Abstract
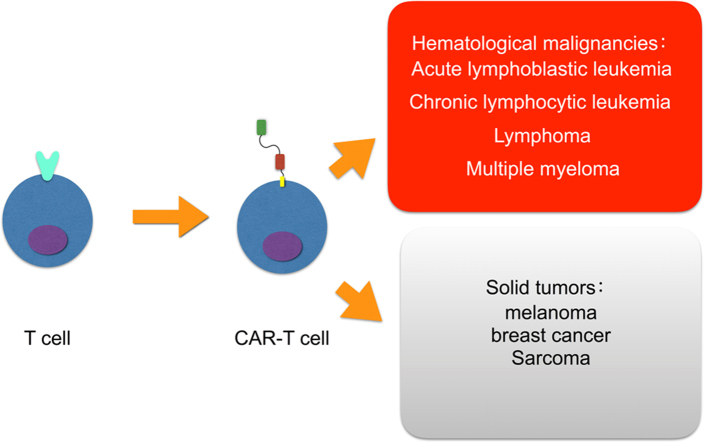
Chimeric antigen receptor T cell (CAR-T cell) therapy is a novel adoptive immunotherapy where T lymphocytes are engineered with synthetic receptors known as chimeric antigen receptors (CAR). The CAR-T cell is an effector T cell that recognizes and eliminates specific cancer cells, independent of major histocompatibility complex molecules. The whole procedure of CAR-T cell production is not well understood. The CAR-T cell has been used predominantly in the treatment of hematological malignancies, including acute lymphoblastic leukemia, chronic lymphocytic leukemia, lymphoma, and multiple myeloma. Solid tumors including melanoma, breast cancer and sarcoma offer great promise in CAR-T cell research and development. CD19 CAR-T cell is most commonly used, and other targets, including CD20, CD30, CD38 and CD138 are being studied. Although this novel therapy is promising, there are several disadvantages. In this review we discuss the applications of CAR-T cells in different hematological malignancies, and pave a way for future improvement on the effectiveness and persistence of these adoptive cell therapies.
KEY WORDS: Chimeric antigen receptor T cell, Clinical applications, Immunotherapy, Malignancies
Graphical abstract
Chimeric antigen receptor T cells (CAR-T cells) are effector T cells processed from natural T cells by replacing the T cell receptor (TCR) part to CAR part which can recognize and eliminate tumor cells specifically. Their application has been made great progress in hematological malignancies, whereas various difficulties should be overcame in treating solid tumors. This review mainly focuses on the applications of CAR-T cells in different hematological malignancies.
1. Introduction
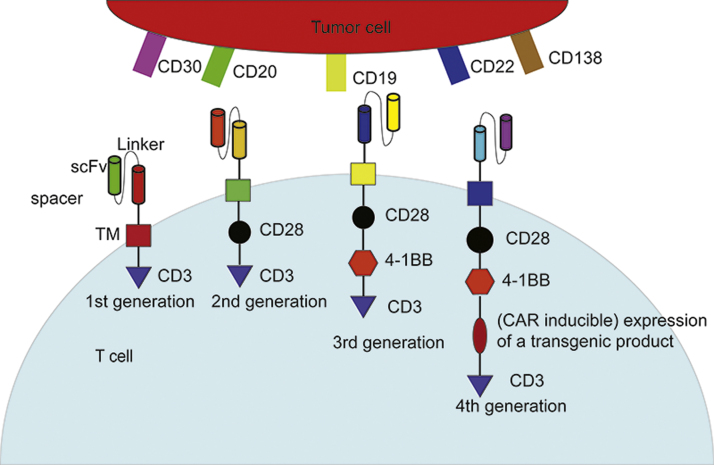
T lymphocyte cells (T cells) play a key role in cell-mediated immune response. These cells are involved in monitoring and killing tumor cells or potentially malignant cells. During past years many therapies have been developed to culture, redirect, and/or enhance T cells against tumors1. Among them is the T cell-based adoptive immunotherapy, which is developing new means to deal with malignancies, especially hematologic cancers. This emerging therapy includes three models: tumor infiltrating lymphocytes, T cell receptor (TCR)-modified T cells and chimeric antigen receptor T cells (CAR-T cell)2. The first two techniques, compared with CAR-T therapy do not make a huge modification of the T cell per se, and therefore the efficacy is not substantial. Also, the process of production, the poor success rate, and the dependence upon vaccination limit their development3, 4. As a promising therapeutic regimen, CAR-T cell therapy has stood the test of time for over 25 years5. Its TCR part is replaced by CAR which includes two domains: an extracellular and an intracellular domain. The extracellular domain is typically an antibody single-chain fragment (scFv) specifically against a cell surface antigen, while the intracellular domain includes fused signaling domains from a natural TCR complex and costimulatory molecules6, 7. Different intracellular sections represent various CAR-T cell generations. The structure ranges from CD3z signaling domain alone in first generation CARs (lack of costimulatory signal) to those that possess the signaling endo-domains of costimulatory molecules like CD28, CD134 (OX40) or CD137 (4-1BB), which are fused with CD3z, in second and third generation CARs (Fig. 1). This structure imitates the costimulation signal when TCR combines with antigen-presenting cells to complete the process of activation6, 8, 9. All of these generate CAR-T cell specificity for a certain type of cancer cell and leads to their elimination10. Because a monoclonal antibody against a tumor antigen offers novel T cell specificity for certain types of cancer cells and bypasses the established antigen-presenting process, an important strength of this method is that the recognition is independent of the major histocompatibility complex6, 11. Nevertheless, a novel generation of CARs has proven attractive to scientists. In addition to costimulatory signal(s) like CD28 and (or) CD137, this so-called “fourth generation of CARs” is also equipped with a “nuclear factor of activated T cell-responsive expression” element for an inducible transgenic product like IL-12 or other cytokine (Fig. 1). The specific recognition of a carcinogenic target by CAR-CD3 signaling stimulates the nuclear factor of activated T cell minimal promoter so IL-12 production and release result12. To avoid interaction between the promoter of CAR and inducible box, the two trans-genes are separated into different genomic sites12. The newest version is being tested in solid tumors, but current records of clinical trials are insufficient.
Figure 1.
Illustration of basic structure of 4 generations of chimeric antigen receptor T cells (CAR-T cell) and common targets on tumor cells. The whole structure of CARs consisted of an antibody single-chain fragment (scFv, extracellular segment) specifically against a cell surface antigen as well as one or several fused signaling domain(s) from natural TCR complex and costimulatory molecules (intracellular segment). Different intracellular segments represent various CAR-T cell generations. scFv, single-chain fragment. TM, transmembrane region.
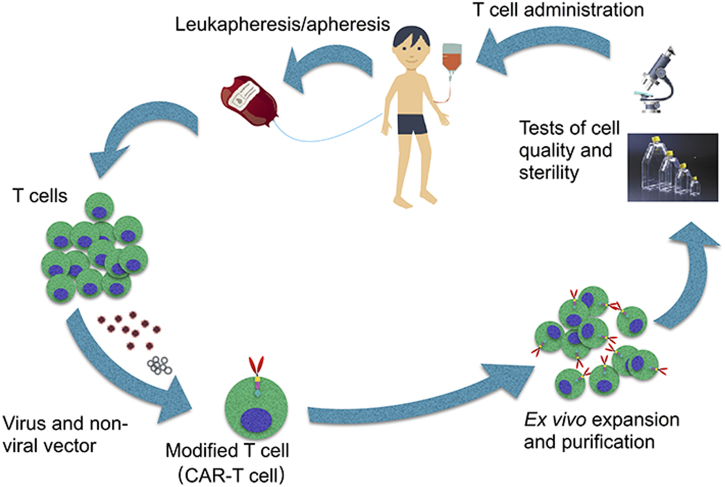
The whole procedure of CAR-T cell production is complicated13 (Fig. 2). Firstly, T cells from peripheral blood are collected by phlebotomy or leukapheresis, followed by apheresis without addition of granulocyte colony stimulating factor14. The reason why granulocyte colony stimulating factor is excluded is that it may disrupt T-cell proliferation and responsiveness15, 16. The separated T cells then are transfected with a CAR viral (retroviral or lentiviral) or nonviral vector, where a section of genome DNA is inserted artificially14, 16. T-cell ex vivo expansion and purification is the subsequent and key step, determining the efficacy of this novel adoptive immunotherapy14. The ideal dose is 1 to 5 × 108 cells which, however, is not equal to the CAR-T cell count in human bodies17, 18. Finally, tests of cell quality and sterility are necessary, which take 2–4 weeks to complete16. Before the transduced T cells are administered a conditioning treatment, including lymphodepleting, should be done 2 days ahead for a greater T cell expansion14, 16.
Figure 2.
