Abstract
Parthenocarpy, or pollination-independent fruit set, is an attractive trait for fruit production and can be induced by increased responses to the phytohormone gibberellin (GA), which regulates diverse aspects of plant development. GA signaling in plants is negatively regulated by DELLA proteins. A loss-of-function mutant of tomato DELLA (SlDELLA), procera (pro) thus exhibits enhanced GA-response phenotypes including parthenocarpy, although the pro mutation also confers some disadvantages for practical breeding. This study identified a new milder hypomorphic allele of SlDELLA, procera-2 (pro-2), which showed weaker GA-response phenotypes than pro. The pro-2 mutant contains a single nucleotide substitution, corresponding to a single amino acid substitution in the SAW subdomain of the SlDELLA. Accumulation of the mutated SlDELLA transcripts in wild-type (WT) resulted in parthenocarpy, while introduction of intact SlDELLA into pro-2 rescued mutant phenotypes. Yeast two-hybrid assays revealed that SlDELLA interacted with three tomato homologues of GID1 GA receptors with increasing affinity upon GA treatment, while their interactions were reduced by the pro and pro-2 mutations. Both pro and pro-2 mutants produced higher fruit yields under high temperature conditions, which were resulted from higher fruit set efficiency, demonstrating the potential for genetic parthenocarpy to improve yield under adverse environmental conditions.
Introduction
Fruit set, the developmental transition of the ovary into fruit, is critical for determining yield in fruit-bearing crops. However, the efficiency of fruit set is often inhibited by a failure of pollination and/or fertilization due to unfavorable environmental conditions. For instance, low or high temperature stress during flower development inhibits pollen production and fertilization, leading to a critical reduction of the yield performance in tomato (Solanum lycopersicum)1–3. Parthenocarpy, the production of seedless fruit or pollination-independent fruit set, can potentially increase the efficiency of fruit set even under unfavorable conditions during reproductive phase; it is thus recognized as an attractive trait for many fruit crops to stabilize or even enhance yield performance. Despite its potential value, the adoption of parthenocarpy has nevertheless been limited to only some fruit crops including tomato, as it is usually accompanied with unfavorable traits, such as reduced fruit quality4–6. Therefore, the acquisition of novel genetic parthenocarpic resources without such unfavorable traits as well as understanding the molecular mechanism underlying parthenocarpy would broaden the possibility to breed novel parthenocarpic varieties.
There are a number of unambiguous pieces of evidence pointing to plant hormones such as auxin, gibberellin (GA) and cytokinin as positive regulators of fruit set initiation7–9. Indeed, the ovaries of naturally parthenocarpic tomato mutants including pat, pat-2 and pat-3/pat-4 contain high amounts of GAs10–12. GA is a tetracyclic diterpenoid that regulates diverse aspects of plant development including stem elongation, seed germination, the transition to flowering and fruit set; and a large body of evidence has demonstrated that the degree of GA responses results from the coordination between GA metabolism and its signaling pathway13. In higher plants, the GA signaling pathway is activated by the degradation of a negative regulator of GA signaling, known as DELLA, through the ubiquitin 26S proteasome pathway, thus triggering GA responses14. The DELLA protein consists of an N-terminal DELLA regulatory domain that is important for binding to the GIBBERELLIN INSENSITIVE DWARF 1 (GID1) GA receptors; and a C-terminal GRAS domain that is suggested to function in the repression of GA responses by interacting with downstream components14,15. The GRAS domain further consists of several conserved subdomains including LHR1, VHIID, LHR2, PFYRE and SAW; mutations in the GRAS domain often result in loss-of-function of DELLA that causes enhanced GA phenotypes. One exceptional gain-of-function mutation in the rice DELLA (slr1-d4) diminishes the stability of the GID1-GA-DELLA complex but retains the suppressor activity16.
In tomato, PROCERA/SlDELLA is believed to be the solo DELLA gene, and the loss-of-function procera (pro) mutation corresponding to a single nonsynonymous substitution in the GRAS domain of the SlDELLA displays enhanced GA phenotypes including parthenocarpy17,18. Therefore, this mutation is the subject of much attention in studies seeking to explore the mechanism of fruit set, as well as for breeding purposes. However, the pro mutant also shows some disadvantages for such a breeding program, such as elongated stem, reduction of flower number and an increase in malformed fruit production. Recently, other loss-of-function mutants of SlDELLA produced through targeted mutagenesis or transposon-based mutagenized populations were reported; those mutants showed even more severe phenotypes than pro19,20. In this study, we identified a new, milder hypomorphic allele of tomato DELLA, designated pro-2, whose mutation is located in the SAW subdomain of the GRAS domain. The pro-2 plants showed a potential for high yield performance in optimal (climate-controlled) and unfavorable reproductive conditions with less qualitative fruiting drawbacks compared to pro. Both pro and pro-2 mutations led to reduced interaction with homologues of the GID1 GA receptors, while the impact varied depending on the counterpart GID1 family members. These results indicate that the newly identified pro-2 mutant can be a candidate breeding resource for parthenocarpic varieties as well as a useful tool to analyze functional associations of tomato DELLA and specific GID1 family members in the GA response in developmental processes including fruit set.
Results
Identification of a new recessive mutant allelic to procera
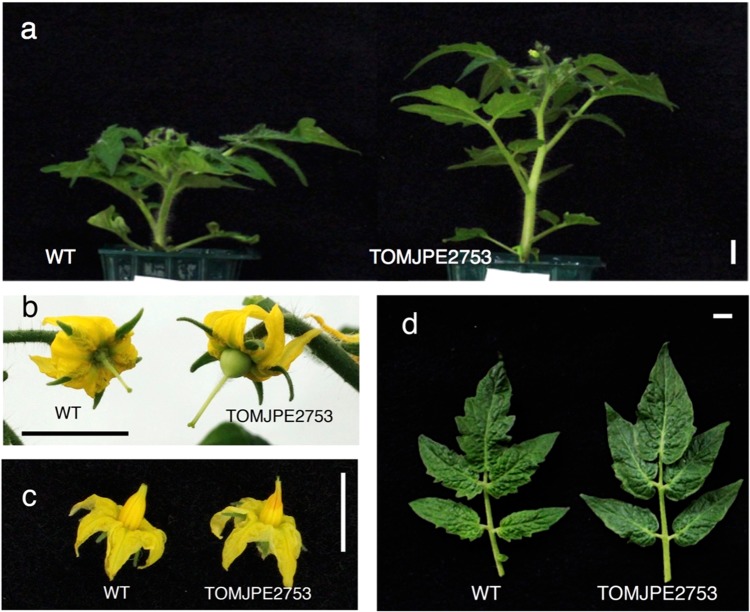
We have previously produced a large-scale collection of Micro-Tom mutagenized populations21,22. From the ethyl methanesulfonate (EMS)-treated mutagenized M2 populations grown in the greenhouse, a visual screening found a mutant (TOMJPE2753) exhibiting greater plant height than wild-type (WT) and parthenocarpy (Fig. 1a,b). This mutant produced flowers that contained an elongated style protruding from the anther (Fig. 1c), and leaflets with smoother margins (Fig. 1d) compared to the WT. These vegetative and reproductive phenotypes resembled those of a constitutive GA response mutant, procera (pro), conferred by a loss-of-function of SlDELLA17,18. We thus directly sequenced the SlDELLA gene in the TOMJPE2753 mutant and found a C to T single nucleotide transition, known as a major nucleotide substitution induced by EMS23, at the 1699th nucleotide (c1699t) from the translational start site. This transition results in a nonsynonymous amino acid mutation from Leu (L) to Phe (F) at the 567th position (L567F) in the SAW subdomain of the GRAS domain (Fig. 2a). The L567 in the SlDELLA corresponds to a highly conserved amino acid residue among homologous DELLA proteins of various species (Fig. 2b).
Figure 1.
