Abstract
Overexpressing of ATP-binding cassette (ABC) transporters is the essential cause of multidrug resistance (MDR), which is a significant hurdle to the success of chemotherapy in many cancers. Therefore, inhibiting the activity of ABC transporters may be a logical approach to circumvent MDR. Olmutinib is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), which has been approved in South Korea for advanced EGFR T790M-positive non-small cell lung cancer (NSCLC). Here, we found that olmutinib significantly increased the sensitivity of chemotherapy drug in ABCG2-overexpressing cells. Furthermore, olmutinib could also increase the retention of doxorubicin (DOX) and rhodamine 123 (Rho 123) in ABC transporter subfamily G member 2 (ABCG2)-overexpressing cells. In addition, olmutinib was found to stimulate ATPase activity and inhibit photolabeling of ABCG2 with [125I]-iodoarylazidoprazosin (IAAP). However, olmutinib neither altered ABCG2 expression at protein and mRNA levels nor blocked EGFR, Her-2 downstream signaling of AKT and ERK. Importantly, olmutinib enhanced the efficacy of topotecan on the inhibition of S1-MI-80 cell xenograft growth. All the results suggest that olmutinib reverses ABCG2-mediated MDR by binding to ATP bind site of ABCG2 and increasing intracellular chemotherapeutic drug accumulation. Our findings encouraged to further clinical investigation on combination therapy of olmutinib with conventional chemotherapeutic drugs in ABCG2-overexpressing cancer patients.
Abbreviations: ABC, adenosine triphosphate (ATP)-binding cassette; ABCG2, ABC transporter subfamily G member 2; DDP, cisplatin; DMEM, Dulbecco׳s modified Eagle׳s medium; DMSO, dimethyl sulfoxide; DOX, doxorubicin; FTC, fumitremorgin C; IAAP, iodoarylazidoprazosin; MDR, multidrug resistance; MTT, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazoliumbromide; MX, methotrexate; PCR, polymerase chain reaction; Rho 123, rhodamine 123; TKI, tyrosine kinase inhibitor; VRP, verapamil
KEY WORDS: Olmutinib, ABCG2, Multidrug resistance, Tyrosine kinase inhibitor, Chemotherapy, ATPase
Graphical abstract
Olmutinib could bind to ATP-binding cassette transporter subfamily G member 2 (ABCG2) ATP binding site, inhibit its drug efflux function, increase intracellular chemotherapeutic drug accumulation, and then restore sensitivity of multidrug resistance cells to ABCG2 substrate drug.
1. Introduction
Intrinsic or acquired multidrug resistance (MDR) is one of the most common reasons for failure of chemotherapy1, 2. The common mechanisms that produced MDR in cancer cells include the overexpressing of adenosine triphosphate (ATP)-binding cassette (ABC) transporters, changing in drug targets, alterations in membrane lipids, DNA repair, drug metabolic enzymes, and inhibiting signal pathways of apoptosis3, 4, 5. One of the predominant mechanisms for MDR is the overexpressing of efflux pumps, which actively extrude a variety of chemotherapeutic drugs from the cancer cells, resulting in drug resistance. To date, in the human genome, 49 members ABC transporters family have been discovered and classified into 7 subfamilies (A–G) based on the sequence similarities as well as structural organization6. Among them, ABCB1, ABCC1 and ABCG2 are considered to be accountable for MDR in human cancers7.
These ABC transporters could utilize the energy to pump out conventional chemotherapeutic drugs, contributing to the failure of chemotherapy. The ABCB1 transporter confers by ATP hydrolysis to reducing the intracellular anticancer drug concentration, resulting in resistance to vincristine, doxorubicin, taxanes and some tyrosine kinase inhibitors (TKIs)8. The ABCC1 transporter can pump out a wide spectrum of compounds including anthracyclines, methotrexate, vincristine, epipodophyllotoxins and camptothecins9. The ABCG2 transporter was identified from human placenta10, 11. The ABCG2 expression has been reported in various tumors, such as gastric cancer, colon cancer, lung cancer and melanoma12. It could transport a lot of cytotoxic compounds out of cells, such as methotrexate, topotecan and methotrexate13. In addition, compared to irinotecan-naive metastases, the ABCG2 mRNA significantly increased in irinotecan treated hepatic metastases14.
Developing inhibitors targeted ABC transporters is a promising strategy to overcome MDR. So far, many modulators of ABCG2 have been found and continue to increase. However, there is no commercial available ABCG2 modulator in clinic due to unpredictable adverse reactions and additional toxicity15. TKIs are a new class of anticancer drugs that inhibit cancer development, proliferation, metastasis, invasion, angiogenesis. But new resistance to TKIs has been well documented owing to clinical application in great quantities16. Some studies have shown that overexpressing ABC transporters were not only developed to MDR but also affected pharmacokinetics (absorption, distribution, metabolism, and excretion) and toxicity of various antineoplastic agents, including TKIs17. Recent reports have demonstrated that at clinically accomplishable concentration, some TKIs could inhibit drug efflux function of ABC transporters by directly binding to the drug-binding sites on these transporters, thereby reversing ABC transporter-mediated MDR to conventional chemotherapeutic drugs in cancer cells18, 19. Therefore, TKIs is possible to be developed as a novel, potent and nontoxic inhibitors of the efflux protein, providing a promising clinical approach to reverse MDR and thereby increasing the success of chemotherapy.
Olmutinib (HM61713) is an EGFR TKI that binds to cysteine residue near the kinase domain. Olmutinib also potently inhibits the growth of cell lines and xenograft tumors harboring EGFR T790M and EGFR del19, while having little effect on cell lines with EGFRwt20, 21. In May 2016, olmutinib was approved for advanced EGFR T790M-positive NSCLC patients who were pretreated with EGFR TKIs in South Korea22. But there is no previous study reporting that olmutinib could interact with ABC transporters. Here, for the first time, we investigated the chemo-sensitizing effect of olmutinib in conjunction with conventional chemotherapeutic to overcome ABCG2-mediated multidrug resistance in vitro and in vivo.
2. Materials and methods
2.1. Chemicals and reagents
The anti-ABCG2 monoclonal antibody (BXP-21) was purchased from Santa Cruz Biotechnology (Paso Robles, USA). Anti-AKT, anti-phospho AKT, anti-phospho ERK and anti-ERK antibodies were obtained from Santa Cruz Biotechnology (Paso Robles, USA). The antibody against GAPDH was from Kangcheng (Shanghai, China). The MX, DOX, Rho 123, topotecan, DDP, VRP, FTC, dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-yl)-2,5-diphenyltetrazolium bromide (MTT) were products of Chemical Co. (St. Louis, USA). Olmutinib (HM61713) was purchased from MCE (New Jersey, USA). Dulbecco׳s modified Eagle׳s medium (DMEM), RPMI-1640 and fetal bovine serum (FBS) were products of Gibco BRL (Gaithersburg, USA). Penicillin, streptomycin and trypsin were obtained from Hyclone Thermo Scientific (Logan, UT, USA).
