Abstract
Whether and how garlic-derived S-allylmercaptocysteine (SAMC) inhibits hepatocellular carcinoma (HCC) is largely unknown. In the current study, the role of low-density lipoprotein receptor (LDLR)-related protein 6 (LRP6) in HCC progression and the anti-HCC mechanism of SAMC was examined in clinical sample, cell model and xenograft/orthotopic mouse models. We demonstrated that SAMC inhibited cell proliferation and tumorigenesis, while induced apoptosis of human HCC cells without influencing normal hepatocytes. SAMC directly interacted with Wnt-pathway co-receptor LRP6 on the cell membrane. LRP6 was frequently over-expressed in the tumor tissue of human HCC patients (66.7% of 48 patients) and its over-expression only correlated with the over-expression of β-catenin, but not with age, gender, tumor size, stage and metastasis. Deficiency or over-expression of LRP6 in hepatoma cells could partly mimic or counteract the anti-tumor properties of SAMC, respectively. In vivo administration of SAMC significantly suppressed the growth of Huh-7 xenograft/orthotopic HCC tumor without causing undesirable side effects. In addition, stable down-regulation of LRP6 in Huh-7 facilitated the anti-HCC effects of SAMC. In conclusion, LRP6 can be a potential therapeutic target of HCC. SAMC is a promising specific anti-tumor agent for treating HCC subtypes with Wnt activation at the hepatoma cell surface.
Abbreviations: Axin1, axis inhibition protein 1; DKK-1, Dickkopf Wnt signaling pathway inhibitor 1; DVL2, disheveled 2; FADD, Fas-associated protein with death domain; HCC, hepatocellular carcinoma; KD, knock-down; LDH, lactate dehydrogenase; LRP6, low-density lipoprotein receptor (LDLR)-related protein 6; MCL-1, myeloid cell leukemin-1; NAFLD, non-alcoholic fatty liver disease; PCNA, proliferating cell nuclear antigen; SAC, S-allylcysteine; SAMC, S-allylmercaptocysteine; SPR, surface plasmon resonance; TCF/LEF, T-cell factor/lymphoid enhancing factor; TSA, thermal shift assay; Tm, melting temperature
KEY WORDS: S-allylmercaptocysteine, HCC, Wnt, LRP6, Human, Nude mice
Graphical abstract
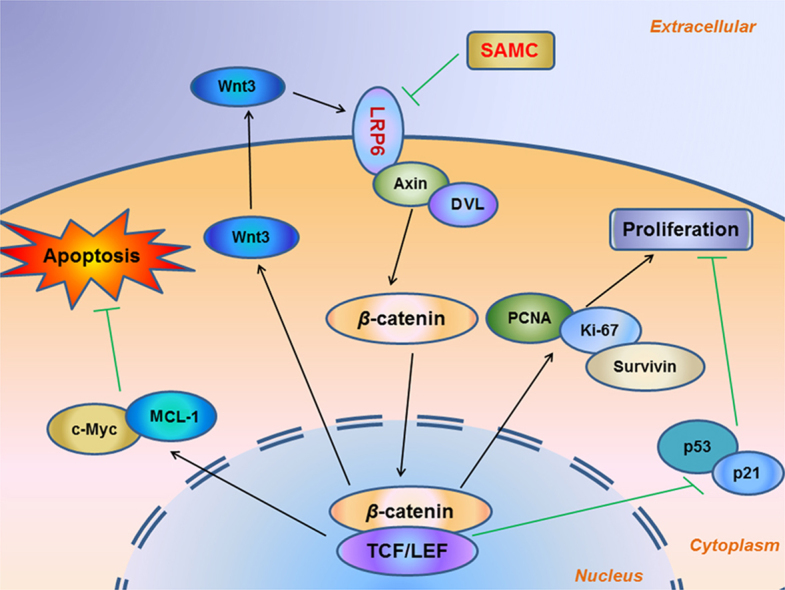
Garlic-derived S-allylmercaptocysteine (SAMC) directly interacts with cancer cell membrane-bound receptor low-density lipoprotein receptor-related protein 6 (LRP6) to modulate the activation of the Wnt/β-catenin pathway. The apoptosis of cancer cell is promoted through the c-Myc- and Mcl-1-mediated pathways, while the proliferation of cancer cell is inhibited via the converged PCNA/p53/p21 regulations.
1. Introduction
Currently, liver cancer is the sixth most common malignant disease and the second leading cause of cancer death worldwide. There are approximately 50.5% of new liver cancer cases in China in each year, ~75% of which is hepatocytes-derived hepatocellular carcinoma (HCC)1. The survival rate after the onset of HCC symptoms is generally less than one year and as to date no effective clinical therapeutic strategy with desirable effects has been developed2. Therefore, elucidating the molecular mechanisms on the initiation and progression of HCC is critical for the control of this fatal disease.
The canonical Wnt/β-catenin pathway is aberrantly activated in HCC3. Activation of the Wnt pathway is through the binding of Wnt family proteins (e.g., Wnt3a) to the cell surface receptors low-density lipoprotein receptor (LDLR)-related protein 5 (LRP5) and/or LRP6. After that, phosphorylated receptors recruit disheveled homologue proteins, e.g., disheveled 2/3 (DVL2/3), and axis inhibition protein 1 (axin1) to stabilize and promote the nuclear translocation of β-catenin, which acts as a transactivator of T-cell factor/lymphoid enhancing factor (TCF/LEF) transcription factors to regulate the expression of key genes for cell proliferation, differentiation, and tumorigenesis4. In the liver, many temporal roles of the Wnt/β-catenin pathway have been identified during its development and maintenance of physiological homeostasis5. Emerging evidence suggests that dysregulated signaling of the Wnt/β-catenin pathway lead to hepatic carcinogenesis6, 7, 8. Recently, LRP6 has been identified as a novel nutritional therapeutic target for several liver diseases, including non-alcoholic fatty liver disease (NAFLD) and hyperlipidemia9 while another study also found that up-regulation of LRP6 was associated with enhanced hepatic carcinogenesis and cell invasion10. Therefore, we hypothesized that LRP6 might be a direct target for nutraceutical agents with anti-HCC properties.
Garlic is used as a medicinal food for its anti-bacterial, immunoregulatory and anti-tumor properties in many countries for more than 2000 years11. Epidemiological studies indicate an association between garlic consumption and decreased risk of gastrointestinal tract cancers11. S-allylmercaptocysteine (SAMC) is a water-soluble active compound derived from aged garlic. We have demonstrated its potent hepato-protective properties and mechanisms in acute liver injury and NAFLD12, 13. Its anti-tumor effects have been demonstrated in colon cancer14, prostate cancer15, 16, bladder cancer17, breast cancer18, and gastric cell cancer19. A very recent study found that SAMC induced apoptosis in human HepG2 cell through targeting the cross-talk between the transforming growth factor-β and the mitogen-activated protein kinase pathways20. However, mechanistic data regarding the detailed anti-HCC functions of SAMC, particularly its “immediate receptor” when in contact with the tumor cell, is lacking. Therefore, in the current study, we aimed to investigate the anti-tumor effects and mechanisms of SAMC in human and mouse HCC cell lines and xenograft/orthotopic models, with emphasis in its direct target on the cell membrane.
2. Materials and methods
2.1. Patient samples and analysis
Use of human tissue samples in this project was approved by the Ethical Committee of Shenzhen Third People's Hospital. All patients were given formal notification and written consent on the use of the clinical specimens for research. Forty-eight pairs of HCC tissues and their corresponding non-tumorous liver tissues (1 cm away from the tumor), as well as 6 liver tissues from healthy people, were employed for analyses. The clinicopathological features of all these patients are listed in Supplementary Information Table S1.
2.2. Generation of LRP6 rescue and over-expressed constructs
The cloning and generation of a codon-modified shRNA-resistant LRP6 (LRP6 rescue) construct was conducted as previously reported21.
2.3. GST–E-cadherin pull-down assay
The GST–E-cadherin pull-down assay was performed as previously described22. Western blotting was performed using an antibody to β-catenin.
2.4. Surface plasmon resonance (SPR) and thermal shift assay (TSA)
Analysis of direct binding between SAMC and LRP6 protein was performed by using SPR and TSA as previously described23. Apparent equilibrium dissociation constants (Kd) were then calculated as the ratio of kd/ka. In TSA, we used 0.04 mg/mL of recombinant human LRP6 protein with or without 0.2 mmol/L of SAMC in phosphate buffered saline (PBS). Data were analyzed with the differential scanning fluorimetry analysis tool (Excel based) using the curve-fitting software XLfit 5 (ID Business Solutions Ltd., Bridgewater, NJ, USA)23.
2.5. Xenograft and orthotopic HCC nude mice model
Male nude mice (Athymic nu/nu, 5–6 weeks, 20–25 g) were purchased from Guangdong Medical Laboratory Center (Guangdong, China). For the establishment of a subcutaneous (s.c.) xenograft HCC model, mice were s.c. injected at the dorsal region with 1×106/150 μL (low dose group) or 4×106/150 μL (high dose group) viable normal or LRP6 knockdown Huh-7 cells. Seven days after Huh-7 injection, 300 mg/kg SAMC was treated by daily oral gastric lavage feeding (n=6 for each group of mice)16. Tumor volume was assessed with digital calipers at days 8, 13, 18, 23, and 28 post Huh-7 injection. The tumor volume was calculated using the formula: π/6 × larger diameter × smaller diameter23.