Flow chart of the whole procedure of chimeric antigen receptor T cell (CAR-T cell) production. Firstly, T cells from peripheral blood are collected via leukapheresis, followed by apheresis. Then the T cells are transduced by viral (retroviral or lentiviral) or nonviral vector loading genes of CAR inserted artificially. Next step, the cultured T cells are expanded and purified. Ultimately, cell quality and sterility will be examined before the cell products are infused into patients.
This kind of immunotherapy is commonly used in hematological malignancies such as acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), lymphoma, and multiple myeloma (MM)19. The most common target is CD19 and the total response is optimistic for ALL20, 21. Other targets such as CD20, CD30, CD138 are showing some success as well22, 23, 24. Solid tumors are becoming another battleground for CAR-T cell regimen, including melanoma, sarcoma and breast cancer25, 26, 27. Contrary to hematologic tumors, the majority of treatment in solid tumors is unsuccessful due to insufficient and untypical molecular targets for CAR-T cells to attack and control the microenvironment of tumor28, 29, 30, 31. Despite many issues about safety and efficacy, this technique is indisputably a promising tool for the future adoptive cancer immunotherapy. Here, we provide a framework mainly for understanding the applications of CAR-T cells in different hematological cancers, and also discuss future directions that will undoubtedly inform the improvement of the effectiveness of these adoptive cell therapies.
2. Applications of CAR-T cells in various hematological malignancies
2.1. CAR-T cell in acute lymphoblastic leukemia and chronic lymphocytic leukemia
2.1.1. CAR-T cell therapy in acute lymphoblastic leukemia
So far treatment of ALL, especially fatal relapsed/refractory (r/r) B-ALL is the most suitable for CAR-T therapy32. During the treatment of ALL, the most effective CAR is anti-CD19, an essential biomarker of B cell lineage showing higher expression in B-ALL, while anti-CD20 and immunoglobulin light chains are also potential targets6, 33, 34, 35, 36 (Fig. 1). The first generation of CAR incorporated only a CD3ζ chain and failed to generate potent antitumor effects37 with relatively short persistence38. This prompted scientists to upgrade, triggering creation of the second generation of CAR. Despite a better efficacy of the second generation CAR-T cell with either CD28 or 4-1BB, combining them might be a superior choice, which may give rise to a third generation of CAR-T cell.
Studies have reported data from clinical trials with CD19-targeted CAR-T cells for adults and children inflicted by r/r B-ALL17, 20, 39, 40, 41. All showed promising complete remission (CR) and partial remission (PR) rates. In one clinical study, following conditioning therapy (cyclophosphamide), CD19 CAR-T cells were infused, and 15 out of 16 patients required a qualified amount of T cells; the CR rate was 88%39. Delightfully, the CR was of high quality as few detectable disease indicators were detected by high-sensitive molecular assays such as deep-sequencing or real-time polymerase chain reaction32. Studies involving children and young adult patients (aged 1—30 years old) have found that the CR rate for the 20 B-ALL patients was 70% and the molecular CR rate was 60%. The limited persistence of CAR-T cells (approximately 2 months) is counterbalanced by the rapid remission of patients and post-treatment allogeneic stem-cell transplant17, 32. In another clinical trial20, 41, patients received conditioning treatment, including both fludarabine and cyclophosphamide completed 1 week ahead of adoptive transfer of CAR-T cells. The CR rate was 90% and the molecular CR rate was 73%. Other research teams have also carried out clinical studies of ALL42, 43.
In addition, some studies have suggested that the defined composition of CD4+ and CD8+ CAR-T cell in one intravenous infusion can reveal factors that facilitate the evaluation of efficacy, adverse effects, cell expansion and the persistence of mixed products21, 44, 45. This is important for additional therapies such as lymphodepletion and anti-tumor drug use21, 44, 46, 47, which may clarify the relationship between CAR-T cell dose, cell expansion in vivo and the toxicity risk in order to adjust infusing dose to reduce the possibility of cytokines release syndrome (CRS) and neurotoxicity48. Although CD19 is an ideal target for CAR-T cell treatment in ALL, studies have found that “antigen escape” is a potential obstacle in the development of immunotherapy, thereby making it imperative to optimize, including recognition and identification of additional targets49, 50. Fortunately, CD22 is another potential target for CAR-T cell, and recently, two different anti-CD22 agents have been tested against B-ALL in clinical trials to make up the deficiency of anti-CD19 therapy37.
2.1.2. CAR-T cells therapy in chronic lymphocytic leukemia
CLL is a chronic malignancy with a varying clinical course and prognosis for chemotherapy6. However, the only current approach to curing CLL is allogeneic stem-cell transplantation51. Recently, CD19 CAR-T cells were used to treat patients with relapsed and risky CLL and some response to the CAR-T cell in CLL patients with equal CR and PR rate have been reported52, 53. In addition to CD19, several other targets such as the tyrosine-protein kinase trans-membrane receptor have been explored36. In the past several years several studies have explored the effect of CAR-T cells in CLL patients18, 48, 53, 54, 55, 56.
Because CLL pathogenesis leads to early immune deficiency, the efficacy of CAR-T cell therapy will be limited by difficulties in expansion of T cells ex vivo from CLL patients and their proliferative response in vivo. Finding agents that enhance the ability to prevent the above phenomenon is of necessity55, 57. Ibrutinib, an irreversible inhibitor of bruton tyrosine kinase, may not only avoid negative effects on the T cell but could also improve its antitumor capability57. To test the effect of ibrutinib on the T cell in CLL patients, Fraietta et al.57 tested the phenotype and function of T cells in a cohort study of CLL patients during their treatment course with ibrutinib. The result showed that five cycles of ibrutinib therapy enhanced the expansion of CD19-directed CAR-T cells (CTL019, a second-generation of CD19 CAR-T cell), and decreased the expression of programmed cell death protein 1 (an immunosuppressive molecule) on T cells; and increased the expression of CD200 in B-cell CLL57. CD200/CD200 receptor is important to regulate antitumor immunity58, 59, 60. CD200 overexpression in CLL leads to the functional impairment of CD8+ T-cell responses61. Importantly, when CAR-T cell is exposed to ibrutinib, its function is not altered in vitro but survival, CAR-T cell engraftment, and tumor clearance are indeed improved in human xenograft models of resistant ALL and CLL when both the cells and agent are administered concurrently57. Nevertheless, a case in which ibrutinib was ineffective and CAR-T cell was less effective in treating CLL patients has been reported62.
Additionally, studies have found that CAR-T cells can be used to treat patients with a relapse of B-cell malignancies after allogeneic hematopoietic stem cell transplantation (Allo-HSCT). Traditionally, donor lymphocyte infusions of natural allogeneic lymphocytes derived from transplant donors are used to treat B-cell malignancies after allo-HSCT63, 64. However, the main side effect is graft-versus-host disease (GVHD) and the acute type occurs in about 1/3 of the patients receiving donor lymphocyte infusions. GVHD is a predominant cause of the 6—11% mortality rate from donor lymphocyte infusion64, 65. Thus, studies have tested the effect of CAR-T cell infusion in patients suffering from relapsed B-ALL and CLL after allo-HSCT66, 67. Both of the trials suggested that donor-derived CAR-T cell infusion seems to be effective and safe for relapsed B cell malignancies after allo-HSCT, although larger clinical studies are needed. All the studies reviewed above and others involved in ALL and CLL are summarized in Table 117, 20, 32, 39, 41, 42, 43, 48, 66 and Table 218, 48, 53, 54, 55, 62, 67.
Table 1.
Selected clinical trials of CAR-T cell therapy in ALL.
| Institute | CAR (target & generation) | Sample |
Effective No. of participants | Outcomea | Publishing Year and Ref. | |
|---|---|---|---|---|---|---|
| Number (M/F) | Age* | |||||
| Memorial Sloan-Kettering Cancer Center | CD19 2nd CD28 | 16 (12/4) | Adult | 15b | CR rate: 88% | 201439 |
| Fred Hutchinson Cancer Research Center | CD19 2nd 4-1BB | 29c | Not available | 26 | CR rate: 93% | 201548 |
| National Cancer Institute | CD19 2nd CD28 | 21d (14/7) | 14.71 ± 6.64 | 21 | CR rate :66.7% | 201417, 32 |
| University of Pennsylvania | CD19 2nd 4-1BB | 30 (18/12) | Children & Adult | 30 | CR rate: 90% | 201420, 41 |
| University of Pennsylvania | CD19 2nd 4-1BB | 27c | Adult | 27 | 3 CR in cohort 1 and 2; 3 CR in cohort 3; 75%CR and 8.3%PR in cohort 4 | 201643 |
| Hebei Yanda Lu Daopei Hospital | CD19 2nd 4-1BB | 42 (28/14)e | Children & adult | 40f | CR rate: 90% | 201742 |
| 9 (4/5)e | Children & adult | 9 | All patients achieved MRD- | 201742 | ||
| Peking University People's Hospital | CD19 4th (CD28/4-1BB/CD27/inducible apoptotic caspase9 | 6 (1/5) | 26.50 ± 13.62 | 5g | 5 achieved minimal residual disease (MRD)-negative remissionh | 201766 |
Abbreviations: aGVHD, acute Graft-versus-host disease; ALL, acute lymphoblastic leukemia; BM, bone marrow; CAR T cell, chimeric antigen receptor T cell; CR, complete remission; M/F, male and female; MRD, minimal residual disease; PR, partial remission; Ref, reference; SEM, standard error of mean.