2.2. Cell lines and cell culture
H460/MX20 (methotrexate-selected ABCG2-overexpressing cells) and its parental cells H46023; KBv200 (vincristine-selected ABCB1-overexpressing cells) and its parental cells KB; S1-MI-80 (methotrexate-selected ABCG2-overexpressing cells) and its parental cells S124; HEK293 and its stable HEK293/pcDNA3.1, wild-type ABCG2–482-R2 and mutant ABCG2–482-T7 cells were from the laboratory of Susan Bates (Columbia University/New York Presbyterian Hospital, Manhattan, NY, USA). H460, H460/MX20, S1 and S1-MI-80 were maintained in DMEM. KB and KBv200 were maintained in RPMI-1640 medium. The DMEM and RPMI-1640 contain 10% fetal bovine serum and 1% penicillin/streptomycin. All cell lines were cultured in a humidified incubator at 37 °C containing 5% CO2.
2.3. Cell proliferation assay
The reversal experiments and drugs cytotoxicity tests in vitro were evaluated using the MTT colorimetric assay as described previously25. Briefly, cells were seeded in 96-well plates at appropriate density, incubating for 24 h at 37 °C. The toxicity of olmutinib indicated that more than 80% cells survived at the olmutinib 1 μmol/L, so we used olmutinib 1 μmol/L for the reversal experiments. Olmutinib, FTC or VRP were added 1 h before adding different concentrations of conventional anti-cancer drugs. The 20 μL of MTT solution (5 mg/mL) was added to each well after 68 h of incubation. The MTT-medium was discarded and the resulting formazan crystals were dissolved with DMSO. The cytotoxicity was measured by a Model 550 Microplate Reader (Bio-Rad, Hercules, CA, USA). The half maximal (50%) inhibitory concentration (IC50) was calculated from survival curves using GraphPad Prism 4.0 software. The effect of the MDR-reversal by olmutinib was calculated by dividing the IC50 of cells treated with the anti-cancer drugs alone or by the IC50 of cells treated with anti-cancer drugs in the presence of olmutinib. VRP was used as a positive control for ABCB1-overexpressing cell line26. FTC was used as the positive control for ABCG2-overexpressing cell line27. All experiments were repeated at least three times, and the mean value ± SD was calculated.
2.4. Nude mice MDR xenograft model
The S1-MI-80 cell xenograft model was established as previously described to examine the reversal activity of olmutinib in ABCG2-mediated MDR in vivo with slight modifications28. Athymic nude mice (BALB/c-nude, 4 to 6 weeks old, 16 to 18 g in weight) was purchased from Vital River (Beijing, China). Briefly, S1-MI-80 cells (1×107) were subcutaneously injected into the right flank of athymic nude mice. When xenograft size reached mean volume of about 100 mm3, the 24 mice were randomized into four groups and received various treatments: (1) saline (every 5 day); (2) olmutinib (30 mg/kg, p.o., every 5 day); (3) topotecan (2 mg/kg, i.p., every 5 day) and (4) olmutinib (30 mg/kg, p.o., every 5 day, given 1 h before topotecan administration) plus topotecan (2 mg/kg, i.p., every 5 day). The weight of mice and the two perpendicular tumor diameters (A and B) were measured every 5 day, and the tumor volume (V) was calculated according to the following formula:
| (1) |
When the mean of tumor weight was over 1 g in the saline group, the mice were euthanized and the xenografts were excised and weighed. The ratio of tumor growth inhibition (IR) was estimated according to the following formula:
| (2) |
2.5. DOX and Rho 123 accumulation assay
We examined whether olmutinib could affect the accumulation of DOX and Rho 123 in ABCG2-overexpressing cells by flow cytometry29. The logarithmically growing cells were seeded in six-well plates overnight and were incubated with different concentrations of olmutinib or FTC at 37°C for 3 h, and 5 μmol/L Rho 123 or 10 μmol/L DOX were added to the medium and incubated for additional 0.5 or 3 h, respectively. The cells were collected, centrifuged, and washed 3 times with chilled PBS. Finally, the cells were resuspended in 500 μL PBS and analyzed by flow cytometry (CytomicsFC500, Beckman Coulter Inc., Brea, CA, USA).
2.6. Rho 123 efflux assay
To evaluate the effect of olmutinib on the efflux of Rho 123, S1 and S1-MI-80 cells were pre-treated with 5 μmol/L Rho 123 for 0.5 h30. Then, the cells were collected, washed three times with cold PBS and subsequently incubated at 37 °C with culture medium in the presence or absence of 1 μmol/L olmutinib. Subsequently cells were harvested at the various times (0, 15, 30, 60, 90 and 120 min). Finally, the cells were resuspended in chilled PBS and analyzed by flow cytometry.
2.7. Examination of cell surface ABCG2 expression
We studied whether olmutinib could alter the cell surface expression of ABCG2 by low cytometry-based assay as described previously30. After the treatment with 1 μmol/L olmutinib for 48 h, cells were prepared and washed three times with chilled PBS (supplemented with 0.5% bovine serum albumin). Then, 25 μL of cells (4×106 cells/mL) was mixed with 10 μL of FITC-conjugated ABCG2 antibody and incubated in the dark for 45 min at 4 °C. The cells were washed twice with chilled PBS (supplemented with 0.5% bovine serum albumin) and were resuspended in 400 μL of PBS for flow cytometric analysis. Isotype control samples were prepared in the same manner but with FITC-conjugated Mouse IgG2b/κ antibody.
2.8. Photoaffinity labeling of ABCG2 with [125I]-IAAP
Crude membrane from High Five insect cells expressing ABCB1 (50 μg protein) was incubated with 0–5 μmol/L olmtinib for 5 min at room temperature in 50 mmol/L Tris–HCl (pH 7.5). [125I]-IAAP (2200 Ci/nmole, 3 nmol/L) was added and incubation was continued for a further 5 min under subdued light. The samples were then cross-linked by UV illumination (365 nm) on ice. The labeled ABCG2 was immunoprecipitated using the BXP21 antibody. The samples were then subjected to SDS-PAGE using a 7% Tris-acetate NuPAGE gel, dried and exposed to Bio-Max MR film (Eastman Kodak Co., Rochester, NY, USA) at –80 °C for 4 h. The radioactivity incorporated into the transporter protein was quantified using the Storm 860 PhosphorImager system (Molecular Dynamics, Sunnyvale, CA, USA).
