Abstract
HER3 belongs to the human epidermal growth factor receptor (HER) family which also includes HER1/EGFR/erbB1, HER2/erbB2, and HER4/erbB4. As a unique member of the HER family, HER3 lacks or has little intrinsic tyrosine kinase activity. It frequently co-expresses and forms heterodimers with other receptor tyrosine kinases (RTKs) in cancer cells to activate oncogenic signaling, especially the PI-3K/Akt pathway and Src kinase. Elevated expression of HER3 has been observed in a wide variety of human cancers and associates with a worse survival in cancer patients with solid tumors. Studies on the underlying mechanism implicate HER3 expression as a major cause of treatment failure in cancer therapy. Activation of HER3 signaling has also been shown to promote cancer metastasis. These data strongly support the notion that therapeutic inactivation of HER3 and/or its downstream signaling is required to overcome treatment resistance and improve the outcomes of cancer patients.
Abbreviations: Ab, antibody; ADCC, antibody-dependent cell-mediated cytotoxicity; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition; FDA, Food and Drug Administration; HER, Human epidermal growth factor receptor; HRG, heregulin; IGF-1R, insulin-like growth factor-I receptor; lncRNA, long ncRNA; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; miRNA, microRNA; ncRNA, noncoding RNA; NSCLC, non-small cell lung cancer; OS, overall survival; PI-3K, phosphoinositide 3-kinase; RTK, receptor tyrosine kinase; TKI, tyrosine kinase inhibitor
Key words: HER3, Dimerization, Cell signaling, Therapeutic resistance, Tumor metastasis, Targeted therapy
Graphical abstract
HER3 belongs to the human epidermal growth factor receptor (HER) family. HER3 lacks or has little intrinsic tyrosine kinase activity, but it frequently co-expresses and forms heterodimers with other receptor tyrosine kinases (RTKs) to activate oncogenic signaling in cancer cells. Therapeutic inactivation of HER3 and/or its downstream signaling is required to overcome treatment resistance and improve the outcomes of cancer patients.
1. Introduction
Human epidermal growth factor receptor (HER) family includes the epidermal growth factor receptor (EGFR), HER2 (also known as erbB2/neu), HER3 (erbB3), and HER4 (erbB4). It is arguably the most important family of receptor tyrosine kinase (RTK) in normal development and tumorigenesis1, 2. These receptors are widely expressed in epithelial, mesenchymal, and neuronal cells3. Abnormal expression of HER family members is involved in carcinogenesis and progression of diverse types of human cancer4, 5. While EGFR, HER3, and HER4 have ligands, HER2 has no known ligand. When a ligand binds to the extracellular region of EGFR, HER3, or HER4 (domains I and III), the dimerization arm in domain II is exposed leading to receptor-receptor interaction6. Dimerization is an essential step for the receptor function and activation of the cytoplasmic signaling, including PI-3K/Akt, MEK/MAPK, Jak/Stat pathways, Src kinase, etc.5, 7. EGFR, HER3, and HER4 normally exist as inactive molecularly folded monomers to prevent dimerization8, 9, whereas HER2 is always in a constitutively active conformation with its dimerization arm opening even without ligand binding8. Accumulating evidence indicates that HER3 frequently co-expresses and interacts with other RTKs to form a heterodimeric complex, which subsequently activates oncogenic signaling, especially the PI-3K/Akt pathway and Src kinase to promote cancer cell survival, proliferation, and progression10, 11, 12. Studies on the underlying mechanisms demonstrate that HER3 signaling plays a major role causing treatment failure in cancer therapy13, 14. Recent reports reveal that enhanced HER3 signaling facilitates tumor cell motility and intravasation in breast cancer lung metastasis15; and a HER3-lncRNA (long noncoding RNA) axis regulates bone metastasis in breast cancer16, 17. Increased expression and activation of HER3 has also been observed in brain metastasis of breast cancer resistance to PI-3K inhibition18, 19. Collectively, these data support the importance of developing effective therapeutics to inhibit HER3 signaling for cancer treatment. A number of anti-HER3 monoclonal antibodies are actively under preclinical studies and clinical evaluation in cancer patients. There is currently no HER3-targeted therapy approved by the FDA for cancer treatment. This review summarizes our understanding of the unique biology of HER3 in cancer progression and discusses the latest advances in identifying therapeutic antibodies against HER3 for cancer treatment.
2. Unique biology of HER3-initiated signaling in human cancer
HER3 is a unique member of the HER family as it has been considered as an inactive receptor20, 21, although a recent study suggests that HER3 contains weak kinase activity22. Sequence comparison of tyrosine kinase domains among the HER receptors reveals that certain residues, including Cys-721, His-740, and Asn-815, in HER3 have non-conservative substitutions. These changes significantly reduce the kinase activity of HER323. Thus, to fully transduce signaling, HER3 has to form dimers with other receptors and be phosphorylated by its interactive partners, with HER2 being the most important one24. Of the four HER receptors, HER3 is best suited to induce activation of the PI-3K/Akt pathway, which is a well-known survival signaling pathway in normal development and tumorigenesis25. This is likely due to the C-terminal tail of HER3 having multiple tyrosine residues, whose phosphorylation is able to bind to the p85 subunit of PI-3K24. It is thought that, among all the homo- and hetero-dimerization complexes potentially formed by HER receptors, the HER2/HER3 heterodimer is the most biologically active and potent to activate the PI-3K/Akt signaling cascade26, 27.
Overexpression of HER3 is frequently observed in a wide variety of human malignancies, including colorectal carcinoma, head and neck squamous cell carcinoma, melanoma, and breast, gastric, ovarian, prostate, and bladder cancers28, 29, 30. Moreover, it has been shown that HER3 is a more potent partner than other HER receptors for the oncogenic activity of HER2 in HER2-overexpressing tumors29, 31, 32, 33. Especially in erbB2-amplified breast cancers, preferential phosphorylation of HER3, but not EGFR, is found29. Indeed, most metastatic breast cancers have expression of either EGFR or HER2, and rarely express both34. In contrast, HER2 and HER3 commonly co-express in breast cancer tissues35 and breast cancer cell lines36. Elevated expression of the endogenous mouse HER3 and its association with the transgene encoded erbB2 promote mammary tumorigenesis in erbB2/neu-transgenic mice37, 38. Despite its lack of20, 21 or weak kinase activity22, HER3 serves as a critical co-receptor of HER2 and its expression is essential for HER2-mediated breast cancer cell survival and proliferation10, 11. These data have been supported by a recent meta-analysis of 12 clinical studies of human cancers, including colorectal cancer, gastric cancer, breast cancer, melanoma, ovarian cancer, head and neck cancer, pancreatic cancer, and cervical cancer39. It concludes that expression of HER3 is associated with worse survival in solid tumors, and the impact of HER3 on clinical outcome is greater in those tumors where HER2 is also overexpressed39.
