Abstract
Niches are specialized microenvironments that regulate stem cells’ activity. The neural stem cell (NSC) niche defines a zone in which NSCs are retained and produce new cells of the nervous system throughout life. Understanding the signaling mechanisms by which the niche controls the NSC fate is crucial for the success of clinical applications. In a recent study, Sato and colleagues, by using state-of-the-art techniques, including sophisticated in vivo lineage-tracing technologies, provide evidence that endothelial amyloid precursor protein (APP) is an important component of the NSC niche. Strikingly, depletion of APP increased NSC proliferation in the subventricular zone, indicating that endothelial cells negatively regulate NSCs’ growth. The emerging knowledge from this research will be important for the treatment of several neurological diseases.
Keywords: neural stem cells, vascular niche, microenvironment, endothelial cells
INTRODUCTION
Tremendous advances have been made in our understanding of the signals that promote stem cell quiescence in various tissues (Birbrair and Frenette, 2016; Borges et al., 2017). Nevertheless, deciphering the complex mechanisms involved in this process may be very challenging in the brain. In a recent article in Development, Sato and colleagues revealed an important component of the neural stem cell (NSC) niche: an endothelial cell-derived molecule that keeps NSCs’ quiescence in the subventricular zone in vivo (Sato et al., 2017). The authors identified by in vitro assays soluble amyloid precursor protein (APP) as an extrinsic cue that suppresses NSCs’ growth, and enhances neurosphereforming ability, while retaining their multipotency. Furthermore, Sato and colleagues investigated the role of APPs on NSCs in vivo by using APP-null mouse model. The authors discovered that, in the absence of APP, NSCs’ proliferation in the subventricular zone increases, suggesting that APP is important for NSCs’ quiescence in their niche (Sato et al., 2017).
Additionally, using state-of-the-art lineage-tracing Cre/loxP-mediated technologies, the authors deleted APP specifically in endothelial cells, or in NSCs and astrocytes. These experiments revealed that endothelial cells, but not astrocytes, regulate NSCs’ proliferation in the subventricular zone via APP (Sato et al., 2017). This study uncovers an important molecular component of the NSC microenvironment: APP derived from endothelial cells.
The main findings from this study are based on the data obtained from Tie2-Cre/APP-floxed mice (Sato et al., 2017). It is known that Tie2-Cre mice exhibit Cre recombinase activity in both hematopoietic and endothelial cells (Tang et al., 2010). Thus, it is possible that the effect on NSCs could be due to a specific hematopoietic cell in which APP was deleted in Tie2-Cre/APP-floxed mice. To perform endothelial-specific gene targeting, a more specific mouse model should be used in future studies, i.e. VE-Cadherin-CreERT2 mice (Park et al., 2017). In VE-Cadherin-CreERT2/APP-floxed mice will be possible to temporally control APP expression in the endothelium. Interestingly, endothelial cells are heterogeneous in their distribution and function. Thus, endothelial cells may vary between different segments of the vasculature within the same organ (Aird, 2007; Paiva et al., 2017). It remains unknown, for instance, whether all endothelial cells are important as a niche for NSCs or a specific subtype (arteriolar, venular, or capillary). Deciphering the molecular differences of endothelial cells in the brain may bring novel concepts about their role as NSC niche cells.
As the molecular functions of proteins may depend on the specific area of the tissue in analysis in which they are produced, restricting gene manipulation to specific brain regions and time will be very useful in understanding the function of specific genes in complex microenvironments, such as the brain. In this scenario, the use of viral vectors to deliver transgenes, such as APP floxed, into CreER-bearing mice comes as a powerful tool. Viral vectors may integrate into both postmitotic and dividing cells, generating little or no immune response, and expressing stably these transgenes over several months (Lai and Brady, 2002).
In the adult brain, an arterial network covers its surface, and terminates in capillary beds; blood vessels supply the brain with oxygen and glucose, and assure that metabolic end products are removed to maintain tissue homeostasis (Anstrom et al., 2002). The subventricular zone is extensively vascularized by a rich plexus of blood vessels (Ihrie and Alvarez-Buylla, 2011). Vascular niches play important roles supporting stem cells in different organs (Kunisaki et al., 2013; Birbrairand Frenette, 2016; Khan et al., 2016; Asada et al., 2017; Borges et al., 2017; Lousado et al., 2017), including the brain (Goldberg and Hirschi, 2009). The vascular niches themselves are heterogeneous and contain distinct cell populations besides endothelial cells (Sena et al., 2017a,b), including vascular smooth muscle cells (Wanjare et al., 2013), perivascular microglia (Guillemin and Brew, 2004), perivascular adventitial cells (Crisan et al., 2012), perivascular fibroblasts (Soderblom et al., 2013), perivascular macrophages (Bechmann et al., 2001; Prazeres et al., 2017), and pericytes subpopulations (Almeida et al., 2017; Birbrair et al., 2017a,b, 2011, 2014a,b,c, 2013a,b,c,d, 2015; Birbrair and Delbono, 2015; Dias Moura Prazeres et al., 2017; Coatti et al., 2017; Santos et al., 2017). Interestingly, possibly perivascular macrophages were also targeted in Tie2-Cre/APP-floxed mice. Future studies will elucidate what is the relationship between distinct components of the vascular NSC niche. It remains unknown whether those other vascular cells also produce APP. Notice that APP is also produced in large quantities in neurons (Lee et al., 2008), and hypothalamic neurons are a key component of the SVZ NSC niche (Andreotti et al., 2017). Future studies should analyze the influence of neuronal-derived APP on NSCs. What effects does the endothelial APP produce on the other vascular cellular components of the niche? Is there a cross-talk between the different vascular components of the NSC microenvironment? Also, it will be essential and urgent to identify the receptor through which APP acts on NSCs. Possible candidates are lipoprotein receptors which interact with APP to control developmental processes (Pohlkamp et al., 2017). Interestingly, the APP itself was proposed to be able to act as a receptor (Deyts et al., 2016).