2.9. ABCG2 ATPase assay
A colorimetric ABCG2 ATPase assay was carried out as described previously with minor modifications31. Briefly, crude membranes isolated from ABCG2-overexpressing MCF7/FLV1000 cells (100 g protein/mL) were incubated with olmutinib (0–5 μmol/L) at 37 °C in the presence or absence of 1.2 mmol/L sodium orthovanadate in an assay buffer (50 mmol/L KCl, 5 mmol/L sodium azide, 2 mmol/L EDTA, 10 mmol/L MgCl2, 1 mmol/L DTT, pH 6.8) for 5 min. 5 mmol/L Mg-ATP (concentration in a final volume of 60 μL) was then added to start the ATP hydrolysis reaction and the reaction mixture was allowed to incubate for 10 min. The reaction was terminated by the addition of SDS solution (30 μL of 10% SDS). Absorbance was subsequently measured at 750 nm after the addition of a detection reagent (35 mmol/L ammonium molybdate, 15 mmol/L zinc acetate, 10% ascorbic acid) and incubation at 37 °C for 20 min. The amount of inorganic phosphate released was quantified by reading from a standard curve. Olmutinib-stimulated ABCG2 ATPase activity (i.e. vanadate-sensitive) was determined as the difference between the amounts of inorganic phosphate released from ATP in the absence and presence of sodium orthovanadate.
2.10. Western blot assay
The protein expression of ABCG2 was evaluated in H460/MX20 and S1-MI-80 cells after treatment with olmutinib in the different concentrations. The protein expression levels of AKT, ERK and their phosphorylations were evaluated in the S1 and S1-MI-80 with 1 µmol/L olmutinib for 48 h. Western blot analysis was conducted as previously described32. After blocking with 5% nonfat milk for 2 h at room temperature, the membranes were incubated by using antibodies including ABCG2, AKT, p-AKT, ERK and p-ERK overnight at 4 °C. The membranes were then washed three times with TBST and incubated with HRP conjugated secondary antibody at 1:5000 dilution for 2 h at room temperature. After three times washed with TBST, proteins were detected by the enhanced chemiluminescence solutions (#1705061, Bio-Rad, CA, USA) and exposed to a Kodak medical X-ray processor (Kodak, Rochester, NY, USA). The expression of GAPDH was used as a loading control.
2.11. Reverse transcriptase qPCR
Reverse transcriptase qPCR was used to determine ABCG2 mRNA levels in the presence or absence of olmutinib as described previously33. In brief, H460/MX20 and S1-MI-80 cells were treated with different concentrations of olmutinib for 48 h, then total cellular RNA was isolated by Trizol reagent RNA extraction kit (Molecular Research Center, Cincinnati, OH, USA). The qPCR primers were as follows: 5′-TGGCTGTCATGGCTTCAGTA-3′ (forward) and 5′-GCCACGTGATTCTTCCACAA-3′ (reverse) for ABCG2, 5′-CTTTGGTATCGTGGAAGGA-3′ (forward) and 5′-CACCCTGTTGCTGTAGCC-3′ (reverse) for GAPDH. The qPCR reactions were conducted using Multi qPCR kit in a Veriti 96-well Thermal Cycler System (AppliedB iosystems, USA) following the manufacturer׳s protocol. Data was analyzed using the 2–ΔΔCt method after normalization with the GAPDH expression level in each sample.
2.12. Statistics
All experiments were repeated at least three times. All data was presented as mean±standard deviations (SD). The SPSS statistical software (SPSS 16.0) was used in Statistical analyses. Student׳s t test was used for the statistical analysis. P<0.05 or P<0.01 was considered statistically significant. All the data have been recorded at Sun Yat-sen University Cancer for future reference (number RDDB2018000358).
3. Results
3.1. Olmutinib potentiated the anticancer activity of chemotherapeutic agents in ABCG2-overexpressing cells in vitro
To examine the reversal activity of olmutinib in ABCG2-mediated MDR in cancer cells, we first examined the cytotoxic activity of olmutinib in different cell lines by MTT assay. As shown in Fig. 1, more than 80% of cells survived when treated with olmutinib alone up to 1.0 μmol/L in KB, KBv200, H460, H460/MX20, S1, S1-M1–80, HEK293/pcDNA3.1, HEK293/ABCG2–482-R2 and HEK293/ABCG2–482-T7 cells. Therefore, we used ≤1 μmol/L olmutinib for further reversal experiments in vitro. To study the reverse effect of olmutinib, a few chemotherapeutic agents (DOX, MX, topotecan) combination with it were evaluated in KBv200, H460/MX20, S1-MI-80, HEK293/ABCG2–482-R2 and HEK293/ABCG2–482-T7 and their parental cells (KB, H460, S1 and HEK293/pcDNA3.1) by the MTT cell proliferation assay in vitro. We observed that olmutinib (1 μmol/L) significantly potentiated the anticancer activity of ABCG2 substrate anticancer drugs (MX and topotecan) in ABCG2-overexpressing H460/MX20 and S1-MI-80 cells, while neither prominent enhancement effect was found in the parental H460 and S1 cells nor in ABCB1-overexpressing KBv200 cells (Table 1). The anticancer activity of non-ABCG2 or non-ABCB1 substrate anticancer drug (DDP, 10 μmol/L) was not affected by olmutinib in both parental and ABCG2-overexpressing cells (Table 1). The powerful ABCG2 inhibitor (FTC, 2.5 μmol/L) was used as a positive control for comparison of the reversal effect.
Figure 1.
The structure of olmutinib and cytotoxicity of olmutinib. (A) The structure of olmutinib. MTT cytotoxicity assay was assessed in ABCG2 and ABCB1-overexpressing cells and their parental sensitive cells: (B) ABCB1-negative KB and ABCB1-overexpressing KBv200 cells; (C) ABCG2-negative H460 and ABCG2-overexpressing H460/MX20 cells; (D) ABCG2-negative S1 and ABCG2-overexpressing S1-MI-80 cells; (E) ABCB1-negative HEK293/pcDNA3.1 and ABCG2-overexpressing Wild-type ABCG2–482-T7 and; (F) ABCG2-negative HEK293/pcDNA3.1 and ABCG2-overexpressing mutant ABCG2–482-R2 cells. Cells were treated with varying concentrations of olmutinib for 72 h. Results from three independent experiments are expressed as the mean ± SD.
Table 1.