Overexpression of HER3 has been reported in 50%–70% of human breast cancers40, 41, 42 and appears to be associated with prognostic factors, such as distant metastasis, tumor size, risk of local recurrence, and etc.43, 44, although the prognostic value of HER3 in breast cancer is not well documented and the currently available data are inconsistent42, 43, 44, 45, 46. Some studies show that elevated expression of HER3 significantly correlates with reduced overall survival and disease-free survival35, 47, 48, whereas others report HER3 expression as a favorable prognostic factor of overall survival in breast cancer patients49, 50. Several theories have been proposed to explain the controversial findings, such as the potential influence of HER3 ligand—heregulin (HRG) and subcellular distribution of HER314. The fact that we do not have a unified methodology to detect HER3 expression in clinical samples may also account for the inconsistent data, as each laboratory uses different antibodies and probes to detect the expression of HER3 protein and mRNA. In addition, breast cancer is a heterogenous disease with several intrinsic subtypes, including luminal, HER2-enriched, and triple negative breast cancer (TNBC)51. It is possible that HER3 exhibits distinct influences on patient survival in different subtypes of breast cancer. Thus, detailed evaluation of HER3 expression and its interactive partners in a specific subtype is warranted to define the prognostic value of HER3 signaling in such subtype of breast cancer patients.
3. Mechanism of HER3-mediated cancer progression
Accumulating evidence emphasizes the critical role of HER2/HER3 heterodimer-mediated PI-3K/Akt signaling in cancer development39, 52. Basic research on the underlying mechanisms indicates that HER2 contributes to breast carcinogenesis potentially via two major mechanisms—increased therapeutic resistance and enhanced metastatic potential53, 54. Thus, it is conceivable to hypothesize that HER3 signaling-mediated cancer progression is likely through its capability to induce therapeutic resistance and promote tumor metastasis.
3.1. HER3 and cancer treatment resistance
A recent report implicates HER3 activation as a major cause of treatment failure in cancer therapy13. It has been shown that HER3 signaling plays a crucial role in the development of various human cancers, including HER2-overexpressing breast cancer10, 11, castration-resistant prostate cancer55, platinum-resistant/refractory ovarian cancer56, 57, and non-small cell lung cancer (NSCLC) resistance to EGFR tyrosine kinase inhibitor (TKI)58, 59. A number of studies reveal that compensatory upregulation of HER3 along with the sustained PI-3K/Akt signaling is implicated as an important mechanism resulting in resistance to EGFR-targeted therapy60, 61, 62, 63. In addition, elevated expression of the HER3 ligand (HRG) is a possible mechanism of resistance to anti-EGFR antibody (Ab)-cetuximab in the treatment of patients with colorectal cancer64. Furthermore, HER3 may work in concert with other RTKs, such as hepatocyte growth factor receptor (HGFR or MET)65. Amplification of MET oncogene may also result in resistance to EGFR-TKI (gefitinib). Phosphorylated HER3 was able to interact with the p85 subunit of PI-3K in a MET kinase-dependent manner in NSCLC, suggesting a role of HER3 in MET-induced resistance to gefitinib65. In squamous cell carcinomas of head and neck cancer cell lines sensitive to the dual EGFR/HER2 inhibitor lapatinib, increased HRG and activated HER3 strongly correlated with lapatinib sensitivity66. However, the potential mechanism by which HER3 may be a valuable biomarker for lapatinib sensitivity and gefitinib resistance remains unclear. It may be through distinct activation mechanisms that need to be further investigated.
Studies in our laboratory have been focusing on the biologic function of HER3 in the progression of erbB2-aberrant breast cancer. We show that elevated expression of HER3 in HER2-overexpressing breast cancer cells results in resistance to hormone therapy (tamoxifen), HER2-targeted therapy (trastuzumab and lapatinib), and chemotherapy (paclitaxel)67, 68, 69, 70, 71. Our data demonstrate the crucial role of HER3 signaling in HER2-mediated therapeutic resistance to tamoxifen, trastuzumab, and paclitaxel in breast cancer12, 14. One innovative finding comes from our studies on the underlying mechanism of HER3-mediated resistance to the anti-HER2 antibody trastuzumab (also known as Herceptin). It was reported that both HER3 and the insulin-like growth factor-I receptor (IGF-1R)-mediated signaling contributed to trastuzumab resistance72, 73, 74, whereas the relationship between HER3 and IGF-IR in trastuzumab resistance was less understood. Our studies uncovered that HER2 interacted with both HER3 and IGF-1R to form a heterotrimeric complex in the trastuzumab-resistant breast cancer cells we tested. In fact, it was the heterotrimer of HER2/HER3/IGF-1R, not the heterodimer of HER2/HER3 or IGF-1R/HER2, that played a causal role leading to trastuzumab resistance67. Further studies on downstream signaling revealed that HER3 and IGF-1R triggered different signaling pathways contributing to trastuzumab resistance - HER3 activated both PI-3K/Akt signaling and Src kinase, whereas IGF-1R mainly elicited Src activation67. Interestingly, our recent data show that HER3 and IGF-1R exhibit distinct effects on the sensitivity of HER2-overexpressing breast cancer cells to lapatinib, another HER2-targeted therapy71. While HER3 signaling also induces lapatinib resistance in the trastuzumab-resistant breast cancer cells, IGF-1R signaling did not alter lapatinib sensitivity71.
