Abstract
Increasing evidence suggests that cancer stem cell (CSC) theory represents an important mechanism underlying the observed failure of existing therapeutic modalities to fully eradicate cancers. In addition to their more established role in maintaining minimal residual disease after treatment and forming the new bulk of the tumor, CSCs might also critically contribute to tumor recurrence and metastasis. For this reason, specific elimination of CSCs may thus represent one of the most important treatment strategies. Emerging evidence has shown that CSCs have a different metabolic phenotype to that of differentiated bulk tumor cells, and these specific metabolic activities directly participate in the process of CSC transformation or support the biological processes that enable tumor progression. Exploring the role of CSC metabolism and the mechanism of the metabolic plasticity of CSCs has become a major focus in current cancer research. The targeting of CSC metabolism may provide new effective therapies to reduce the risk of recurrence and metastasis. In this review, we summarize the most significant discoveries regarding the metabolism of CSCs and highlight recent approaches in targeting CSC metabolism.
Keywords: Cancer Metabolism, Cancer Stem Cell, Glycolysis, Mitochondria, OXPHOS
INTRODUCTION
Otto Warburg, a German biochemist who won the Nobel Prize in the 1930s, first discovered that tumor cells use a different metabolic pathway than normal cells. Since then, the field of cancer metabolism has become a new area of interest, especially in the last decade. Through the technical development of new biochemical tools such as metabolomics, studies of cancer cell metabolism have extended our knowledge of the mechanisms and role of metabolic reprogramming in cancer for tumor growth, metastasis, and drug resistance (1). Genomic instability and the diverse microenvironment condition contribute to the heterogeneity of tumors, and the existence of small subpopulations of cancer cells with high capacity for self-renewal and the ability to initiate tumorigenesis found in primary tumors, which are referred to as cancer stem cells (CSCs) (2, 3). CSCs are considered as the source from which cancer cells arise, and they are therapy resistant and responsible for metastatic dissemination, thereby CSC-targeted therapy would be an important challenge in cancer research (3). It is well-known that the metabolic reprogramming of pluripotent stem cells is essential for stem cell function (4, 5). Similar to normal stem cells, recent studies suggest that CSCs undergo metabolic changes including mitochondrial respiration and glycolysis, and this transition is critical for the function of CSCs (6). Therefore, targeting of the CSC metabolism may provide new therapies to reduce the risk of recurrence and metastasis (Fig. 1). However, results regarding the metabolic phonotype of CSCs, which mainly utilize glycolytic or mitochondrial respiration, are contradictory, and the exact role of the precise metabolic reprogramming in cancer and underlying detailed mechanisms regulating this metabolic plasticity need to be elucidated. This review highlights the role of the metabolic reprogramming of CSCs and the differences in the metabolic pathway between CSCs and normal tissues. This review also explores the potential for the regulation of the metabolic pathway via new anti-cancer drugs.
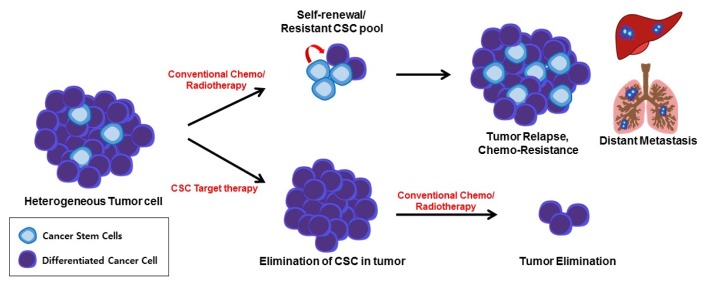
Fig. 1.
Impact of CSCs on the effectiveness of anticancer therapy. CSCs are a small sub population of bulk tumor cells that are highly chemo-resistant and play a prominent role in tumor relapse. While conventional therapy results in a transient reduction in the tumor by killing non-stem cancer cells (differentiated cancer cells), the remaining CSCs can form recurring tumors, and metastasis is induced by the formation of a secondary colony in distant organs. The use of CSC-specific inhibitors would reduce therapy resistance and relapse, and prevent metastasis, with a loss of stem cell properties.