The denominator in the calculation is the total sample number.
One patient had only gross extramedullary disease (no detectable disease in the BM).
No gender indicated.
Twenty ALL patients.
This clinical trial has two groups: one includes 42 primary refractory/hematological relapsed and 9 refractory minimal residual disease (MRD) by flow cytometry B-ALL patients.
Two patients died from treatment-related mortality early in the trial (on days 21 and 24).
One patient was discharged automatically without evaluation after developing severe thrombotic microangiopathies.
Four of five responsive patients relapsed after 2–7 months, and one died of sepsis following MRD-negative remission after a second infusion. None of the other second infusion recipients achieved a second complete remission. Two and one patient developed grade 2 and 3 aGVHD, respectively.
Ages of patients are expressed as mean ± SEM if the data are available.
Table 2.
Selected clinical trials of CAR-T cell therapy in CLL.
| Institute | CAR (target & generation) | Sample |
Effective No. of participants | Outcomeb,d | Publishing year and Ref. | |
|---|---|---|---|---|---|---|
| Number (M/F) | Age* | |||||
| University of Pensylvania | CD19 2nd 4-1BB | 14 (12/2) | 66.90±8.10 | 14 | CR rate: 28%c; PR rate: 28%b,d | 201548, 55 |
| University of Pensylvania | CD19 2nd 4-1BB | 30h | Adult | 23 | CR rate: 22%; PR rate: 17% | 201418 |
| National Cancer Institute | CD19 2nd CD28 | 15e (8/7) | 51.67±11.22 | 15e | CR rate: 53% | 201453 |
| Memorial Sloan Kettering Cancer Center | CD19 2nd CD28 | 10f (8/2) | 63.90±8.49 | 9a,f | No CR | 201154 |
| National Cancer Institute | CD19 2nd CD28 | 20 (11/9) | 50.93±12.86 | 20g | CR rate: 30%; PR rate: 10%, no aGVHD by CAR-T cell infusion | 201667 |
| Fred Hutchinson Cancer Research Center | CD19 3rd CD28/4-1BB | 24h | 59.54±7.87 | 24 | CR rate: 16.7%; PR rate: 54.2% | 201762 |
Abbreviations: a GVHD, acute Graft-versus-host disease; ALL, acute lymphoblastic leukemia; CAR-T cell, chimeric antigen receptor T cell; CLL, chronic lymphocytic leukemia; CR, complete remission; M/F, male and female; PR, partial remission; Ref, reference; SEM, standard error of mean.
One patient (ALL) was yet to be treated with modified T cells.
The denominator in the calculation is the total sample number.
One died after 21 months post-treatment period.
Only one is alive with disease.
Including 4 CLL patients.
There were 8 CLL patients.
There are 5 CLL patients.
No gender indicated.
Ages of patients are expressed as mean ± SEM if the data are available.
2.2. CAR-T cell therapy in lymphoma
Patients with disease deterioration after primary and secondary therapies for lymphoma have been found to have a poor prognosis even though they have shown improvements in cytotoxic chemotherapy regimens and monoclonal antibody therapies68. However, there is a novel and effective treatment alternative for patients who have shown no resolution after multiple lines of chemotherapy16. CAR-T cells are among the latest advanced immunotherapies for relapsed or chemotherapy-refractory B-cell non-Hodgkin lymphoma (NHL)69.
There are several types of CARs modified on the surface of T cells either autonomously or allogeneically, and the anti-CD19 CAR-T cell is the earliest and the most traditional. For lymphoma, the first-generation of CAR-T cells were not effective in preventing proliferation, persistence and homing compared to the second and third generation68, 70, 71. Two different studies have reported preclinical results showing superior proliferative and antitumor activities of the second and third generation T cells, with the CD28 or 4-1BB cytoplasmic signaling domains, both in vitro and in vivo72, 73, 74. Despite a positive effect of CD28 as an early signal in improving cell expansion and persistence, some trials have suggested that using CAR containing 4-1BB as a late costimulatory signal yields more remarkable expansion and anti-tumor activity in indolent B cell malignancies56. For example, CTL019 therapy has shown great effect in some patients with advanced r/r follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). Among 8 eligible patients, 4 people had responses at different levels: 3 CRs (CR rate is 13%) and 1 PR (PR rate is 4%), with a 50% 3-month overall response rate. Four patients with DLBCL had progressive disease before or at initial response assessment, and there was no treatment-related mortality14, 75. The longest ongoing clinical response in this study was more than 350 days for FL, and 400 days for DLBCL. CD28-containing CD19 CAR-T cells have continued to show promise for patients. For instance, studies have shown that 75% of patients who suffered from splenic marginal zone lymphoma achieved PR76, 77, and this was also shown in other corroborative studies53, 78, 79. These patients were all maintained for several months (ranging from 7 to over 18 months)77.
Besides CD19, other surface biomarkers are also essential. CD20, a tetra-trans-membrane protein presents in more than 90% of B-cell lymphomas and is a well-established target for NHL treatment80. Currently, first–generation anti-CD20 CAR-T cell therapy has been used in several studies70. In one clinical trial study 7 participants suffering from lymphomas were treated. Two patients in this study achieved CR, 1 subject got a PR while the disease in another 4 patients was stable 81. To test whether the second-generation of CAR-T treatment is effective in DLBCL patients, a study in 2014 using anti-CD20 CAR-T cell with 4-1BB reported a promising effect of this novel treatment80. In this study, 7 patients with refractory advanced CD20+ DLBCL were recruited. Among them, 5 patients were burdened with bulky tumors and the other 2 were not. Except for 1 subject, the other 6 patients received preconditioning chemotherapy for disease control or the tumor was debulked before anti-CD20 CAR-T cell infusion. One of the two patients without a bulky tumor burden achieved a 14-month long lasting and ongoing CR without preconditioning regimen, and another gained a 6-month tumor regression. 3 of 5 with bulky tumors got 3- to 6-month tumor regression80.
CD30, another potential target, is a member of the TNFR superfamily. It is an antigen to the Ki-1 antibody which binds to Reed-Sternberg cells in Hodgkin lymphoma (HL)82, 83. CD30-positive lymphocytes are mainly found around the follicular areas of lymphoid tissues, but is less common in germinal centers84. For lymphoma, CD30 is expressed in classical HL, anaplastic large cell lymphoma, DLBCL, primary mediastinal B-cell lymphoma, and peripheral T-cell lymphoma83, 85, 86. Recently, Wang et al.23 reported that 18 patients with r/r HL, who received a conditioning chemotherapy prior to the CD30 CAR-T cell infusion showed progression in regard to their conditions before CAR-T cell infusion. Almost 7 patients achieved PR and 6 maintained stable disease (SD). In all these patients their lymph node lesions responded better than extra-nodal lesions, and the response in lung lesions seemed to be relatively poor. Analysis of biopsy tissues by real-time quantitative polymerase chain reaction and immunohistochemistry revealed CAR-T cells migrating into the targeted sites, and reduction in the expression of CD30 in tumors23. Moreover, introduction of EBV-specific CAR-T cells that recognize and kill Epstein-Barr virus (EBV)-infected cells may secure against the relapse of EBV-related B-cell NHLs, including BL and DLBCL87.
In regard to κ-light chain, it is a feasible choice for B-cell lymphoma in that mature malignant B cells express either a κ or λ light immunoglobulin chain. Anti-κ/λ CAR-T cells, hence, can target one kind of malignant B cell and avoid damaging normal B cells70. In one dose-escalation study, 10 r/r κ+ NHL, or CLL patients were infused with autologous CAR-κ T cells and any other treatments were discontinued at least 4 weeks ahead of T-cell infusion. This study reported that among the 5 NHL patients, 2 entered CR (after 2 and 3 infusions at dose level 1 and 3, respectively), 1 patient achieved PR, and 2 other subjects progressed88. The clinical trials aforementioned are summarized in Table 314, 23, 53, 75, 76, 77, 78, 79, 81, 88.
Table 3.
