Graphical abstract
Keywords: High intensity focused ultrasound (HIFU), Low intensity focused ultrasound (LIPUS), Pulsed magnetic field, Static magnetic field, Cancer, Hyperthermia
Abstract
Current popular cancer treatment options, include tumor surgery, chemotherapy, and hormonal treatment. These treatments are often associated with some inherent limitations. For instances, tumor surgery is not effective in mitigating metastases; the anticancer drugs used for chemotherapy can quickly spread throughout the body and is ineffective in killing metastatic cancer cells. Therefore, several drug delivery systems (DDS) have been developed to target tumor cells, and release active biomolecule at specific site to eliminate the side effects of anticancer drugs. However, common challenges of DDS used for cancer treatment, include poor site-specific accumulation, difficulties in entering the tumor microenvironment, poor metastases and treatment efficiency. In this context, non-invasive cancer treatment approaches, with or without DDS, involving the use of light, heat, magnetic field, electrical field and ultrasound appears to be very attractive. These approaches can potentially improve treatment efficiency, reduce recovery time, eliminate infections and scar formation. In this review we focus on the effects of magnetic fields and ultrasound on cancer cells and their application for cancer treatment in the presence of drugs or DDS.
Introduction
American Cancer society estimated that about 1.7 million new cancer cases and 609,640 deaths occurred in 2018 in US [1]. Lung cancer is the leading cause of cancer death due to established risk factors such as smoking, overweight, physical inactivity, and changing reproductive patterns associated with urbanization and economic development [1]. The common type of cancer deaths include lung (1.69 million), liver (788,000), colorectal (774,000), stomach (754,000) and breast (571,000) cancer [2]. Annual healthcare cost for treating cancer in 2010 has been approximately US$ 1.16 trillion [3]. These figures clearly indicate that the economic impact of cancer is very high. Table 1 provides brief summary of cancer severity in the US population estimated in 2018 [1].
Table 1.
Estimated new cancer cases and deaths in the United States, 2018 (compiled from [1] with permission from American Cancer Society. Modified Cancer Facts and Figures 2018. Atlanta: American Cancer Society, Inc.).
| Organ specific cancer | New cases |
Estimated deaths |
||||
|---|---|---|---|---|---|---|
| Both sex | Male | Female | Both sex | Male | Female | |
| Tongue | 17,110 | 12,490 | 4620 | 2510 | 1750 | 760 |
| Esophagus | 17,290 | 13,480 | 3810 | 15,850 | 12,850 | 3000 |
| Stomach | 26,240 | 16,520 | 9720 | 10,800 | 6510 | 4290 |
| Small intestine | 10,470 | 5430 | 5040 | 1450 | 810 | 640 |
| Colon and Rectum | 140,250 | 75,610 | 64,640 | 50,630 | 27,390 | 23,240 |
| Lung and Bronchus | 234,030 | 121,680 | 112,350 | 154,050 | 83,550 | 70,500 |
| Melanoma (skin) | 91,270 | 55,150 | 36,120 | 9320 | 5990 | 3330 |
| Ovary | 22,240 | 22,240 | 14,070 | 14,070 | ||
| Prostate | 164,690 | 164,690 | 29,430 | 29,430 | ||
| Acute myeloid leukemia | 19,520 | 10,380 | 9140 | 10,670 | 6180 | 4490 |
| Eye and orbit | 3540 | 2130 | 1410 | 350 | 190 | 160 |
| Urinary System | 150,350 | 107,600 | 42,750 | 33,170 | 23,110 | 10,060 |
Current cancer treatments such as tumor surgery, chemotherapy, immunotherapy, hormonal treatment are inherently associated with some limitations. For example, tumor surgery is not effective in mitigating metastases, radiation therapy is expensive as well as time consuming. In chemotherapy the anticancer drugs can quickly spread throughout the body and is ineffective in killing metastatic cancer cells. Moreover, these drugs are highly toxic to healthy cells and can potentially decrease patient’s survival rates. While the drugs used for immunotherapy are known to develop toxicities and adverse events (in 1–95% of patients) related to skin, gastrointestinal, endocrine, hepatic, pulmonary, and renal [4]. To address these limitations drug delivery systems (DDS) have been developed to target specific tumor cells and release active biomolecules at specific site of infection thus eliminating the side effects of these drugs. However, DDS often use nanoparticles (NPs) as drug carriers, which pose risks of toxicity and solubility in the biological matrices. Earlier investigations revealed that excessive exposure of NPs can cause pulmonary inflammation, immune adjuvant effect, and blood coagulation [5]. Another important limitation of NPs based DDS is their entrapment in the mononuclear based phagocytic system of liver and spleen. The inherent agglomeration potential of NPs restricts their systemic circulation. Therefore, appropriate surface modification is required to reduce their agglomeration and associated cytotoxicity. Other common challenges of NPs based DDS for cancer therapy include poor site-specific accumulation, production cost, inability to cross Blood-Brain barrier for neurodegenerative diseases and brain tumors, difficulties in entering the tumor microenvironment, poor metastases. Some of the important issues related to NPs based DDS for cancer treatment are summarized in Table 2.