For the orthotopic HCC model, a single tumor nodule could be observed in the liver after 6 days of an injection of 2×106 wild-type or LRP6 knock-down Huh-7 cells into the left liver lobe of nude mice. Then mice received daily oral gastric lavage feeding of 300 mg/kg SAMC or saline (n=10 for each group of mice). Median survival analysis was conducted using the Kaplan–Meier methods in GraphPad Prism v6.0 software (GraphPad Software, San Diego, CA, USA)24. Mice were subjected to anesthesia and then killed if moribund. The total observation duration was 70 days. Tumor nodule was collected for the intra-tumor expression check of key proteins.
2.6. Statistical analysis
Data from each group were expressed as mean ± standard error of the mean (SEM). Statistical comparisons between groups were done using the Kruskal–Wallis test followed by Dunn's post hoc test to detect differences in all groups. Clinical data were used Fisher's exact test to compared and calculate P-values. A P<0.05 was statistically significant (Prism 5.0, Graphpad software, Inc., San Diego, CA, USA).
Full methods and any associated references are available in supplementary Information.
3. Results
3.1. LRP6 is frequently over-expressed in human HCC samples
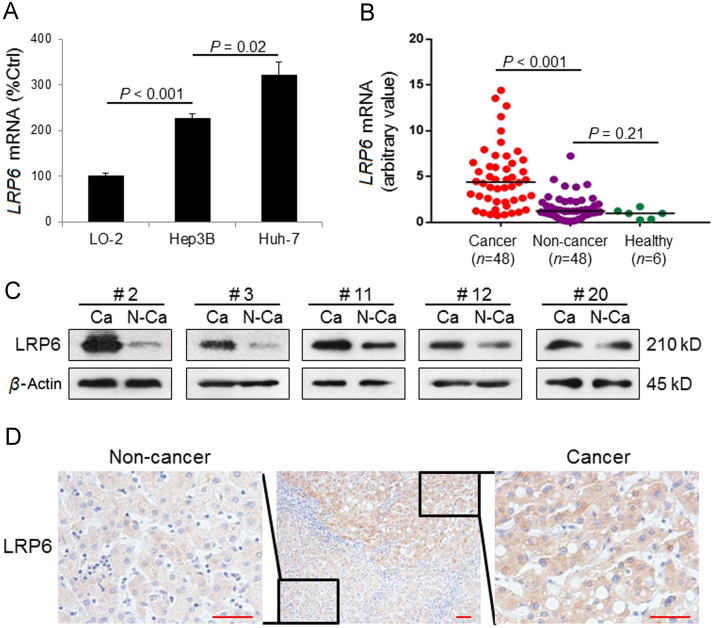
It was shown that the mRNA level of LRP6 was significantly higher in both hepatoma cell lines (Hep3B and Huh-7) than normal cell line LO-2 (P<0.001). Interestingly, Huh-7 expressed a higher LRP6 level than Hep3B (P=0.02, Fig. 1A). Then we examined its transcriptional level in the liver tissues of healthy subjects (n=6), non-cancerous sections (n=48) and cancerous sections (n=48) of HCC patients (Fig. 1B), whose clinicopathological characteristics were presented in Supplementary Information Table S1. LRP6 transcripts were frequently and significantly up-regulated (P<0.001) in the cancerous liver tissue sections of patients than their corresponding non-cancerous tissues. Quantitative PCR showed that LRP6 mRNA expression was elevated in 32 out of 48 (66.7%) cancerous liver tissues of HCC patients (defined as a 2-fold expressional elevation). Five representative Western blot results were presented in Fig. 1C. Immunohistochemistry results indicated obvious over-expression of LRP6 protein in the cytoplasmic part of tumor cells while the non-cancerous liver tissue only showed relatively lower LRP6 signals (Fig. 1D). In addition, clinicopathological correlation analysis exhibited that the association between LRP6 over-expression and β-catenin over-expression was statistically significant in those patients (P=0.0039, Supplementary Information Table S2).
Figure 1.
LRP6 was frequently over-expressed in HCC. (A) The basal mRNA expression of LRP6 was highest in Huh-7 HCC cell line, midst in Hep3B HCC cell line, and lowest in normal human hepatocyte cell line LO-2 (n=4 for each cell line). (B) The LRP6 mRNA level was significantly higher in human HCC cancerous tissue (n=48) than that in non-cancerous tissue (n=48) or liver tissue from healthy human (n=6). (C) Representative Western blot results of LRP6 from human HCC cancerous tissue and their corresponding non-cancerous tissues, showing that the protein level of LRP6 was frequently higher in HCC tumor than non-tumor area. (D) Representative immunohistochemistry results of LRP6 in a human liver section showing both cancerous and non-cancerous areas (Scale bars, 20 μm). Data are presented in means±SEM.
3.2. SAMC selectively inhibited cell proliferation of hepatoma cells
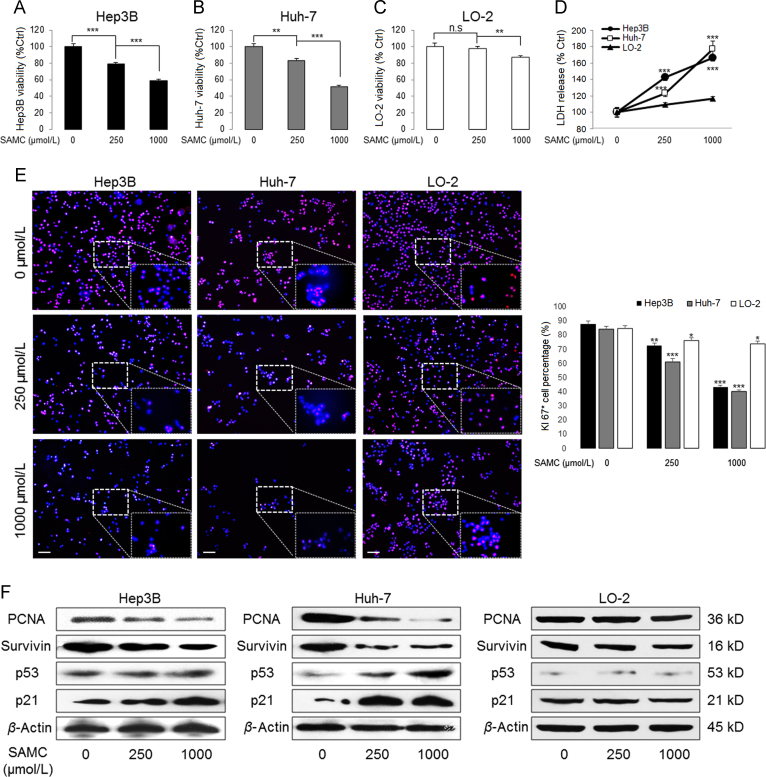
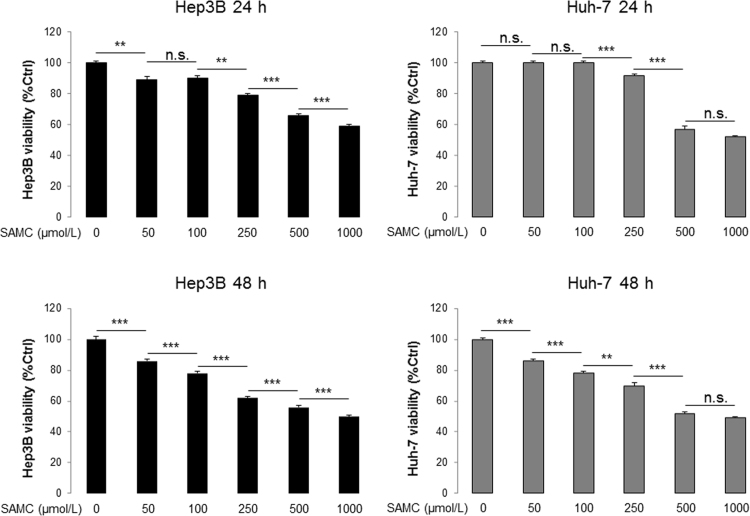
After 24-h incubation, SAMC significantly reduced the cell viability of both Hep3B and Huh-7 cells in a dose-dependent manner. The dose of 250 μmol/L showed intermediate inhibitory effects (Fig. 2A, B, and Supplementary Information Fig. S1). The cell damaging effects of SAMC mostly occurred in the first 24 h of treatment since the inhibitory results of both 24-h and 48-h incubation were similar (Supplementary Information Fig. S1). Thus, we used 250 μmol/L/1 mmol/L SAMC and 24 h as the optimal in vitro treatment conditions.
Figure 2.