Selected clinical trials of CAR-T cell therapy in lymphoma.
| Institute | CAR (target & generation) | Sample |
Effective No. of participants | Outcomea | Publishing year & Ref. | |
|---|---|---|---|---|---|---|
| Number (M/F) | Age* | |||||
| University of Pennsylvania | CD19 2nd 4-1BB | 23 (14/9) | Adult | 20b | CR rate: 13%; PR rate: 4%; | 201414, 75 |
| National Cancer Institute | CD19 2nd CD28 | 8c,d | 55.88±5.77 | 8c | 5 PRse; 1 CRe; 1 SDe | 201276, 77 |
| National Cancer Institute | CD19 2nd CD28 | 15f (8/7) | 51.67±11.22 | 15f | CR rate: 53%; PR rate: 26%; SD rate: 7% | 201453 |
| National Cancer Institute | CD19 2nd CD28 | 9 (8/1) | Adult | 9 | 1 CRe; 5 PRse | 201478 |
| Fred Hutchinson Cancer Research Center & National Cancer Institute | CD20 1st CD3z | 9 (8/1) | Adult | 7g | 2 CRse; 1 PRe; 4SDse | 200881 |
| Fred Hutchinson Cancer Research Center | CD19 (the generation is unknown) | 28h,d | Adult | 24i | In 12 patients received lymphodepletion with Cy-based regimens without fludarabine, the CR rate is 8.3% and PR rate is 41.7%; In 16 patients received lymphodepletion with addition of fludarabine, the CR rate is 42% and PR rate is 25% | 201579 |
| 6h,d | Adult | 6 | 3 CR ;1 PR | 201579 | ||
| Chinese PLA General Hospital | CD20 2nd 4-1BB | 7 (6/1) | 65 | 6j | 1 CRg; 3PRsg; 2 PDsg | 201480 |
| Chinese PLA General Hospital | CD30 2nd 4-1BB | 18 (13/5) | 31 | 18 | PR rate: 39%; SD rate: 33% | 201623 |
| Baylor College of Medicine | κ-Lightchain | 13d | Not available | 10c | CR rate: 15%; PR rate: 30% | 201388 |
Abbreviations: CLL, chronic lymphocytic leukemia; CR, complete remission; M/F, male and female; PD, progress disease; PR, partial remission; Ref, reference; SD, stable disease; SEM, standard error of mean.
The denominator in the calculation is the total sample number.
Three patients were removed from the trial before therapy due to progressive disease.
There are 5 lymphoma patients.
No gender indicated.
In this study, we use the absolute value instead of rate in that the response rate is meaningless (sample size is less than 10).
There are 11 lymphoma patients.
The reason why 2 of nine patients was untreated is unknown.
In this clinical trial, there are two main groups, one is non-Hodgkin lymphoma and the other one is chronic lymphacytic leukemia.
Four patients were not available, among which 2 died early.
One participant died of severe hemorrhage of the alimentary tract.
Ages of patients are expressed as mean ± SEM if the data are available.
2.3. CAR-T cell in multiple myeloma
Multiple myeloma (MM) is a bone-marrow-derived refractory malignancy leading to anemia, immunosuppression with repeated infections, hypercalcemia, bone lesions and renal failure89. Despite chemotherapy, autologous hematopoietic stem cell transplantation or the use of other immune-modulatory agents, this disease remains incurable because of the heterogeneous cytogenetic and molecular abnormalities of myeloma90, 91. However, the fact that myeloma can subside as a result of the graft-versus-myeloma effect in allogeneic stem cell transplantation provides insight into the role of T-cell-based immunotherapy92, 93. Studies have found a lower, but a more frequent expression of CD19 on myeloma cells94, 95. Because myeloma cells hardly express CD19 on their surface, anti-CD19 CAR-T cells cannot perform well to kill the malignant cells; instead, they harm some healthy tissues other than tumors96. Two studies targeted this population via the use of CTL019 cells, reporting a remission in a 43-year-old patient with 9 prior lines of treatment with nearly no sign of CRS94, 97. It is therefore evident that more specific targets presented on tumor cells versus those on non-vital, healthy tissues should be explored to achieve a broader application in MM91, 96.
Another molecule involved in this event is CD138, which is a membrane protein and belongs to the Syndecan family of heparan sulfate proteoglycans98. In hematopoietic tissues, malignant and differentiated plasma cells express this biomarker99. It is also present in neoplastic, mature epithelial cells and other normal tissues100. By virtue of its expression in nearly all MM patients, CD138 is used as a primary diagnostic marker101. The result of a clinical trial using second-generation of anti-CD138 CAR-T cell treatment of 5 refractory MM subjects showed that, after 7-month follow-up treatment, the conditions of at least 4 patients were stable and one patient with advanced plasma cell leukemia had a reduction of myeloma cells in peripheral blood24. This shows that the CAR-T cells had entered the bone marrow98. Thus, CD138 CAR-T cell therapy for MM is well-tolerated and potentially has anti-tumor immunity24.
B-cell maturation antigen (BCMA, CD269) is another molecule that has been identified and is a promising immunotherapeutic target in MM102, 103. Expressed only in lymphoid tissue of healthy individuals and on mature B cells or plasma cells, BCMA enhances the survival of long-lived plasma cells in MM patients102, 104. Patients that were recently enrolled in an anti-BCMA/CD269 CAR-T cell treatment in MM received CAR-BCMA T cells in a dose-escalation trial105. In addition, this study reported that all patients were pretreated with cyclophosphamide and fludarabine to enhance the activity of adoptive transferred T cells as previously reported105, 106, 107, 108. In these 6 patients treated at the lowest 2 dose levels, low anti-myeloma activity or toxicity was noticed. Moreover, at the third dose level 1 patient obtained a very-good PR. Furthermore, two chemotherapy-resistant patients were treated on the fourth dose level with anti-BCMA CAR-T and achieved stringent CR lasting for 17 weeks before relapse. Ongoing promising PR105 corroborative studies have also shown a similar therapeutic outcome109, 110. The above mentioned studies and the 2 corroborative studies are further summarized Table 424, 105, 109, 110. Some bench studies have brought exciting stories of new approaches to treat MM111, 112, and progress have been achieved in vitro and hopefully further experimental and clinical trials will usher in a new era of MM immunotherapy.
Table 4.
Selected clinical trials of CAR-T cell therapy in multiple myeloma.
| Institute | CAR (target & generation) | Sample size |
Effective No. of participants | Outcome | Publishing year & Ref. | |
|---|---|---|---|---|---|---|
| Number (M/F) | Age* | |||||
| Chinese PLA General Hospital | CD138 2nd 4-1BB | 5(1/4) | Adult | 5 | 4SDa | 201524 |
| National Cancer Institute | BCMA 2nd CD28 | 12d | Not available | 12 | 1PR and 2 SD in group of “0.3 × 106/kg CAR-T cell”; 3 SD in group of “1 × 106/kg CAR-T cell”; 1PR and 2 SD in group of “3 × 106/kg CAR-T cell”; 1 CR, 1 PR and 1 SD in group of “9 × 106/kg CAR-T cell”b | 2016105 |
| Bluebird Bio | BCMA 2nd 4-1BB(bb2121) | 9d | Not available | 9 | 2CRs in a cohort of 15*107 CAR-T cells; 1 PR in a cohort of 5.0*107 CAR-T cell, 1 PR in the cohort of 15*107 CAR-T cells and 2 PR in a cohort of 45*107 CAR-T cell; 1 SD in the cohort of 5.0*107 CAR-T cell and another one is in the cohort of 45*107 CAR-T cell | 2017109 |
| University of Pennsylvania | BCMA 2nd 4-1BB | 11d | Not available | 6c | 1CR ;1PR ;1 SD | 2016110 |
Abbreviations: BCMA, B cell maturation antigen; CAR-T cell, chimeric antigen receptor T cell; CR, complete remission; M/F, male and female; PR, partial remission; Ref, reference; SD, stable disease.
In this study, we use the absolute value instead of rate in that the response rate is meaningless (sample size is less than 10).
One patient only accepted the dose of 3 × 106/kg CAR-T cell.
Five patients not receiving treatment because of screen fail (n = 2), rapid multiple myeloma progression/renal failure (n = 2), and self choice (n = 1).
No gender indicated.
Ages of patients are expressed as mean ± SEM if the data are available.
3. Advantages and disadvantages in the application of CAR-T cell therapy in hematological malignancies
3.1. Advantages of CAR-T cell therapy in hematological malignancies
In contrast with common adaptive immune cells, CAR-T cells have unique specificity and can eliminate cancer cells containing the corresponding TAAs. To some extent this technique will avoid unnecessary killing of healthy tissues. Furthermore, CAR-T cells can recognize cell surface molecules without the help of HLA expression. Its advantage over the former one is that tumors often avoid T cell immune surveillance by hiding HLA or other molecules involved in antigen processing and presentation70, 113, 114. In addition, the flexibility of intracellular signaling domains within CARs permits the cell to counteract the down-regulation of co-stimulatory molecules directly or indirectly caused by cancer cells115. It is worthwhile to mention that CAR-T cell can recognize potential antigens in nearly all forms including carbohydrate, lipid, protein antigens, which can be combined specifically by antibodies30.