Characteristic mutant phenotypes in the TOMJPE2753 line. (a) Six-week-old plants. (b) Parthenocarpic fruit initiation from emasculated flower in TOMJPE2753 at 4 days after anthesis. (c) Flowers with stamen and style. (d) Leaflets of the 6th node. Bars = 1 cm.
Figure 2.
A mutation in the SlDELLA SAW subdomain of the TOMJPE2753. (a) Schematic structure of SlDELLA. Each subdomain is indicated by white box. An L567F substitution in the TOMJPE2753 is indicated by black arrow head. The mutation site of a classic Sldella mutant, pro, in the VHIID subdomain is indicated by white arrow head. (b) Sequence comparison of the C terminus region of DELLA proteins from various plant species including Arabidopsis thaliana (AtGAI, AtRGA, At RGL1 and AtRGL2), Zea mays (ZmD8), Oryza sativa (SLR1, SLN1 and SLRL1), Triticum aestivum (RHT1) and Selaginella moellendorffii (SmDELLA1).
To examine whether the identified TOMJPE2753 mutant is allelic to the known pro mutant, allelism tests were conducted for the reproductive phenotypes in the greenhouse. Both TOMJPE2753 and pro in the Micro-Tom background produced flowers with elongated and protruding styles and parthenocarpic fruit (Supplementary Fig. S1). When the TOMJPE2753 was crossed with the pro mutant, the F1 plants also showed these phenotypes. The F1 progenies between WT and TOMJPE2753 or pro produced normal flowers (Supplementary Fig. S1a). The TOMJPE2753 and pro both showed high parthenocarpic rates of 75% and 81%, respectively (Supplementary Fig. S1b). The F1 plants between TOMJPE2753 and pro (TOMJPE2753 × pro) showed a high rate of parthenocarpic fruit formation (90%). Although WT and F1 plants between WT and TOMJPE2753 (TOMJPE2753 × WT) or pro (pro × WT) showed certain proportions of parthenocarpy (5, 8 and 20%, respectively) under our greenhouse condition, those values were clearly lower than the TOMJPE2753, pro or their F1 progeny (TOMJPE2753 × pro). These results revealed that the TOMJPE2753 and pro mutants are allelic, and that the mutation in TOMJPE2753 is recessive. Analysis of additional phenotypes of the TOMJPE2753 × WT F1 plants, including plant height and leaf shape (Supplementary Fig. S2), further supports that TOMJPE2753 is a recessive mutant.
The pro has been identified as a monogenic recessive mutant17. To further understand the genetic mode of inheritance of the allelic TOMJPE2753 mutant, qualitative and quantitative mutant phenotypes and their linkage with the c1699t mutation were investigated under greenhouse conditions. In the F2 progeny of TOMJPE2753 backcrossed with the WT, the segregation ratio of normal and mutant phenotype for the flower morphology was 31:13, which corresponded to the expected 3:1 for a single recessive gene at the 5% level by the chi-square test (χ2 = 0.485). A derived cleaved amplified polymorphic sequence (dCAPS) marker was designed to genotype the WT and c1699t mutation allele in the SlDELLA. The normal and mutant flower phenotypes were co-segregated in the F2 population with the homozygous WT or heterozygous alleles and the homozygous mutant allele of SlDELLA, respectively (data not shown). The averaged values of the quantitative plant height and parthenocarpic rate traits were compared among different genotypes. In the F2 progeny, the heterozygous plants exhibited a comparable height (Supplementary Table S1) and parthenocarpic rate (Supplementary Table S2) to plants with homozygous WT allele or the original Micro-Tom WT plants, while homozygous mutant plants showed significantly greater plant height and parthenocarpic rate. These results indicate that the monogenic recessive mutation in SlDELLA gene was tightly linked with vegetative and reproductive phenotypic alteration.
Transgenic complementation and induction of mutant phenotypes observed in TOMJPE2753
To confirm that the TOMJPE2753 is a new Sldella mutant, complementation rescue of the mutant phenotypes was conducted by introducing the WT SlDELLA under the control of its native promoter (pSlDELLA::SlDELLAWT) into TOMJPE2753 mutant plants. Initial screening at the T0 generation found that the plant heights of nine out of ten independent transgenic lines were normal, and three of them barely produced parthenocarpic fruit. Two screened independent lines, c6 and c7, with normal height and fruit production were selected for further analysis at the T2 generation. Compared to the azygous (AZ) sibling plants (c6-#5 and c7-#8) that do not carry transgene, morphological phenotypes, including plant height (Fig. 3a), leaf shape (Fig. 3b) and stylar length (Fig. 3c), in transgenic plants of both lines were restored and comparable with those of WT. The transgenic plants showed largely reduced parthenocarpic ability compared to the corresponding nontransgenic AZ plants in the TOMJPE2753 mutant background (Fig. 3d). These results demonstrate that SlDELLA is responsible for the mutant phenotypes in TOMJPE2753.
Figure 3.
Effect of SlDELLA expression on vegetative and reproductive development in TOMJPE2753 background. Representative pictures of (a) eight-week-old plants, (b) leaflets of the 6th node and (c) flowers of transgenic and nontransgenic azygous plants. (d) Parthenocarpic rate in transgenic lines. Two transgenic plants (c6-#2 and c6-#7) from line c6 and two transgenic plants (c7-#2 and c7-#6) from line c7 were used along with their corresponding azygous siblings c6-#5 and c7-#8, respectively. AZ, azygous. Bars = 1 cm.
In rice, the DELLA protein forms a homodimer and the overexpression of a loss-of-function DELLA with a domain required for dimerization but lacking the GRAS domain exerts a dominant-negative effect and makes rice plants slender due to increased GA sensitivity24. Since the TOMJPE2753 mutation locates within the GRAS domain, the mutant form (SlDELLAL567F) under the control of the 35S promoter (p35S::SlDELLAL567F) was introduced into the WT background plants to assess its dominant-negative effect. We generated three independent transgenic lines that showed parthenocarpy and two representative transgenic lines, m2 and m4, both of which were confirmed to express the mutated SlDELLAL567F transcripts and were thus selected for further analysis (Supplementary Fig. S3a). The transgenic plants carrying the transformation construct displayed mutant phenotypes observed in TOMJPE2753, including elongated plant stature (Supplementary Fig. S3b), attenuation of the serrated edge of the leaf (Supplementary Fig. S3c), longer style (Supplementary Fig. S3d) and parthenocarpic fruit formation (Supplementary Fig. S3e and f). These results indicate that ectopically expressed SlDELLAL567F can confer constitutive GA response phenotypes including parthenocarpy. Most likely, this is via an underlying dominant-negative mechanism that may mask normal function of endogenous SlDELLA.
Phenotypic comparison of the identified pro-2 with WT and pro mutant
The M3 population of the TOMJPE2753 mutant was backcrossed to WT Micro-Tom, followed by two self-pollinations, to eliminate the mutagen-induced background mutations and obtain a BC1S2 population harboring homozygous mutated SlDELLA, renamed here as procera-2 (pro-2), which was used for a further phenotypic comparison with WT and the pro mutant. The comparison of the vegetative development revealed that the pro-2 mutant was intermediate in height between the WT and pro mutant (Fig. 4a and b). The leaf margin of pro-2 was smoother compared to WT, but the phenotypic change was less severe than pro that completely lacked the serrated edge (Fig. 4c). It has been shown that the branching architecture is altered in pro, in which growth of axillary buds is highly repressed17,25. We found that the pro-2 produced axillary buds that grow to normal branches, which was barely found in pro, particularly at far nodes from apex (Fig. 4d). It has been shown that GA overproduction or increased GA signaling suppresses root growth, particularly the lateral roots, through interactions with auxin and other hormones in Populus26. Such a side effect was clearly observed in both pro and pro-2 (Fig. 4e).
Figure 4.