TUMOR CELL METABOLISM
In non-transformed or quiescent somatic cells, mitochondria are the subcellular organelle that produce the principal source of energy through the tricarboxylic acid (TCA) cycle linked with oxidative phosphorylation (OXPHOS). Cells uptake carbon fuels such as glucose, fatty acids, and glutamine that enter into the TCA cycle to produce 36 ATPs with maximum efficiency in the mitochondria. Normal cells have a metabolic pathway designed to minimize the use of energy. However, highly proliferating cancer cells use glycolysis rather than OXPHOS for ATP generation, despite the presence of sufficient oxygen concentrations in the tumor microenvironment (1, 7). Aerobic glycolysis in many cancers is caused by various factors, such as the hypoxic tumor microenvironment, activation of oncogenes and loss of tumor suppressors, and mitochondrial DNA mutation. While cancer cell metabolism does not efficiently produce ATP, it allows cancer cells to rapidly divide and grow. Consequently, cancer cells produce a substantial portion of their energy from aerobic glycolysis which is more rapid than OXPHOS, although the aerobic glycolysis is considerably less efficient in terms of the amounts of ATP produced per unit of glucose consumed. This inefficient metabolic process is useful for producing the nucleic acids, amino acids, and lipids necessary for rapid cell growth via glycolysis and OXPHOS intermediates (8, 9). To compensate for this inefficient metabolic process for energy production per unit of glucose consumed, cancer cells uptake glucose and glutamate at a rate of more than about 200 times that of normal cells. In addition to glucose and glutamate as the core metabolic sources, increasing body of evidence suggests that various nutrients and metabolic pathways support the altered energy metabolism of cancer cells. Various metabolic fuel sources have been identified in cancer cells. These include acetate, lactate, fatty acids, serine, glycine, and branched chain amino acids.
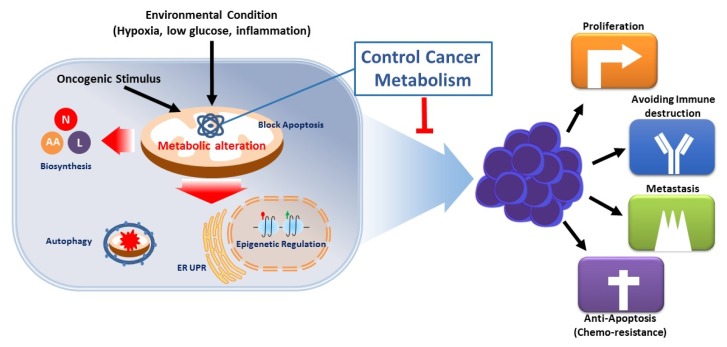
The metabolic alteration of cancer cells also has a beneficial effect on cancer survival and resisting cell death. The environmental conditions of cancer cells compared to those of normal cells is spatially and temporally heterogeneous and frequently sparse in levels of glucose, glutamine, and oxygen (10). The altered metabolic pathway of cancer cells enables cancer cells to survive in these metabolically stressful conditions found in the tumor microenvironment (such as low oxygen or nutrient levels) (11). It is known that the mitochondrial membrane permeabilization process, which is a mitochondrial apoptosis control mechanism, is inactivated in most cancer cells. These cytopathic mechanisms are known to be regulated by mitochondrial metabolism, especially hexokinase related with the glycolysis pathway; changes in cancer metabolism are therefore closely related to the anti-apoptotic property of cancer cells (12). In addition, changes in the metabolic pathway have been shown to be involved in gene expression by regulating the activity of epigenetic modification enzymes or by controlling the amount of substrate for epigenetic modification. Somatic mutations in IDH1 and IDH2 occur in up to 70% of glioma as well as in 20% of leukemia, and these IDH mutants acquire a neomorphic activity to convert α-ketoglutarate to (D)-2-hydroxyglutarate. The subsequent accumulation of 2-hydroxyglutarate results in epigenetic dysregulation via inhibition of α-ketoglutarate-dependent histones and DNA demethylases, and suppress expression of many tumor suppressor gene (13–15). Lactate, a final product of glycolysis, acidifies the surrounding environment of cancer cells, inhibits the activation of NK and CTL cells, and plays an important role in the growth of cancer (16). Therefore, the reprogramed metabolic pathway of cancer plays an important role not only in tumor growth, but also in metastasis and chemo resistance through energy supply, survival under unfavorable environmental conditions, immune avoidance, and epigenetic modification (Fig. 2).