3.2. Disadvantages of CAR-T cell therapy in hematological malignancies
When treating hematological malignancies using CAR-T cells, it turns out that activating the antitumor immunity of engineered T cell results in broad and strong cytokine-driven effects, including CRS, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis. Among them, CRS is a clinical response to raise cytokine levels and includes symptoms such as hypotension, fevers, neurological changes and hypoxia52, 56, 77, 116. Risk-adapted CAR-T cell dosing strategies have been carried out in response to the greater incidence of severe CRS observed among patients who showed greater baseline disease burden, and were treated with higher doses of CAR-T cells21, 43, 117. Systemic corticosteroids are used as first-aid treatment for patients with life-threatening CRS to lessen or even eliminate the hyper-proliferative activated CAR-T cells. But the downside has been revealed as well: rapid ablation of engineered T cells will depress the antitumor efficacy of CD19 CAR-T cells and trigger subsequent disease progression or relapse39, 49, 118. The investigation of cytokines in several studies surprisingly identified IL-6 as a major cytokine induced by CAR therapy. Meanwhile, IL-6 also stems from apoptotic B cells or activated macrophages and moves to lysed tumor cells via chemotaxis11. Thus, the anti-IL-6 receptor antagonist antibody tocilizumab was successfully designated to weaken the cytokine-induced side effect, a treatment now being applied more systematically to counteract cytokine-release syndrome33, 39.
Neurotoxicity is another serious potential toxicity arising from CAR-T cell therapy and has been observed in several patients treated with CD19 CAR-T cells119. Symptoms of neurotoxicity include visual hallucinations, delirium, dysphasia, epilepsy or seizure120. Endothelial dysfunction, including vascular instability, capillary leak, blood-brain barrier disruption and disseminated intravascular coagulation are clinical evidence of neurotoxicity121. For medical imaging results, an abnormal brain MRI scan can suggest neurotoxicity by showing vasogenic edema, leptomeningeal enhancement, and/or multifocal microhemorrhages121. Similar to CRS, IL6, IFN-γ and TNF are prime culprits in inducing acute neurotoxicity so treatment can also apply tocilizumab to suppress IL6-mediated inflammatory pathways121.
Although CAR-T cell has relative specificity as noted above, every regimen may result in tissue damage at different levels because the molecular biomarkers can be expressed in some normal tissues or organs, especially the lymphatic tissues. This phenomenon is referred to as on-target/off–tumor toxicity, which includes B cell aplasia in anti-CD19/CD20 CAR-T cell treatment20, 77, 122. Also, antigen escape, typically CD19-negative relapse of B cell malignancy, may challenge the success rate of CARs in blood cancer17, 20, 21, 41.
4. Outlook
CAR-T cell therapy has made great progress in recent decades, however, several challenges still need to be solved. The main issues are how to improve the effectiveness and persistence of adoptive cells and to reduce the adverse effects of treatment.
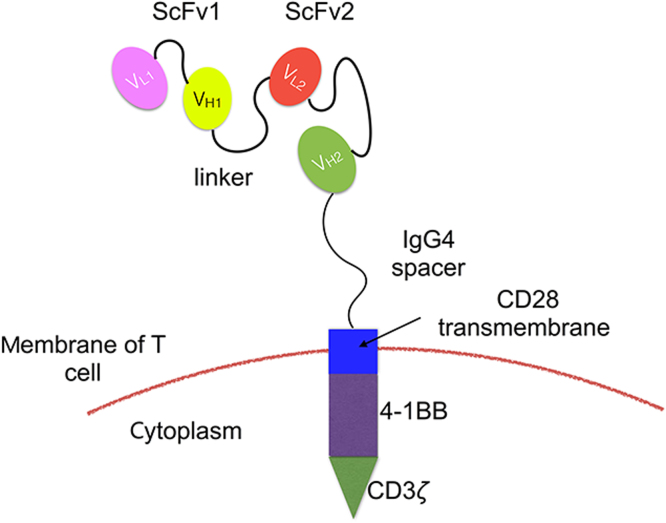
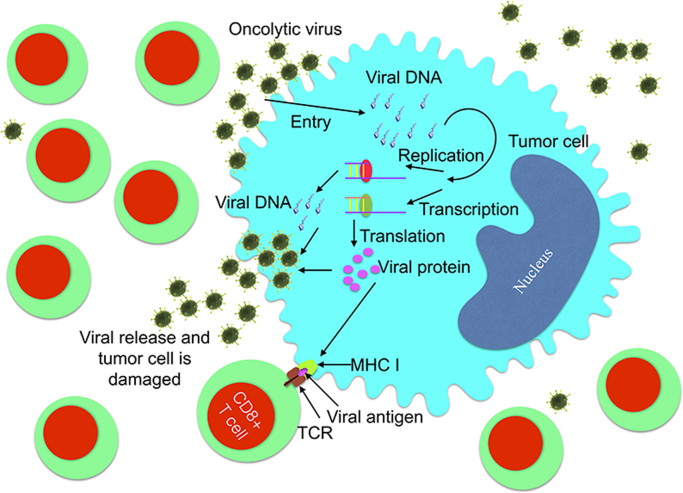
On one hand, to improve the treatment effect, CAR-T cells can be further equipped to improve their effectiveness and persistence by adding new co-stimulatory domains or CAR parts123. Since glucocorticoid-inducible tumor necrosis factor receptor-related protein (GITR, CD357) and 4-1BB are both nuclear factor-κB (NF-κB)-inducing members of the TNFR family and GITR is a costimulatory molecule, we suggest that further research is needed on the effect of adding this domain, and combining them with CD28 and 4-1BB to evaluate the effect of T-cells124. Plus, reconstructing the CAR part is a new approach to antigen escape or coexistence of multiple tumor antigens in lymphoma, MM and other type of malignancies. Bi-specific CAR-T cell is a promising treatment and it includes two formations: First, one CAR is composed of two different scFvs “hand-in-hand” (TanCAR) (Fig. 3) or two distinct CARs with different scFvs on one single T cell (dual-signaling CAR)125, 126; second, T cell pools with different typical CARs infused sequentially or simultaneously127. Nevertheless, challenges still exist in recognizing the coexisting targets on a tumor cell and selecting the proper epitope in TanCAR therapy128, 129. In addition, oncolytic viruses can infect the host, replicate and damage certain tumor cells without harming normal cells130, 131. Evidence has shown that tumor cells can be killed via the new viral proteins integrated in its cytoplasm presented by the major histocompatibility complex class I molecules, and thereafter being recognized and destroyed by CD8 T cells 130. In addition, tumor cells can also be killed by the release of progeny viral particles130 (Fig. 4). Applications for this type of tumor cell killing using viruses such as Myxoma virus, adenovirus serotype 5 and Coxsackievirus A21 have been reported in MM132, 133, 134. B- and T-lymphoma or leukemia-derived cells/xenografts have also been used as preclinical models135, 136, 137. Studies have reported the use of oncolytic virus equipped with chemokine genes to recruit CAR-T cells, prolong their persistence and directly attack tumor cells138, 139. This is a promising approach which can be tested during hematological malignancies.
Figure 3.
The structure of TanCAR with two different scFvs “hand-in-hand”. The TanCAR is a type of bispecific T cell dealing with antigens escaping or multiple tumor antigens in relapsed B cell malignancies. The two antibodies on the CAR part are connected in tandem so that one CAR-T cell can combine two kinds of tumor antigen at the same time or binds to the tumor cell expressing either kinds of targeted antigen.
Figure 4.
Oncolytic virus can help T cells to kill tumor cells in two ways. 1. Direct attack through viral entry, replication, transcription, and release, leading to tumor lysis while sparing normal cells; 2. Indirect damaging via integrating its genome to that of tumor cells for further expression on surface of tumor cells and the cell can be recognized by T cell through MHC (major histocompatibility complex) molecule. Moreover, these viruses can be equipped with chemokine genes to “attract” CAR-T cells, prolong their persistence and let them complete directly attack tumor cell.
On the other hand, avoiding uncontrolled T cell proliferation, cytokine storm, or “on-target/off-tumor” effects during CAR-T cell treatment is essential. Some researchers suggest that suicide-gene (also known as “elimination genes”) systems can be activated to destroy infused CAR-T cells to regulate the expansion of CAR-T cells and restrict toxicity123, 140. To date, some methods have been proved: suicide gene therapy with the Herpes simplex thymidine kinase/ganciclovir suicide system in the context of allo-HSCT has shown its safety and effectiveness141, and other non-immunogenic suicide systems have been developed, especially through a modified caspase-9 member of the intrinsic apoptosis pathway. A new version of FK506-binding protein, demonstrating a propyl isomerase activity has been combined with caspase-9142, 143. However, such suicide strategy will shorten the duration of treatment by thoroughly eliminating CAR-T cell144. Switchable CAR-T cell can make up this defect by inserting a small molecular complex between the scFv and T cell surface to “turn on” the cell function and discontinue adding it to switch off144. Another way to switch the cell between “on and off” would be to engineer a soluble intercellular molecule that contains a tumor antigen-specific antibody, a secondary moiety binding to CARs. This structure allows the researcher to switch the cell function by changing the concentration of the intermediary molecules. This novel format is being tested145.