Vegetative and root phenotypes of pro-2 compared with WT and pro. (a) Representative 6-week-old plants. (b) Plant height at 6 weeks old. Values are mean ± SE (n = 5). Different letters indicate significant differences (P < 0.05, Tukey–Kramer test). (c) Representative leaves of 3rd to 7th node from cotyledons at 8 weeks old. (d) Representative axillary buds of 4th to 6th node at 9 weeks old. (e) Representative root growth at 6 weeks old. Scale bars = 1 cm.
Comparison of reproductive phenotypes in the optimal growth condition (climate controlled condition) showed that both Sldella mutants produced more leaves before the first inflorescence than WT (Table 1). On the other hand, the pro mutant produces a decreased number of flowers in a truss18, which was confirmed in our study (Table 1). The pro-2 also produced fewer flowers per truss than WT, but more than that in pro. Under spontaneous self-pollination condition, both pro and pro-2 produced more fruit than WT, which most likely resulted from higher fruit set rate, while most of the fruits were smaller and seedless, i.e. parthenocarpic (Table 1 and Fig. 5a). It is interesting, however, that pro-2 exhibited significantly higher yield than pro, resulting from higher number of fruits. Although pro and pro-2 displayed efficient parthenocarpy, these mutants also produced normal seeded fruit when they were manually pollinated (Fig. 5a), although the number of seeds were reduced to approximately half of WT (Table 1). Almost all fruits spontaneously produced by pro and pro-2 were seedless, most likely due to a failure in self-pollination due to the protruded stigma from the anther.
Table 1.
Comparison of reproductive phenotypes among WT, pro-2 and pro in the optimal condition.
| WT | pro-2 | pro | |
|---|---|---|---|
| Leaves to first inflorescence (n)z | 8.4 ± 0.3a | 10.2 ± 0.2b | 9.8 ± 0.2b |
| Flowers in the first two inflorescences (n)z | 14.4 ± 1.0a | 12.0 ± 0.4b | 9.6 ± 0.5c |
| Fruit per plant (n)z | 10.0 ± 0.3a | 32.6 ± 0.7c | 23.8 ± 1.8b |
| Fruit weight (g)y | 4.8 ± 1.5a | 1.9 ± 0.2b | 2.1 ± 0.2b |
| Fruit production (g per plant)z | 44.6 ± 2.7a | 61.2 ± 3.1b | 38.7 ± 3.2a |
| Seedless fruit (%)y | 0 | 89.1 | 100 |
| Seeds per manually pollinated fruit (n)x | 43.1 ± 5.6a | 19.7 ± 5.1b | 22.2 ± 4.6b |
zTen plants. yThirty to fifty fruit. xTen fruit. Values are mean ± SE. Different letters indicate significant differences (P < 0.05; Tukey–Kramer test). Pollination occurred through spontaneous self-pollination.
Figure 5.

Fruit phenotypes of WT, pro-2 and pro. (a) Representative unpollinated and manually pollinated fruit. Comparison of (b) Brix value and (c) sugar contents. (d) Representative orange patched pro fruit. (e) Comparison of carotenoid contents. Values are mean ± SE of (b) 20, (c) 3 and (e) 5 fruit. Different letters indicate significant differences (P < 0.05; Tukey–Kramer test). Poll, pollinated; Unpoll, unpollinated; ns, not significant. Scale bars = 1 cm.
A previous study showed that the Brix value, the indicative of levels of soluble solids content, of pro mature fruit is higher than that in WT18. We found that mature parthenocarpic fruit produced by both pro-2 and pro mutants had higher Brix values than seeded WT fruit (Fig. 5a,b). The quantitative analysis of sucrose, fructose and glucose in mature red fruit revealed that contents of glucose and fructose in these mutants were nine times and two to three times higher, respectively, than in WT (Fig. 5c). However, the sucrose content was comparable among the three genotypes, suggesting that the high Brix value was derived from high hexose content. In addition, we found that pro often produced mature fruit with an orange patch, which was barely observed in WT and pro-2 (Fig. 5d). This partially orange-coloration of fruits did not appear to be associated with β-carotene levels but rather with reduced levels of lycopene compared to WT pollinated and pro-2 parthenocarpic mature fruits that showed equivalent carotenoid contents (Fig. 5e).
High yield potential of Sldella mutants during a high temperature growth condition
Since parthenocarpy is considered to be a beneficial tool for improving the yield of fruit crops under severe environmental conditions9, WT and two Sldella mutants were used to investigate productivity in the greenhouse during four months of the summer period in year 2014. One-week-old seedlings of all genotypes were planted on June 1st and then mature ripe and immature fruits were harvested during the 1st week of September. The day and night temperature variation throughout the experiment is shown in Fig. 6a. After week 6 when all plants commenced flowering, the averaged daily mean temperature remained above 27 °C for each week with the exception of week 7 and two weeks before harvesting; and remained above 29 °C at weeks 8 through 10. The averaged day and night temperature for each week ranged from 26 to 33 °C and 23 to 27 °C, respectively. The vegetative growth phenotypes observed in the optimal (climate-controlled) conditions were all reproduced in this greenhouse condition, including the attenuated axillary branching in pro, which led to a decrease in the fresh weight of the aerial part of the plant, and the suppression of root growth (dry weight of root) in both Sldella mutants (Table 2 and Fig. 6b). The total number of flowers largely varied between WT and pro, while that of pro-2 was intermediate between WT and pro. The fruit set rate was calculated by the ratio of the number of fruits to total flowers per plant under spontaneous self-pollination condition and this was the highest in pro (87.9%) followed by pro-2 (51.8%) and was much smaller in WT (10.9%). The yield of each genotype was compared by the averaged fruit production per plant (Table 2). At harvesting, the immature fruit yield was similar among WT and Sldella mutants. Clearly, fruit formation in WT was severely suppressed and the average yield of the mature fruit was limited to 24.3 ± 2.9 g per plant, due to a failure in fruit set under the high temperature stress, although cultivation of WT under optimal conditions produced 44.6 ± 2.7 g per plant. The average yield in both Sldella mutants was 1.9 (pro) to 2.5 times (pro-2) higher than in WT; it was not associated with fruit weight but with increased number of fruit per plant. The pro-2 produced more fruit than pro, which was again most likely due to the less severe attenuation of axillary branching and flower production. The percentages of seedless fruit production by pro and pro-2 were 100% and 99.1%, respectively (Table 2). Unexpectedly, WT also produced seedless fruit under heat stress, but the ratio was much lower than Sldella mutants.
Figure 6.
Plants and fruit of WT, pro-2 and pro produced in greenhouse at high temperature. (a) Averaged daily, day (5 am to 7 pm) and night (7 pm to 5 am) mean temperature by each week. All plants opened the first flower during week 6 and vegetative and reproductive traits were quantified during week 14, which corresponds to during 6- and 14-week-old plant age, respectively. (b) Representative aerial plants at week 14. (c) Representative harvested fruit. Scale bars = 5 cm in panel b and 1 cm in panels c.
Table 2.
Vegetative and reproductive production of WT, pro-2 and pro in the greenhouse during summer.
| WT | pro-2 | pro | |
|---|---|---|---|
| Plant height (cm) | 11.8 ± 0.3a | 15.2 ± 0.5b | 17.9 ± 0.8c |
| Leaves to first inflorescence (n) | 8.0 ± 0.0a | 9.8 ± 0.4b | 9.3 ± 0.3b |
| Fresh weight of aerial part (g) | 46.1 ± 3.9a | 38.3 ± 3.2a | 27.7 ± 1.7b |
| Dry weight of root (g) | 0.39 ± 0.06a | 0.27 ± 0.03b | 0.23 ± 0.02b |
| Flowers per plant (n) | 95.0 ± 8.5a | 52.2 ± 5.5b | 23.5 ± 2.2c |
| Fruit set rate (%) | 10.9 ± 1.8a | 51.8 ± 1.9b | 87.9 ± 4.7c |
| Mature fruitz | |||
| Fruits per plant (n) | 4.4 ± 0.6a | 18.6 ± 1.2c | 11.8 ± 2.0b |
| Fruit weight (g) | 5.6 ± 0.3a | 3.3 ± 0.3b | 3.9 ± 0.2b |
| Fruit production (g per plant) | 24.3 ± 2.9a | 61.1 ± 2.8c | 45.5 ± 5.2b |
| Seedless fruit (%) | 20.0 | 99.1 | 100.0 |
| Immature fruit | |||
| Fruits per plant (n) | 5.6 ± 0.7ns | 7.4 ± 2.6ns | 6.4 ± 2.3ns |
| Fruit production (g per plant) | 10.9 ± 3.0ns | 8.8 ± 2.9ns | 10.4 ± 4.2ns |
| Malformed fruit | |||
| Fruits per plant (n) | 0.0 ± 0.0a | 1.0 ± 0.3b | 3.2 ± 0.6c |
| Malformed fruit per mature fruit (%) | – | 5.1y | 16.9x |
zNormal shape fruit fully or partially turned to red. yFive out of ninety-eight fruit.
xTwelve out of seventy-one fruit. All data were obtained from five plants of each genotype. Values are mean with or without ±SE. Different letters indicate significant differences (P < 0.05; Tukey–Kramer test). ns, not significant. Pollination occurred through spontaneous self-pollination.