3.2. HER3 and tumor metastasis
HER3 frequently co-expresses and interacts with HER2 to activate oncogenic signaling, especially the PI-3K/Akt pathway and Src kinase, and promote cancer cell survival, proliferation, and progression10, 11, 12. We have shown that elevated expression of HER3 confers resistance to several commonly used therapeutics against HER2-overexpressing breast cancer67, 68, 69, 70, 71. Drug-resistant tumors likely recur and metastasize to distant organs. Thus, it is generally believed that overexpression of HER3 and its downstream signaling can promote tumor metastasis. Activation of HER3 signaling facilitates tumor cell motility and intravasation in lung metastasis of human breast cancer15. Our analysis of clinical database reveals that increased HER3 expression leads to a worse overall survival (OS) in lymph node positive breast cancer patients. Especially in HER2-overexpressing breast cancer, the patients with higher expression of HER3 show poorer OS and distant metastasis-free survival (Liu laboratory unpublished data). In addition, the HER3 ligand, HRG can stimulate chemotaxis and invasion via HER2/HER3 heterodimers75. Recent studies suggest that the HRG-HER3 signaling axis plays a crucial role in the brain metastasis of breast cancer18, 19. While overexpression of HER3 is found in the brain metastatic legions of breast cancer19, 76, activation of HER3 and its downstream signaling has also been observed in breast cancer brain metastasis likely via increased HRG production by the stromal cells in brain microenvironment18, 19, 77. Activation of the downstream signaling, such as the PI-3K/Akt and MEK/MAPK pathways can be critical for cell motility and chemotaxis75, 78, 79, 80, 81, 82. PI-3K is capable of regulating cytoskeleton through Rho family G proteins and Akt activation83, 84, 85. MAPKs can influence adhesion dynamics directly and control gene expression patterns essential for motility and invasion86, 87, 88. It is possible that HER3-dependent motility contributes to cancer metastasis independent of its effects on tumor growth89. Studies on the underlying mechanisms involved in ovarian cancer spread to the omentum shows that elevated expression of HER3 in ovarian cancer cells and increased HRG in the omentum allows for cancer cell localization and growth in the omentum. These findings suggest that the HRG-HER3 signaling axis is also a dominant mechanism responsible for ovarian cancer metastasis via blood stream90.
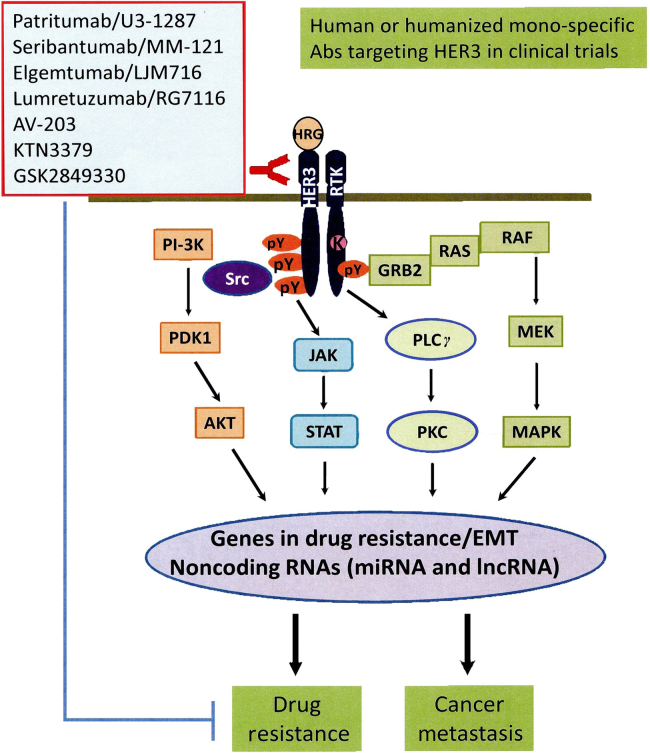
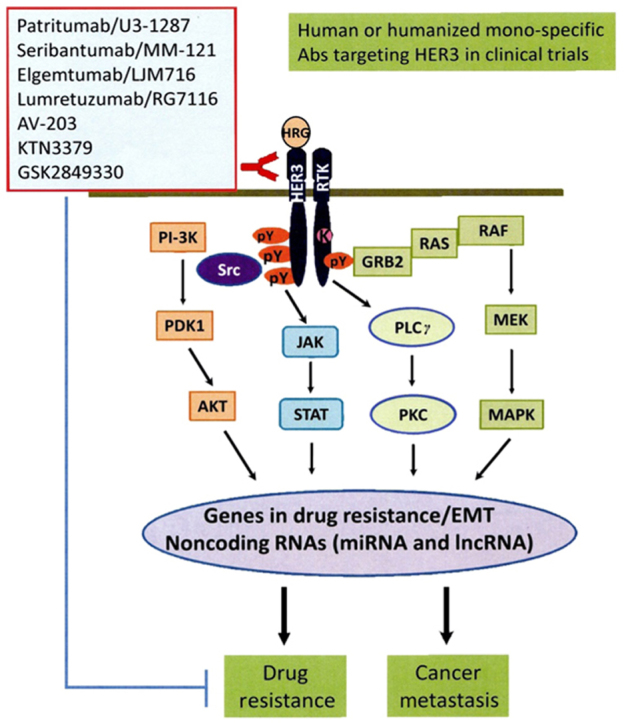
Interestingly, noncoding RNA (ncRNA), including the long ncRNA (lncRNA) MAYA also plays an important role in HER3-mediated tumor metastasis17. It has been reported that a ROR1-HER3-lncRNA axis regulates bone metastasis in breast cancer16, 17. In our efforts to identify key downstream mediators of HER3 signaling in breast cancer metastasis, we found that HER3 signaling specifically downregulates expression of the tumor suppressive miR-203 and miR-542-3p in HER2-overexpressing breast cancer cells91. Bioinformatics analyses reveal that miR-203 and miR-542-3p target several genes, including Survivin, ZEB1, ZEB2, Snail1, and/or Slug, which are critical for drug resistance, epithelial-mesenchymal transition (EMT), and tumor metastasis (Liu's laboratory unpublished data). These data support the notion that HER3 signaling regulates expression of lncRNAs and miRNAs to promote cancer metastasis. Studies in this innovative area will not only further our understanding of HER3 signaling in cancer biology, but may also provide a new avenue for identification of novel therapeutic approaches to abrogate HER3-mediated treatment resistance and tumor metastasis. Figure 1 shows a simple diagram depicting that activation of HER3 and its major downstream signaling induces expression of a cohort of critical molecules, including some EMT markers and ncRNAs, responsible drug resistance and cancer metastasis.
Figure 1.
A diagram showing the major signaling pathways of HER3 during cancer progression and the mono-specific HER3 blocking Abs currently in clinical trilas of cancer patients. The ligand, HRG bound HER3 recruits another RTK to form a heterodimer, which subsequently triggers activation of multiple signaling pathways, including PI-3K/Akt, MEK/MAPK, Jak/Stat pathways, and Src kinase. The downstream signaling will further induces expression of a cohort of crucial genes responsible for drug resistance and cancer metastasis. HER3 signaling is also able to regulate expression of some ncRNAs, including miRNAs and lncRNAs. Currently, there are several human or humanized anti-HER3 mono- and bi-specific Abs in clinical trilas testing their therapeutic activity to abrogate drug resistance and inhibit cancer metastasis.