5. Conclusions
CAR-T cell therapy has made great progress to deal with hematological malignancies, especially ALL, CLL and lymphoma while the effectiveness, cell persistence and adverse effects become bottlenecks to the widespread use of this approach. Innovative bench research is required to strengthen the effectiveness of this therapy; that will give physicians and patients the information and therapeutics to eliminate these malignancies.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 31670880 and 31370868), the Guangdong Natural Science Fund for Distinguished Young Scholars (No. 2016A030306004), Guangdong Special Support Program for Youth Science and Technology Innovation Talents (No. 2015TQ01R473), Guangzhou Pearl River New Star Program (No. 201610010064), Guangdong Innovative Research Team Program (No. 2011Y035).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Yuanqing Zhang, Email: zhangyq65@mail.sysu.edu.cn.
Minhao Wu, Email: wuminhao@mail.sysu.edu.cn.
References
- 1.Houot R., Schultz L.M., Marabelle A., Kohrt H. T-cell-based immunotherapy: adoptive cell transfer and checkpoint inhibition. Cancer Immunol Res. 2015;3:1115–1122. doi: 10.1158/2326-6066.CIR-15-0190. [DOI] [PubMed] [Google Scholar]
- 2.Barrett D.M., Grupp S.A., June C.H. Chimeric antigen receptor-and TCR-modified T cells enter main street and wall street. J Immunol. 2015;195:755–761. doi: 10.4049/jimmunol.1500751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiwara H. Adoptive immunotherapy for hematological malignancies using T cells gene-modified to express tumor antigen-specific receptors. Pharmaceuticals. 2014;7:1049–1068. doi: 10.3390/ph7121049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chmielewski M., Hombach A.A., Abken H. Antigen-specific T-cell activation independently of the MHC: chimeric antigen receptor-redirected T cells. Front Immunol. 2013;4:371. doi: 10.3389/fimmu.2013.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enblad G., Karlsson H., Loskog A.S. CAR-T-cell therapy: the role of physical barriers and immunosuppression in lymphoma. Hum Gene Ther. 2015;26:498–505. doi: 10.1089/hum.2015.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang H., Qiao J., Fu Y.X. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370:85–90. doi: 10.1016/j.canlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava S., Riddell S.R. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Advani A. Antibodies: immunoconjugates and autologous cellular therapy in acute lymphoblastic leukemia. Best Pract Res Clin Haematol. 2015;28:116–123. doi: 10.1016/j.beha.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Doyle C. CAR-T cells: the transplants of the future. Am Health Drug Benefits. 2015;8:14. [PMC free article] [PubMed] [Google Scholar]
- 11.Lorentzen C.L., Straten P.T. CD19-chimeric antigen receptor T cells for treatment of chronic lymphocytic leukaemia and acute lymphoblastic leukaemia. Scand J Immunol. 2015;82:307–319. doi: 10.1111/sji.12331. [DOI] [PubMed] [Google Scholar]
- 12.Chmielewski M., Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 13.Levine B.L. Performance-enhancing drugs: design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer Gene Ther. 2015;22:79–84. doi: 10.1038/cgt.2015.5. [DOI] [PubMed] [Google Scholar]
- 14.Maus M.V., Levine B.L. Chimeric antigen receptor T-cell therapy for the community oncologist. Oncologist. 2016;21:608–617. doi: 10.1634/theoncologist.2015-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka J., Mielcarek M., Torok-Storb B. Impaired induction of the CD28-responsive complex in granulocyte colony-stimulating factor mobilized CD4 T cells. Blood. 1998;91:347–352. [PubMed] [Google Scholar]
- 16.Shank B.R., Do B., Sevin A., Chen S.E., Neelapu S.S., Horowitz S.B. Chimeric antigen receptor T cells in hematologic malignancies. Pharmacotherapy. 2017;37:334–345. doi: 10.1002/phar.1900. [DOI] [PubMed] [Google Scholar]
- 17.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase I dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter D.L., Frey N.V., Melenhorst J.J., Hwang W.T., Lacey S.F., Shaw P. Randomized, phase II dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Blood. 2014;124:1982. [Google Scholar]
- 19.Landoni E., Savoldo B. Treating hematological malignancies with cell therapy: where are we now? Expert Opin Biol Ther. 2018;18:65–75. doi: 10.1080/14712598.2018.1384810. [DOI] [PubMed] [Google Scholar]
- 20.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F., Fan C., Gu X., Zhang H., Liu Q., Gao X. Construction of anti-CD20 single-chain antibody-CD28-CD137-TCR ζ recombinant genetic modified T cells and its treatment effect on b cell lymphoma. Med Sci Monit. 2015;21:2110–2115. doi: 10.12659/MSM.893791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C.M., Wu Z.Q., Wang Y., Guo Y.L., Dai H.R., Wang X.H. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res. 2017;23:1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 24.Guo B., Chen M., Han Q., Hui F., Dai H., Zhang W. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother. 2016;2:28–35. [Google Scholar]
- 25.Gargett T., Yu W., Dotti G., Yvon E.S., Christo S.N., Hayball J.D. GD2-specific CAR-T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24:1135–1149. doi: 10.1038/mt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tchou J., Zhao Y., Levine B.L., Zhang P.J., Davis M.M., Melenhorst J.J. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res. 2017;5:1152–1161. doi: 10.1158/2326-6066.CIR-17-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of her2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakarla S., Gottschalk S. CAR-T cells for solid tumors: armed and ready to go? Cancer J. 2014;20:151–155. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamers C.H., Sleijfer S., Van Steenbergen S., Van Elzakker P., Van Krimpen B., Groot C. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hillerdal V., Essand M. Chimeric antigen receptor-engineered T cells for the treatment of metastatic prostate cancer. BioDrugs. 2015;29:75–89. doi: 10.1007/s40259-015-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You F., Jiang L., Zhang B., Lu Q., Zhou Q., Liao X. Phase I clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified anti-MUC1 chimeric antigen receptor transduced T cells. Sci China Life Sci. 2016;59:386–397. doi: 10.1007/s11427-016-5024-7. [DOI] [PubMed] [Google Scholar]
- 32.Davila M.L., Sadelain M. Biology and clinical application of CAR-T cells for B cell malignancies. Int J Hematol. 2016;104:6–17. doi: 10.1007/s12185-016-2039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maus M.V., Fraietta J.A., Levine B.L., Kalos M., Zhao Y., June C.H. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill S., Maus M.V., Porter D.L. Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev. 2016;30:157–167. doi: 10.1016/j.blre.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Ruella M., Kalos M. Adoptive immunotherapy for cancer. Immunol Rev. 2014;257:14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 36.Jain N., O'Brien S. Targeted therapies for CLL: practical issues with the changing treatment paradigm. Blood Rev. 2016;30:233–244. doi: 10.1016/j.blre.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Shalabi H., Angiolillo A., Fry T.J. Beyond CD19: opportunities for future development of targeted immunotherapy in pediatric relapsed-refractory acute leukemia. Front Pediatr. 2015;3:80. doi: 10.3389/fped.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kershaw M.H., Westwood J.A., Parker L.L., Wang G., Eshhar Z., Mavroukakis S.A. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K. Efficacy and toxicity management of 19-28z CAR-T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan J., Yang J.F., Deng B.P., Zhao X.J., Zhang X., Lin Y.H. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia. 2017;31:2587–2593. doi: 10.1038/leu.2017.145. [DOI] [PubMed] [Google Scholar]
- 43.Frey N.V., Shaw P.A., Hexner E.O., Gill S., Marcucci K., Luger S.M. Optimizing chimeric antigen receptor (CAR) T cell therapy for adult patients with relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL) J Clin Oncol. 2016;34:7002. [Google Scholar]
- 44.Sommermeyer D., Hudecek M., Kosasih P.L., Gogishvili T., Maloney D.G., Turtle C.J. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner R.A., Finney O., Annesley C., Brakke H., Summers C., Leger K. Intent-to-treat leukemia remission by CD19 CAR-T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terakura S., Yamamoto T.N., Gardner R.A., Turtle C.J., Jensen M.C., Riddell S.R. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X., Berger C., Wong C.W., Forman S.J., Riddell S.R., Jensen M.C. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117:1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turtle C.J., Riddell S.R., Maloney D.G. CD19-Targeted chimeric antigen receptor-modified T-cell immunotherapy for B-cell malignancies. Clin Pharmacol Ther. 2016;100:252–258. doi: 10.1002/cpt.392. [DOI] [PubMed] [Google Scholar]