Table 2.
Important limitations of popular drug delivery systems (DDS).
| Drug delivery system | Limitations | Ref. |
|---|---|---|
| Polymeric micelles | Low drug loading, reduced stability, limited targeting ability | [6] |
| Dendrimer | Low encapsulation efficiency, poor storage stability. | [7] |
| Solid Lipid NPs | Insufficient drug loading and relatively high water content of the dispersions. | [8] |
| Liposome | Expensive, leakage and fusion of encapsulated drug/molecules, short half-life and stability issues. | [9] |
| Quantum Dots | Rapid clearance, complex synthesis process, poor localization. | [10] |
| Inorganic DDS | ||
| Layered double hydroxide (LDH) | Poor target recognition, low efficiency, uncontrolled particle size and its distribution can lead to in-vivo tissue damage. | [11] |
| Gold NPs | Uncertain in-vivo kinetics, tumor target efficiency, acute and chronic toxicity. | [12] |
| Iron Oxide | Reunion phenomenon | [13] |
| MSN (Mesoporous Silica NPs) | Hemolysis and melanoma promotion. | [14] |
Alternative cancer treatments involving the use of non-invasive approaches can potentially eliminate infections and scar formation associated with surgery, as well as minimizes the side effects of chemotherapeutic drug overdose. Non-invasive cancer treatment approaches, with or without DDS, typically use various physical stimuli such as light, heat, magnetic field, electrical field, ultrasound [15]. These approaches have shown good potential to improve treatment efficiency, reduce treatment costs, eliminate infections and scar formation. Important mechanisms associated with these non-invasive approaches in inhibiting cancer cell growth include hyperthermia, controlled drug release, mechanical stress, changing membrane permeability, etc. [16], [17]. For example, the use of ultrasound increased reactive oxygen species (ROS) production inside the tumor of a mice administered with TiO2 NPs and then suppressed the tumor growth [18]. Further, inherent electrical characteristics of cells (responding to external electrical fields due to the presence of ions, charged molecules, membranes and organelles) have been effectively exploited to inhibit cancer cells using external electrical fields [19]. Some of these external stimuli have also been used to alter membrane permeability thereby improving the efficiency of DDS based cancer treatments. However, in this review we focus on the effects of magnetic fields and ultrasound on cancer cells, and their application for cancer treatment in the presence of anticancer drugs and DDS.
Biological effects of magnetic fields
Magnetic fields are well known to boost blood circulation in tissues and stimulate body metabolism. Proper blood circulation is extremely important to provide oxygen to different organs, muscles and tissues thus ensuring their healthy function. Generally wounds and painful areas of the body suffer from lack of oxygen and poor blood circulation. Low-frequency pulsed magnetic therapy is widely being used to induce detoxification (cleansing) effect and enhanced metabolism. Typically magnetic therapy induces weak electrical currents in the tissues, which enhances surface potential of cells leading to enhanced blood circulation, oxygenation, nutrient supply and better removal of metabolic waste from the exposed body tissues [20]. Magnetic fields have also been used as natural pain killers, to promote repair and healing, reduce swelling, stiffness and acidity from the wounds. Magnetic fields have been found to stimulate collagen density in and around the joints, and help to trigger Ca2+ flow to the defect site resulting in faster bone healing [21]. Studies on blood microcirculation revealed that magnetic fields have strong influence on relaxation and constriction of capillary blood vessels which alters the blood flow. In vivo experiments performed on rats with 70 mT magnetic field demonstrated clear increase in the blood flow due to dilation of blood vessels [22]. The magnetic field assisted enhancement of blood flow reduced the swelling (up to 50%) in rat paws when the magnetic field was applied immediately after injury [22]. At molecular level static magnetic field (SMF) appears to change several cytokines and interleukin from lymphocytes and macrophages. The anti-inflammatory activity of SMF has also been demonstrated via controlling secretion of pro-inflammatory cytokines (IL-6, IL8, and TNF-α) and enhanced anti-inflammatory cytokines production (IL-10) [23]. Since inflammation is closely linked to cancer and likely increase in the cancer risk due to chronic inflammation, the SMF exposure could be a potential approach to treat cancer.
Alternating magnetic fields (AMF)/pulsed magnetic fields (PFM) can induce small electric currents, in conducting tissues, directly proportional to the field frequency. At very high frequencies or amplitudes, induced currents can generate excessive heat in the tissues and cause thermal damage. On the other hand, at extremely low frequencies (∼0–300 Hz) and very low frequencies (∼300–100,000 Hz) the tissue heating is negligible, but the induced currents, if sufficiently strong, can stimulate electrically excitable cells such as neurons for their treatment. The AMF generated heat can also be used for physiotherapy and other treatments [24]. In general, the glycolysis and glucose oxidations are decreased in diabetic patients leading to lower ATP production. However, increase in the insulin, glycogen as well as decrease in the glucose level were observed in a diabetic rat exposed to AMF [25]. Further, it was found that the blood cholesterol, glucose and triglyceride levels of diabetic rats were lowered with AMF exposure. Gordon [25] showed the selective effect of AMFs on atherosclerotic lesions without harming blood vessels. Reported biophysical changes caused by electromagnetic fields on atherosclerotic plaques can aid further developments in selective treatment of atherosclerosis.