Effect of olmutinib on enhancement of conventional chemotherapeutic agents.
| Compound | IC50 (μmol/L)a (fold-reversal) |
|
|---|---|---|
| KB | KBv200 (ABCB1) | |
| DOX | 0.078 ± 0.010 (1.00) | 0.521 ± 0.087 (1.00) |
| +0.25 μmol/L olmuitinb | 0.069 ± 0.002 (1.12) | 0.493 ± 0.128 (1.06) |
| +0.5 μmol/L olmuitinb | 0.082 ± 0.007 (0.95) | 0.424 ± 0.411 (1.23) |
| +1 μmol/L olmuitinb | 0.083 ± 0.002 (0.99) | 0.546 ± 0.105 (0.78) |
| +2.5 μmol/L VRP | 0.083 ± 0.002 (0.99) | 0.144 ± 0.003 (2.94)** |
| DDP | 2.433 ± 0.025 (1.00) | 14.173 ± 0.698 (1.00) |
| +1 μmol/L olmuitinb | 2.269 ± 0.058 (1.07) | 13.651 ± 0.220 (1.04) |
| H460 | H460/MX20 (ABCG2) | |
| MX | 0.034 ± 0.000 (1.00) | 0.666 ± 0.142 (1.00) |
| +0.25 μmol/L olmuitinb | 0.026 ± 0.003 (1.29) | 0.366 ± 0.073 (1.82)* |
| +0.5 μmol/L olmuitinb | 0.024 ± 0.002 (1.41) | 0.275 ± 0.009 (2.42)** |
| +1 μmol/L olmuitinb | 0.033 ± 0.002 (1.03) | 0.150 ± 0.009 (4.56)** |
| +2.5 μmol/L FTC | 0.036 ± 0.005 (0.93) | 0.031 ± 0.001 (21.39)** |
| DDP | 3.624 ± 0.835(1.00) | 12.567 ± 0.297 (1.00) |
| +1 μmol/L olmuitinb | 4.822 ± 0.983 (0.75) | 12.763 ± 0.656 (0.98) |
| S1 | S1-MI-80 (ABCG2) | |
| MX | 0.013 ± 0.000 (1.00) | 40.066 ± 1.776 (1.00) |
| +0.25 μmol/L olmuitinb | 0.009 ± 0.000 (1.46) | 5.531 ± 0.100 (7.24)** |
| +0.5 μmol/L olmuitinb | 0.016 ± 0.001 (0.84) | 1.339 ± 0.137 (29.9)** |
| +1 μmol/L olmuitinb | 0.012 ± 0.002 (1.08) | 1.272 ± 0.565 (31.49)** |
| +2.5 μmol/L FTC | 0.013 ± 0.001 (1.01) | 1.481 ± 0.002 (27.21)** |
| Topotecan | 1.164 ± 0.123 (1.00) | 83.190 ± 2.467 (1.00) |
| +0.25 μmol/L olmuitinb | 1.037 ± 0.143 (1.12) | 72.294 ± 8.059 (1.15) |
| +0.5 μmol/L olmuitinb | 1.082 ± 0.125 (1.07) | 15.988 ± 1.951 (5.18)** |
| +1 μmol/L olmuitinb | 0.988 ± 0.205 (1.17) | 11.464 ± 1.060 (7.24)** |
| +2.5 μmol/L FTC | 1.396 ± 0.301 (0.84) | 7.716 ± 0.587 (10.75)** |
| DDP | 2.963 ± 0.050 (1.00) | 6.344 ± 0.152 (1.00) |
| +1 μmol/L olmuitinb | 2.610 ± 0.061 (1.13) | 6.484 ± 0.271 (0.90) |
| MX | 0.034 ± 0.000 (1.00) | 0.666 ± 0.142 (1.00) |
| +0.25 μmol/L olmuitinb | 0.0260 ± 0.003 (1.29) | 0.366 ± 0.073 (1.82)* |
| +0.5 μmol/L olmuitinb | 0.024 ± 0.002 (1.41) | 0.275 ± 0.008 (2.42)** |
| +1 μmol/L olmuitinb | 0.033 ± 0.002 (1.03) | 0.150 ± 0.009 (4.56)** |
| +2.5 μmol/L FTC | 0.036 ± 0.005 (0.93) | 0.031 ± 0.001 (21.39)** |
| DDP | 3.624 ± 0.835 (1.00) | 12.567 ± 0.297 (1.00) |
| +1 μmol/L olmuitinb | 4.822 ± 0.983 (0.75) | 12.763 ± 0.656 (0.98) |
Cell survival was performed by MTT assay as described in Section Materials and methods. VRP, (verapamil, specific inhibitor of ABCB1) and FTC (fumitremorgin C, specific inhibitor of ABCG2) were used as the positive control. The fold reversal of MDR (values given in parentheses) was calculated by dividing the IC50 value for cells with the anticancer agent in the absence of olmutinib by that obtained in the presence of olmutinib. DOX; DDP; MX.
Data were shown as the mean±standard deviation (SD) of at least three independent experiments performed in triplicate.
P<0.05;
P<0.01 significantly different from control group.
Furthermore, we also determined the reversal effect of olmutinib in ABCG2-transfected MDR cell lines (Table 2). As shown in Table 2, the ABCG2–482-R2 and ABCG2–482-T7 cells showed higher resistance to their substrates MX than those in their parental cell line HEK293/pcDNA3.1. Olmutinib at 1 μmol/L significantly reduced the IC50 values of MX, which have shown similar trend to that of the specific ABCG2 inhibitor FTC at 2.5 μmol/L, in either wild-type or mutated ABCG2-overexpressing cells. However, there were no significant differences in the IC50 values for MX with or without olmutinib in HEK293/pcDNA3.1 cells (Table 2). Meanwhile, no change in the IC50 value of DDP, a non-substrate of ABCG2, was seen with or without the combination of olmutinib. Taken together, our data suggested that olmutinib might reversed MDR in ABCG2-overexpressing cells.
Table 2.