SAMC inhibited human hepatoma cell proliferation but not normal hepatocytes in vitro. (A)–(C) SAMC significantly reduced cell viability of human hepatoma cell lines Hep3B and Huh-7 at 250 μmol/L and 1 mmol/L without evident influence in human normal hepatocyte cell line LO-2 (n=4). (D) Release of LDH (lactate dehydrogenase) was significantly higher in Hep3B and Huh-7 than LO-2 after SAMC incubation (n=4; Scale bars, 100 μm). (E) Representative immuno-fluorescent images of Ki-67 protein in three cell lines after SAMC incubation. (F) Representative Western blot showing change of protein levels of PCNA, survivin, p53 and p21 in three cell lines after SAMC incubation. Data are presented in means±SEM. **P<0.01 and ***P<0.001, respectively between indicated groups (in panel D means comparison with the LO-2 group).
For normal hepatocytes LO-2, only the higher dose of SAMC (1 mmol/L) reduced its viability from ~100% to ~87% (Fig. 2C). This observation was further confirmed by the release of lactate dehydrogenase (LDH) from these three kinds of cells (Fig. 2D). To study the effects of SAMC on cell proliferation, we stained cells with Ki-67, a cell proliferation marker, after 24-h incubation of SAMC. SAMC reduced the cell number and Ki-67 fluorescent signal density in a dose-dependent manner in both Hep3B and Huh-7 cells. However, both doses of SAMC did not significantly alter the LO-2 cell number (Fig. 2E). In addition, SAMC treatments down-regulated the protein expressions of proliferation markers—proliferating cell nuclear antigen (PCNA) and survivin, and up-regulated the protein levels of tumor suppressor genes P53 and P21 in Hep3B and Huh-7 cells (Fig. 2F)25. Accordingly, SAMC did not affect the protein expression of these markers in LO-2 cells. Furthermore, SAMC strongly disrupted the cell cycle distribution of both Hep3B and Huh-7 cells via reducing the S phase percentage but increasing the G0/G1 phase percentage (Supplementary Information Table S3). Collectively, we found that SAMC specifically inhibited the proliferation of hepatoma cells without significantly influencing the normal hepatocytes.
3.3. SAMC induced apoptosis of hepatoma cells through both Intrinsic and extrinsic apoptotic pathways
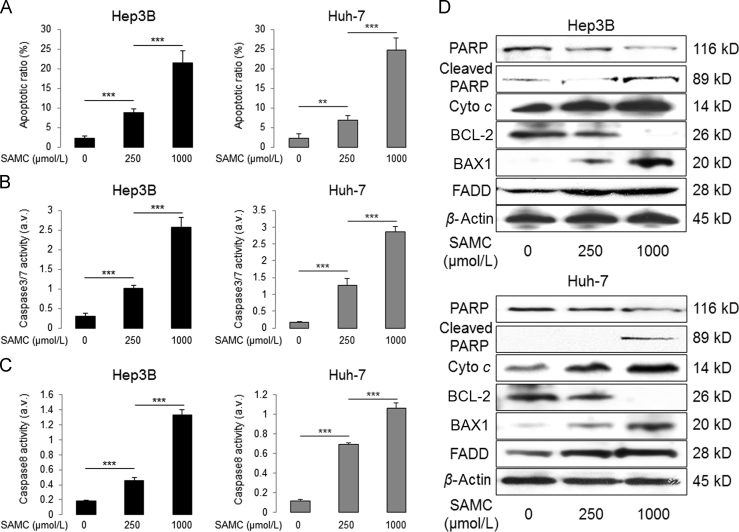
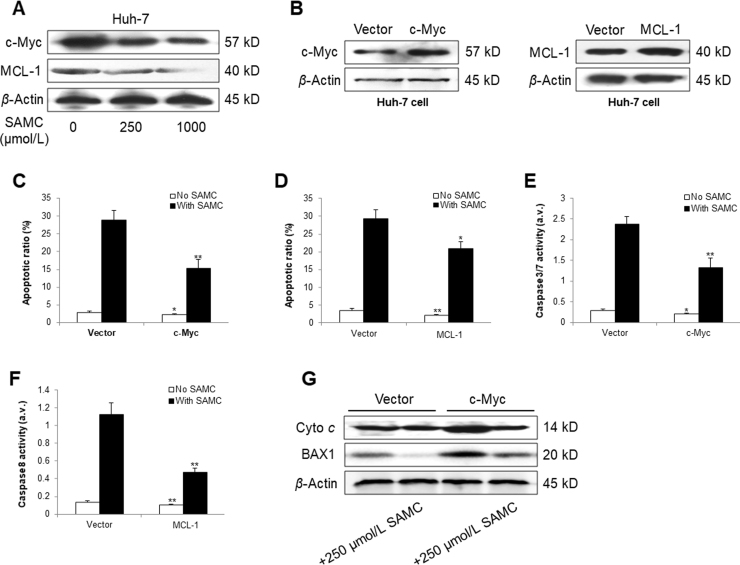
To further investigate the anti-tumor ability of SAMC, we tested its apoptosis-inducing effects on Hep3B and Huh-7 cells. As expected, SAMC significantly increased the apoptotic ratio of both hepatoma cells in a dose-dependent manner, with a similar increasing pattern in the cellular activities of both caspase-3/7 and caspase-8 (Fig. 3A–C). Additionally, SAMC up-regulated the protein expressions of cleaved poly(ADP-ribose) polymerase, cytochrome c, BAX1, and FAS-associated protein with death domain (FADD) while down-regulating the BCL-2 protein level, indicating that SAMC caused hepatoma cell apoptosis through both the intrinsic and extrinsic apoptotic pathways (Fig. 3D). Although SAMC treatment slightly increased the apoptotic cell number and caspase-8 activity in LO-2, the apoptotic-inductive effects of SAMC on LO-2 were significantly less than those of hepatoma cells (Supplementary Information Fig. S2). To further investigate the apoptotic inducing mechanisms of SAMC, we then checked the expressional changes of c-Myc and myeloid cell leukemin-1 (MCL-1), key mediators of intrinsic and extrinsic apoptotic pathways, respectively26, 27. Their protein expressions were dose-dependently inhibited by SAMC incubation in Huh-7 cell (Supplementary Information Fig. S3A). When their endogenous expressions were over-expressed by corresponding plasmids, the basal and SAMC-induced apoptosis levels were significantly suppressed, with inhibited activity of caspase-3/7/8 (Supplementary Information Fig. S3B–F). Furthermore, over-expression of c-Myc partly counter-acted the intrinsic apoptotic induction effect of SAMC through elevating the protein levels of both cytochrome c and BAX1 (Supplementary Information Fig. S3G).
Figure 3.
SAMC induced apoptosis through both apoptotic pathways in human hepatoma cell lines in vitro. (A) Change of apoptotic ratio after two doses of SAMC incubation in human hepatoma cell lines Hep3B and Huh-7 (n=4). (B) Change of caspase-3/7 activity after SAMC incubation in Hep3B and Huh-7 (n=4). (C) Change of caspase-8 activity after SAMC incubation in Hep3B and Huh-7 (n=4). (D) Representative Western blot results of protein level change of cleaved PARP, total PARP, cytochrome c (cyto c), BCL-2, BAX1, and FADD after SAMC incubation in both Hep3B and Huh-7. Data are presented in means±SEM. **P<0.01 and ***P<0.001, respectively between indicated groups.
3.4. SAMC reduced the tumorigenic ability of hepatoma cells
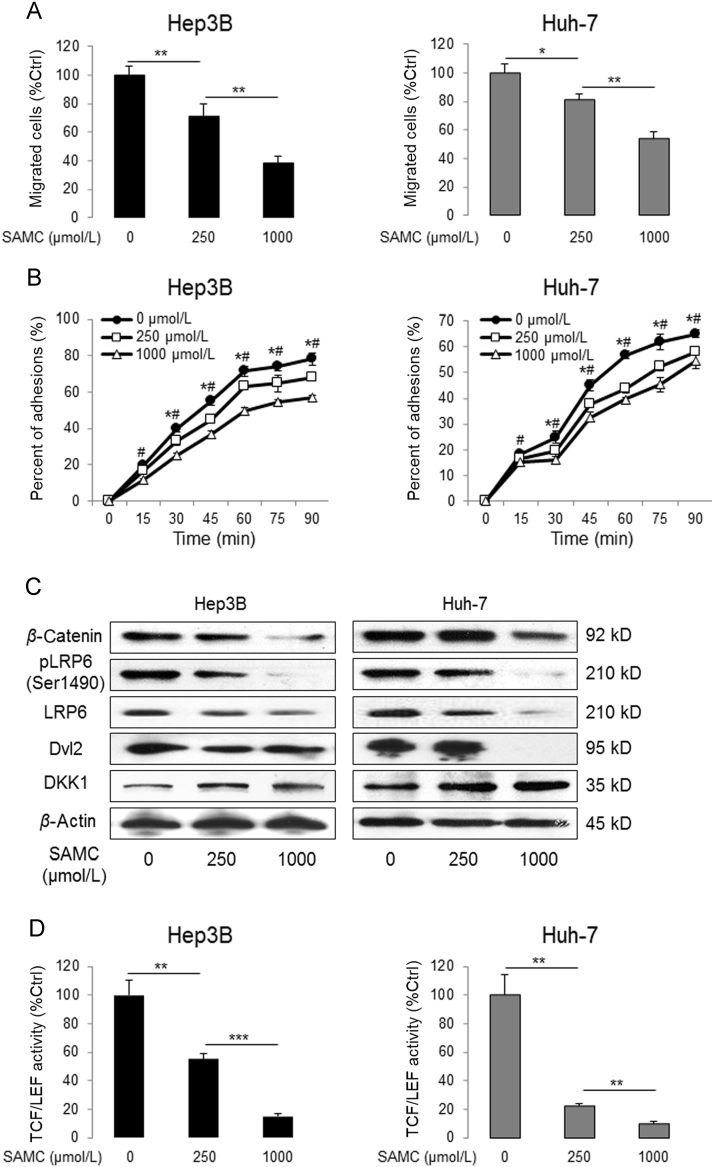
To further investigate the effects of SAMC on the tumorigenic ability of hepatoma cells, we measured the changes of cellular migration and adhesion in both Hep3B and Huh-7 cells with or without SAMC incubation. We found that SAMC significantly reduced their migration ability (Fig. 4A). It is well known that cell adhesion is a vital step in cell migration process. Thus, as shown in Fig. 4B, SAMC decreased the cellular adhesion ability on fibronectin in both Hep3B and Huh-7 cells.