Importantly, the NSCs of the walls of the lateral ventricle are heterogeneous in their origin, transcriptional profiles and functions (Chaker et al., 2016). Little is known about the extrinsic regulation of NSCs’ subpopulations. For instance, it is unclear whether there are distinct niches in the subventricular zone. The functional heterogeneity of NSCs points to the potential for matching heterogeneity in the microenvironmental influences that support the function and behavior of these NSC subsets. Sato and colleagues analyzed the subventricular zone NSCs as one whole population (Sato et al., 2017). Nonetheless, it will be interesting in future experiments to distinguish the various NSC subsets, and determine whether they respond differently to APP.
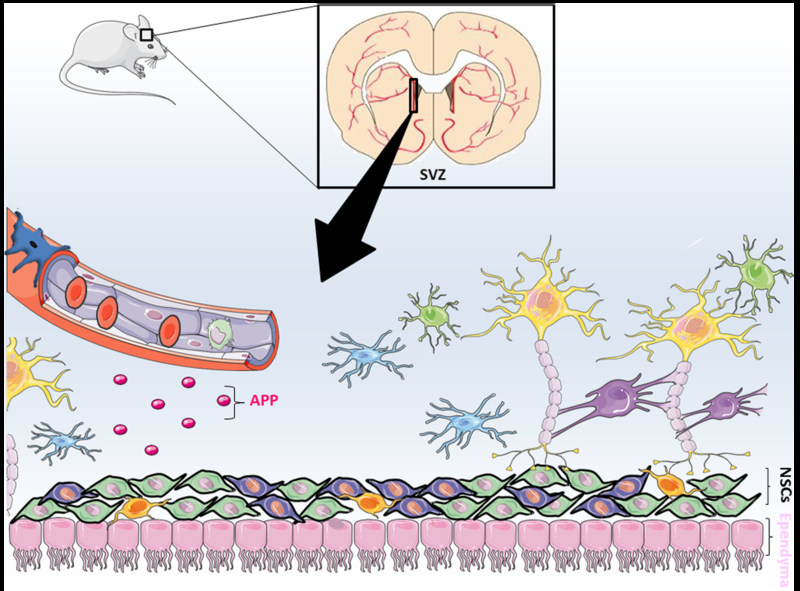
Additionally, neurogenesis in adults is not limited to the subventricular zone of the lateral ventricle as it has a niche also in the subgranular zone of the dentate gyrus in the hippocampus (Ming and Song, 2011). Adult NSCs of the subgranular zone proliferate and differentiate into neuroblasts that migrate to the granule layer of the dentate gyrus, and form mature granule cell neurons, which make functional synapses with other neurons of the hippocampal network. Hippocampal neurogenesis is strongly implicated in protection against cognitive dysfunction (Zhao et al., 2008). In Tie2-Cre/APP-floxed mice, recombinase expression should be also present in the hippocampal endothelium. Therefore, future studies will focus on investigating the role of endothelial cells in the hippocampal NSC niche. As endothelial cells produce several biologically active proteins, other molecules produced by the endothelium in vivo may also be important in the regulation of the NSCs’ niche. A big challenge for the future will be to translate animal research into humans Fig. 1.
Fig. 1.
Endothelial cells create a stem cell niche in the subventricular zone. Neural stem cell (NSC) niche is a specialized microenvironment that regulates NSCs’ activity. Sato and colleagues now suggest that endothelial cells present in the subventricular zone secrete soluble amyloid precursor protein (sAPP) which inhibits NSC proliferation (Sato et al., 2017).
Acknowledgments—
Alexander Birbrair is supported by a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); Akiva Mintz is supported by the National Institute of Health (1R01CA179072-01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13-121-01-CDD).
Abbreviations:
- APP
amyloid precursor protein
- NSC
neural stem cell
Footnotes
DISCLOSURES
The authors indicate no potential conflicts of interest.