Fig. 2.
Functions of metabolic alteration in cancer. Genetic mutations and growth signals in cancer cells and microenvironments within large tumors can dynamically alter metabolic pathways and modulate the regulation of metabolic pathways. This results in increased biosynthesis and abnormal bioenergy production, both of which promote cell proliferation, avoidance of immune-based destruction, metastasis, and survival. Furthermore, metabolic remodeling regulates tumor epigenetic alterations by regulating the activity of epigenetic modification enzymes because of the effect on gene expression in cancer.
CANCER STEM CELL
The heterogeneous nature of cell populations within a tumor has been recognized for several decades (3, 17). CSCs are defined as undifferentiated, slow-cycling cells that are able to form tumor tissue even from a single cell. In accordance with CSC model, heterogeneous and hierarchical cellular organization have been found in most tumors, with a group of undifferentiated cells at the apex of the hierarchy. CSCs typically exist as minority subpopulation within the entire tumor mass (0.001–0.1%) and are responsible for the generation of highly proliferative cancer cells forming the bulk of the tumor, even in the recurrence of cancer after therapy (17–19). After prospective identification of CSCs in leukemia for the first time in 1994 (20), CSCs have been continuously identified in various solid tumors including those of breast cancer (21, 22), brain tumors (23, 24), colorectal cancer (25), prostate cancer (26), lung cancer (27), and melanoma (28). This new concept for intraclonal and functional heterogeneity of cancer cells can fundamentally change the way we diagnose and treat cancer. Accumulating evidence suggests that CSCs are responsible for metastasis, chemoresistance, and tumor relapse, and the elimination of CSCs may thus represent one of the most important challenges in treatment of cancer (3, 29, 30).
CSCs possess various biological features of normal stem cells: the self-renewal ability; the expression of surface markers such as CD44, CD133, and aldehyde dehydrogenase; the activation of particular signaling pathways such as Wnt, Hedgehog, or Notch (17). Not only stem cells but also CSCs require a finely-tuned balance between self-renewal and differentiation. While the origin of CSCs is still unclear, CSCs are known to remain in the G0 phase, a quiescent phase, and express a high drug efflux transport system. CSCs, especially since they are in a dormant state, are almost impossible to eliminate by general anti-cancer drugs, which usually target proliferating cancer cells, and targeted therapies for CSCs are therefore needed (33, 34). An important feature of embryonic stem cells is their special metabolic phenotype when compared with differentiated progenies (35). Similar to cancer, it is well-known that metabolic alterations regulate stem cell self-renewal, and stem cell function is also regulated by bioenergetic signaling, such as the AKT-mTOR pathway, glutamine metabolism, and fatty acid metabolism (31). Emerging evidence strongly suggests that CSCs also undergo metabolic alterations (including mitochondrial respiration, glycolytic activity, and altered lipid metabolism) that are critical for CSC function. Furthermore, low oxygen tension (hypoxia) contributes to the maintenance of an undifferentiated state and influences proliferation and cell-fate commitment in normal stem cells. It is also well-known that hypoxia is a critical factor for malignancy, chemoresistance, and poor survival rate of cancer patients (32). Thus, exploiting the metabolism changes required for CSC self-renewal, cell division, and quiescence may provide effective therapies and diminish the risk of recurrence and metastasis.