Figure 4.
SAMC reduced human hepatoma cell tumorigenesis and Wnt signaling in vitro. (A) Change of migrated cell number after SAMC treatment, examined by Transwell assay, in human hepatoma cell lines Hep3B and Huh-7 (n=4). (B) Change in percentage of adhered cells after SAMC treatment in Hep3B and Huh-7 (n=4). (C) Representative Western blot results of protein level change of β-catenin, phosphorylated LRP6 at ser1490, total LRP6, DVL2, and DKK1 after SAMC incubation in both Hep3B and Huh-7. (D) Change of Wnt signaling target TCF/LEF activity after SAMC treatment in Hep3B and Huh-7 (n=4). Data are presented in means±SEM. *P<0.05, **P<0.01 and ***P<0.001, respectively between indicated groups (in panel C and D, * and # indicate a P<0.05 when compared with 250 μmol/L and 1 mmol/L groups, respectively).
Since the modulation of the Wnt pathway evidently influences the tumorigenic processes of many types of tumor cells, we tested the protein level changes of key markers in this pathway. It was demonstrated that SAMC down-regulated the protein levels of β-catenin, phosphorylated LRP6, total LRP6, and DVL2 in both Hep3B and Huh-7 cells. Consistently, SAMC elevated DKK-1 (Dickkopf Wnt signaling pathway inhibitor 1) protein level in a dose-dependent manner (Fig. 4C). Then we found that treatments with both concentrations of SAMC led to significant decreases in TCF/β-catenin reporter activity up to 45% and 85% for Hep3B cells and 77% and 90% for Huh-7 cells, respectively (Fig. 4D).
3.5. SAMC directly targeted LRP6 on the hepatoma cell membrane
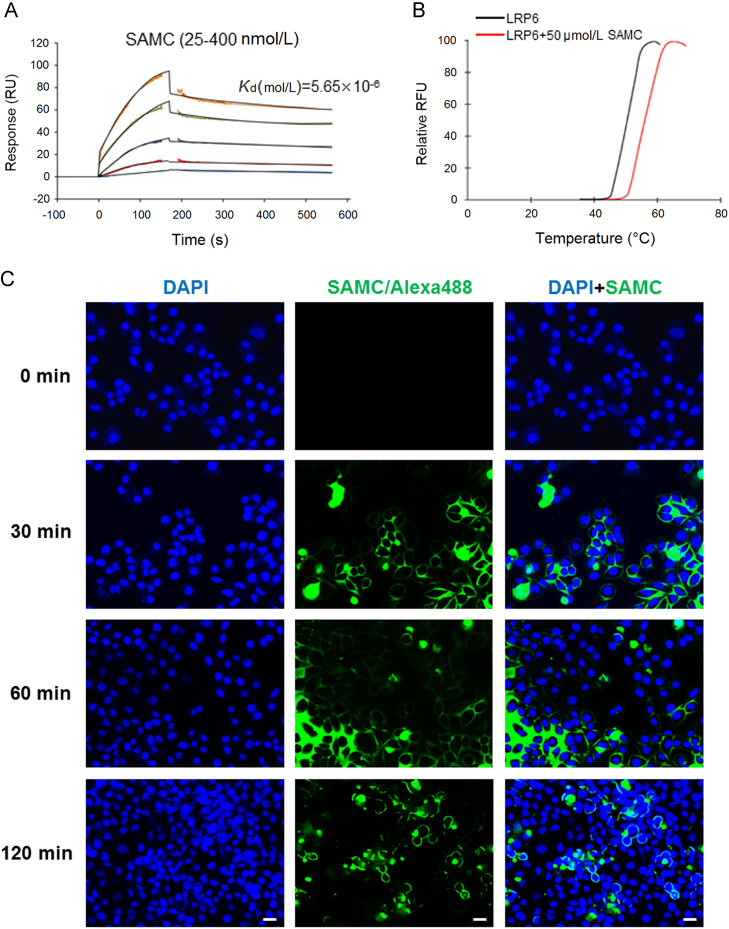
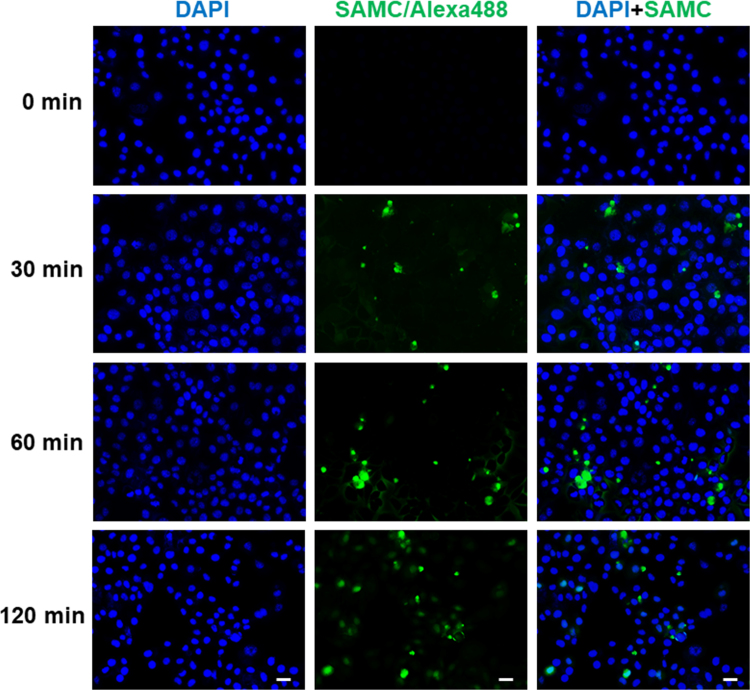
To confirm the hypothesis that LRP6 is the direct acting target of SAMC, we performed SPR using recombinant human LRP6 protein and dissolved SAMC. SAMC bound to LRP6 tightly in a dose-dependent manner (from 25 to 400 nmol/L) with a dissociation constant of Kd=5.65×10−6 mol/L (Fig. 5A). To further confirm this result and to investigate whether SAMC influences the LRP6 protein through the Wnt pathway of modulating its protein structure, we used TSA and found that SAMC treatment increased the melting temperature (Tm) of LRP6 for ~4 °C (Fig. 5B). In addition, we labeled SAMC with Alexa Fluo 488 TFP ester and examined the time-lapse distribution of SAMC after its addition to the culture medium. As shown in Fig. 5C, 30 or 60 min after the treatment, green-fluorescent-labeled SAMC was distributed around a number of Huh-7 cell membranes. Two hours later, the membrane-bound SAMC green signals were intensified and concentrated, with less unbound signals in the medium. It should be noted that in some cells, SAMC penetrated into the cytoplasmic part, which was consistent with the finding that the subcellular localization of LRP6 could be modulated by external treatment (e.g., Wnt) from the cell membrane to the cytoplasm28. Conversely, time-lapse experiments using LO-2 cells showed less SAMC-positive signals around cells and in the culture medium (Supplementary Information Fig. S4). Collectively, our data demonstrated that SAMC directly targeted LRP6 on the cell membrane of hepatoma cells.
Figure 5.
SAMC directly interacted with cell membrane receptor LRP6 in vitro. (A) SPR analysis of the binding of SAMC to immobilized recombinant human LRP6 protein. A gradient of SAMC was applied to quantify the binding affinity (25–400 nmol/L). The Kd (mol/L) value is 5.65×10–5. (B) The interaction of LRP6 with SAMC was detected by thermal shift assay. Black line: basal LRP6 melting curve. Red line: LRP6+50 μmol/L SAMC melting curve. (C) Representative fluorescent images of cultured Huh-7 cells after 0, 30, 60, and 120 min incubation of SAMC which was conjugated with Alexa 488 TFP ester dye (Scale bars, 50 μm).