Effect of olmutinib on reversing and ABCG2-mediated MDR in stable-transfected cells.
| Compound | IC50 (μmol/L)a (fold-reversal) |
||
|---|---|---|---|
| HEK293/pcDNA3.1 | ABCG2–482-R2 | ABCG2–482-T7 | |
| MX | 0.016 ± 0.002 (1.00) | 0.127 ± 0.003 (1.00) | 0.059 ± 0.004 (1.00) |
| +0.25 μmol/L olmuitinb | 0.010 ± 0.001 (1.62) | 0.042 ± 0.009 (3.03)** | 0.023 ± 0.001 (2.60)** |
| +0.5 μmol/L olmuitinb | 0.012 ± 0.013 (0.80) | 0.031 ± 0.002 (4.09)** | 0.013 ± 0.001 (4.56)** |
| +1 μmol/L olmutinib | 0.010 ± 0.000 (0.62) | 0.023 ± 0.020 (5.44)** | 0.012 ± 0.001 (4.83)** |
| +2.5 μmol/L FTC | 0.014 ± 0.002 (0.91) | 0.008 ± 0.002 (15.08)** | 0.005 ± 0.004 (11.92)** |
| DDP | 2.034 ± 0.111 (1.00) | 0.858 ± 0.089 (1.00) | 1.023 ± 0.060 (1.00) |
| +1 μmol/L olmuitinb | 1.601 ± 0.031 (1.27) | 0.662 ± 0.013 (1.30) | 1.048 ± 0.214 (0.98) |
Cell survival was performed by MTT assay as described in Section Materials and methods. FTC (fumitremorgin C, specific inhibitor of ABCG2) was used as the positive control. The fold reversal of MDR (values given in parentheses) was calculated by dividing the IC50 value for cells with the anticancer agent in the absence of olmutinib by that obtained in the presence of olmutinib. DDP; MX.
Data were shown as the mean±SD of at least three independent experiments performed in triplicate.
P<0.01, significantly different from control group.
3.2. Olmutinib reversed ABCG2-mediated MDR in vivo
The ABCG2-overexpressing multidrug-resistant S1-MI-80 cell xenograft model in nude mice was established to evaluate whether olmutinib could reverse the resistance to traditional anti-cancer drugs in vivo. There was no significant difference in tumor weight between animals receiving saline, topotecan or olmutinib alone (Fig. 2). However, the tumor weight of olmutinib plus topotecan group reduced to a large extent and the inhibition rate was 65%. Furthermore, no appreciable weight loss or mortality was observed in the athymic nude mice, indicating that olmutinib effectually enhanced the antitumor activity of topotecan without causing additional toxicity. These results suggested that olmutinib had a significant reversal effect on ABCG2-mediated MDR in vivo without additional side effect.
Figure 2.
Olmutinib enhanced the anticancer effect of topotecan in the S1-MI-80 cell xenograft model in nude mice. (A) The changes in tumor volume over time after the S1-MI-80 cell implantation (n = 6). Data shown are mean ± SD of tumor volumes for each group. (B) The image of tumors size in four groups excised from the mice on the 75th day after implantation. (C) Average percentage change in bodyweight after treatments. (D) Mean tumor weight after excising from the mice on the 75th day after implantation (n = 6). The fourtreatment groups were: (1) control (vehicle of olmutinib, p.o., every 5 day and saline i.p. every 5 day); (2) olmutinib (30 mg/kg, p.o., every 5 day); (3) topotecan (2 mg/kg, i.p., every 5 day) and (4) topotecan (2 mg/kg, i.p., every 5 day)+olmutinib (30 mg/kg, p.o., every 5 day given 1 h before giving topotecan). Results are presented as the mean±SD. **P<0.01 significantly different from control group.
3.3. Olmutinib significantly enhanced the accumulation of Rho 123 and doxorubicin in ABCG2-overexpressing cancer cells
The remarkable synergistic cytotoxic effect from combination of olmutinib with ABCG2-substrate anticancer drugs in vitro and in vivo suggested the reversal of ABCG2-mediated multidrug resistance. In order to further clarify the reversal mechanism, we performed accumulation assay of DOX and Rho 123. Intracellular DOX and Rho 123 were measured in ABCG2-overexpressing cells in the presence or absence of olmutinib, and the results were shown in Fig. 3. After 2 or 0.5 h of incubation, the intracellular level of DOX and Rho 123 in ABCG2-overexpressing S1-MI-80 cells were significantly lower than that of the parental S1 cells. Olmutinib at 1 μmol/L significantly increased the intracellular level of DOX and Rho 123, which were close to the effect of FTC at 2.5 μmol/L in S1-MI-80 cells. However, neither olmutinib nor FTC altered the intracellular levels of DOX and Rho 123 in parental S1 cells (Fig. 3). Therefore, olmutinib could increase intracellular accumulation of chemotherapeutic agents in ABCG2-overexpressing cells.
Figure 3.
Effect of olmutinib on the intracellular accumulation of DOX, Rho 123 in MDR cells and their parental cells. The accumulation of DOX (A), Rho 123 (B) in S1 and S1-MI-80 cells was measured by flow cytometric analysis as described in Section Material and methods. The results were presented as fold change in fluorescence intensity relative to control MDR cells. Data are expressed as mean ± SD from three independent experiments; **P<0.01 significantly different from control group.
3.4. Olmutinib decreased the efflux of Rho 123 in ABCG2-overexpressing cells
To determine whether olmutinib could inhibit the efflux of Rho 123, thus increasing the intracellular accumulation of Rho 123, an efflux assay was performed. The results showed that the ABCG2-overexpressing S1-MI-80 cells extruded a greater amount of Rho 123 at various time points (0, 15, 30, 60, 90 and 120 min) than the parental S1 cells (Fig. 4A). While after pre-incubating with olmutinib (1 μmol/L), the efflux of Rho 123 was significantly inhibited in ABCG2-overexpressing S1-MI-80 cells, but not significantly changed in S1 cells. These data indicated that olmutinib strongly increased the inhibitory of ABCG2-mediated activity on the efflux of Rho 123 in S1-MI-80 cells, but not in S1 cells.
Figure 4.
Effect of olmutinib on the efflux of Rho 123, the ATPase activity and the [125I]-IAAP photoaffinity labeling of ABCG2. (A) Time course (0, 30, 60, 90, 120 min) of Rho 123 efflux was measured in S1 and S1-MI-80 cells, with or without 1 µmol/L olmutinib. Data are expressed as mean±standard. (B) Olmutinib competed for photolabeling of ABCG2 by [125I]-IAAP crude membranes from ABCG2-overexpressing MCF7/FLV1000 cells were incubated with [125I]-IAAP and a range of different concentration (0–5 µmol/L) of olmutinib. The samples were then cross-linked by UV illumination, and subjected to SDS-PAGE. A representative autoradiogram from three independent experiments was shown. The relative amount of [125I]-IAAP incorporated is plotted against the concentration of olmutinib used in the competition. 100% incorporation refers to the absence of olmutinib. (C) Effect of olmutinib on ABCG2 ATPase activity. The vanadate-sensitive ABCG2 ATPase activity in the presence of the indicated concentrations of olmutinib was evaluated. Data are expressed as mean±SD from three independent experiments.