One of the drawbacks of the use of pro for practical breeding is the production of malformed fruit with a bubbling structure at the tip of the fruit (Fig. 6c). Although both Sldella mutants produced such malformed fruit, its production rate was attenuated in the pro-2 mutant (5.1%) compared to the pro mutant (16.9%) (Table 2). In addition, most of the pro mature fruits have faded orange color (Fig. 6c). Such fruit was also found in pro-2 but with much less frequency, and barely found in WT (Fig. 6c).
Seedling growth of pro-2 showed moderate resistance to paclobutrazol
The loss-of-function of DELLA results in enhanced responses to endogenous GA and a decreasing sensitivity to the GA biosynthesis inhibitor paclobutrazol (PAC)18,20. To compare sensitivities to PAC, 3-day-old germinated seedlings of WT, pro-2 and pro were grown in MS medium with different concentrations of PAC (0, 1 and 10 µM) for 10 days, and then the lengths of the root and shoot were measured (Supplementary Fig. S4). Although the 1 µM PAC significantly inhibited the root growth in WT, its inhibitory effect was not observed in pro-2 and pro mutants. The 10 µM PAC treatment decreased the root length to a similar extent in all genotypes (Supplementary Fig. S4a). In WT, the shoot length of seedlings was also significantly decreased by 42% at the 1 µM PAC treatment compared to the control 0 µM PAC condition and no further reduction was found with 10 µM PAC. In contrast, the 1 µM PAC treatment decreased the shoot length of pro-2 and pro by 22% and 10%, respectively, compared to the control condition. The 10 µM PAC treatment further inhibited their shoot growth, while the shoot length in pro was taller than pro-2 seedlings grown with 1 or 10 µM PAC (Supplementary Fig. S4b). These results indicate that the seedling growth rates of these Sldella mutants were more resistant to PAC than WT, and that the pro-2 mutant showed intermediate sensitivity to the PAC between the WT and pro.
pro-2 and pro mutant proteins impact their interactions with SlGID1 family members
Previous studies showed that GA-dependent protein interaction between the DELLA domain and the GA receptor GID1 is further stabilized by the GRAS domain of DELLA16,27. We used yeast two-hybrid (Y2H) assays to investigate the effect of the SlDELLA mutations of pro-2 and pro on the basis of the interactions between SlDELLA and GID1 proteins. In the tomato genome, three GID1 family genes, consisting of two GID1b group members (SlGID1b-1 [Solyc09g074270] and SlGID1b-2 [Solyc06g008870]) and one GID1ac group member (SlGID1ac [Solyc01g098390]) have been identified28 (Supplementary Fig. S5a). We confirmed that these SlGID1s possess most of conserved amino acids essential for binding to GA or DELLA proteins29,30, suggesting that they are functional GA receptors (Supplementary Fig. S5b). Expression data based on transcriptome profiling of samples from various tissues31–33 showed that SlGID1b-1 and SlGID1b-2 exhibit higher expression levels than SlGID1ac across most of the tissues, including pistils/ovaries and young fruit (Supplementary Fig. S6).
The full-length SlDELLA protein was strongly auto-activated when it was fused with the GAL4 DNA-binding domain as a bait, which is consistent with previous reports in other plant species34 (Supplementary Fig. S7). SlDELLA and its mutant forms were thus fused with the GAL4 activating domain and used as a prey, and the three full-length SlGID1 proteins, SlGID1b-1, SlGID1b-2 and SlGID1ac, were used as bait in this study. The Y2H assay indicated that the normal SlDELLA (SlDELLAWT) could interact with all of these GID1 proteins, while the affinity varied depending on the combinations (Fig. 7). In the absence of GA, the yeast transformed with SlDELLAWT and each of the SlGID1s could grow in moderate selection medium without 3-AT application (Fig. 7a and b). SlGID1ac appeared to show the strongest interaction with the SlDELLAWT since the SlGID1ac-transformed yeast grew even under severely selective conditions (50 mM 3-AT), while the growth of yeast transformed with SlGID1b-1 or SlGID1b-2 was inhibited by the application of 3-AT (Fig. 7b). SlDELLAL567F corresponding to the pro-2 mutation could interact with SlGID1b-2 or SlGIDac, while SlDELLAV302E corresponding to the pro mutation did not show any interaction with SlGID1s.
Figure 7.
Effect of pro-2 and pro mutations in SlDELLA on interactions between SlDELLA and SlGID1 family members in yeast two-hybrid assays. (a) Growth of yeast strain PJ69-4A transformants on SD-LW (control) and SD-LWHA plates. The growth status of PJ69-4A transformants was also observed on SD-LWH plates containing different concentrations of 3-amino-1,2,4-triazole (3-AT) without (b) or with (c) 100 μM GA3. SlGID1 family members were used as bait, and SlDELLA and its mutants were used as prey. Pictures of plates incubated for 5 days at 30 °C are shown.
In the presence of GA, the interaction of SlGID1b-1 or SlGID1b-2 with SlDELLAWT was strengthened, as indicated by strong yeast growth even with 20 and 50 mM 3-AT application, while such a positive effect was not clearly evident for SlGID1ac compared with SlGID1b-1 or SlGID1b-2 (Fig. 7c). GA application enabled the SlDELLAL567F to interact with SlGID1b-1 in the absence of 3-AT, although their interaction was weaker than the SlDELLAWT-SlGID1b-1 interaction since the growth of yeast transformed with SlDELLAL567F and SlGID1b-1 was inhibited in the presence of 3-AT, unlike SlDELLAWT. Furthermore, the positive effect of GA application was not observed for the interaction between SlDELLAL567F and SlGID1b-2 or SlGID1ac. GA application promoted the interactions between SlDELLAV302E and the three SlGID1s but only under the condition without 3-AT application (Fig. 7c). It is of note that SlDELLAL567F interacted with all SlGID1, although SlDELLAV302E did not interact with any SlGID1 under the condition of 20 mM 3-AT in the presence of GA. These results suggested that both SlDELLAL567F and SlDELLAV302E reduced the capacity to interact with the three SlGID1s compared to SlDELLAWT; and SlDELLAL567F attained a higher affinity with the three SlGID1 than SlDELLAV302E.