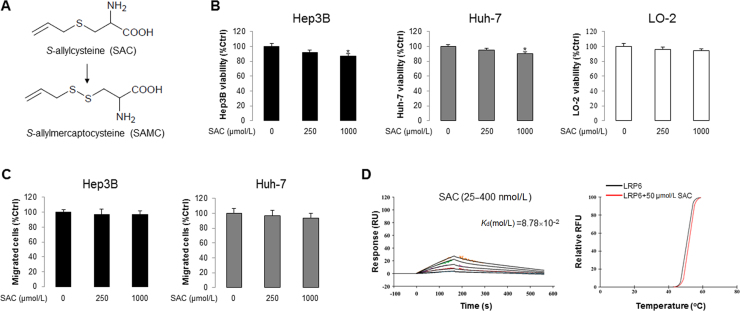
To prove that the potent anti-HCC effect and direct interaction with LRP6 is specific to SAMC, we tested the precursor substance of SAMC during garlic aging–S-allylcystein (SAC)–for its anti-HCC properties and LRP6 interactions (Supplementary Information Fig. S5A). It was found that, unlike SAMC, 250 µmol/L and 1 mmol/L SAC could not effectively inhibit Hep3B and Huh7 proliferation and migration (Supplementary Information Fig. S5B and C). Moreover, the interactions between SAC and LRP6 was loose, with a dissociation constant of Kd=8.78×10−2 mol/L from SPR and ~0.5 °C difference of melting temperature from TSA (Supplementary Information Fig. S5D). This result was in line with a previous report that at least 5 mmol/L of SAC was needed to significantly inhibit HCC cell growth29.
3.6. Down-regulation of LRP6 in hepatoma cells attenuated Wnt pathway signaling and induced apoptosis
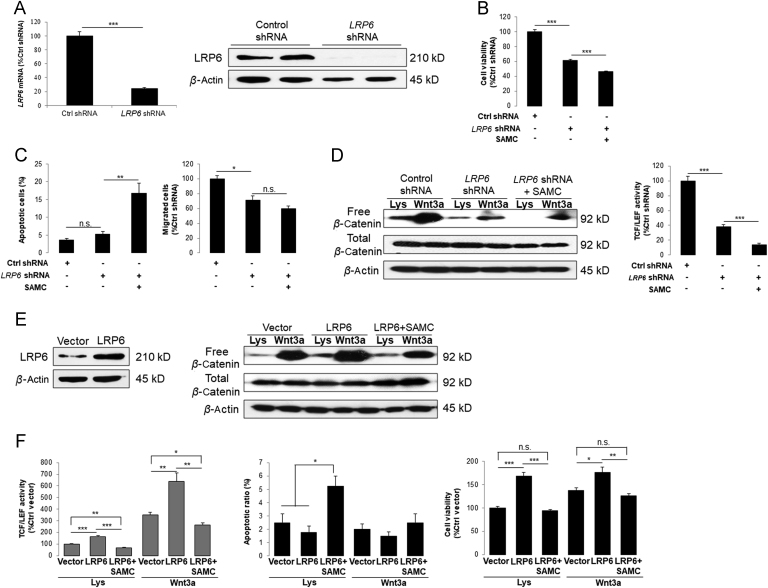
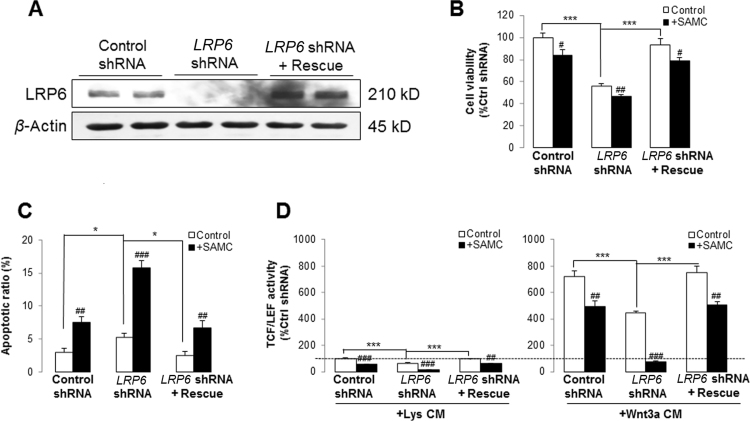
To further study the role of LRP6 on HCC development and SAMC-mediated anti-tumor effects, we then knocked-down the endogenous expression of LRP6 by using specific shRNA in Huh-7 cells, which showed relatively higher level of LRP6 than Hep3B (Figs. 1A and 6A). Cell viability and apoptosis were reduced and increased by the knock-down of LRP6, respectively. Importantly, addition of SAMC further enhanced these effects of LRP6 knock-down on Huh-7 cells (Fig. 6B). In addition, deficiency of LRP6 itself significantly attenuated the migration ability of Huh-7, which was slightly strengthened by SAMC co-treatment (Fig. 6C). Free β-catenin pool and TCF/LEF reporter activity were significantly reduced when LRP6 was knocked-down in Huh-7. Addition of SAMC in the culture medium further enhanced such effects (Fig. 6D).
Figure 6.
Deficiency and over-expression of LRP6 positively and negatively influenced the anti-HCC properties of SAMC in vitro, respectively. (A) Verification of LRP6 shRNA transfection by detecting its mRNA and protein level change before and after the transfection in Huh-7 hepatoma cell line in vitro. (B)–(C) When LRP6 was knocked-down, the cell viability and migration was inhibited, which was further potentiated by 250 μmol/L SAMC incubation. The apoptosis ratio change showed inverse pattern (n=4). (D) LRP6 down-regulation suppressed Wnt signaling examined by free β-catenin pull-down and TCF/LEF reporter assays in the absence and presence of Wnt3a ligands. Incubation of 250 μmol/L SAMC further enhanced such effects (n=4). (E) LRP6 protein level change after transfection of LRP6 plasmid. (F) Over-expression of LRP6 significantly increased the Wnt signaling examined by free β-catenin pull-down and TCF/LEF reporter assays in the absence and presence of Wnt3a ligands, which was attenuated by 250 μmol/L SAMC incubation (n=4). Over-expression of LRP6 slightly reduced the basal apoptotic ratio of Huh-7 cells, which were significantly reversed by 250 μmol/L SAMC incubation (n=4). Over-expression of LRP6 significantly increased cell viability ability of Huh-7, which was re-balanced by SAMC treatment (n=4). Data are presented in means±SEM. *P<0.05, **P<0.01 and ***P<0.001, respectively between indicated groups. Lys, lysates.
3.7. Over-expression of shRNA-resistant LRP6 rescued Wnt signaling and cancer cell growth impaired by SAMC
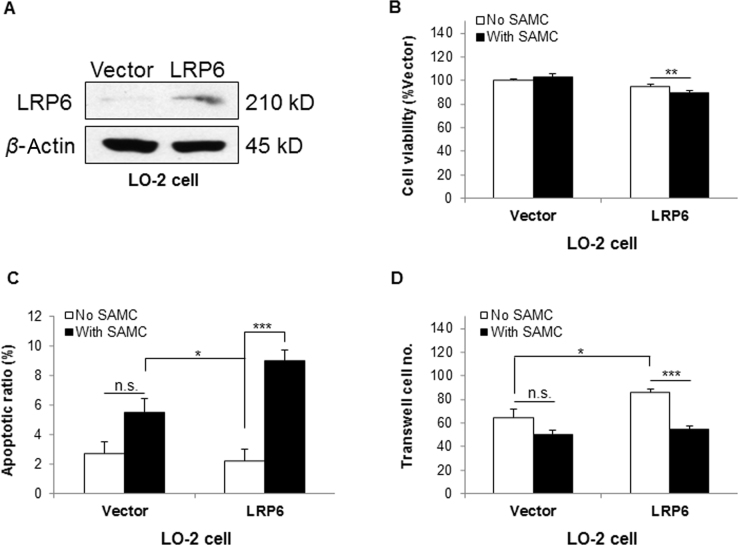
To confirm that the above observations on Huh-7 cells were specifically attributed to the knock-down of LRP6, we synthesized a shRNA-resistant LRP6 (LRP6 rescue) construct to repeat those experiments21. Transfection of LRP6 rescue restored the basal protein expression level down-regulated by LRP6 shRNA in Huh-7 cells (Supplementary Information Fig. S6A). It also rescued the cell viability and apoptosis modulated by the deficiency of LRP6 to a control comparable level (Supplementary Information Fig. S6B and C). More importantly, over-expression of LRP6 rescue markedly restored the TCF/LEF reporter activity of Wnt pathway, with or without Wnt3a conditioned medium (CM, Supplementary Information Fig. S6D). It was interesting that when LRP6 was over-expressed in normal hepatocyte line LO-2, cells gained sensitivity to SAMC-induced viability reduction and apoptosis induction. Additionally, the over-expression of LRP6 significantly increased the migration ability of LO-2, when examined using Matrigel, which could be counter-acted by SAMC (Supplementary Information Fig. S7).