3.5. Olmutinib inhibited the photolabeling of ABCB1 with [125I]-IAAP
To further investigate the interaction of olmutinib with the substrate binding sites of ABCG2, we examined the photo-labeling of ABCG2 with [125I]-IAAP by incubating membrane vesicles in the presence of a range of different concentrations of olmutinib. The autoradiogram showed that olmutinib strongly inhibited the [125I]-IAAP photolabeling of ABCG2 in a concentration-dependent manner (Fig. 4B). The concentration of olmutinib required for 100% inhibition of photolabeling of ABCG2 with [125I]-IAAP was approximately 5 μmol/L. These suggested that olmutinib, like [125I]-IAAP, might complete with substrates and incorporate into the substrate-binding sites of ABCG2.
3.6. Olmutinib modulated the ATPase activity of ABCG2
ABCG2 utilized the energy of ATP hydrolysis for efflux of drug-substrates, and substrates either stimulated or inhibited the ATPase activity. The drug-efflux function of ABCG2 was associated with ATP hydrolysis, which was stimulated in the presence of the transporter substrates. To understand whether olmutinib affected the ATPase activity of ABCG2, we measured the vanadate-sensitive ATPase activity of ABCG2 in the presence of a range of different concentrations of olmutinib. Olmutinib was found to stimulate the ATPase activity of ABCG2 in a concentration-dependent manner (Fig. 4C). The result suggested that olmutinib might be a substrate of ABCG2.
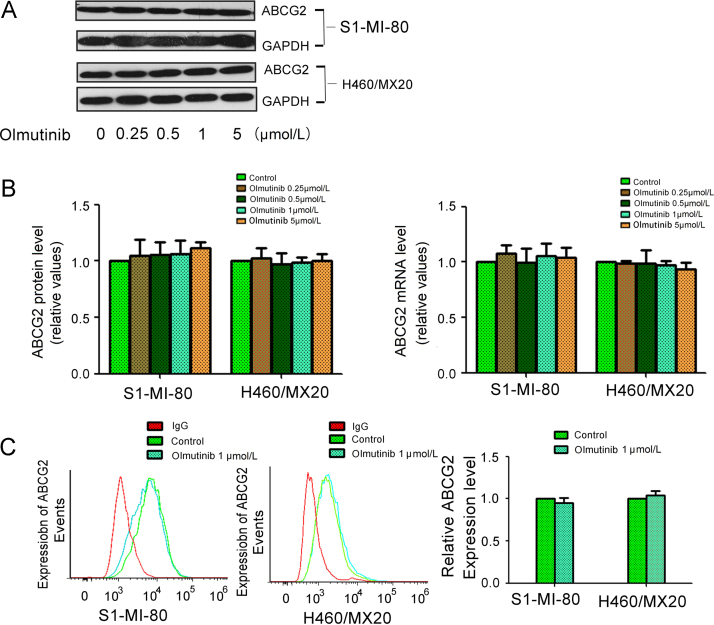
3.7. Olmutinib did not alter the expression of ABCG2
The reversal of ABCG2-mediated MDR could be achieved by either antagonizing their function or downregulating their expression level. Therefore, the effect of olmutinib on the expression of ABCG2 was detected by Western blot and qPCR, respectively (Fig. 5). It turned out that olmutinib did not alter the protein or mRNA level of ABCG2, even up to the concentration of 5 μmol/L in S1-MI-80 cells and H460/MX20 cells. Thus, the reversal of MDR by olmutinib was possible through inhibiting the transport function of ABCG2, instead of regulating their expression.
Figure 5.
Effect of olmutinib on the expression of ABCG2 in MDR cells. (A) The protein levels of ABCG2 in MDR cells after different concentrations olmutinib stimulation for 48 h were measured by Western blot analysis (GAPDH as loading control). (B) Olmutinib did not alter the mRNA and protein levels in MDR cells in concentration dependent manners. Real time-PCR was further applied to confirm the unaltered mRNA levels in MDR cells. (C) The cell surface expression of ABCG2 were measured by fow cytometry before and after olmutinib stimulation on MDR cells and their parental cells. All these experiments were repeated at least three times, and representative images and densitometry results are shown in each panel.
3.8. Olmutinib did not significantly change the cell surface localization of ABCG2
To further evaluate whether the localization of ABCG2 was influenced by olmutinib, the cell surface expression of ABCG2 was analyzed in the presence 1 μmol/L olmtinib in ABCG2-overexpressing H460/MX20 and S1-MI-80 cells. The surface expression of ABCG2 was not obviously changed in H460/MX20 and S1-MI-80 cells (Fig. 5C). These results suggested that olmutinib did not alter the cell surface localization of ABCG2.
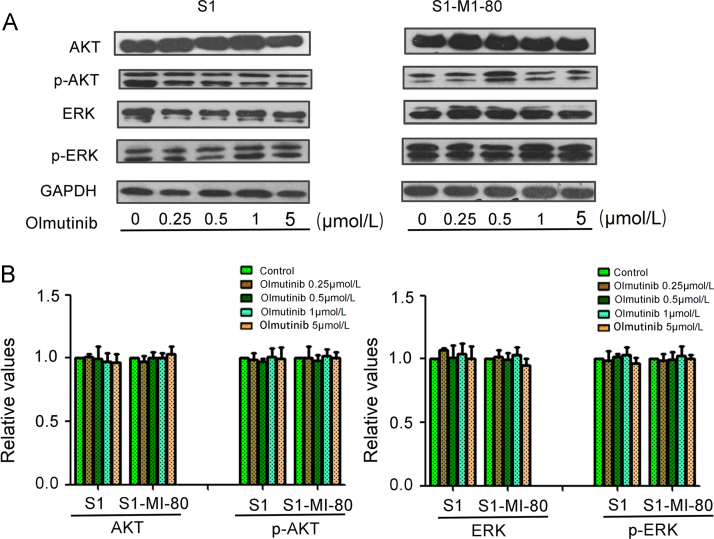
3.9. Olmutinib had no effect on the phosphorylation of AKT and ERK at the concentration of MDR reversal
To determine whether the enhancement effect of olmutinib was related to inhibition of AKT, ERK and their phosphorylation in S1 and S1-MI-80 cells, the expression of total and phosphorylation AKT and ERK were tested after treating the cells with 0, 0.25, 0.5, 1 μmol/L olmutinib for 48 h. As shown in Fig. 6, there was no significant alteration in the total and phosphorylated of AKT and ERK in MDR reversal concentration. These data suggested that the enhancement effect of olmutinib on ABCG2-overexpressing cells was independent on the inhibition of AKT or ERK pathway.
Figure 6.
Effect of olmutinib on AKT, ERK, and their phosphorylations in MDR and the parental cells. S1-MI-80 and S1 cells were treated with different concentrations of olmutinib for 48 h. (A) The total and the phosphorylation protein level of AKT and ERK were detected by Western blot (GAPDH as loading control). All these experiments were repeated at least three times. (B) Quantitative analysis of AKT, ERK and their phosphorylation.