4. Therapeutic antibody against HER3 for cancer treatment
Elevated expression of HER3 plays an essential role in human cancer progression and correlates with a worse overall survival in many solid tumors13, 25, 39, emphasizing the importance in developing novel effective strategic targeting of HER314, 52, 92. Inhibition of HER3 is believed to be required to overcome resistance and effectively treat cancer patients. Because of its lack of or low kinase activity21, 22, targeting HER3 with a blocking Ab is the only strategy under preclinical studies93, 94 and clinical evaluations in patients with advanced solid tumors (http://www.clinicaltrials.gov). Advances have been made to identify HER3-targeted therapy95, and several anti-HER3 monoclonal Abs exhibit antitumor activity in vivo and show promise as novel cancer therapeutics96, 97. Recent studies have identified bispecific Abs dual-targeting EGFR/HER359 or HER2/HER398, that exert potent antitumor activities in both laboratory studies and clinic testing95. The HER3 inhibitors based on a novel biologic scaffold termed surrobody have been developed and display anti-proliferative effects on cancer cells in vitro and in vivo99.
MM-121 (also known as seribantumab, Merrimack Pharmaceuticals, Cambridge, MA), a human anti-HER3 monoclonal IgG2 Ab, blocks ligand-induced HER2/HER3 dimerization and subsequently inhibits downstream signaling. MM-121 exerts antitumor activity in preclinical studies of various human cancers93, 94. We have tested the hypothesis that MM-121 may be able to abrogate HER3 signaling-mediated resistance to trastuzumab and paclitaxel in HER2-overexpressing breast cancer cells via inactivation of HER3 and its downstream PI-3K/Akt signaling. We reported that MM-121 was able to overcome paclitaxel resistance and significantly enhanced paclitaxel-induced apoptosis in the otherwise resistant breast cancer cell lines100. We also showed that MM-121 dramatically inhibited PI-3K/Akt signaling in HER2-overexpressing breast cancer cells refractory to trastuzumab, and significantly enhanced trastuzumab-induced growth inhibition101. MM-121 in combination with trastuzumab mainly induced cell cycle G1 arrest in vitro, whereas the combinations of MM-121 and trastuzumab potently inhibited tumor growth in vivo likely due to induction of both growth inhibition and apoptosis101. Our data strongly support the initiation of clinical trials to evaluate the efficacy of MM-121 in combination with trastuzumab or paclitaxel in HER2-overexpressing breast cancer patients who have developed resistance to the therapeutics. Interestingly, recent studies suggest that higher HRG mRNA expression and low HER2 levels predict a clinical benefit from the addition of seribantumab (MM-121) to standard of care therapies in patients with platinum-resistant/refractory ovarian cancer, hormone receptor-positive HER2-negative breast cancer, and EGFR wild-type NSCLC102, 103. MM-111 (Merrimack Pharmaceuticals, Cambridge, MA) is a bispecific antibody, dual-targeting HER2/HER3, inhibiting the PI-3K/Akt signaling98. The safety and clinical activity of MM-111 is now being tested in several phase I clinical trials. Another HER3-targeted drug, U3–1287/AMG-888 (originally developed by Amgen Inc., Thousand Oaks, CA; later acquired by Daiichi Sankyo Co., Ltd., Tokyo, Japan and re-named as patritumab) is the first fully human anti-HER3 monoclonal Ab and currently under phase III clinical investigations in patients with advanced solid tumors104. This Ab has been shown to inhibit proximal and distal HER signaling and induces rapid internalization of HER3105. Patritumab induces growth inhibition in various cancer cell lines (breast, lung, colorectal) that are resistant to other HER inhibitors105. It significantly decreases colony formation in pancreatic cancer cells and tumor growth in tumor xenograft models of pancreatic cancer, NSCLC, and colorectal cancer55. Interestingly, patritumab is also able to overcome HRG-dependent resistance to EGFR inhibitors in NSCLC in vitro and in vivo, suggesting that patritumab may be useful in combination with EGFR TKIs, such as erlotinib to treat the NSCLC patients with high expression of HRG106, 107. Lumretuzumab (RG7116) is a humanized anti-HER3 IgG1 monoclonal Ab developed by Roche Diagnostics GmbH (Penzberg, Germany). It binds to the extracellular domain of HER3 with high affinity to prevent HRG binding108. As a glycoengineered monoclonal Ab, lumretuzumab displayed an enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) activity as compared with the non-glycoengineered parental antibody109. Although lumretuzumab was well tolerated and showed evidence of clinical activity in a phase I trial110, two recent phase Ib studies suggest otherwise. The toxicity profile of lumretuzumab in combination with the EGFR-targeted therapies cetuximab and erlotinib was managable, but it exerted little clinical benefit in various cancers111. The therapeutic window of lumretuzumab in combination with the anti-HER2 Ab pertuzumab and chemotherapeutic drug paclitaxel for HER3-positive metastatic breast cancer was too narrow to warrant further clinical development112. Several anti-HER3 mono-specific Abs currently under clinical trials with a hope to abrogate HER3-mediated drug resistance and cancer metastasis are shown in Figure 1. Recently, a new anti-HER3 Ab (MP-RM-1) and its humanized version (EV20) exhibit potent antitumor effects in several cancer types in vitro and in vivo113, 114. Because of EV20׳s capability to inhibit both ligand-dependent and -independent activation of HER3113, 114, it is speculated that EV20 may have a broader effect on blocking HER3 signaling than the Abs (like MM-121) which can only block ligand-induced HER3 activation.