3.8. Over-expression of LRP6 antagonized the anti-tumor effects of SAMC
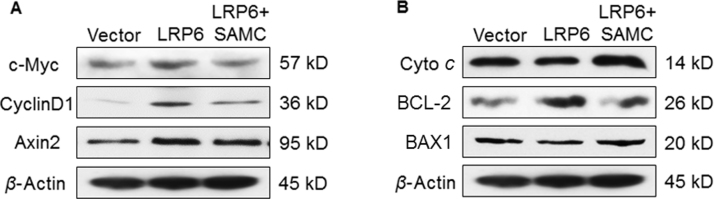
Since deficiency of LRP6 partly mimicked the anti-tumor abilities of SAMC treatment, it is interesting to find out whether over-expression of endogenous LRP6 is able to enhance Wnt signaling in hepatoma cells (Fig. 6E). It was found that over-expression of LRP6 increased the free β-catenin pool and TCF/LEF reporter activity which were re-balanced to the control level by the co-treatment with SAMC (Fig. 6F). In addition, the protein expression of key Wnt targets, including c-Myc, cyclin D1 and axin2, were up-regulated by LRP6 over-expression and then down-regulated by SAMC, indicating that LRP6 was important for the signal transduction of Wnt-mediated cell cycle regulation (Supplementary Information Fig. S8A). We found that LRP6 was important for the tumorigenesis of Hep3B since its over-expression increased its basal viability (Fig. 6F) and transwell ability (data not shown), which were further strengthened by the addition of Wnt3a and attenuated by SAMC. At the apoptotic level, LRP6 over-expression down-regulated pro-apoptotic markers cytochrome c and BAX1, up-regulated the anti-apoptotic marker BCL-2, suggesting that LRP6 could stabilize cell from programmed cell death, although the change of apoptotic ratio was not very evident (Fig. 6F and Supplementary Information Fig. S8B).
3.9. LRP6 was vital for the tumor growth and SAMC treatment efficacy in vivo
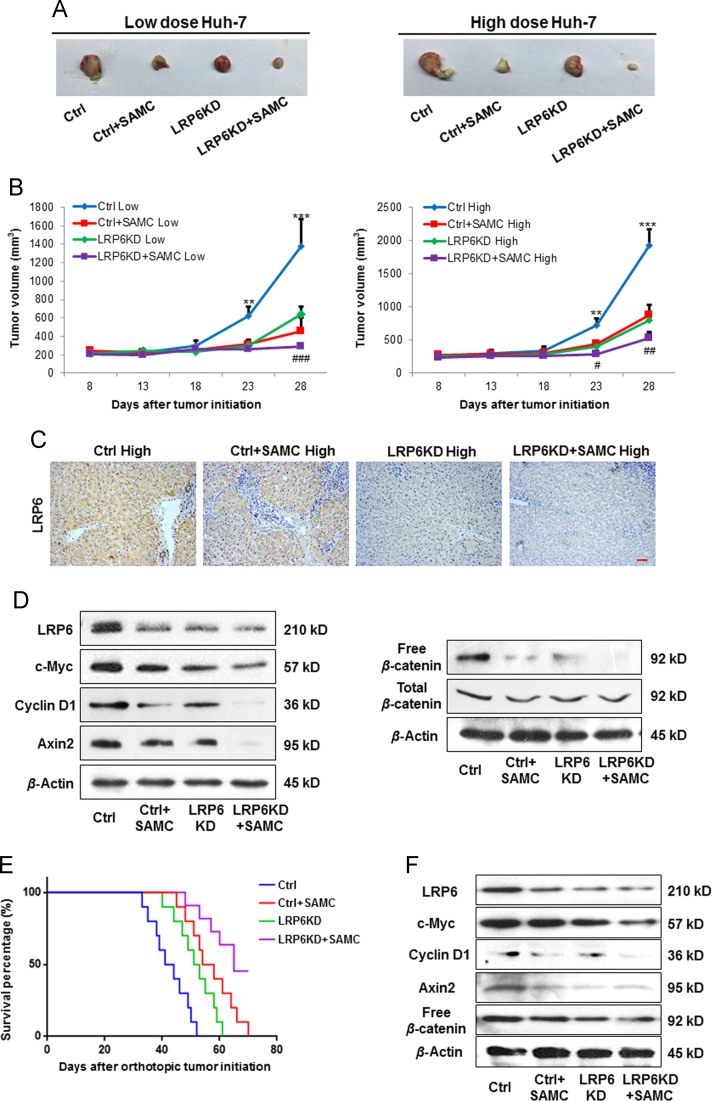
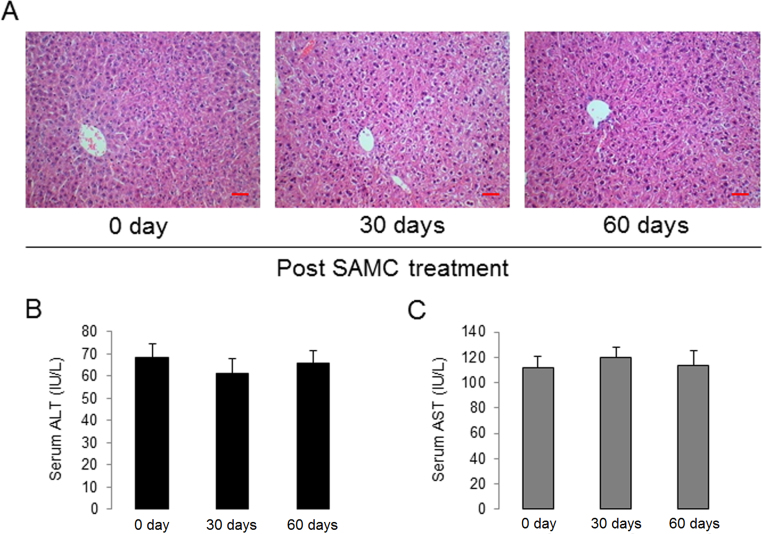
To investigate whether SAMC is effective in suppressing the tumor growth and the role of LRP6 in vivo, we used a Huh-7 xenograft animal model (two concentrations, 1×106 per injection as the low dose group and 5×106 per injection as the high dose group) with or without a stable down-regulation of LRP6 (LRP6-KD). Strikingly, tumors in both Huh-7 concentrations derived from cells with stable down-regulation of LRP6 grew substantially slower than those derived from control cells as evaluated by tumor volume calculation (Fig. 7A and B). Daily oral gastric lavage feeding with 300 mg/kg SAMC further suppressed the tumor growth, when compared with corresponding control groups (Fig. 7A and B). Gross examination of tumors from each group at necropsy also showed that LRP6-KD tumors were significantly smaller than those of control tumors at both Huh-7 treated concentrations. Treatment with SAMC further reduced the tumor sizes (Fig. 7A). Consistent with the in vitro data, knockdown of endogenous LRP6 decreased the level of Wnt signaling and target gene expressions (c-Myc, cyclin D1, and axin2), which were further inhibited by SAMC administration (Fig. 7C and D). To further validate the role of LRP6 and the efficacy of SAMC, we used an orthotopic HCC model through the injection of 2×106 Huh-7 cells. Treatment with SAMC significantly increased survival of tumor-bearing animals compared with animals receiving saline. As expected, knockdown of endogenous LRP6 showed similar rescue effects with SAMC consumption (Fig. 7E). The protein expressional changes of Wnt pathway members were also similar with the results from the s.c. model (Fig. 7F). Taken together, these results demonstrated that SAMC inhibited hepatoma s.c./orthotopic tumor growth partly through the LRP6/Wnt pathway. It should be noted that both 30-day and 60-day of SAMC treatment did not pose any evident toxicity to the mice liver, suggesting it to be an acceptable and bio-safe product when used as a food supplement (Supplementary Information Fig. S9).
Figure 7.
Down-regulation of LRP6 and SAMC treatment significantly inhibits HCC tumor growth in vivo. Huh-7 cells (pooled clones) stably expressing control or LRP6 were subcutaneously injected at the dorsal region with 1×106/150 μL (low dose group) or 4×106/150 μL (high dose group). (A) Gross specimen anatomy of xenograft tumors. (B) Measurements of tumor volume for 33 days with or without SAMC treatment (n=5). (C) Immunohistochemical analysis of LRP6 level in control and LRP6-KD xenograft tumors (with or without SAMC treatment) with anti-LRP6 antibody (Abcam) (Scale bars, 20 μm). (D)–(E) Levels of LRP6 and Wnt target protein expressions (cyclin D1, c-Myc, and axin2) in control and LRP6-KD xenograft tumors detected by Western blot analysis. Western blot analysis shows a decrease in total and free β-catenin in LRP6-KD tumors, which were further reduced by SAMC treatment. Another orthotopic HCC model was established by the injection of 2×106 wild-type or LRP6 knock-down Huh-7 cells into the left liver lobe of nude mice. The animals were treated by oral administration of 300 mg/kg SAMC. (E) Survival of animals was monitored daily for 70 day. (F) intratumor expression of LRP6, c-Myc, cyclin D1, axin2 and free β-catenin were measured by Western blot when tumor tissue was collected at day 50. Data are presented in means±SEM. **P<0.01, ***P<0.001 compared with control group; #P<0.05, ##P<0.01 compared with SAMC group.