4. Discussion
It was a promising strategy to reverse drug resistance in cancer treatment by inhibiting the drug transport function of ABCG234. Since the discovery of ABCG2, so many compounds have been found to inhibit the activity of ABCG2, such as the tyrosine kinase inhibitors gefitinib and dacomitinib35, FTC, some herbal extracts36 and so on. But none of them was in clinical use due to their toxicity and unpredictable pharmacokinetic. Hence it is still urgent to search for a safe and specific inhibitor of the ABCG2 transporter. We have been studying the use of molecularly targeted TKIs to inhibit ABC transporters in the circumvention of MDR. We found that some TKIs could block ABC transporters and inhibit their drug efflux function, thereby reversing MDR to conventional chemotherapeutic drugs in cancer cells. Therefore, the use of TKIs to inhibit ABC drug transporter mediated-MDR in patients is a promising approach for treating drug resistant cancers. So, combined use of TKI and chemotherapy drugs may be an effective clinical treatment strategy, and encourage further study for combination therapy in clinic.
Olmutinib is a third-generation EGFR TKI exhibiting potent effect in treatment of NSCLC with EGFR T790M, and having little effect on the EGFR wild-type allele. In the first phase I/II study (Clinical Trials.gov, NCT01588145) conducted in South Korea, the progression-free survival (PFS) was significantly prolonged in EGFR T790M-positive patients, who orally administered olmutinib at 800 mg/day37, 38. Olmutinib was also first globally approved in South Korea for locally advanced or metastatic EGFR T790M mutation-positive NSCLC patients in May 201622. In our paper, we evaluated whether olmutinib could circumvent ABCG2-mediated MDR for the first time.
Originally, we found that olmutinib at concentration below 1 μmol/L did not produce significant toxic effect on either parental or drug-selected cells. We chose the noncytotoxic concentrations (1, 0.5, and 0.25 μmol/L) for investigation in the following reversal experiments. The results showed that olmutinib significantly reduced resistance to MX and topotecan in ABCG2-overexpressing S1-MI-80 and H460/MX20 cells as compared to their parental S1 and H460 cells. But olmutinib did not potentiate effects of MX, topotecan or doxorubicin in S1, H460, KB and KBV200 cells, which did not express ABCG2. Olmutinib also did not significantly change IC50 values of DDP, which was not ABCG2 substrate. To further test their specificity, we also determined the effects of olmutinib in ABCG2-transfected cell lines (expressing either the wild type ABCG2-482-R2 or the mutant ABCG2-482-T7). The similar results were observed in the ABCG2-transfected cell lines. Collectively, these results suggested that olmutinib could increase the sensitivity of traditional anticancer drugs in ABCG2-overexpressing cells in vitro.
To verify the promising data in vivo, we established an ABCG2-overexpressing tumor xenograft mouse model. We found that combination treatment exhibited more potent inhibitory effect than topotecan alone on the growth of S1-MI-80 tumors, without remarkable toxicity among this cohort. Nude mice treated with olmutinib alone had no effect on the tumor growth or body weight. These results suggest that olmutinib (30 mg/kg) is well tolerated in vivo and may be a good candidate for reversal of ABCG2-mediated MDR.
To further understand the mechanism of olmutinib׳s reversal effect, we performed accumulation assays to evaluate the intracellular level of DOX and Rho 123. ABCG2-overexpressing S1-MI-80 cells when treated with olmutinib exhibited increased intracellular levels of DOX and Rho 123, whereas no change in the intracellular levels of DOX and Rho 123 in the parental S1 cells was observed. The increasing in accumulation could be either due to increased intracellular levels of DOX and Rho 123 or due to inhibited outflow of the DOX and Rho 123 by inhibiting the efflux function of the ABCG2 transporter. So in another assay, the same cells were treated with olmutinib and collected with different time and analyzed for the efflux levels of Rho 123. The results demonstrated that olmutinib proficiently retained the Rho 123 in S1-MI-80 cells. So, we could deduce that olmutinib inhibited the efflux function of the ABCG2 transporter and did not affect the intracellular uptake.
The drugs having inhibitory capacity towards ABC transporters often affected the expression levels of the transporters. Therefore, we investigated whether olmutinib effected on the ABCG2 expression. Western blotting and real-time quantitative RT-PCR analysis were performed. In our study, expression level of the ABCG2 protein or mRNA and localization of ABCG2 were not significantly affected by olmutinib in S1-MI-80 and H460/MX20 cells. Thus, we arrived at the conclusion that olmutinib reversed ABCG2-mediated drug resistance by inhibiting the function of the ABCG2 pump rather than altering the protein expression level of ABCG2. In addition, the treatment with low concentrations of olmutinib for 48 h did not block the phosphorylation of AKT and ERK. Accordingly, the MDR reversal activity of olmutinib was independent of the blockage of EGFR or Her-2 receptors and their downstream signaling pathways. This is beneficial because the inhibition of wild-type EGFR or Her-2 may produce non-specific cytotoxic effect and unwanted side effect.
ABCG2 utilized energy from hydrolysis of ATP to exhibit drug-efflux function and substrates could stimulate or inhibit the activity of transporter. Therefore, we detected the activities of ABCG2 ATPase in the presence or absence of olmutinib. We found that olmutinib could markedly stimulate the activity of ABCG2 ATPase in a concentration-dependent fashion, indicating that it should be a substrate of ABCG2. [125I]-IAAP photo-affinity labeling assay can be used to further explain the binding sites of olmutinib on ABCG2.
5. Conclusions
This is the first study to demonstrate that olmutinib may be a reversal agent of ABCG2-mediated MDR through directly inhibiting the drug efflux function of ABCG2 at non-cytotoxic concentrations. These findings reveal that olmutinib may have a high clinical value for combination with conventional antineoplastic drugs (ABCG2 substrates) to augment the effectiveness of the conventional chemotherapeutic drugs in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81473233), Guangzhou Technology Program Foundation (No. 201604020079); The Science and Technology Project of Guangdong Province (No. 2016A030312014), The Scientific and Technological Leading Talent Project of Guangdong Province (2015).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Shirong Yan, Email: graceyan@163.com.
Liwu Fu, Email: Fulw@mail.sysu.edu.cn.