METABOLIC STATE OF CANCER STEM CELLS
It has been well verified that pluripotent stem cells mainly utilize glycolysis for energy production, whereas normal cells rely on OXPHOS (4). In induced pluripotent stem cells (iPS cells) as a model of stem cell reprogramming, glycolytic metabolic changes occur from OXPHOS prior to their acquisition of the pluripotent state, and this process is essential for stem cell reprogramming (36). These findings suggest that metabolic reprogramming and stemness are closely linked, and the glycolytic switch could play a critical role in CSCs rather than the consequence of acquiring pluripotency. Many studies have been supported the hypothesis that CSCs are more glycolytic than normal cancer cells. Similar to normal stem cells, glucose is an essential nutrient for CSCs, and its presence in the microenvironment significantly increases the number of stem-like cancer cells in the cancer cell population. Glucose induces the expression of specific genes in CSCs associated with the glucose metabolism pathway (c-Myc, Glut-1, HK-1, HK-2, and PDK-1), which contributes to the increase in the CSC population (37). Furthermore, glycolysis inhibition or deprivation of glucose leads to a decline in the CSC population. Small cell populations with stem-like properties from glioblastoma, ovarian cancer, breast cancer, lung cancer, and colon cancer cell lines rely more on glycolysis than on the bulk of differentiated progeny (38–41). As a typical glycolytic cell, glucose uptake, lactate production, glycolytic enzyme expression, and ATP content are significantly increased in CSCs compared to non-CSCs. The stemness marker CD44 is crucial for the regulation of glycolytic metabolism (42). Additionally, CSCs of glioblastoma that are highly dependent on glycolysis show increased migration in hypoxic conditions. Glycolysis was found to be the preferred metabolic state in radiotherapy-resistant stem cells in nasopharyngeal carcinoma and hepatocellular carcinoma. Therefore, glycolytic metabolic reprogramming is critical for the maintenance of CSCs and is associated with the progression of cancer.
While the above reported studies show that CSCs mainly rely on glycolysis, several other studies showed that CSCs possess a preference for mitochondrial oxidative metabolism. Growing evidence has demonstrated that quiescent or slow-cycling tumor-initiating CSCs are less glycolytic, consume less glucose, and produce less lactate, whereas they contain higher ATP levels than their differentiated cancer progeny cells in many other tumor types including lung cancer, breast cancer, glioblastoma, and pancreatic cancer (43–46). Moreover, it has been reposted that CSCs have an increased mitochondrial mass and membrane potential with enhanced oxygen consumption rates. Additionally, invasive cancer cells show high mitochondrial metabolism through the expression of transcription factor PGC1α, the master regulator of mitochondrial biogenesis (47, 48). PGC1α has also been found to be overexpressed in circulating tumor cells, and the inhibition of PGC1α reduces the stemness properties of breast CSCs (49). In addition, NANOG, a pluripotency gene, induces tumorigenesis through metabolic reprogramming to OXPHOS and fatty acid metabolism (50). The increased OXPHOS phenotype and expression of PGC1α seems to be related to the capacity for chemoresistance in CSCs (51–54). Surviving cells from chemotherapy rely on oxidative phosphorylation through increased mitochondrial activity. It has been reported that MYC and MCL1 cooperatively promote chemotherapy-resistant breast CSC via the regulation of mitochondrial OXPHOS (54). A recent study showed that the mitochondrial DNA transfer from host cells to tumor cells re-establishes the tumor-initiating and drug resistance capacity of the tumor calls (55). In contrast to normal stem cells and iPS cells, which mainly utilize glycolysis, CSCs show a distinct metabolic phenotype that can be glycolytic or OXPHOS-dependent. However, growing evidence strongly suggests that in either case, mitochondrial function is critical and plays a crucial role in CSC functions such as stemness, migration, and drug resistance (Fig. 3).
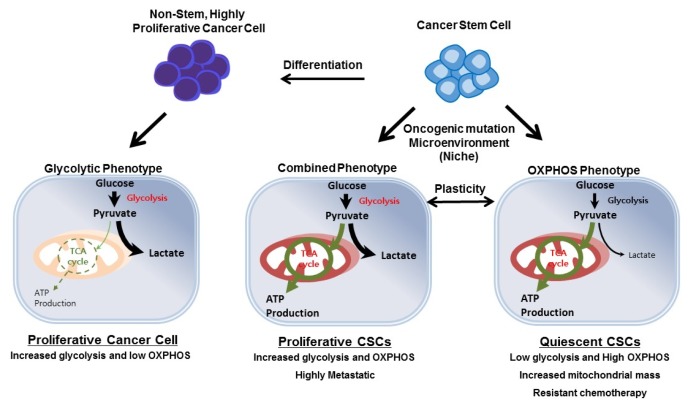
Fig. 3.