4. Discussion
Aberrant Wnt activation is frequently found in ~50% of HCC patients30. As in other forms of cancer, dysregulation of this pathway can be broadly divided into two categories—ligand-dependent disorders (e.g., Wnt protein, LRP and Frizzled receptors) and ligand-independent disorders (e.g., mutations of axin, β-catenin and TCF)31. Since receptor–drug interaction is an important basis for drug screening and discovery, the role of Wnt pathway receptors in cancer progression and drug therapy received much attention recently. For example, Frizzled3, -6 and -7 were found to be overexpressed in HCC32, 33. Additional study confirmed that activated Wnt3/Frizzled7 could promote early carcinogenic process through promoting the acquisition of malignant phenotypes33. Thus, a soluble ectodomain of Frizzled7 was developed to inhibit the Wnt signaling, which sensitized HCC cells to chemotherapy drugs (e.g., doxorubicin)34. In the current study, we identified that LRP6 was frequently up-regulated in human HCC samples and cell lines, when compared with non-tumor liver tissue and normal hepatocytes, respectively. This finding was consistent with a pilot study showing that LRP6 over-expression in HCC promoted cell proliferation, cell migration and hyperactivation of the Wnt signaling10.
SAMC was found to be a tumor suppressor in several kinds of tumors. Although the “down-stream” anti-tumor events, such as promotion of apoptosis, inhibition of proliferation and cell cycle, and suppression of tumorigenesis, were entirely or partially reported in these studies, and there was no study that addressed these two basic questions: (1) What is the immediate molecule(s) that mediate(s) the anti-tumor signaling from SAMC?; and (2) Can SAMC specifically antagonize tumor cell proliferation? This study not only found that LRP6 was over-expressed in HCC cells, but we also identified the direct interaction between SAMC, but not its natural precursor SAC, and LRP6 in vitro, through the experiments done on SPR, TSA and time-lapse fluorescent imaging (Fig. 5 and Supplementary Information Figs. S4 and 5). More importantly, our results demonstrated that knockdown of LRP6 was capable of inhibiting hepatoma cell proliferation, tumorigenesis, Wnt signaling and inducing apoptosis, which could be further potentiated by the co-treatment with SAMC both in in vitro and in vivo models. These findings strongly supported our initial concepts about the crucial role of LRP6 in HCC progression and the receptor signaling used by SAMC. Consistently, when LRP6 was further over-expressed in hepatoma cells, the basal tumorigenic ability and Wnt signaling were elevated, which could also be attenuated by SAMC co-treatment (Figure 6, Figure 7). Furthermore, when LRP6 was over-expressed in normal hepatocytes (LO-2), it enhanced proliferative and tumorigenic phenotypes, which could be reduced by SAMC treatment (Supplementary Information Fig. S7). Considering that after SAMC treatment, the change of LO-2 proliferation, apoptosis and survival markers (surviving and P53/P21) was not evident (Fig. 2 and Supplementary Information Fig. S2), these results were vitally important because they suggested that LRP6 might be one of the molecular pathways for the anti-tumor specificity of SAMC in HCC therapy.
Since basic normal Wnt signaling is indispensable for several physiological functions, an ideal LRP6/Wnt inhibitor should significantly reduce its signaling to inhibit the tumor growth without influencing the physiological homeostasis. We demonstrated in the current study that SAMC treatment in healthy mice did not influence normal liver histology nor caused any evident adverse effect (Supplementary Information Fig. S9). In our previous studies, the hepato-protective roles and possible toxicity of SAMC in several liver diseases have been mechanistically investigated12, 13. Thus, our work introduces SAMC as a promising nutraceutical antitumor agent that can be further developed for HCC targeted therapy, since there is virtually no treatment for advanced HCC.
Acknowledgments
Authors thank the contribution of Wakunaga Pharmaceutical Co., Ltd., Japan, for the free supply of pure form of SAMC and SAC. This study was supported by Foundation of Pearl River Science and Technology New Star (grant number 201506010087) and Basic Research Fund of Shenzhen City (JCYJ20150402111430633) to Jia Xiao Health Medical Research Fund (HMRF, No. 12133881), General Research Fund and Small Project Funding, University Research Committee, The University of Hong Kong. to George L. Tipoe National Natural Science Foundation of China (No. 81570552) to National Program on Key Basic Research Project of China (973 Program, 2014CB542205), Funds of Leading Talents of Guangdong (2013), Programme of Introducing Talents of Discipline to Universities (B14036) to Kwok-Fai So and National Health and Medical Research Council (1031221 & 1031228) to Ming-Tat Ling
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.apsb.2017.10.003.
Contributor Information
Kwok-Fai So, Email: hrmaskf@hku.hk.
George L. Tipoe, Email: tgeorge@hku.hk.
Appendix A. Supplementary material
Fig. S1.
SAMC dose-dependently reduced cell viability of hepatoma cell lines primarily in the first 24 h. Data are presented in means±SEM. **P<0.01 and ***P<0.001, respectively between indicated groups.
.
Fig. S2.
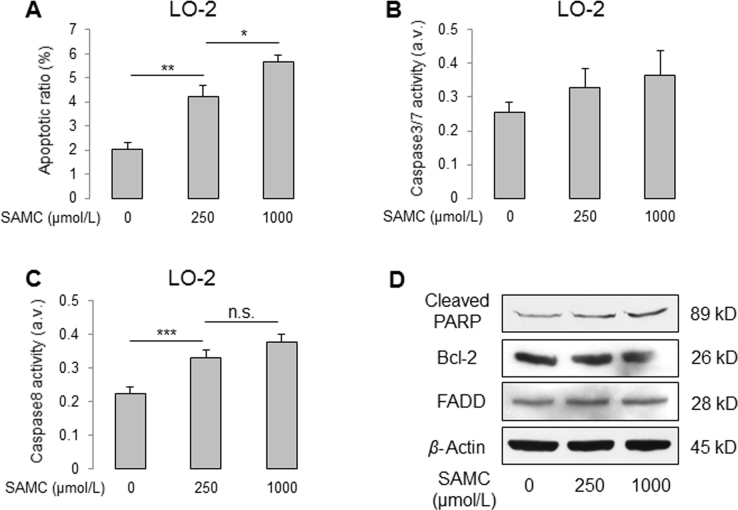
SAMC slightly increased the apoptosis of normal hepatocyte cell line LO-2. (A)–(C) Change of apoptotic ratio and activity of caspase-3, 7, 8 after two doses of SAMC incubation for 24 h. (D) Representative Western blot images for the protein changes of key apoptotic markers, cleaved PARP, BCL-2, and FADD in Huh-7 cells. Data are presented in means±SEM. *P<0.05, **P<0.01 and ***P<0.001, respectively between indicated groups.
.
Fig. S3.
SAMC induced hepatoma cell apoptosis via c-Myc and MCL-1. (A) SAMC treatment dose-dependently inhibited the protein expression of both c-Myc and MCL-1 in Huh-7 cells. (B) c-Myc or MCL-1 were over-expressed in Huh-7 cells by specific plasmid transfection. (C)–(F) Changes of Huh-7 apoptotic ratio and caspase-3/7/8 activity after the endogenous over-expression of c-Myc or MCL-1 with or without SAMC co-treatment. (G) Protein expressional changes of cytochrome c (Cyto c) and BAX1 after c-Myc over-expression with or without SAMC co-treatment. Data are presented in means±SEM. *P<0.05, **P<0.01 and ***P<0.001, respectively between indicated treatment and corresponding control groups. a.v., arbitrary value.
.
Fig. S4.
Representative fluorescent images of cultured LO-2 cells after 0, 30, 60, and 120 min incubation of SAMC which was conjugated with Alexa 488 TFP ester dye (Scale bars, 50 μm).
.
Fig. S5.
The anti-HCC ability and LRP6 interactions of SAMC precursor, S-allylcysteine (SAC), were weak. (A) Natural metabolic pathway from SAC to SAMC during garlic aging. (B) SAC slightly reduced cell viability of Hep3B and Huh-7 at 1 mmol/L without evident influence in human normal hepatocyte cell line LO-2 (n=4). (C) Change of migrated cell number after SAC treatment, examined by Transwell assay, in human hepatoma cell lines Hep3B and Huh-7 (n=4). (D) SPR and TSA analyses of the binding of SAC to recombinant human LRP6 protein. Data are presented in means±SEM. *P<0.05 between indicated group and control group.
.
Fig. S6.
shRNA-resistant LRP6 rescued cell growth and Wnt signaling in Huh-7 cells against the anti-tumor action of SAMC. (A) Protein level of LRP6 after its specific shRNA with or without shRNA-resistant (rescue) transfection. (B)–(C) Change in cell viability and apoptotic ratio after LRP6 specific shRNA with or without shRNA-resistant (rescue) transfection, along with 250 μmol/L SAMC incubation (n=4). (D) Change in TCF/LEF activity after LRP6 specific shRNA with or without shRNA-resistant (rescue) transfection, along with 250 μmol/L SAMC incubation, in the absence and presence of Wnt3a ligands (n=4). Data are presented in means±SEM. *P<0.05, **P<0.01 and ***P<0.001, respectively between indicated groups. #P<0.01 and ##P<0.001 compared with corresponding control group, respectively. Lys, lysate.