5. Perspectives
Research on HER receptors has been focusing on the dysregulation of EGFR and HER2 in human malignancies. The importance of HER3 as an obligate partner for receptor dimerization and in resistance to HER2- or EGFR-targeted therapy and other therapeutics has drawn a lot of attention to define HER3 as a molecular target for cancer treatment. Increased awareness of HER3 function in drug resistance and tumor metastasis has critical implications in the directions of future studies. First, the crucial downstream mediators of HER3 signaling in cancer progression remain elusive. Basic research deciphering the molecular basis of HER3-mediated drug resistance and tumor metastasis is essential to improve our understanding of the unique biology of HER3 in human cancer. Such studies will also facilitate the development of novel therapeutic approaches inhibiting the key downstream mediators against those cancers driven by HER3 signaling. Second, although several anti-HER3 Abs are actively under clinical evaluations in various human cancers, to date no HER3-targeted therapy has been approved by the FDA for cancer treatment. This is possibly due to the uniqueness of HER3 receptor, which may influence the antitumor activity of anti-HER3 Abs. Since HER3 has to form heterodimer or heterotrimer complexes with other RTKs in order to fully transduce signaling10, 11, 67, anti-HER3 monotherapy is unlikely to show significant efficacy against human cancer. We must consider effective combination strategies with a HER3-targeted therapy plus other targeted therapies or chemotherapeutic agents for cancer treatment. Third, it has been shown that HRG expression at tumor sites predicts efficacy of seribantumab (MM-121) in the treatment of human cancers102, 103, suggesting that identification of predictive biomarkers will stratify the usage of anti-HER3 Abs for effective cancer treatment. Indeed, a new anti-HER3 Ab, 9F7-F11, which does not compete with the ligand (HRG), shows higher efficacy than the Abs that compete with the ligand for binding to HER3115. In human tumor cell xenograft models, 9F7-F11 exerts an enhanced antitumor activity in the presence of HRG and thus represents a novel treatment strategy for HRG-addicted tumors115. We believe that HER3 is a focal point in HER receptors-mediated tumorigenesis and plays an essential role in cancer progression. Thus, HER3 constitutes a unique biomarker and molecular target for effective treatment of human cancer.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (NIH), USA (R01CA201011 to BL) and a grant from the National Natural Science Foundation of China (81472763 to BL).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Baselga J., Swain S.M. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 2.Hynes N.E., MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Hynes N.E., Lane H.A. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 4.DeFazio A., Chiew Y.E., Sini R.L., Janes P.W., Sutherland R.L. Expression of c‐erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer. 2000;87:487–498. [PubMed] [Google Scholar]
- 5.Olayioye M.A., Neve R.M., Lane H.A., Hynes N.E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogiso H., Ishitani R., Nureki O., Fukai S., Yamanaka M., Kim J.-H. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/s0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson K.M., Berger M.B., Mendrola J.M., Cho H.-S., Leahy D.J., Lemmon M.A. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11:507–517. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 8.Burgess A.W., Cho H.-S., Eigenbrot C., Ferguson K.M., Garrett T.P., Leahy D.J. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 9.Cho H.-S., Leahy D.J. Structure of the extracellular region of HER3 reveals an interdomain tether. Sci Signal. 2002;297:1330–1333. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 10.Holbro T., Beerli R.R., Maurer F., Koziczak M., Barbas C.F., 3rd, Hynes N.E. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: erbb2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee-Hoeflich S.T., Crocker L., Yao E., Pham T., Munroe X., Hoeflich K.P. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y., Ma J., Lyu H., Huang J., Kim A., Liu B. Role of erbB3 receptors in cancer therapeutic resistance. Acta Biochim Biophys Sin (Shanghai) 2014;46:190–198. doi: 10.1093/abbs/gmt150. [DOI] [PubMed] [Google Scholar]
- 13.Amin D.N., Campbell M.R., Moasser M.M. The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin Cell Dev Biol. 2010;21:944–950. doi: 10.1016/j.semcdb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J., Lyu H., Huang J., Liu B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer. 2014;13:105. doi: 10.1186/1476-4598-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue C., Liang F., Mahmood R., Vuolo M., Wyckoff J., Qian H. ErbB3-dependent motility and intravasation in breast cancer metastasis. Cancer Res. 2006;66:1418–1426. doi: 10.1158/0008-5472.CAN-05-0550. [DOI] [PubMed] [Google Scholar]
- 16.Li C., Wang S., Xing Z., Lin A., Liang K., Song J. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19:106–119. doi: 10.1038/ncb3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuo W., Kang Y. Lnc-ing ROR1-HER3 and Hippo signalling in metastasis. Nat Cell Biol. 2017;19:81–83. doi: 10.1038/ncb3467. [DOI] [PubMed] [Google Scholar]
- 18.Kabraji S., Ni J., Lin N.U., Xie S., Winer E.P., Zhao J.J. Drug resistance in HER2-positive breast cancer brain metastases: blame the barrier or the brain? Clin Cancer Res. 2018;24:1795–1804. doi: 10.1158/1078-0432.CCR-17-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodack D.P., Askoxylakis V., Ferraro G.B., Sheng Q., Badeaux M., Goel S. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci Transl Med. 2017;9:eaal4682. doi: 10.1126/scitranslmed.aal4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boudeau J., Miranda-Saavedra D., Barton G.J., Alessi D.R. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Citri A., Skaria K.B., Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 22.Shi F., Telesco S.E., Liu Y., Radhakrishnan R., Lemmon M.A. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A. 2010;107:7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plowman G.D., Whitney G.S., Neubauer M.G., Green J.M., McDonald V.L., Todaro G.J. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc Natl Acad Sci U S A. 1990;87:4905–4909. doi: 10.1073/pnas.87.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulze W.X., Deng L., Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1(2005):0008. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell M.R., Amin D., Moasser M.M. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res. 2010;16:1373–1383. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattoon D., Lamothe B., Lax I., Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2004;2:24. doi: 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suenaga A., Takada N., Hatakeyama M., Ichikawa M., Yu X., Tomii K. Novel mechanism of interaction of p85 subunit of phosphatidylinositol 3-kinase and ErbB3 receptor-derived phosphotyrosyl peptides. J Biol Chem. 2005;280:1321–1326. doi: 10.1074/jbc.M410436200. [DOI] [PubMed] [Google Scholar]