Metabolic features of CSCs. In non-stem/highly proliferative cancer cells, glycolysis is a predominant metabolic phenotype contributing to tumor growth. CSCs rely on OXPHOS metabolism or combined metabolism with high glycolysis, depending on the oncogenic background and surrounding microenvironment conditions such as hypoxia or nutrient supplementation. CSCs also show metabolic plasticity between two metabolic phenotypes. However, in either case, mitochondrial function is critical for CSC functions such as stemness, migration, and drug resistance.
GLUTAMINE AND LIPID METABOLISM IN CSCs
It has become increasingly clear that the unique model of cancer metabolic reprogramming can neither be universally applied to the entire spectrum of cancer types, nor to the various intrinsic subtypes of cancer cells in tumors. Glycolysis and OXPHOS alone cannot accurately account for cancer stem cell metabolism because the metabolic pathway is intricately intertwined. In addition to glucose metabolism, CSCs also rely on glutamine, which provides the carbon and amino-nitrogen needed for biosynthesis of amino-acid, nucleotide, and lipids (56). Therefore, glutamine metabolism is intertwined with glucose metabolism and can complement each other. In some cancers, glutamine helps to compensate for glucose shortages (57, 58). Moreover, emerging evidence suggests that alterations in lipid- and cholesterol-associated pathways are also essential for the maintenance of CSCs (59). In cancer cells, various metabolic intermediates are generated that can be utilized in anabolic processes for membrane building blocks. It is known that the lipid metabolic pathway is flexible and closely linked to the glucose and amino acid metabolic pathways in order to meet the increasing bioenergetics requirements of CSCs. The altered lipid metabolism may also affect the cytosolic oncogenic signaling pathway. Lipid rafts, which are rich in sphingolipids and cholesterol, are unique small lipid domains within the cell membrane in cancer cells and contain a set of receptors and signaling proteins involved in cell survival, adhesion, metastasis, and tumor progression (60–62). Lipid metabolic alteration in cancer can have an effect on cytosolic signaling changes via regulating lipid raft dynamics and components in CSCs. High lipid droplets and stored-cholesteryl ester content have been observed in circulating tumor cells, colorectal CSCs, and breast CSCs (63, 64). Actually, lipid droplets content and CD133 expression are directly correlated, and cancer cells with high lipid droplets have greater clonogenic potential and in vivo tumor-forming ability (63). Ovarian CSCs have high levels of unsaturated lipids, and blocking lipid desaturation impairs cancer stemness and tumor initiation capacity (65). It has been demonstrated that fatty acid oxidation pathways are critical for both hematopoietic stem cells, leukemia-initiating cells, and breast cancer stem cell functions (66, 67). The inhibition of fatty acid β-oxidation preferentially eliminates CSC population. Furthermore, altered lipid metabolism has been shown to influence the aggressiveness and progression of cancer. Lipid droplet-rich colon cancer cells are more resistant to chemotherapy. It has also been found that fatty acid β-oxidation is critical for self-renewal and chemoresistance of breast CSCs (66). In addition, lipid metabolism is closely associated with tumor metastasis (68).