Discussion
pro-2 is a novel and mild hypomorphic allele of SlDELLA gene
This study identified pro-2, a new mutant tomato allele of the GA signaling component, DELLA, whose mutation was localized in the SAW subdomain within the GRAS domain. Previous studies demonstrated that mutations in the SAW subdomain of rice DELLA (SLR1) lead to either reduced (slr1–2, slr1–3, slr1–4 and slr1–7) or increased (slr-d4) repression activity depending on the site and/or type of mutation16,35,36. The pro-2 mutant harbored a single amino acid substitution at the 567th Leu that is highly conserved within the DELLA proteins of plant species (Fig. 2b); and this conferred enhanced GA phenotypes similar to previously reported loss-of-function mutants of SlDELLA such as pro, proTALEN_2, proΔGRAS and SlDELLA-silenced plants using an antisense strategy17,19,20,37. This study demonstrated that the transgenic tomato plants in which the mutated SlDELLAL567F transcripts were expressed showed enhanced GA phenotypes including increased stem elongation, reduced leaf serration, stigma protrusion and parthenocarpy, as observed in the pro-2 mutant (Supplementary Fig. S3). Furthermore, introduction of the full length of the SlDELLA coding sequence under the control of the native promoter fully rescued the pro-2 phenotypes (Fig. 3). Therefore, the conserved Leu plays an important role for conferring repression activity of SlDELLA and its substitution to Phe was responsible for pro-2 mutant phenotypes.
The pro-2 mutant displayed milder GA-response phenotypes except for root growth, compared to the pro mutant (Fig. 4 and Table 1). Additionally, the pro-2 seedling was more sensitive to a GA biosynthesis inhibitor, PAC, compared to the pro seedlings as indicated by the shoot growth response (Supplementary Fig. S4). It has been previously reported that proΔGRAS or proTALEN_2, which completely lack the GRAS domain, show exaggerated GA-induced phenotypes as well as nearly complete resistance to PAC17,20. These and our results suggest that the pro-2 mutant is a novel weak hypomorphic Sldella allele.
pro-2 may reduce stabilization of the GA-GID1-DELLA complex
In the current model, the molecular mechanism of de-repression of the DELLA inhibitory effect is mediated through the reception of GA by GID1 followed by the formation of the GID1-GA-DELLA complex, which is subsequently recognized by the E3 ubiquitin ligase F-box protein that marks DELLA with ubiquitination and subsequent degradation, hence inducing the GA responses14. However, the role of the SAW subdomain in the GRAS domain’s repressor-related activity and interaction with GID1 is poorly understood in tomato.
Previously, Hirano et al.16 showed that an amino acid substitution of rice DELLA (SLR1), from Gly to Val at position 576, in the SAW subdomain reduced its interaction capacity with GID1 (OsGID1)-GA without affecting the repressor activity of SLR1, while the substitution of the Leu-Phe at positions 589–590 to Ala-Ala reduced both the affinity with GID1-GA and the repressor activity. In contrast, artificial residue substitutions by an Ala scanning experiment at another seven conserved positions in the SAW subdomain, including Arg at position 621 that corresponds to just two residues upstream from the pro-2 mutation, did not influence the OsGID1-GA-SLR1 interaction16, implying that the important amino acids in the SAW subdomain that stabilize the interaction are not widely scattered, but rather specifically localized. Our study reveals that the substitution of a highly conserved Leu at position 567 (L567) in the SAW subdomain to Phe (SlDELLAL567F) which corresponds to the pro-2 weak loss-of-function mutation, caused the reduction of the GA-independent and -dependent affinity of SlDELLA with two GID1 homologues, SlGID1b-1 and SlGID1b-2 (Fig. 7c). Furthermore, we found that SlDELLAV302E, which corresponds to the pro mutation located in the VHIID subdomain, also caused both the attenuation of the repressor activity and the reduction of the affinity with SlGID1 proteins. These data suggest that the highly conserved Val at the 302nd amino acid in the VHIID subdomain and the Leu at the 567th amino acid in SAW subdomain, both play important bifunctional roles in the stabilization of the DELLA-GA-GID1 complex and the repressor activity of DELLA.
This study also showed strong GA-independent interaction between WT SlDELLA and a GID1ac-group member (SlGID1ac), even under highly stringent conditions (50 mM 3-AT) (Fig. 7). Similarly, GA-independent interactions of GID1-DELLA have been reported in other species. For instance, Arabidopsis has two GID1ac- (AtGID1a and AtGID1c) and one GID1b-group member (AtGID1b), the latter of which can interact with DELLA proteins in the absence of GA38. Brassica and soybean GID1b-group members homologous to AtGID1b can interact with their own DELLA orthologues in the absence of GA, most likely through an unconserved loop region that may contribute to the stabilization of the GID1-GA interaction39. The present Y2H assays showed that GID1ac-group member SlGID1ac, rather than GID1b-group members, had a highly stable GA-independent interaction with SlDELLA (Fig. 7). It is possible that the strong stability of SlGID1ac-SlDELLA interaction is associated with the loop region whose sequence is well conserved between SlGID1b-1 and SlGID1b-2 but not between these and SlGID1ac (Supplementary Fig. S5b). Also, the L567F mutation did not influence the GA-independent interaction with SlGID1ac, whereas it reduced the interaction with SlGID1b-1 and SlGID1b-2 (Fig. 7). Furthermore, SlGIDac transcripts were much less abundant throughout tissues in tomato (Supplementary Fig. S6). These results suggested that the SlGID1b-1 and SlGID1b-2 mainly contribute to a de-repression mechanism of GA signaling.
High yield potential of pro-2 under heat stress condition through improved fruit set efficiency
In fruit crops, the number and weight of fruit essentially determine the yield and thus high fruit set efficiency is an important factor to achieve high yield performance. Since harsh temperature conditions often cause male sterility leading to a failure in fruit set initiation, fruit set induced by parthenocarpy could be useful to improve yield under such stressed conditions9. It has been reported that the daily mean temperature is particularly critical for fruit set, e.g., the setting rate and number of fruit were largely decreased from 25 °C to 27 °C and further declined at 29 °C2. In our greenhouse experiment, the daily mean temperature averaged by week remained above 27 °C after flowering at week 6, with the exception of week 7 and two weeks before harvesting, and above 29 °C at weeks 8 through 10. This temperature condition indeed suppressed pollination-dependent fruit production in WT, compared to the optimal condition (Tables 1 and 2). This experiment demonstrated that the two different alleles of Sldella mutants exhibited higher yield than WT under this natural heat stress condition (WT, 24.3 ± 2.9 g; pro-2, 61.1 ± 2.8 g; pro, 45.5 ± 5.2 g) (Table 2). Although the average fruit weight produced by Sldella mutants was lower than WT (WT, 5.6 ± 0.3 g; pro-2, 3.3 ± 0.3 g; pro, 3.9 ± 0.2 g), these Sldella mutants showed higher yield potential due to their higher fruit set rate through parthenocarpy (Table 2). This demonstrates the potential of genetic parthenocarpy under unfavorable growth conditions to enhance fruit productivity. Interestingly, although the average fruit weight produced by pro-2 mutant was equivalent to the pro mutant, the normal red fruit yield of the pro-2 mutant was higher than the pro mutant, which was also observed in the optimal condition (Table 1). This superior outcome of the milder pro-2 allele might be related to its higher number of flowers per plant, which is most likely due to less severe reduction in the number of flowers per truss and/or axillary branches (Table 1 and Figs 4d and 6b), rather than the fruit set rate that was higher in pro than pro-2 (Table 2), suggesting that the weaker Sldella allele is more suitable for a breeding program. Indeed, this study also demonstrated that pro-2 exhibited less unfavorable fruit and vegetative characteristics than the pro mutant, including a decreased rate of malformed fruit formation and the attenuation of increased stem elongation (Table 2 and Fig. 4b). Additionally, pro occasionally produced partially orange mature fruit that is associated with less lycopene content most likely resulting from decreased sensitivity to a ripening hormone ethylene due to the antagonistic effect of GA40; whereas the pro-2 mutant produced mature red fruit with the same level of lycopene accumulation as the WT (Fig. 5e). Since tomato fruit constitutes a major natural source of lycopene, considered to have a range of benefits to human health due to its strong antioxidant property41, the mild pro-2 allele could be more suitable than the pro allelic mutant from the view point of productivity as well as quality of the fruit.
Finally, we observed that mature red fruit of both Sldella mutants contained higher sugar contents estimated by Brix value, most likely due to higher levels of glucose and fructose (Fig. 5b and c), which are important components for the nutritional value and for the taste of the fruit. Thus, in terms of both yield and quality (representing market value), the use of Sldella mutants for breeding varieties with high sugar contents is attractive.