- 28.Beji A., Horst D., Engel J., Kirchner T., Ullrich A. Toward the prognostic significance and therapeutic potential of HER3 receptor tyrosine kinase in human colon cancer. Clin Cancer Res. 2012;18:956–968. doi: 10.1158/1078-0432.CCR-11-1186. [DOI] [PubMed] [Google Scholar]
- 29.Lee-Hoeflich S.T., Crocker L., Yao E., Pham T., Munroe X., Hoeflich K.P. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 30.Maurer C.A., Friess H., Kretschmann B., Zimmermann A., Stauffer A., Baer H.U. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Human Path. 1998;29:771–777. doi: 10.1016/s0046-8177(98)90444-0. [DOI] [PubMed] [Google Scholar]
- 31.Alimandi M., Romano A., Curia M.C., Muraro R., Fedi P., Aaronson S.A. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- 32.Hsieh A., Moasser M. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453–457. doi: 10.1038/sj.bjc.6603910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallasch C., Weiss F., Niederfellner G., Jallal B., Issing W., Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grupka N.L., Lear-Kaul K.C., Kleinschmidt-DeMasters B.K., Singh M. Epidermal growth factor receptor status in breast cancer metastases to the central nervous system. Comparison with HER-2/neu status. Arch Pathol Lab Med. 2004;128:974–979. doi: 10.5858/2004-128-974-EGFRSI. [DOI] [PubMed] [Google Scholar]
- 35.Bieche I., Onody P., Tozlu S., Driouch K., Vidaud M., Lidereau R. Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer. 2003;106:758–765. doi: 10.1002/ijc.11273. [DOI] [PubMed] [Google Scholar]
- 36.deFazio A., Chiew Y.E., Sini R.L., Janes P.W., Sutherland R.L. Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer. 2000;87:487–498. [PubMed] [Google Scholar]
- 37.Siegel P.M., Ryan E.D., Cardiff R.D., Muller W.J. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim A., Liu B., Ordonez-Ercan D., Alvarez K.M., Jones L.D., McKimmey C. Functional interaction between Mouse erbB3 and wild type rat c-neu in transgenic mouse mammary tumor cells. Breast Cancer Res. 2005;7:R708–R718. doi: 10.1186/bcr1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ocana A., Vera-Badillo F., Seruga B., Templeton A., Pandiella A., Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst. 2013;105:266–273. doi: 10.1093/jnci/djs501. [DOI] [PubMed] [Google Scholar]
- 40.Barnes N.L., Khavari S., Boland G.P., Cramer A., Knox W.F., Bundred N.J. Absence of HER4 expression predicts recurrence of ductal carcinoma in situ of the breast. Clin Cancer Res. 2005;11:2163–2168. doi: 10.1158/1078-0432.CCR-04-1633. [DOI] [PubMed] [Google Scholar]
- 41.Naidu R., Yadav M., Nair S., Kutty M. Expression of c-erbB3 protein in primary breast carcinomas. Br J Cancer. 1998;78:1385–1390. doi: 10.1038/bjc.1998.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinn C., Ostrowski J., Lane S., Loney D., Teasdale J., Benson E. c‐erbB‐3 protein expression in human breast cancer: comparison with other tumour variables and survival. Histopathology. 1994;25:247–252. doi: 10.1111/j.1365-2559.1994.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 43.Lemoine N., Barnes D., Hollywood D., Hughes C., Smith P., Dublin E. Expression of the ERBB3 gene product in breast cancer. Br J Cancer. 1992;66:1116–1121. doi: 10.1038/bjc.1992.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Travis A., Pinder S., Robertson J., Bell J., Wencyk P., Gullick W. C-erbB-3 in human breast carcinoma: expression and relation to prognosis and established prognostic indicators. Br J Cancer. 1996;74:229–233. doi: 10.1038/bjc.1996.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasparini G., Pozza F., Bevilacqua P., Gullick W., Lemoine N., Maluta S. c-erbB-3 and c-erbB-2 protein expression in node-negative breast carcinoma—an immunocytochemical study. Eur J Cancer. 1994;30:16–22. doi: 10.1016/s0959-8049(05)80010-3. [DOI] [PubMed] [Google Scholar]
- 46.Pawlowski V., Révillion F., Hebbar M., Hornez L., Peyrat J.-P. Prognostic value of the type I growth factor receptors in a large series of human primary breast cancers quantified with a real-time reverse transcription-polymerase chain reaction assay. Clin Cancer Res. 2000;6:4217–4225. [PubMed] [Google Scholar]
- 47.Sassen A., Rochon J., Wild P., Hartmann A., Hofstaedter F., Schwarz S. Cytogenetic analysis of HER1/EGFR, HER2, HER3 and HER4 in 278 breast cancer patients. Breast Cancer Res. 2008;10:R2. doi: 10.1186/bcr1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witton C.J., Reeves J.R., Going J.J., Cooke T.G., Bartlett J.M. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 49.Koutras A.K., Kalogeras K.T., Dimopoulos M.A., Wirtz R.M., Dafni U., Briasoulis E. Evaluation of the prognostic and predictive value of HER family mRNA expression in high-risk early breast cancer: a Hellenic Cooperative Oncology Group (HeCOG) study. Br J Cancer. 2008;99:1775–1785. doi: 10.1038/sj.bjc.6604769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y., Cho S., Seo J.H., Shin B.K., Kim H.K., Kim I. Correlated expression of erbB-3 with hormone receptor expression and favorable clinical outcome in invasive ductal carcinomas of the breast. Am J Clin Pathol. 2007;128:1041–1049. doi: 10.1309/GA5VRFQFY5D0MVKD. [DOI] [PubMed] [Google Scholar]
- 51.Russnes H.G., Lingjaerde O.C., Borresen-Dale A.L., Caldas C. Breast cancer molecular stratification: from intrinsic subtypes to integrative clusters. Am J Pathol. 2017;187:2152–2162. doi: 10.1016/j.ajpath.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 52.Mujoo K., Choi B.K., Huang Z., Zhang N., An Z. Regulation of ERBB3/HER3 signaling in cancer. Oncotarget. 2014;5:10222–10236. doi: 10.18632/oncotarget.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu D., Hung M.C. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115–6121. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 54.Eccles S.A. The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol. 2011;55:685–696. doi: 10.1387/ijdb.113396se. [DOI] [PubMed] [Google Scholar]