.
Fig. S7.
Normal hepatocyte LO-2 gained part of hepatoma cell phenotypes and sensitivity to SAMC treatment when LRP6 was over-expressed. (A) Western blot results showing that LRP6 was over-expressed in LO-2 cells. (B)–(C) LO-2 became sensitive to 250 μmol/L SAMC-induced reduction of cell viability (B) Increase of apoptotic ratio (C) Over-expression of LRP6 (n=4). (D) The invasion ability of Huh-7 cells was increased when LRP6 was over-expressed, which was reduced to a control-comparable level after SAMC incubation (n=4). Data are presented in means±SEM. *P<0.05, **P<0.01 and ***P<0.001, respectively between indicated groups.
.
Fig. S8.
Over-expression of LRP6 regulated Wnt and apoptotic pathway proteins in Huh-7 cells. (A) Protein expressional changes of key oncogenes and Wnt pathway components (c-Myc, cyclinD1 and axin2) and (B) apoptotic cytochrome c (cyto c), BCL-2 and BAX1 after LRP6 over-expression with or without SAMC co-treatment.
.
Fig. S9.
Long-term treatment with SAMC did not affect normal liver function of nude mice. (A) Representative H&E staining images showing that 30 days or 60 days of 300 mg/kg daily SAMC treatment did not affect the normal hepatic histology of nude mice (Scale bars, 20 μm). (B)–(C) Serum ALT and AST level changes when nude mice received 30 days or 60 days of 300 mg/kg daily SAMC treatment (n=5). Data are presented in means±SEM.
.
Supplementary material
.
Supplementary material
.
References
- 1.Theise N.D., Chen C.J., Kew M.C. Liver cancer. In: Stewart B., Wild C., editors. World Cancer Report 2014. International Agency for Research on Cancer; Lyon: 2014. pp. 577–593. [Google Scholar]
- 2.Hong Y., Huang J. Autoantibodies against tumor-associated antigens for detection of hepatocellular carcinoma. World J Hepatol. 2015;7:1581–1585. doi: 10.4254/wjh.v7.i11.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monga S.P. β-Catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148:1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gough N.R. Focus issue: wnt and β-catenin signaling in development and disease. Sci Signal. 2012;5:eg2. doi: 10.1126/scisignal.2002806. [DOI] [PubMed] [Google Scholar]
- 5.Monga S.P. Role and regulation of β-catenin signaling during physiological liver growth. Gene Expr. 2014;16:51–62. doi: 10.3727/105221614X13919976902138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pez F., Lopez A., Kim M., Wands J.R., Caron de Fromentel C., Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107–1117. doi: 10.1016/j.jhep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Bogaerts E., Heindryckx F., Vandewynckel Y.P., van Grunsven L.A., van Vlierberghe H. The roles of transforming growth factor-β, Wnt, Notch and hypoxia on liver progenitor cells in primary liver tumours. Int J Oncol. 2014;44:1015–1022. doi: 10.3892/ijo.2014.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercer K.E., Hennings L., Ronis M.J. Alcohol consumption, Wnt/β-catenin signaling, and hepatocarcinogenesis. Adv Exp Med Biol. 2015;815:185–195. doi: 10.1007/978-3-319-09614-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go G.W. Low-density lipoprotein receptor-related protein 6 (LRP6) is a novel nutritional therapeutic target for hyperlipidemia, non-alcoholic fatty liver disease, and atherosclerosis. Nutrients. 2015;7:4453–4464. doi: 10.3390/nu7064453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung E.K., Wong B.Y., Yau T.O., Ng I.O. Upregulation of the Wnt co-receptor LRP6 promotes hepatocarcinogenesis and enhances cell invasion. PLoS One. 2012;7:e36565. doi: 10.1371/journal.pone.0036565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicastro H.L., Ross S.A., Milner J.A. Garlic and onions: their cancer prevention properties. Cancer Prev Res. 2015;8:181–189. doi: 10.1158/1940-6207.CAPR-14-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao J., Liong E.C., Ling M.T., Ching Y.P., Fung M.L., Tipoe G.L. S-Allylmercaptocysteine reduces carbon tetrachloride-induced hepatic oxidative stress and necroinflammation via nuclear factor κB-dependent pathways in mice. Eur J Nutr. 2012;51:323–333. doi: 10.1007/s00394-011-0217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao J., Guo R., Fung M.L., Liong E.C., Chang R.C., Ching Y.P. Garlic-derived S-allylmercaptocysteine ameliorates nonalcoholic fatty liver disease in a rat model through inhibition of apoptosis and enhancing autophagy. Evid Based Complement Altern Med. 2013;2013:642920. doi: 10.1155/2013/642920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao D., Pinto J.T., Soh J.W., Deguchi A., Gundersen G.G., Palazzo A.F. Induction of apoptosis by the garlic-derived compound S-allylmercaptocysteine (SAMC) is associated with microtubule depolymerization and c-Jun NH2-terminal kinase 1 activation. Cancer Res. 2003;63:6825–6837. [PubMed] [Google Scholar]
- 15.Chu Q., Ling M.T., Feng H., Cheung H.W., Tsao S.W., Wang X. A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis. 2006;27:2180–2189. doi: 10.1093/carcin/bgl054. [DOI] [PubMed] [Google Scholar]
- 16.Howard E.W., Ling M.T., Chua C.W., Cheung H.W., Wang X., Wong Y.C. Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clin Cancer Res. 2007;13:1847–1856. doi: 10.1158/1078-0432.CCR-06-2074. [DOI] [PubMed] [Google Scholar]
- 17.Hu H., Zhang X.P., Wang Y.L. Identification of a novel function of Id-1 in mediating the anticancer responses of SAMC, a water-soluble garlic derivative, in human bladder cancer cells. Mol Med Rep. 2011;4:9–16. doi: 10.3892/mmr.2010.380. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Wang K., Lin G., Chua C.W., Luk S.U., Wong Y.C. Antitumor mechanisms of S-allyl mercaptocysteine for breast cancer therapy. BMC Complement Altern Med. 2014;14:270. doi: 10.1186/1472-6882-14-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y., Kim H., Lee J., Kim K. Anticancer activity of S-allylmercapto-l-cysteine on implanted tumor of human gastric cancer cell. Biol Pharm Bull. 2011;34:677–681. doi: 10.1248/bpb.34.677. [DOI] [PubMed] [Google Scholar]
- 20.Tong D., Qu H., Meng X., Jiang Y., Liu D., Ye S. S-Allylmercaptocysteine promotes MAPK inhibitor-induced apoptosis by activating the TGF-β signaling pathway in cancer cells. Oncol Rep. 2014;32:1124–1132. doi: 10.3892/or.2014.3295. [DOI] [PubMed] [Google Scholar]
- 21.Liu C.C., Prior J., Piwnica-Worms D., Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci U S A. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang S., Song X., Wang Y., Ru H., Shaw N., Jiang Y. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36:1073–1086. doi: 10.1016/j.immuni.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llovet J.M., Bustamante J., Castells A., Vilana R., Ayuso Mdel C., Sala M. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 24.Hu J., Dong A., Fernandez-Ruiz, Shan J., Kawa M., Martínez-Ansó E. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res. 2009;69:6951–6959. doi: 10.1158/0008-5472.CAN-09-0541. [DOI] [PubMed] [Google Scholar]
- 25.Liang X., Da M., Zhuang Z., Wu W., Wu Z., Wu Y. Effects of Survivin on cell proliferation and apoptosis in MG-63 cells in vitro. Cell Biol Int. 2009;33:119–124. doi: 10.1016/j.cellbi.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Ladu S., Calvisi D., Conner F., Farina M., Factor V.M., Thorgeirsson S.S. E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer. Gastroenterology. 2008;135:1322–1332. doi: 10.1053/j.gastro.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas L.W., Lam C., Edwards S.W. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 28.Kagermeier-Schenk B., Wehner D., Özhan-Kizil G., Yamamoto H., Li J., Kirchner K. Waif1/5T4 inhibits Wnt/β-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev Cell. 2011;21:1129–1143. doi: 10.1016/j.devcel.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Ng K.T., Guo D.Y., Cheng Q., Geng W., Ling C.C., Li C.X. A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma. PLoS One. 2012;7:e31665. doi: 10.1371/journal.pone.0031655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lachenmayer A., Alsinet C., Savic R., Cabellos L., Toffanin S., Hoshida Y. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-2322. :4997-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klaus A., Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 32.Bengochea A., de Souza M.M., Lefrancois L., Le Roux E., Galy O., Chemin I. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nambotin S.B., Tomimaru Y., Merle P., Wands J.R., Kim M. Functional consequences of WNT3/Frizzled7-mediated signaling in non-transformed hepatic cells. Oncogenesis. 2012;1:e31. doi: 10.1038/oncsis.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei W., Chua M.S., Grepper S., So S.K. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. doi: 10.1186/1476-4598-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material