- 49.Maude S.L., Barrett D., Teachey D.T., Grupp S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zah E., Lin M.Y., Silva-Benedict A., Jensen M.C., Chen Y.Y. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mewawalla P., Nathan S. Role of allogeneic transplantation in patients with chronic lymphocytic leukemia in the era of novel therapies: a review. Ther Adv Hematol. 2014;5:139–152. doi: 10.1177/2040620714550773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P., Carpenter R.O., Stetler-Stevenson M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fraietta J.A., Beckwith K.A., Patel P.R., Ruella M., Zheng Z., Barrett D.M. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–1127. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coles S.J., Wang E.C., Man S., Hills R.K., Burnett A.K., Tonks A. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25:792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong K.K., Brenneman F., Chesney A., Spaner D.E., Gorczynski R.M. Soluble CD200 is critical to engraft chronic lymphocytic leukemia cells in immunocompromised mice. Cancer Res. 2012;72:4931–4943. doi: 10.1158/0008-5472.CAN-12-1390. [DOI] [PubMed] [Google Scholar]
- 60.Kretz-Rommel A., Qin F., Dakappagari N., Ravey E.P., McWhirter J., Oltean D. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol. 2007;178:5595–5605. doi: 10.4049/jimmunol.178.9.5595. [DOI] [PubMed] [Google Scholar]
- 61.Wong K.K., Khatri I., Shaha S., Spaner D.E., Gorczynski R.M. The role of CD200 in immunity to B cell lymphoma. J Leukoc Biol. 2010;88:361–372. doi: 10.1189/jlb.1009686. [DOI] [PubMed] [Google Scholar]
- 62.Turtle C.J., Hay K.A., Hanafi L.A., Li D., Cherian S., Chen X. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35:3010–3020. doi: 10.1200/JCO.2017.72.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pavletic S.Z., Kumar S., Mohty M., De Lima M., Foran J.M., Pasquini M. NCI first international workshop on the biology, prevention, treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on the epidemiology and natural history of relapse following allogeneic cell transplantation. Biol Blood Marrow Transplant. 2010;16:871–890. doi: 10.1016/j.bbmt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roddie C., Peggs K.S. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11:473–487. doi: 10.1517/14712598.2011.554811. [DOI] [PubMed] [Google Scholar]
- 65.Frey N.V., Porter D.L. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol. 2008;21:205–222. doi: 10.1016/j.beha.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y., Cheng Y., Suo P., Yan C., Wang Y., Chen Y. Donor-derived CD19-targeted T cell infusion induces minimal residual disease-negative remission in relapsed B-cell acute lymphoblastic leukaemia with no response to donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation. Br J Haematol. 2017;179:598–605. doi: 10.1111/bjh.14923. [DOI] [PubMed] [Google Scholar]
- 67.Brudno J.N., Somerville R.P., Shi V., Rose J.J., Halverson D.C., Fowler D.H. Allogeneic T cells that express an Anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu Y., Tan Y., Ou R., Zhong Q., Zheng L., Du Y. Anti-CD19 chimeric antigen receptor-modified T cells for B-cell malignancies: a systematic review of efficacy and safety in clinical trials. Eur J Haematol. 2016;96:389–396. doi: 10.1111/ejh.12602. [DOI] [PubMed] [Google Scholar]
- 69.Turtle C.J., Hanafi L.A., Berger C., Gooley T., Chaney C., Cherian C. Rate of durable complete response in ALL, NHL, and CLL after immunotherapy with optimized lymphodepletion and defined composition CD19 CAR-T cells. J Clin Oncol. 2016;34:102. [Google Scholar]
- 70.Ramos C.A., Heslop H.E., Brenner M.K. CAR-T cell therapy for lymphoma. Annu Rev Med. 2016;67:165–183. doi: 10.1146/annurev-med-051914-021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Savoldo B., Ramos C.A., Liu E., Mims M.P., Keating M.J., Carrum G. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brentjens R.J., Santos E., Nikhamin Y., Yeh R., Matsushita M., La Perle K. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 73.Imai C., Mihara K., Andreansky M., Nicholson I.C., Pui C.H., Geiger T.L. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 74.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schuster S.J., Svoboda J., Dwivedy Nasta S., Porter D.L., Chong E.A., Mahnke Y. Phase iia trial of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. Blood. 2014;124:3087. [Google Scholar]
- 76.Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kochenderfer J.N., Somerville R., Lu L., Iwamoto A., Yang J.C., Klebanoff C. Anti-CD19 CAR-T cells administered after low-dose chemotherapy can induce remissions of chemotherapy-refractory diffuse large B-cell lymphoma. Blood. 2014;124:550. [Google Scholar]
- 79.Turtle C.J., Berger C., Sommermeyer D., Hanafi L.A., Pender B., Robinson E.M. Anti-CD19 chimeric antigen receptor-modified T cell therapy for B cell non-hodgkin lymphoma and chronic lymphocytic leukemia: fludarabine and cyclophosphamide lymphodepletion improves in vivo expansion and persistence of CAR-T cells and clinical outcomes. Blood. 2015;126:184. [Google Scholar]
- 80.Wang Y., Zhang W.Y., Han Q.W., Liu Y., Dai H.R., Guo Y.L. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol. 2014;155:160–175. doi: 10.1016/j.clim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Till B.G., Jensen M.C., Wang J., Chen E.Y., Wood B.L., Greisman H.A. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982;299:65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- 83.Stein H., Mason D.Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–858. [PubMed] [Google Scholar]
- 84.Pierce J.M., Mehta A. Diagnostic, prognostic and therapeutic role of CD30 in lymphoma. Expert Rev Hematol. 2017;10:29–37. doi: 10.1080/17474086.2017.1270202. [DOI] [PubMed] [Google Scholar]
- 85.Horie R., Watanabe T. CD30: expression and function in health and disease. Semin Immunol. 1998;10:457–470. doi: 10.1006/smim.1998.0156. [DOI] [PubMed] [Google Scholar]
- 86.Falini B., Pileri S., Pizzolo G., Dürkop H., Flenghi L., Stirpe F. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- 87.Onea A.S., Jazirehi A.R. CD19 chimeric antigen receptor (CD19 CAR)-redirected adoptive T-cell immunotherapy for the treatment of relapsed or refractory B-cell Non-Hodgkin's lymphomas. Am J Cancer Res. 2016;6:403–424. [PMC free article] [PubMed] [Google Scholar]
- 88.Ramos C.A., Savoldo B., Liu E., Gee A.P., Mei Z., Grilley B.J. Clinical responses in patients infused with T lymphocytes redirected to target κ-light immunoglobulin chain. Blood. 2013;122:506. [Google Scholar]
- 89.The International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 90.Chung C. Role of immunotherapy in targeting the bone marrow microenvironment in multiple myeloma: an evolving therapeutic strategy. Pharmacotherapy. 2017;37:129–143. doi: 10.1002/phar.1871. [DOI] [PubMed] [Google Scholar]
- 91.Zhang K., Desai A., Zeng D., Gong T., Lu P., Wang M. Magic year for multiple myeloma therapeutics: key takeaways from the ASH 2015 annual meeting. Oncotarget. 2017;8:10748–10759. doi: 10.18632/oncotarget.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verdonck L.F., Lokhorst H.M., Dekker A.W., Nieuwenhuis H.K., Petersen E.J. Graft-versus-myeloma effect in two cases. Lancet. 1996;347:800–801. doi: 10.1016/s0140-6736(96)90871-5. [DOI] [PubMed] [Google Scholar]
- 93.Tricot G., Vesole D.H., Jagannath S., Hilton J., Munshi N., Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. [PubMed] [Google Scholar]
- 94.Garfall A.L., Maus M.V., Hwang W.T., Lacey S.F., Mahnke Y.D., Melenhorst J.J. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hajek R., Okubote S.A., Svachova H. Myeloma stem cell concepts, heterogeneity and plasticity of multiple myeloma. Br J Haematol. 2013;163:551–564. doi: 10.1111/bjh.12563. [DOI] [PubMed] [Google Scholar]
- 96.Atanackovic D., Radhakrishnan S.V., Bhardwaj N., Luetkens T. Chimeric antigen receptor (CAR) therapy for multiple myeloma. Br J Haematol. 2016;172:685–698. doi: 10.1111/bjh.13889. [DOI] [PubMed] [Google Scholar]
- 97.Kocoglu M., Badros A. The role of immunotherapy in multiple myeloma. Pharmaceuticals. 2016;9:3. doi: 10.3390/ph9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Hujaily E.M., Oldham R.A., Hari P., Medin J.A. Development of novel immunotherapies for multiple myeloma. Int J Mol Sci. 2016;17:1506. doi: 10.3390/ijms17091506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O'Connell F.P., Pinkus J.L., Pinkus G.S. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am J Clin Pathol. 2004;121:254–263. doi: 10.1309/617D-WB5G-NFWX-HW4L. [DOI] [PubMed] [Google Scholar]