- 55.Jathal M.K., Chen L., Mudryj M., Ghosh P.M. Targeting ErbB3: the new RTK(id) on the prostate cancer block. Immunol Endocr Metab Agents Med Chem. 2011;11:131–149. doi: 10.2174/187152211795495643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mills G.B., Yarden Y. The rebirth of a phoenix: ovarian cancers are addicted to ErbB-3. Cancer Cell. 2010;17:217–218. doi: 10.1016/j.ccr.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 57.Sheng Q., Liu X., Fleming E., Yuan K., Piao H., Chen J. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell. 2010;17:298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 59.Huang S., Li C., Armstrong E.A., Peet C.R., Saker J., Amler L.C. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2013;73:824–833. doi: 10.1158/0008-5472.CAN-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baselga J., Swain S.M. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 61.Bianco R., Shin I., Ritter C.A., Yakes F.M., Basso A., Rosen N. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 62.Sergina N.V., Rausch M., Wang D., Blair J., Hann B., Shokat K.M. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.She Q.-B., Solit D., Basso A., Moasser M.M. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/Akt pathway signaling. Clin Cancer Res. 2003;9:4340–4346. [PubMed] [Google Scholar]
- 64.Yonesaka K., Zejnullahu K., Okamoto I., Satoh T., Cappuzzo F., Souglakos J. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 66.Wilson T.R., Lee D.Y., Berry L., Shames D.S., Settleman J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell. 2011;20:158–172. doi: 10.1016/j.ccr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Huang X., Gao L., Wang S., McManaman J.L., Thor A.D., Yang X. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-I receptor in breast cancer cells resistant to herceptin. Cancer Res. 2010;70:1204–1214. doi: 10.1158/0008-5472.CAN-09-3321. [DOI] [PubMed] [Google Scholar]
- 68.Liu B., Ordonez-Ercan D., Fan Z., Edgerton S.M., Yang X., Thor A.D. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int J Cancer. 2007;120:1874–1882. doi: 10.1002/ijc.22423. [DOI] [PubMed] [Google Scholar]
- 69.Liu B., Ordonez-Ercan D., Fan Z., Huang X., Edgerton S.M., Yang X. Estrogenic promotion of ErbB2 tyrosine kinase activity in mammary tumor cells requires activation of ErbB3 signaling. Mol Cancer Res. 2009;7:1882–1892. doi: 10.1158/1541-7786.MCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 70.Wang S., Huang X., Lee C.K., Liu B. Elevated expression of erbB3 confers paclitaxel resistance in erbB2-overexpressing breast cancer cells via upregulation of Survivin. Oncogene. 2010;29:4225–4236. doi: 10.1038/onc.2010.180. [DOI] [PubMed] [Google Scholar]
- 71.Lyu H., Yang X.H., Edgerton S.M., Thor A.D., Wu X., He Z. The erbB3- and IGF-1 receptor-initiated signaling pathways exhibit distinct effects on lapatinib sensitivity against trastuzumab-resistant breast cancer cells. Oncotarget. 2016;7:2921–2935. doi: 10.18632/oncotarget.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agus D.B., Akita R.W., Fox W.D., Lewis G.D., Higgins B., Pisacane P.I. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 73.Lu Y., Zi X., Zhao Y., Mascarenhas D., Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin).[comment] J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 74.Nahta R., Yuan L.X., Zhang B., Kobayashi R., Esteva F.J. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 75.Spencer K.S., Graus-Porta D., Leng J., Hynes N.E., Klemke R.L. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saunus J.M., Quinn M.C., Patch A.M., Pearson J.V., Bailey P.J., Nones K. Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol. 2015;237:363–378. doi: 10.1002/path.4583. [DOI] [PubMed] [Google Scholar]
- 77.Da Silva L., Simpson P.T., Smart C.E., Cocciardi S., Waddell N., Lane A. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010;12:R46. doi: 10.1186/bcr2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adam L., Vadlamudi R., Kondapaka S.B., Chernoff J., Mendelsohn J., Kumar R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 79.Chausovsky A., Waterman H., Elbaum M., Yarden Y., Geiger B., Bershadsky A. Molecular requirements for the effect of neuregulin on cell spreading, motility and colony organization. Oncogene. 2000;19:878–888. doi: 10.1038/sj.onc.1203410. [DOI] [PubMed] [Google Scholar]
- 80.Hinton D.R., He S., Graf K., Yang D., Hsueh W.A., Ryan S.J. Mitogen-activated protein kinase activation mediates PDGF-directed migration of RPE cells. Exp Cell Res. 1998;239:11–15. doi: 10.1006/excr.1997.3873. [DOI] [PubMed] [Google Scholar]
- 81.Summy J.M., Gallick G.E. Src family kinases in tumor progression and metastasis. Cancer Metastas- Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 82.Tan M., Yao J., Yu D. Overexpression of the c-erbB-2 gene enhanced intrinsic metastasis potential in human breast cancer cells without increasing their transformation abilities. Cancer Res. 1997;57:1199–1205. [PubMed] [Google Scholar]
- 83.Fukata M., Nakagawa M., Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 84.Katso R., Okkenhaug K., Ahmadi K., White S., Timms J., Waterfield M.D. Cellular function of phosphoinositide 3-kinases: implications for development, immunity, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 85.Merlot S., Firtel R.A. Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J Cell Sci. 2003;116:3471–3478. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- 86.Reddy K.B., Nabha S.M., Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastas- Rev. 2003;22:395–403. doi: 10.1023/a:1023781114568. [DOI] [PubMed] [Google Scholar]
- 87.Webb D.J., Donais K., Whitmore L.A., Thomas S.M., Turner C.E., Parsons J.T. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 88.Xia Y., Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 89.Xue C., Liang F., Mahmood R., Vuolo M., Wyckoff J., Qian H. ErbB3-dependent motility and intravasation in breast cancer metastasis. Cancer Res. 2006;66:1418–1426. doi: 10.1158/0008-5472.CAN-05-0550. [DOI] [PubMed] [Google Scholar]