METABOLIC COMPLEXITY OF CANCER STEM CELLS
CSCs may have highly glycolytic or OXPHOS phenotypes, depending on the cancer type. However, contradictory results regarding the metabolic phenotype in the same type of cancer have been reported. One possible reason for this type of contradictory result is the high flexibility of the metabolic phenotype of the CSCs between OXPHOS and glycolytic phenotypes depending on the environment and cellular signaling pathway. In support of this metabolically flexible scenario, it has been reported that CSCs are able to switch to a glycolytic metabolism when OXPHOS is blocked (69, 70). And most recently, it has been shown that MYC/PGC-1α balance determines the metabolic phenotype and plasticity of pancreatic CSCs (45). It is well-known that the microenvironment around CSCs plays an important role in maintaining stemness. Different types of cancer in diverse tissues exist in considerably different microenvironments (different oxygen tension and glucose concentrations), so each CSC can show a different metabolic phonotype to adapt to the different environment condition. In glucose-rich environments, proliferating CSCs primarily utilize aerobic glycolysis for their energy production, while in glucose-deprived conditions, CSCs shift to a quiescent and slow cycling state while relying on mitochondrial oxidative metabolism for ATP generation. Moreover, it has been shown that CSCs can adapt to hypoxia by upregulating the glucose metabolic enzymes and switching to a more glycolytic phenotype (70). Another factor that causes CSCs to have various metabolic phenotypes is the different signaling contexts and oncogenic mutations within cancer cells. The glycolytic metabolism phenotype and stemness of normal stem cells and iPS cells is mediated by OCT4, KLF4, SOX2, and MYC; however, NOTCH, WNT/β-catenin, PI3K/Akt, PTEN, NF-kB, KRAS, HIF, TP53, and many oncogenic pathways are involved in retaining the stemness of CSCs. This can affect the metabolic phenotype of CSCs. Moreover, a subpopulation of CSCs (heterogeneity) exists in tumors, and these can exhibit different metabolic patterns with different genetic alterations. In summary, CSCs are able to dynamically transition between different metabolic states, and the role of diverse genetic backgrounds and microenvironments in the metabolic state of CSCs and tumorigenic potential still needs to be elucidated.
TARGETING CELLULAR METABOLISM
The metabolic targeting of CSCs has become a very important emerging area to address effective cancer therapy for the elimination of CSCs, which are responsible for chemo-resistance and tumor relapse. To inhibit glycolysis metabolism, glucose transporter and glycolytic enzymes such as GLUT1-4, Hexokinase1-2, Pyruvate kinase M2, and lactate dehydrogenase have been suggested as targets (71). Another potential target is an adaptive mechanism of CSCs within the tumor microenvironment. CSCs rapidly transit their metabolism under heterogeneous environmental conditions (such as hypoxia, glucose deprivation, and low pH); this adaptive metabolic response by CSCs plays a pivotal role in cancer metastasis or chemo-resistance. Hypoxic inducible factor HIF1-2α is a key enzyme for metabolic adaptation in hypoxia and is involved in angiogenesis, metastasis, and cell survival (72). Pyruvate dehydrogenase kinase 1 has been demonstrated to regulate the metabolic transition in hypoxia via regulating the amount of acetyl-coA, which is then oxidized in the mitochondria to produce energy in the TCA cycle (73). Furthermore, pyruvate dehydrogenase kinase 1 is enriched in breast CSCs and is critical for metastasis in hypoxia (74, 75). mTOR controls energy homeostasis and involves cell survival during cellular metabolic stress such as nutrient and energy depletion. The downregulation of mTOR signaling reduces CSC properties in pancreatic, breast, and colorectal cancer (76). CSCs dependent on OXPHOS can be targeted by impairing mitochondrial energy metabolism. It has been shown that Metformin and Phenformin inhibit the electron transport chain complex I and cause cell death by energy crisis in CSCs (45, 77). Inhibition of mitochondrial protein biosynthesis can block mitochondrial OXPHOS by the inhibition of mitochondrial ribosomes using Tetracyclines, which has toxicity against CSCs (78). Similarly, mitochondrial metabolism can also be targeted by the mitochondrial chaperone TRAP1 inhibitor, Gamitrinib, which induces the impairment of protein folding in mitochondria (79, 80). Growing evidence strongly suggests that targeting mitochondrial OXPHOS could be an effective strategy to target CSCs and to reduce cancer metastasis and chemo-resistance. However, it is necessary to identify specific targets to inhibit OXPHOS only in CSCs and for the metabolic transition of CSCs without affecting normal cells that generally use OXPHOS to produce energy.