Here we isolated a new sldella mutant allele pro-2 that exhibited milder GA-induced phenotypes and a substantial potential for higher yield under heat stress condition compared to pro. These traits resulted from efficient parthenocarpy and most likely the attenuation of the drawbacks that accompany the severe GA responses found in pro. These results demonstrate the potential of the mild mutant allele for breeding of parthenocarpic tomato varieties. However, this study used cv. Micro-Tom as a genetic background which concomitantly harbors a mutation affecting brassinosteroid biosynthesis (dwarf) that possibly influences GA response42. Future study should test the effectiveness of the pro-2 allele in other genetic backgrounds. In addition, pro-2 still carries undesirable traits for breeding (i.e. small size of fruit and reduced seed numbers), although a gene pyramiding strategy using QTL that increases fruit size43 or PAC treatment to promote seed production from parthenocarpic plants44 may alleviate these drawbacks.
Methods
Plant materials and growth conditions
This study used tomato (Solanum lycopersicum) cv. Micro-Tom including the WT, the pro-2 mutant (line name TOMJPE2753) isolated from the previously developed ethyl methanesulfonate (EMS)-mutagenized populations21,45 and the pro mutant46. The M3 generation of the pro-2 mutant line was backcrossed to Micro-Tom WT followed by two self-pollinations, resulting in BC1S2 populations. The homozygous pro-2 allele of this population was used for phenotypic comparison experiments. Seeds were imbibed with deionized water overnight and placed on a filter paper moistened with deionized water for 4–6 d at 25 °C under 16 h light at 100 μmol m−2 s−1. For cultivation in a climate-controlled room (optimal condition), the germinated seedlings were transplanted into rockwool cubes (75 × 75 × 65 mm, Grodan Delta), except when otherwise stated, and grown with a nutrient solution with an electrical conductivity (EC) of 1.6 dS m−1 (Otsuka Chemical) in a photoperiod of 16 h light at 25 °C (light)/20 °C (dark) under fluorescent lights at 150–200 μmol m−2 s−1. To observe the roots grown under the optimal conditions, the plants were cultivated with soil in a cell plant tray (65 × 65 × 55 mm of each cell size). For cultivation in a greenhouse at University of Tsukuba (Tsukuba, Japan), seedlings were grown with the soil and tray, and 1000-fold diluted Hyponex (HYPONeX JAPAN Co., Ltd.) was supplied after the flowering stage twice a week. For the yield tests conducted at the greenhouse from June to September in 2014, WT, homozygous pro and homozygous pro-2 plants were grown in 15-cm pots and the temperature during cultivation was recorded using a data logger TR-72ui (T&D). In both the optimal and greenhouse conditions, flowers were spontaneously self-pollinated, except when otherwise stated, and the presence of seeds within fruit was examined. All selected flowers for the parthenocarpic test or for crossing with other genotypes were emasculated one day before anthesis to prevent self-pollination.
Sequencing and genotyping of the mutation
Genomic DNA of four pro-2 (two M3 and two M4 generation) and two WT plants was extracted from the fresh leaves using a Maxwell 16 DNA purification kit according to the manufacturer’s protocol (Promega). The extracted DNA was used as a template of PCR using specific primers (SlDELLA-orf-forward: ATGAAGAGAGATCGAGATCGAG; SlDELLA-orf-reverse: TTACAACTCGACTTCTCCGGC) and Ex Taq DNA polymerase (TaKaRa) to amplify the coding region of SlDELLA/PROCERA (Solyc11g011260). The SlDELLA PCR products were subjected to agarose gel electrophoresis, and then fragments were purified by the Wizard SV Gel and PCR Clean-Up system kit (Promega). The purified PCR products were used as a template for sequencing using PRISM 3130 (Applied Biosystems) coupled with BigDye Terminator v3.1 Cycle Sequencing Kit (Life technologies).
To determine the genotype of the SlDELLA mutation allele (c1669t) found in pro-2, primers for the derived cleaved amplified polymorphic sequence (dCAPS) analysis were designed (forward: GATTTGGTGATGTCGGAGGTTTATT; reverse: AGCTTCCAGGCGGAGGTAGCTTTAA) by a web-based program (dCAPS Finder 2.0; http://helix.wustl.edu/dcaps/)47 to yield a 284 bp PCR product. Genomic DNA was extracted as described above and used as a template of PCR using GoTaq Green Master Mix (Promega). Thermal cycling conditions were: 95 °C for 2 min followed by 35 cycles of 30 s at 95 °C, 30 s at 54 °C and 20 s at 72 °C, and finally 5 min at 72 °C. The PCR products were digested with DraI (New England Biolabs) and then electrophoresed using 2% agarose gel. DraI can digest only the product derived from the mutant allele, resulting in 261- and 23-bp fragments. The DraI-digested product of heterozygous allele plants thus resulted in three fragments, consisting of the 261-bp, the 23-bp and the 284-bp fragment derived from non-digested WT allele.
Creation of transgenic plants
For transgenic complementation experiments of the pro-2 mutation, the SlDELLA coding genomic region, which includes 2502 bp of upstream promoter sequences from the translation initiation site (without a stop codon) was synthesized and cloned into HindIII-SacI sites of 35S:MIR:HSP48 using the In-Fusion technique (TAKARA). The destination construct (pSlDELLA::SlDELLAWT) was designated to express WT SlDELLA, C-terminally tagged with HA and MYC, under the control of its native promoter. For the creation of transgenic plants expressing the aberrant form of the SlDELLA found in pro-2, the SlDELLA coding genomic region between the start and stop codons was amplified by PCR with SlDELLA-orf-forward and -reverse primers using genomic DNA extracted from pro-2 leaves. The PCR product was cloned into the entry vector pCR8/GW/TOPO (Invitrogen) by TA cloning and then sub-cloned into the pGWB15 vector49 using the Gateway LR Clonase enzyme (Invitrogen). The destination construct (p35S::SlDELLAL567F) was designed to express the pro-2-form mutated Sldella, N-terminally tagged with HA, under the control of 35S promoter.
These complete vectors were introduced into Agrobacterium tumefaciens strain GV2260 by electroporation. Micro-Tom pro-2 and WT were used as the transgenic background for the introduction of pSlDELLA::SlDELLAWT and p35S::SlDELLAL567F, respectively. Transformation of tomato plants was performed using the transformed A. tumefaciens50. Only diploid plants were selected from the regenerated plants that survived on MS plates containing the selection antibiotic, kanamycin (100 mg L−1). T2 and T1 generation plants harboring the pSlDELLA::SlDELLAWT and p35S::SlDELLAL567F, respectively, were used for further analyses. qRT-PCR was conducted51 using the leaves of the transgenic plants introduced with p35S::SlDELLAL567F and the azygous plants. Primer pairs were designed for a region spanning across SlDELLAL567F and the N-terminus HA-tag or conserved region between SlDELLAL567F and endogenous SlDELLA, to measure the expression level of exogenous SlDELLAL567F or encompassing both SlDELLAL567F and endogenous SlDELLA transcripts, respectively. The CAC gene was used as a reference and the expression level was normalized to the maximum expression within the test.
Measurement of Brix value and sugar content
Soluble solids content (Brix values) in the juice of cut fruit were measured with a Brix refractometer (Atago Co., Ltd.). The contents of hexoses were measured in the soluble fraction of an ethanol extract based on methods described previously52–54. Briefly, aliquots of about 20 mg fresh weight of fruit pericarp were fractionated in 96-well microplates using a Star pipetting robot (Hamilton). Soluble sugars were then measured in the supernatant by measuring the absorbance at 340 nm in an MP96 microplate reader (SAFAS), in the presence of glucose-6-phosphate dehydrogenase coupled to hexokinase, phosphoglucose isomerase and invertase, which were sequentially added to determine glucose, fructose, and sucrose contents, respectively.