- 100.Kambham N., Kong C., Longacre T.A., Natkunam Y. Utility of syndecan-1 (CD138) expression in the diagnosis of undifferentiated malignant neoplasms: a tissue microarray study of 1754 cases. Appl Immunohistochem Mol Morphol. 2005;13:304–310. doi: 10.1097/01.pai.0000159773.50905.7b. [DOI] [PubMed] [Google Scholar]
- 101.Lin P., Owens R., Tricot G., Wilson C.S. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121:482–488. doi: 10.1309/74R4-TB90-BUWH-27JX. [DOI] [PubMed] [Google Scholar]
- 102.Carpenter R.O., Evbuomwan M.O., Pittaluga S., Rose J.J., Raffeld M., Yang S. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chu J., He S., Deng Y., Zhang J., Peng Y., Hughes T. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clin Cancer Res. 2014;20:3989–4000. doi: 10.1158/1078-0432.CCR-13-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gill S., June C.H. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263:68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- 105.Ali S.A., Shi V., Maric I., Wang M., Stroncek D.F., Rose J.J. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.North R.J. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kochenderfer J.N., Yu Z., Frasheri D., Restifo N.P., Rosenberg S.A. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gattinoni L., Finkelstein S.E., Klebanoff C.A., Antony P.A., Palmer D.C., Spiess P.J. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ormhøj M., Bedoya F., Frigault M.J., Maus M.V. CARs in the lead against multiple myeloma. Curr Hematol Malig Rep. 2017;12:119–125. doi: 10.1007/s11899-017-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cohen A.D., Garfall A.L., Stadtmauer E.A., Lacey S.F., Lancaster E., Vogl D.T. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Blood. 2016;128:1147. [Google Scholar]
- 111.Gogishvili T., Danhof S., Prommersberger S., Rydzek J., Schreder M., Brede C. SLAMF7-CAR-T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood. 2017;130:2838–2847. doi: 10.1182/blood-2017-04-778423. [DOI] [PubMed] [Google Scholar]
- 112.Hosen N., Matsunaga Y., Hasegawa K., Matsuno H., Nakamura Y., Makita M. The activated conformation of integrin β7 is a novel multiple myeloma-specific target for CAR-T cell therapy. Nat Med. 2017;23:1436–1443. doi: 10.1038/nm.4431. [DOI] [PubMed] [Google Scholar]
- 113.Bonini C., Mondino A. Adoptive T-cell therapy for cancer: the era of engineered T cells. Eur J Immunol. 2015;45:2457–2469. doi: 10.1002/eji.201545552. [DOI] [PubMed] [Google Scholar]
- 114.Catalán E., Charni S., Jaime P., Aguiló J.I., Enríquez J.A., Naval J. MHC-I modulation due to changes in tumor cell metabolism regulates tumor sensitivity to CTL and NK cells. Oncoimmunology. 2015;4:e985924. doi: 10.4161/2162402X.2014.985924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duong C.P., Yong C.S., Kershaw M.H., Slaney C.Y., Darcy P.K. Cancer immunotherapy utilizing gene-modified T cells: from the bench to the clinic. Mol Immunol. 2015;67:46–57. doi: 10.1016/j.molimm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 116.Riches J.C., Gribben J.G. Understanding the immunodeficiency in chronic lymphocytic leukemia: potential clinical implications. Hematol Oncol Clin North Am. 2013;27:207–235. doi: 10.1016/j.hoc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 117.Park J.H., Riviere I., Wang X., Purdon T., Sadelain M., Brentjens R.J.B. Impact of disease burden on long-term outcome of 19-28z CAR modified T cells in adult patients with relapsed B-ALL. J Clin Oncol. 2016;34 Suppl:7003. [Google Scholar]
- 118.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wei G., Ding L., Wang J., Hu Y., Huang H. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Exp Hematol Oncol. 2017;6:10. doi: 10.1186/s40164-017-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Whilding L.M., Maher J. CAR-T-cell immunotherapy: the path from the by-road to the freeway? Mol Oncol. 2015;9:1994–2018. doi: 10.1016/j.molonc.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gust J., Hay K.A., Hanafi L.A., Li D., Myerson D., Gonzalez-Cuyar L.F. Endothelial activation and blood—brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bonifant C.L., Jackson H.J., Brentjens R.J., Curran K.J. Toxicity and management in CAR-T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Batlevi C.L., Matsuki E., Brentjens R.J., Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016;13:25–40. doi: 10.1038/nrclinonc.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Watts T.H. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 125.Grada Z., Hegde M., Byrd T., Shaffer D.R., Ghazi A., Brawley V.S. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruella M., Barrett D.M., Kenderian S.S., Shestova O., Hofmann T.J., Perazzelli J. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Investig. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Z., Guo Y., Han W. Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment. Protein Cell. 2017;8:896–925. doi: 10.1007/s13238-017-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jackson H.J., Brentjens R.J. Overcoming antigen escape with CAR-T-cell therapy. Cancer Discov. 2015;5:1238–1240. doi: 10.1158/2159-8290.CD-15-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sadelain M. Tales of antigen evasion from CAR therapy. Cancer Immunol Res. 2016;4:473. doi: 10.1158/2326-6066.CIR-16-0089. [DOI] [PubMed] [Google Scholar]
- 130.Parato K.A., Senger D., Forsyth P.A., Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 131.Cui B., Cao X., Zou W., Wan Y., Wang N., Wang Y. Regulation of immune-related diseases by multiple factors: a meeting report of 2017 International Workshop of the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine on Tumor Immunology. Acta Pharm Sin B. 2017;7:532–540. [Google Scholar]
- 132.Bartee M.Y., Dunlap K.M., Bartee E. Myxoma virus induces ligand independent extrinsic apoptosis in human myeloma cells. Clin Lymphoma Myeloma Leuk. 2016;16:203–212. doi: 10.1016/j.clml.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Senac J.S., Doronin K., Russell S.J., Jelinek D.F., Greipp P.R., Barry M.A. Infection and killing of multiple myeloma by adenoviruses. Hum Gene Ther. 2010;21:179–190. doi: 10.1089/hum.2009.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Au G.G., Lincz L.F., Enno A., Shafren D.R. Oncolytic Coxsackievirus A21 as a novel therapy for multiple myeloma. Br J Haematol. 2007;137:133–141. doi: 10.1111/j.1365-2141.2007.06550.x. [DOI] [PubMed] [Google Scholar]
- 135.Qian W., Liu J., Tong Y., Yan S., Yang C., Yang M. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia. 2008;22:361–369. doi: 10.1038/sj.leu.2405034. [DOI] [PubMed] [Google Scholar]
- 136.Esfandyari T., Tefferi A., Szmidt A., Alain T., Zwolak P., Lasho T. Transcription factors down-stream of Ras as molecular indicators for targeting malignancies with oncolytic herpes virus. Mol Oncol. 2009;3:464–468. doi: 10.1016/j.molonc.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Castleton A., Dey A., Beaton B., Patel B., Aucher A., Davis D.M. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood. 2014;123:1327–1335. doi: 10.1182/blood-2013-09-528851. [DOI] [PubMed] [Google Scholar]
- 138.Nishio N., Diaconu I., Liu H., Cerullo V., Caruana I., Hoyos V. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74:5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosewell Shaw A., Porter C.E., Watanabe N., Tanoue K., Sikora A., Gottschalk S. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR-T cells against metastatic head and neck cancer. Mol Ther. 2017;25:2440–2451. doi: 10.1016/j.ymthe.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geyer M.B., Brentjens R.J. Review: current clinical applications of chimeric antigen receptor (CAR) modified T cells. Cytotherapy. 2016;18:1393–1409. doi: 10.1016/j.jcyt.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ciceri F., Bonini C., Marktel S., Zappone E., Servida P., Bernardi M. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109:4698–4707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 142.Heiblig M., Elhamri M., Michallet M., Thomas X. Adoptive immunotherapy for acute leukemia: new insights in chimeric antigen receptors. World J Stem Cells. 2015;7:1022–1038. doi: 10.4252/wjsc.v7.i7.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Juillerat A., Marechal A., Filhol J.M., Valton J., Duclert A., Poirot L. Design of chimeric antigen receptors with integrated controllable transient functions. Sci Rep. 2016;6:18950. doi: 10.1038/srep18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ma J.S., Kim J.Y., Kazane S.A., Choi S.H., Yun H.Y., Kim M.S. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A. 2016;113:E450–E458. doi: 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]