CONCLUSION
In various cancers, CSCs have been shown to have a distinct metabolic phenotype and can change their metabolic pathway depending on their microenvironment condition and genetic background. This complexity leads to more intricate variability in the metabolic pathways of CSCs. However, despite the limited research on the mechanism of metabolic plasticity of CSCs, recent studies strongly suggest that metabolic reprogramming in CSCs is crucial for tumorigenesis, metastasis, drug resistance, and tumor relapse. The targeting of the CSC metabolism is suggested as a novel therapeutic approach to eradicate the progression of various cancers. Since traditional anti-cancer drugs are inefficient in eliminating cancer and preventing its recurrence, targeting the metabolism of CSCs could provide a direction for the development of new anti-cancer drugs. A combination treatment with CSC-targeting drugs and conventional anticancer drugs could be a more effective strategy to treat cancer. Finally, to develop drugs that target the metabolic pathway of CSCs, the exact role of precise metabolic plasticity in cancer and the underlying detailed mechanism of regulating this metabolic plasticity should be elucidated. In addition, because of their metabolic similarity, an accurate distinction between CSCs and normal stem cells should be addressed. Once a specific metabolic pathway of CSCs has been identified, new therapies can be developed to eliminate CSCs without damaging normal cells.
ACKNOWLEDGEMENTS
This work was supported by a grant from the 2017 Research Fund (1.170074.01) of Ulsan National Institute of Science and Technology (UNST), the (MRC) program (NRF-2015R1A5A2 009656), the National Research Foundation (NRF) of Korea (2014M3A9D8034459; NRF-2016R1D1A1B03935769) funded by the Ministry of Science and ICT., and the Korea Health Technology R&D Project, Ministry of Health and Welfare (HI17C1635).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 4.Facucho-Oliveira JM, St John JC. The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev. 2009;5:140–158. doi: 10.1007/s12015-009-9058-0. [DOI] [PubMed] [Google Scholar]
- 5.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Zou ZW, Ma C, Medoro L, et al. LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget. 2016;7:61741–61754. doi: 10.18632/oncotarget.11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5:e190. doi: 10.1038/oncsis.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majewski N, Nogueira V, Bhaskar P, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell Metab. 2012;16:9–17. doi: 10.1016/j.cmet.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465:966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 17.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 19.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 25.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 26.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 27.Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 28.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merlos-Suarez A, Barriga FM, Jung P, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis and resistance to therapy. Hypoxia (Auckl) 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleffel S, Schatton T. Tumor dormancy and cancer stem cells: two sides of the same coin? Adv Exp Med Biol. 2013;734:145–179. doi: 10.1007/978-1-4614-1445-2_8. [DOI] [PubMed] [Google Scholar]
- 34.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panopoulos AD, Yanes O, Ruiz S, et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012;22:168–177. doi: 10.1038/cr.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu PP, Liao J, Tang ZJ, et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ. 2014;21:124–135. doi: 10.1038/cdd.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Zhou Y, Shingu T, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286:32843–32853. doi: 10.1074/jbc.M111.260935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciavardelli D, Rossi C, Barcaroli D, et al. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014;5:e1336. doi: 10.1038/cddis.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao J, Qian F, Tchabo N, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9:e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emmink BL, Verheem A, Van Houdt WJ, et al. The secretome of colon cancer stem cells contains drug-metabolizing enzymes. J Proteomics. 2013;91:84–96. doi: 10.1016/j.jprot.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Tamada M, Nagano O, Tateyama S, et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72:1438–1448. doi: 10.1158/0008-5472.CAN-11-3024. [DOI] [PubMed] [Google Scholar]
- 43.Janiszewska M, Suva ML, Riggi N, et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26:1926–1944. doi: 10.1101/gad.188292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sancho P, Burgos-Ramos E, Tavera A, et al. MYC/PGC-1alpha Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015;22:590–605. doi: 10.1016/j.cmet.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Viale A, Pettazzoni P, Lyssiotis CA, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan Z, Luo X, Xiao L, et al. The Role of PGC1alpha in Cancer Metabolism and its Therapeutic Implications. Mol Cancer Ther. 2016;15:774–782. doi: 10.1158/1535-7163.MCT-15-0621. [DOI] [PubMed] [Google Scholar]