Measurement of carotenoid content
The β-carotene and lycopene contents of each fruit were measured based on the method described by Nagata and Yamashita55. Briefly, the frozen fruit pericarp was ground into a fine powder in liquid nitrogen. Carotenoids were then extracted with acetone–hexane (4:6, v/v), and the resulting clear supernatant was used for measurement. The absorbance values at 663 nm (A663), 645 nm (A645), 505 nm (A505) and 453 nm (A453) were measured using a spectrophotometer (ND-2000C, Thermo Fisher Scientific), and the contents of β-carotene (CCAR) and lycopene (CLYC) were calculated from the following equation:
Paclobutrazol treatment
Seeds were sterilized with 10% commercial bleach including a detergent (Kitchen Haiter, Kao) for 20 min and then rinsed with sterile water three times for 5 min each. The seeds were imbibed in sterile water with gentle rotation for 3 d. The germinated seeds with emerged root of 4–6 mm were grown in 1/2 MS medium56 containing 0, 1 or 10 μM paclobutrazol (PAC) (Sigma-Aldrich). The lengths of root and shoot of the grown seedlings were measured at 12 days after planting.
Yeast two hybrid assay
Two Gateway-compatible vectors, pDEST32-HA (bait) with HA-tag and pDEST22-FLAG (prey) with 3xFLAG-tag in the upstream region of the Gateway cassette were used for the yeast two hybrid (Y2H) assay57. Full-length open reading frames of WT SlDELLA proteins (SlDELLAWT), two mutated-type (SlDELLAL567F, SlDELLAV302E) and three GID1-like proteins (SlGID1b-1, SlGID1b-2 and SlGID1ac) were cloned into the entry vector pCR8/GW/TOPO using the In-Fusion system (TaKaRa). The fragments of SlDELLA proteins were then sub-cloned into the pDEST22-FLAG vector that harbors an activation domain (AD) to generate prey vectors via the LR reaction according to the manufacturer’s instructions (Thermo Fisher Scientific). The fragments of three GID1-like proteins were sub-cloned into the pDEST32-HA plasmid that harbors the DNA-binding (DB) domain to generate bait vectors via the LR reaction. Yeast strain PJ69-4A was transformed with a total of 16 possible combinations of DB and AD destination vectors to assess GAL4-based protein-protein interactions (PPIs)58. Cells were grown on SD-Trp-Leu (SD-WL) plates, which lack Trp and Leu, at 30 °C for 4 d. Individual colonies were picked from the plates, streaked to another SD-WL plate and grown at 30 °C for 3 d. Grown cells were suspended in a 0.9% NaCl solution and diluted (optical density at 600 nm = 0.05). Diluted cultured cells were spotted on following plates: (1) SD-WL plates, (2) SD-WLHA plates that additionally lack His and Ade, (3) SD-WLH plates that contain Ade but lack His with 0, 20 or 50 mM 3-amino-1,2,4-triazole (3-AT) and (4) SD-WLH + GA that additionally contains 100 μM GA3. Spotted yeast cells were grown at 30 °C for 3–5 d to evaluate PPIs. The 3-AT was used as a competitive inhibitor of reporter HIS3, an enzyme required for His biosynthesis and the growth of yeast cells on the selection medium in this system.
Electronic supplementary material
Acknowledgements
This work was supported by JSPS KAKENHI, grant no. 15KK0273, Program to Disseminate Tenure Tracking System, and JSPS bilateral program to T.A., Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry, Japan (grant no. 26013A) to H.E. and T.A., a grant from the Japan Society for the Promotion of Science to Y.S. (16J00582) and K.E. (16J00797), and research grants from US Department of Agriculture to T.-p.S. (USDA 2014-67013-21548 and 2018-67013-27395). C.B. and Y.G. acknowledge funding by PHENOME-ANR-INBS-0012. Micro‐Tom WT (TOMJPF00001) and pro-2 (TOMJPE2753) were obtained from the National BioResource Project, Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and Micro-tom pro was kindly provided from Dr. Lázaro Eustáquio Pereira Peres.
Author Contributions
Y.S., Y.O. and T.A. contributed to the mutant screening; Y.S. performed phenotypic, physiological and molecular biological characterization of plants; Y.S., C.B., D.P. and Y.G. contributed to sugar measurement; K.E., Y.S., J.H. and T.-p.S. contributed to Y2H assays; Y.S., K.E., T.A. and H.E. wrote the manuscript. All authors reviewed and approved the final manuscript.
Patents have been filed related to the identified mutant as numbered PCT/JP2014/079552 and JP 2013-231495.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yoshihito Shinozaki and Kentaro Ezura contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30502-w.
References
- 1.Charles WB, Harris RE. Tomato fruit-set at high and low temperatures. Can. J. Plant Sci. 1972;52:497–506. doi: 10.4141/cjps72-080. [DOI] [Google Scholar]
- 2.Peet MM, Willits DH, Gardner R. Response of ovule development and post-pollen production processes in male-sterile tomatoes to chronic, sub-acute high temperature stress. J. Exp. Bot. 1997;48:101–111. doi: 10.1093/jxb/48.1.101. [DOI] [Google Scholar]
- 3.Sato S, Peet MM, Thomas JF. Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant Cell Environ. 2000;23:719–726. doi: 10.1046/j.1365-3040.2000.00589.x. [DOI] [Google Scholar]
- 4.Varoquaux F, Blanvillain R, Delseny M, Gallois P. Less is better: new approaches for seedless fruit production. Trends Biotechnol. 2000;18:233–242. doi: 10.1016/S0167-7799(00)01448-7. [DOI] [PubMed] [Google Scholar]
- 5.Pandolfini T, Rotino GL, Camerini S, Defez R, Spena A. Optimisation of transgene action at the post-transcriptional level: high quality parthenocarpic fruits in industrial tomatoes. BMC Biotechnol. 2002;2:1. doi: 10.1186/1472-6750-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinozaki, Y. & Ezura, K. Tomato fruit set and its modification using molecular breeding techniques in Functional Genomics and Biotechnology in Solanaceae and Cucurbitaceae Crops. (eds Ezura, H., Ariizumi, T., Garcia-Mas, J. & Rose, J.) 93–112 (Springer Berlin Heidelberg, 2016).