References
- 1.Gottesman M.M. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 2.Deeley R.G., Westlake C., Cole S.P. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 3.Quintieri L., Fantin M., Vizier C. Springer; New York: 2007. Identification of molecular determinants of tumor sensitivity and resistance to anticancer drugs. [DOI] [PubMed] [Google Scholar]
- 4.Lowe S.W., Ruley H.E., Jacks T., Housman D.E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 5.Synold T.W., Dussault I., Forman B.M. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 6.Vasiliou V., Vasiliou K., Nebert D.W. Human ATP-binding cassette (ABC) transporter family. Hum Genom. 2009;3:281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 8.Leonard G.D., Fojo T., Bates S.E. The role of ABC transporters in clinical practice. oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 9.Kruh G.D., Belinsky M.G. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 10.Allikmets R., Schriml L.M., Hutchinson A., Romanospica V., Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 11.Miyake K., Mickley L., Litman T., Zhan Z., Robey R., Cristensen B. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 12.Diestra J.E., Scheffer G.L., Català I., Maliepaard M., Schellens J.H., Scheper R.J. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 13.Volk E.L., Farley K.M., Wu Y., Li F., Robey R.W., Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002;62:5035–5040. [PubMed] [Google Scholar]
- 14.Candeil L., Gourdier I., Peyron D., Vezzio N., Copois V., Bibeau F. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer. 2004;109:848–854. doi: 10.1002/ijc.20032. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Sridhar R., Shan L., Sha W., Gu X., Sukumar S. Loperamide, an FDA-approved antidiarrhea drug, effectively reverses the resistance of multidrug resistant MCF-7/MDR1 human breast cancer cells to doxorubicin-induced cytotoxicity. Cancer Investig. 2012;30:119–125. doi: 10.3109/07357907.2011.640653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janne P.A., Gray N., Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8:709–723. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti S., de Vries N.A., Buckle T., Bolijn M.J., van Eijndhoven M.A.J., Beijnen J.H. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in vitro and in vivo pharmacokinetic studies employing Bcrp1–/–/Mdr1a/1b–/– (triple-knockout) and wild-type mice. Mol Cancer Ther. 2008;7:2280–2287. doi: 10.1158/1535-7163.MCT-07-2250. [DOI] [PubMed] [Google Scholar]
- 18.Ozvegy-Laczka C., Cserepes J., Elkind N.B., Sarkadi B. Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resis Updat. 2005;8:15–26. doi: 10.1016/j.drup.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari A.K., Sodani K., Dai C., Ashby C.R.J., Chen Z. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570–594. doi: 10.2174/138920111795164048. [DOI] [PubMed] [Google Scholar]
- 20.Passaro A., Guerinirocco E., Pochesci A., Vacirca D., Spitaleri G., Catania C.M. Targeting EGFR T790M mutation in NSCLC: from biology to evaluation and treatment. Pharmacol Res. 2017;117:406–415. doi: 10.1016/j.phrs.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Liao B.C., Lin C.C., Lee J.H., Yang J.C. Update on recent preclinical and clinical studies of T790M mutant-specific irreversible epidermal growth factor receptor tyrosine kinase inhibitors. J Biomed Sci. 2016;23:86. doi: 10.1186/s12929-016-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim E.S. Olmutinib: first global approval. Drugs. 2016;76:1153–1157. doi: 10.1007/s40265-016-0606-z. [DOI] [PubMed] [Google Scholar]
- 23.Robey R.W., Honjo Y., van de Laar A., Miyake K., Regis J.T., Litman T. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochim Biophys Acta. 2001;1512:171–182. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 24.Robey R.W., Honjo Y., Morisaki K., Nadjem T.A., Runge S., Risbood M. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmichael J., DeGraff W.G., Gazdar A.F., Minna J.D., Mitchell J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 26.Robey R.W., Shukla S., Finley E.M., Oldham R.K., Barnett D., Ambudkar S.V. Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1®. Biochem Pharmacol. 2008;75:1302–1312. doi: 10.1016/j.bcp.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabindran S.K., Ross D.D., Doyle L.A., Yang W., Greenberger L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 28.Chen L., Liang Y., Ruan J., Ding Y., Wang X., Shi Z. Reversal of P-gp mediated multidrug resistance in-vitro and in-vivo by FG020318. J Pharm Pharmacol. 2004;56:1061–1066. doi: 10.1211/0022357043879. [DOI] [PubMed] [Google Scholar]
- 29.Fu L., Liang Y., Deng L., Ding Y., Chen L., Ye Y. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol. 2004;53:349–356. doi: 10.1007/s00280-003-0742-5. [DOI] [PubMed] [Google Scholar]
- 30.Dai C.L., Tiwari A.K., Wu C.P., Su X.D., Wang S.R., Liu D.G. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambudkar S.V. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Method Enzymol. 1998;292:504–514. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 32.Yang K., Chen Y., To K.K., Wang F., Li D., Chen L. Alectinib (CH5424802) antagonizes ABCB1- and ABCG2-mediated multidrug resistance in vitro, in vivo and ex vivo. Exp Mol Med. 2017;49:e303. doi: 10.1038/emm.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukla S., Robey R.W., Bates S.E., Ambudkar S.V. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–8951. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 34.Bumbaca B, Li W.Taxane resistance in castration-resistant prostate cancer: mechanisms and therapeutic strategies. Acta Pharm Sin B 2018. Available from: 〈 10.1016/j.apsb.2018.04.007〉. [DOI] [PMC free article] [PubMed]
- 35.Guo X, To KKW, Chen Z, Wang X, Zhang J, Luo M, et al. Dacomitinib potentiates the efficacy of conventionalchemotherapeutic agents via inhibitingthe drug efflux function of ABCG2 in vitro and in vivo. Journal ofexperimental & clinical cancer research: CR 2018;37: 31. [DOI] [PMC free article] [PubMed]
- 36.Tamaki H., Satoh H., Hori S., Ohtani H., Sawada Y. Inhibitory effects of herbal extracts on breast cancer resistance protein (BCRP) and structure-inhibitory potency relationship of isoflavonoids. Drug Metab Pharmacokinet. 2010;25:170–179. doi: 10.2133/dmpk.25.170. [DOI] [PubMed] [Google Scholar]
- 37.Park K., Lee J.S., Han J.Y., Lee K.H., Kim J.H., Cho E.K. 1300: Efficacy and safety of BI 1482694 (HM61713), an EGFR mutant-specific inhibitor, in T790M-positive NSCLC at the recommended phase II dose. J Thorac Oncol. 2006;11 : S113. [Google Scholar]
- 38.Lee K-O., Cha M.Y., Kim M., Song J.Y., Lee J-H., Kim Y.H. Abstract LB-100: Discovery of HM61713 as an orally available and mutant EGFR selective inhibitor. Cancer Res. 2014 doi: 10.1158/1538-7445.AM2014-LB-100. [DOI] [Google Scholar]