. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McInnes C., Mazumdar A., Mezna M., Meades C., Midgley C., Scaerou F. Inhibitors of Polo-like kinase reveal roles in spindle-pole maintenance. Nat Chem Biol. 2006;2:608–617. doi: 10.1038/nchembio825. [DOI] [PubMed] [Google Scholar]
- 127.Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 128.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 129.Rieder C.L. Mitosis in vertebrates: the G2/M and M/A transitions and their associated checkpoints. Chromosome Res. 2011;19:291–306. doi: 10.1007/s10577-010-9178-z. [DOI] [PubMed] [Google Scholar]
- 130.Baserga R. The relationship of the cell cycle to tumor growth and control of cell division: a review. Cancer Res. 1965;25:581–595. [PubMed] [Google Scholar]
- 131.Komlodi-Pasztor E., Sackett D., Wilkerson J., Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8:244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 132.Komlodi-Pasztor E., Sackett D.L., Fojo A.T. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res. 2012;18:51–63. doi: 10.1158/1078-0432.CCR-11-0999. [DOI] [PubMed] [Google Scholar]
- 133.Dillard P.R., Lin M.F., Khan S.A. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–120. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Titus M.A., Schell M.J., Lih F.B., Tomer K.B., Mohler J.L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 135.Singh P., Uzgare A., Litvinov I., Denmeade S.R., Isaacs J.T. Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr Relat Cancer. 2006;13:653–666. doi: 10.1677/erc.1.00797. [DOI] [PubMed] [Google Scholar]
- 136.Ingersoll M.A., Miller D.R., Martinez O., Wakefield C.B., Hsieh K.C., Simha M.V. Statin derivatives as therapeutic agents for castration-resistant prostate cancer. Cancer Lett. 2016;383:94–105. doi: 10.1016/j.canlet.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Souchek J.J., Davis A.L., Hill T.K., Holmes M.B., Qi B., Singh P.K. Combination treatment with orlistat-containing nanoparticles and taxanes is synergistic and enhances microtubule stability in taxane-resistant prostate cancer cells. Mol Cancer Ther. 2017;16:1819–1830. doi: 10.1158/1535-7163.MCT-17-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang P.Y., Liu K., Ngai M.H., Lear M.J., Wenk M.R., Yao S.Q. Activity-based proteome profiling of potential cellular targets of orlistat --- an FDA-approved drug with anti-tumor activities. J Am Chem Soc. 2010;132:656–666. doi: 10.1021/ja907716f. [DOI] [PubMed] [Google Scholar]
- 139.Qiu L.Y., Yan L., Zhang L., Jin Y.M., Zhao Q.H. Folate-modified poly(2-ethyl-2-oxazoline) as hydrophilic corona in polymeric micelles for enhanced intracellular doxorubicin delivery. Int J Pharm. 2013;456:315–324. doi: 10.1016/j.ijpharm.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 140.Struss W.J., Tan Z., Zachkani P., Moskalev I., Jackson J.K., Shademani A. Magnetically-actuated drug delivery device (MADDD) for minimally invasive treatment of prostate cancer: an in vivo animal pilot study. Prostate. 2017;77:1356–1365. doi: 10.1002/pros.23395. [DOI] [PubMed] [Google Scholar]