- 90.Pradeep S., Kim S.W., Wu S.Y., Nishimura M., Chaluvally-Raghavan P., Miyake T. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26:77–91. doi: 10.1016/j.ccr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lyu H., Wang S., Huang J., Wang B., He Z., Liu B. Survivin-targeting miR-542-3p overcomes HER3 signaling-induced chemoresistance and enhances the antitumor activity of paclitaxel against HER2-overexpressing breast cancer. Cancer Lett. 2018;420:97–108. doi: 10.1016/j.canlet.2018.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gala K., Chandarlapaty S. Molecular pathways: her3 targeted therapy. Clin Cancer Res. 2014;20:1410–1416. doi: 10.1158/1078-0432.CCR-13-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schoeberl B., Faber A.C., Li D., Liang M.C., Crosby K. Onsum M, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70:2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schoeberl B., Pace E.A., Fitzgerald J.B., Harms B.D., Xu L., Nie L. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci Signal. 2009;2:ra31. doi: 10.1126/scisignal.2000352. [DOI] [PubMed] [Google Scholar]
- 95.Jiang N., Saba N.F., Chen Z.G. Advances in Targeting HER3 as an anticancer therapy. Chemother Res Pract. 2012;2012:817304. doi: 10.1155/2012/817304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aurisicchio L., Marra E., Luberto L., Carlomosti F., De Vitis C., Noto A. Novel anti-ErbB3 monoclonal antibodies show therapeutic efficacy in xenografted and spontaneous mouse tumors. J Cell Physiol. 2012;227:3381–3388. doi: 10.1002/jcp.24037. [DOI] [PubMed] [Google Scholar]
- 97.Aurisicchio L., Marra E., Roscilli G., Mancini R., Ciliberto G. The promise of anti-ErbB3 monoclonals as new cancer therapeutics. Oncotarget. 2012;3:744–758. doi: 10.18632/oncotarget.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McDonagh C.F., Huhalov A., Harms B.D., Adams S., Paragas V., Oyama S. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11:582–593. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 99.Foreman P.K., Gore M., Kobel P.A., Xu L., Yee H., Hannum C. ErbB3 inhibitory surrobodies inhibit tumor cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11:1411–1420. doi: 10.1158/1535-7163.MCT-12-0068. [DOI] [PubMed] [Google Scholar]
- 100.Wang S., Huang J., Lyu H., Cai B., Yang X., Li F. Therapeutic targeting of erbB3 with MM-121/SAR256212 enhances antitumor activity of paclitaxel against erbB2-overexpressing breast cancer. Breast Cancer Res. 2013;15:R101. doi: 10.1186/bcr3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang J., Wang S., Lyu H., Cai B., Yang X., Wang J. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer. 2013;12:134. doi: 10.1186/1476-4598-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu J.F., Ray-Coquard I., Selle F., Poveda A.M., Cibula D., Hirte H. Randomized phase II trial of seribantumab in combination with paclitaxel in patients with advanced platinum-resistant or -refractory ovarian cancer. J Clin Oncol. 2016;34:4345–4353. doi: 10.1200/JCO.2016.67.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schoeberl B., Kudla A., Masson K., Kalra A., Curley M., Finn G. Systems biology driving drug development: from design to the clinical testing of the anti-ErbB3 antibody seribantumab (MM-121) NPJ Syst Biol Appl. 2017;3:16034. doi: 10.1038/npjsba.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malm M., Frejd F.Y., Stahl S., Lofblom J. Targeting HER3 using mono- and bispecific antibodies or alternative scaffolds. MAbs. 2016;8:1195–1209. doi: 10.1080/19420862.2016.1212147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arnett SO, Teillaud J-L, Wurch T, Reichert JM, Dunlop DC, Huber M. IBC’s Proceedings of the 21st Annual Antibody Engineering and 8th Annual Antibody Therapeutics International Conferences and 2010 Annual Meeting of The Antibody Society December 5–9, 2010, San Diego, CA USA. MAbs: Landes Bioscience, 2011:133–52. [DOI] [PMC free article] [PubMed]
- 106.Shimizu T., Yonesaka K., Hayashi H., Iwasa T., Haratani K., Yamada H. Phase 1 study of new formulation of patritumab (U3-1287) Process 2, a fully human anti-HER3 monoclonal antibody in combination with erlotinib in Japanese patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2017;79:489–495. doi: 10.1007/s00280-016-3231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yonesaka K., Hirotani K., Kawakami H., Takeda M., Kaneda H., Sakai K. Anti-HER3 monoclonal antibody patritumab sensitizes refractory non-small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib. Oncogene. 2016;35:878–886. doi: 10.1038/onc.2015.142. [DOI] [PubMed] [Google Scholar]
- 108.Mirschberger C., Schiller C.B., Schraml M., Dimoudis N., Friess T., Gerdes C.A. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res. 2013;73:5183–5194. doi: 10.1158/0008-5472.CAN-13-0099. [DOI] [PubMed] [Google Scholar]
- 109.Kawakami H., Yonesaka K. HER3 and its ligand, heregulin, as targets for cancer therapy. Recent Pat Anticancer Drug Discov. 2016;11:267–274. doi: 10.2174/1574892811666160418123221. [DOI] [PubMed] [Google Scholar]
- 110.Meulendijks D., Jacob W., Martinez-Garcia M., Taus A., Lolkema M.P., Voest E.E. First-in-human phase I study of lumretuzumab, a glycoengineered humanized anti-HER3 monoclonal antibody, in patients with metastatic or advanced HER3-positive solid tumors. Clin Cancer Res. 2016;22:877–885. doi: 10.1158/1078-0432.CCR-15-1683. [DOI] [PubMed] [Google Scholar]
- 111.Meulendijks D., Jacob W., Voest E.E., Mau-Sorensen M., Martinez-Garcia M., Taus A. Phase Ib study of lumretuzumab plus cetuximab or erlotinib in solid tumor patients and evaluation of HER3 and heregulin as potential biomarkers of clinical activity. Clin Cancer Res. 2017;23:5406–5415. doi: 10.1158/1078-0432.CCR-17-0812. [DOI] [PubMed] [Google Scholar]
- 112.Schneeweiss A, Park-Simon TW, Albanell J, Lassen U, Cortes J, Dieras V, et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest New Drugs 2018. Available from: <http://dx.doi.org/10.1007/s10637-018-0562-4>. [DOI] [PMC free article] [PubMed]
- 113.Sala G., Traini S., D׳Egidio M., Vianale G., Rossi C., Piccolo E. An ErbB-3 antibody, MP-RM-1, inhibits tumor growth by blocking ligand-dependent and independent activation of ErbB-3/Akt signaling. Oncogene. 2012;31:1275–1286. doi: 10.1038/onc.2011.322. [DOI] [PubMed] [Google Scholar]
- 114.Sala G., Rapposelli I.G., Ghasemi R., Piccolo E., Traini S., Capone E. EV20, a Novel Anti-ErbB-3 humanized antibody, promotes ErbB-3 down-regulation and inhibits tumor growth in vivo. Transl Oncol. 2013;6:676–684. doi: 10.1593/tlo.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le Clorennec C., Bazin H., Dubreuil O., Larbouret C., Ogier C., Lazrek Y. Neuregulin 1 allosterically enhances the antitumor effects of the noncompeting anti-HER3 antibody 9F7-F11 by increasing its binding to HER3. Mol Cancer Ther. 2017;16:1312–1323. doi: 10.1158/1535-7163.MCT-16-0886. [DOI] [PubMed] [Google Scholar]