REFERENCES
- Aird WC (2007) Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 100:174–190. [DOI] [PubMed] [Google Scholar]
- Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, Birbrair A (2017) Pericytes make spinal cord breathless after injury. Neuroscientist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti JP, Mesquita L, Magno LAV, Birbrair A (2017) Hypothalamic neurons take center stage in the neural stem cell niche. Cell Stem Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom JA, Brown WR, Moody DM, Thore CR, Challa VR, Block SM (2002) Anatomical analysis of the developing cerebral vasculature in premature neonates: absence of precapillary arteriole-to-venous shunts. Pediatr Res 52:554–560. [DOI] [PubMed] [Google Scholar]
- Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS (2017) Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R (2001) Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 14:1651–1658. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Delbono O (2015) Pericytes are essential for skeletal muscle formation. Stem Cell Rev 11:547–548. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Frenette PS (2016) Niche heterogeneity in the bone marrow. Ann N Y Acad Sci 1370:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O (2011) Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One 6:e16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013a) Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22:2298–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013b) Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 319:45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013c) Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2013d) Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol 305: C1098–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang Z-M, Messi ML, Mintz A, Delbono O (2014a) Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther 5:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2014b) Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci 6:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O (2014c) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307:C25–C38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2015) Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 128:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O (2017a) How plastic are pericytes? Stem Cells Dev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, Messi ML, Solingapuram Sai KK, Marini FC, Delbono O, et al. (2017b) Novel peripherally derived neural-like stem cells as therapeutic carriers for treating glioblastomas. Stem Cells Transl Med 6:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges IDT, Sena IFG, de Azevedo PO, Andreotti JP, de Almeida VM, de Paiva AE, Pinheiro Dos Santos GS, de Paula Guerra DA, Dias Moura Prazeres PH, Mesquita LL, et al. (2017b) Lung as a niche for hematopoietic progenitors. Stem Cell Rev Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaker Z, Codega P, Doetsch F (2016) A mosaic world: puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdiscip Rev Dev Biol 5:640–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavacana N, et al. (2017) Pericytes extend survival of ALS SOD1 mice and induce the expression of antioxidant enzymes in the murine model and in IPSCs derived neuronal cells from an ALS patient. Stem Cell Rev. 10.1007/s12015-017-9752-2. [DOI] [PubMed] [Google Scholar]
- Crisan M, Corselli M, Chen WC, Peault B (2012) Perivascular cells for regenerative medicine. J Cell Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyts C, Thinakaran G, Parent AT (2016) APP receptor? To be or not to be. Trends Pharmacol Sci 37:390–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro Dos Santos GS, Mintz A, et al. (2017) Pericytes are heterogeneous in their origin within the same tissue. Dev Biol 427:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JS, Hirschi KK (2009) Diverse roles of the vasculature within the neural stem cell niche. Regen Med 4:879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ (2004) Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol 75:388–397. [DOI] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A (2011) Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 70:674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, et al. (2016) Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. (2013) Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z, Brady RO (2002) Gene transfer into the central nervous system in vivo using a recombinant lentivirus vector. J Neurosci Res 67:363–371. [DOI] [PubMed] [Google Scholar]
- Lee J, Retamal C, Cuitino L, Caruano-Yzermans A, Shin JE, van Kerkhof P, Marzolo MP, Bu G (2008) Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. J Biol Chem 283:11501–11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lousado L, Prazeres PHDM, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, Filev R, Mintz A, Birbrair A (2017) Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva AE, Lousado L, Almeida VM, Andreotti JP, Santos GSP, Azevedo PO, Sena IFG, Prazeres PHDM, Borges IT, Azevedo V, et al. (2017) Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone. Neoplasia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DY, Lee J, Kim J, Kim K, Hong S, Han S, Kubota Y, Augustin HG, Ding L, Kim JW, et al. (2017) Plastic roles of pericytes in the blood-retinal barrier. Nat Commun 8:15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlkamp T, Wasser CR, Herz J (2017) Functional roles of the interaction of APP and lipoprotein receptors. Front Mol Neurosci 10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prazeres PHDM Almeida VM, Lousado L Andreotti JP, Paiva AE Santos GSP, Azevedo PO, Souto L, Almeida GG, Filev R, et al. (2017) Macrophages generate pericytes in the developing brain. Cell Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GSP, Prazeres PHDM, Mintz A, Birbrair A (2017) Role of pericytes in the retina. Eye. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Uchida Y, Hu J, Young-Pearse TL, Niikura T, Mukouyama YS (2017) Soluble APP functions as a vascular niche signal that controls adult neural stem cell number. Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena IFG, Prazeres P, Santos GSP, Borges IT, Azevedo PO, Andreotti JP, Almeida VM, Paiva AE, Guerra DAP, Lousado L, et al. (2017a) Identity of Gli1+ cells in the bone marrow. Exp Hematol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena IFG, Prazeres PHDM, Santos GSP, Borges IT, Azevedo PO, Andreotti JP, Almeida VM, Paiva AE, Guerra DAP, Lousado L, et al. (2017b) LepR+ cells dispute hegemony with Gli1+ cells in bone marrow fibrosis. Cell Cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, et al. (2013) Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 33:13882–13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Harrington A, Yang X, Friesel RE, Liaw L (2010) The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis 48:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjare M, Kusuma S, Gerecht S (2013) Perivascular cells in blood vessel regeneration. Biotechnol J 8:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660. [DOI] [PubMed] [Google Scholar]