- 7.Gillaspy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan Y-L, Patrick JW, Bouzayen M, Osorio S, Fernie AR. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012;17:656–665. doi: 10.1016/j.tplants.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Ariizumi T, Shinozaki Y, Ezura H. Genes that influence yield in tomato. Breed. Sci. 2013;63:3–13. doi: 10.1270/jsbbs.63.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fos M, Nuez F, Garcia-Martinez JL. The gene pat-2, which induces natural parthenocarpy, alters the gibberellin content in unpollinated tomato ovaries. Plant Physiol. 2000;122:471–480. doi: 10.1104/pp.122.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fos M, Proano K, Nuez F, Garcia-Martinez JL. Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat‐3/pat‐4 in tomato. Physiol. Plant. 2001;111:545–550. doi: 10.1034/j.1399-3054.2001.1110416.x. [DOI] [PubMed] [Google Scholar]
- 12.Olimpieri I, et al. Tomato fruit set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis. Planta. 2007;226:877–888. doi: 10.1007/s00425-007-0533-z. [DOI] [PubMed] [Google Scholar]
- 13.Richards DE, King KE, Ait-Ali T, Harberd NP. How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:67–88. doi: 10.1146/annurev.arplant.52.1.67. [DOI] [PubMed] [Google Scholar]
- 14.Sun T-p, Gubler F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004;55:197–223. doi: 10.1146/annurev.arplant.55.031903.141753. [DOI] [PubMed] [Google Scholar]
- 15.Pysh LD, Wysocka-Diller JW, Camilleri C, Bouchez D, Benfey PN. The GRAS gene family in Arabidopsis: sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999;18:111–119. doi: 10.1046/j.1365-313X.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- 16.Hirano K, et al. Characterization of the Molecular Mechanism Underlying Gibberellin Perception Complex Formation in Rice. Plant Cell. 2010;22:2680–2696. doi: 10.1105/tpc.110.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassel GW, Mullen RT, Bewley J. D. Procera is a putative DELLA mutant in tomato (Solanum lycopersicum): effects on the seed and vegetative plant. J. Exp. Bot. 2008;59:585–593. doi: 10.1093/jxb/erm354. [DOI] [PubMed] [Google Scholar]
- 18.Carrera E, Ruiz-Rivero O, Peres LEP, Atares A, Garcia-Martinez JL. Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 2012;160:1581–1596. doi: 10.1104/pp.112.204552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lor VS, Starker CG, Voytas DF, Weiss D, Olszewski NE. Targeted mutagenesis of the tomato PROCERA gene using transcription activator-like effector nucleases. Plant Physiol. 2014;166:1288–1291. doi: 10.1104/pp.114.247593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livne S, et al. Uncovering DELLA-independent gibberellin responses by characterizing new tomato procera mutants. Plant Cell. 2015;27:1579–1594. doi: 10.1105/tpc.114.132795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito T, et al. TOMATOMA: a novel tomato mutant database distributing Micro-Tom mutant collections. Plant Cell Physiol. 2011;52:283–296. doi: 10.1093/pcp/pcr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shikata M, et al. TOMATOMA update: phenotypic and metabolite information in the micro-tom mutant resource. Plant Cell Physiol. 2016;57:e11–e11. doi: 10.1093/pcp/pcv194. [DOI] [PubMed] [Google Scholar]
- 23.Greene EA, et al. Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics. 2003;164:731–740. doi: 10.1093/genetics/164.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh H. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell. 2002;14:57–70. doi: 10.1105/tpc.010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lombardi-Crestana S, et al. The tomato (Solanum lycopersicum cv. Micro-Tom) natural genetic variation Rg1 and the DELLA mutant procera control the competence necessary to form adventitious roots and shoots. J. Exp. Bot. 2012;63:5689–5703. doi: 10.1093/jxb/ers221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gou J, et al. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell. 2010;22:623–639. doi: 10.1105/tpc.109.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T, et al. Expression and purification of a GRAS domain of SLR1, the rice DELLA protein. Protein Expr. Purif. 2014;95:248–258. doi: 10.1016/j.pep.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Voegele A, Linkies A, Müller K, Leubner-Metzger G. Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. J. Exp. Bot. 2011;62:5131–5147. doi: 10.1093/jxb/err214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada A, et al. Structural basis for gibberellin recognition by its receptor GID1. Nature. 2008;456:520–523. doi: 10.1038/nature07546. [DOI] [PubMed] [Google Scholar]
- 30.Hirano K, Ueguchi-Tanaka M, Matsuoka M. GID1-mediated gibberellin signaling in plants. Trends Plant Sci. 2008;13:192–199. doi: 10.1016/j.tplants.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature485, 635—641 (2012). [DOI] [PMC free article] [PubMed]
- 32.Zhang S, et al. Spatiotemporal transcriptome provides insights into early fruit development of tomato (Solanum lycopersicum) Sci. Rep. 2016;6:23173. doi: 10.1038/srep23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ezura K, et al. Genome-wide identification of pistil-specific genes expressed during fruit set initiation in tomato (Solanum lycopersicum) PLoS ONE. 2017;12:e0180003. doi: 10.1371/journal.pone.0180003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Lucas M, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda A, et al. Slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirano K, et al. The suppressive function of the rice DELLA protein SLR1 is dependent on its transcriptional activation activity. Plant J. 2012;71:443–453. doi: 10.1111/j.1365-313X.2012.05000.x. [DOI] [PubMed] [Google Scholar]
- 37.Martí C, et al. Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 2007;52:865–876. doi: 10.1111/j.1365-313X.2007.03282.x. [DOI] [PubMed] [Google Scholar]
- 38.Griffiths J, et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell. 2006;18:3399–3414. doi: 10.1105/tpc.106.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto Y, et al. A ricegid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins. Plant Cell. 2010;22:3589–3602. doi: 10.1105/tpc.110.074542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dostal HC, Leopold AC. Gibberellin delays ripening of tomatoes. Science. 1967;158:1579–1580. doi: 10.1126/science.158.3808.1579. [DOI] [PubMed] [Google Scholar]
- 41.Shi J, Kakuda Y, Yeung D. Antioxidative properties of lycopene and other carotenoids from tomatoes: synergistic effects. Biofactors. 2004;21:203–210. doi: 10.1002/biof.552210141. [DOI] [PubMed] [Google Scholar]
- 42.Martí E, Gisbert C, Bishop GJ, Dixon MS, Garcia-Martinez JL. Genetic and physiological characterization of tomato cv. Micro-Tom. J. Exp. Bot. 2006;57:2037–2047. doi: 10.1093/jxb/erj154. [DOI] [PubMed] [Google Scholar]
- 43.Monforte AJ, Diaz A, Caño-Delgado A, van der Knaap E. The genetic basis of fruit morphology in horticultural crops: lessons from tomato and melon. J. Exp. Bot. 2014;65:4625–4637. doi: 10.1093/jxb/eru017. [DOI] [PubMed] [Google Scholar]
- 44.Ohkawa H, Sugahara S, Oda M. Seed formation promoted by paclobutrazol, a gibberellin biosynthesis inhibitor, in pat-2 parthenocarpic tomatoes. J. Japan. Soc. Hort. Sci. 2012;81:177–183. doi: 10.2503/jjshs1.81.177. [DOI] [Google Scholar]
- 45.Okabe Y, et al. Tomato TILLING technology: development of a reverse genetics tool for the efficient isolation of mutants from Micro-Tom mutant libraries. Plant Cell Physiol. 2011;52:1994–2005. doi: 10.1093/pcp/pcr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carvalho RF, et al. Convergence of developmental mutants into a single tomato model system: ‘Micro-Tom’ as an effective toolkit for plant development research. Plant Methods. 2011;7:18. doi: 10.1186/1746-4811-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neff MM, Turk E, Kalishman M. Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 2002;18:613–615. doi: 10.1016/S0168-9525(02)02820-2. [DOI] [PubMed] [Google Scholar]
- 48.Hirai T, et al. The HSP terminator of Arabidopsis thaliana induces a high level of miraculin accumulation in transgenic tomatoes. J. Agric. Food Chem. 2011;59:9942–9949. doi: 10.1021/jf202501e. [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa T, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 50.Sun H-J, Uchii S, Watanabe S, Ezura H. A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 2006;47:426–431. doi: 10.1093/pcp/pci251. [DOI] [PubMed] [Google Scholar]
- 51.Shinozaki Y, et al. Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. Plant J. 2015;83:237–251. doi: 10.1111/tpj.12882. [DOI] [PubMed] [Google Scholar]
- 52.Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M. Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta. 1992;188:238–244. doi: 10.1007/BF00216819. [DOI] [PubMed] [Google Scholar]
- 53.Geigenberger P, Lerchi J, Stitt M, Sonnewald U. Phloem-specific expression of pyrophosphatase inhibits long distance transport of carbohydrates and amino acids in tobacco plants. Plant Cell Environ. 1996;19:43–55. doi: 10.1111/j.1365-3040.1996.tb00225.x. [DOI] [Google Scholar]
- 54.Biais B, et al. Remarkable reproducibility of enzyme activity profiles in tomato fruits grown under contrasting environments provides a roadmap for studies of fruit metabolism. Plant Physiol. 2014;164:1204–1221. doi: 10.1104/pp.113.231241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi. 1992;39:925–928. doi: 10.3136/nskkk1962.39.925. [DOI] [Google Scholar]
- 56.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 57.Hu, J., Israeli, A., Ori, N. & Sun, T.-p. The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell; 10.1105/tpc.18.00363 (2018). [DOI] [PMC free article] [PubMed]
- 58.James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.