- 48.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. 1–15. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peiris-Pages M, Martinez-Outschoorn UE, Pestell RG, Sotgia F, Lisanti MP. Cancer stem cell metabolism. Breast Cancer Res. 2016;18:55. doi: 10.1186/s13058-016-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CL, Uthaya Kumar DB, Punj V, et al. NANOG Metabolically Reprograms Tumor-Initiating Stem-like Cells through Tumorigenic Changes in Oxidative Phosphorylation and Fatty Acid Metabolism. Cell Metab. 2016;23:206–219. doi: 10.1016/j.cmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vazquez F, Lim JH, Chim H, et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yajima T, Ochiai H, Uchiyama T, Takano N, Shibahara T, Azuma T. Resistance to cytotoxic chemotherapy-induced apoptosis in side population cells of human oral squamous cell carcinoma cell line Ho-1-N-1. Int J Oncol. 2009;35:273–280. [PubMed] [Google Scholar]
- 53.Zhang G, Frederick DT, Wu L, et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J Clin Invest. 2016;126:1834–1856. doi: 10.1172/JCI82661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee KM, Giltnane JM, Balko JM, et al. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017;26:633–647.e7. doi: 10.1016/j.cmet.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan AS, Baty JW, Dong LF, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21:81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017;3:169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oburoglu L, Tardito S, Fritz V, et al. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell. 2014;15:169–184. doi: 10.1016/j.stem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Kim JH, Lee KJ, Seo Y, et al. Effects of metformin on colorectal cancer stem cells depend on alterations in glutamine metabolism. Sci Rep. 2018;8:409. doi: 10.1038/s41598-017-18762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancini R, Noto A, Pisanu ME, De Vitis C, Maugeri-Sacca M, Ciliberto G. Metabolic features of cancer stem cells: the emerging role of lipid metabolism. Oncogene. 2018;37:2367–2378. doi: 10.1038/s41388-018-0141-3. [DOI] [PubMed] [Google Scholar]
- 60.Gupta VK, Banerjee S. Isolation of Lipid Raft Proteins from CD133+ Cancer Stem Cells. Methods Mol Biol. 2017;1609:25–31. doi: 10.1007/978-1-4939-6996-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babina IS, McSherry EA, Donatello S, Hill AD, Hopkins AM. A novel mechanism of regulating breast cancer cell migration via palmitoylation-dependent alterations in the lipid raft affiliation of CD44. Breast Cancer Res. 2014;16:R19. doi: 10.1186/bcr3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murai T, Maruyama Y, Mio K, Nishiyama H, Suga M, Sato C. Low cholesterol triggers membrane microdomain-dependent CD44 shedding and suppresses tumor cell migration. J Biol Chem. 2011;286:1999–2007. doi: 10.1074/jbc.M110.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tirinato L, Liberale C, Di Franco S, et al. Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem Cells. 2015;33:35–44. doi: 10.1002/stem.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo X, Cheng C, Tan Z, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16:76. doi: 10.1186/s12943-017-0646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, Condello S, Thomes-Pepin J, et al. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017;20:303–314 e5. doi: 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang T, Fahrmann JF, Lee H, et al. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27:136–150 e5. doi: 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito K, Carracedo A, Weiss D, et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pascual G, Avgustinova A, Mejetta S, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 69.Vlashi E, Lagadec C, Vergnes L, et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci U S A. 2011;108:16062–16067. doi: 10.1073/pnas.1106704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong C, Yuan T, Wu Y, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 72.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Dupuy F, Tabaries S, Andrzejewski S, et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015;22:577–589. doi: 10.1016/j.cmet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Peng F, Wang JH, Fan WJ, et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene. 2018;37:1062–1074. doi: 10.1038/onc.2017.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res. 2015;5:1602–1609. [PMC free article] [PubMed] [Google Scholar]
- 77.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamb R, Ozsvari B, Lisanti CL, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget. 2015;6:4569–4584. doi: 10.18632/oncotarget.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chae YC, Caino MC, Lisanti S, et al. Control of tumor bioenergetics and survival stress signaling by mitochondrial HSP90s. Cancer Cell. 2012;22:331–344. doi: 10.1016/j.ccr.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chae YC, Angelin A, Lisanti S, et al. Landscape of the mitochondrial Hsp90 metabolome in tumours. Nat Commun. 2013;4:2139. doi: 10.1038/ncomms3139. [DOI] [PMC free article] [PubMed] [Google Scholar]