Magnetic field assisted cancer treatment
The effects of magnetic fields on cancer cells/tumors depends on three main mechanisms namely (1) thermic effect, (2) cavitation effect, and (3) non-thermic/non-cavitation effect [26]. PMF have been used to actuate localized hyperthermia in the tissues where magnetic nanoparticles (MNPs) have been accumulated [27]. PMF treatment has also been advocated for advanced stage of cancer (Stage 3 and 4) primarily because of their intolerance towards chemotherapy due to decreased functionality of several organs [28]. Extremely low frequency PMF has also been shown to inhibit murine malignant tumor growth by arresting neoangiogenesis required for tumor growth [29]. However, with repeated use of PMF the cells found to acquire thermo-resistance, as a result the treatment efficiency decreases [30]. In contrast, the use of SMF induces oxidative stress leading to the damage of cancer cellular membrane ion channels followed by apoptosis. Moreover, the interaction between SMF and polar, ionic molecules of cellular compartment produces reactive oxygen species (ROS) because of pro-inflammatory changes inside the cancer cell [30], which inhibit their growth and proliferation. So far the use of magnetic fields towards cancer treatment has shown promising results in animal studies, which demonstrate their application potential as adjuvant therapy. Furthermore, magnetic fields can induce Joule’s heating and expand cancer tumor blood vessels. These expanded blood vessels enable excessive oxygen to enter the tumor and create hindrance to the survival of cancer cells in oxygen-rich tumor environment. The expanded blood vessels also allow more Natural Killer (NK) cells to enter the tumor thus interfering with cancer cell activities [31]. On the other hand, cancer cell eventual distribution, inside the body, requires formation of new blood vessels, which depends on Vascular Endothelial Growth Factor (VEGF) in the blood. Application of magnetic fields can significantly decrease VEGF level and therefore reduces the growth and distribution of cancer to other parts of the body [32]. It has been observed that SMF interacts with the charged molecules (ions, proteins etc.) of biological system through several physical mechanisms and alters the activity, concentration, and life time of paramagnetic free radicals i.e. ROS (reactive oxygen species), RNS (reactive nitrogen species), which causes oxidative stress, genetic mutation, and apoptosis in cancer cells [33]. These ROS and RNS are known to play important roles in natural immunological defense [34] of the body against cancer through intracellular signaling pathways. However, free radical production can also damage ion channels of cancer cells leading to changes in their morphology and apoptosis.
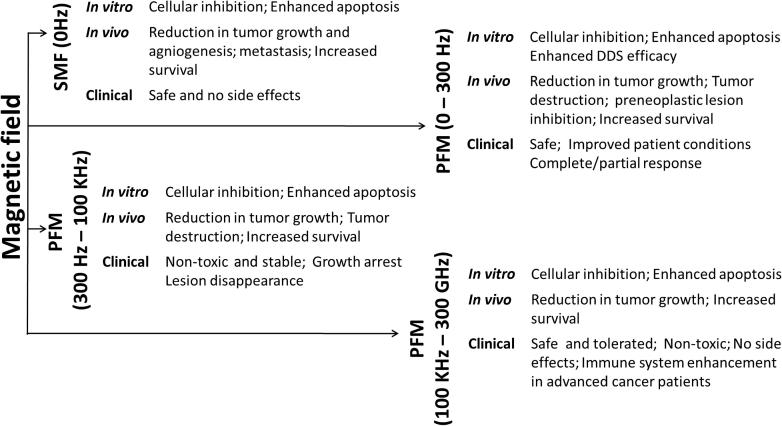
Modern magnetic field assisted cancer therapy uses electromagnetic field (EMF), which can generate much higher hyperthermia in the presence of magnetic NPs. In this treatment, EMF is focused on to a tumor at frequencies that will selectively heat the tumor. However, such hyperthermia based cancer treatments often suffer from low radiation selectivity, long treatment times and potential necrosis in the surrounding healthy tissues. Molecular interactions between the heat and tumor tissues have strong influence on angiogenesis (formation of new blood vessels) and vasculature system, which increased the interest in clinical use of magnetic fields for cancer treatment [35]. The high temperature generated during hyperthermia increases cell membrane fluidity, permeability and activates immune system, which can damage cancer cell DNA by deactivating specific repair proteins (chaperone) [36]. These changes are responsible for the observed disturbances in homeostasis that triggers various signaling cascades and cancer cell apoptosis [37]. In addition to hyperthermia, thermo-ablation based treatments have also been attempted using AMF to treat tumors loaded with iron oxide NPs [38]. Generalized experimental set up used for magnetic field assisted cancer treatment is shown in Fig. 1. In vitro, in vivo, and clinical effects of different magnetic fields on cancer are summarized in Fig. 2.
Fig. 1.
Typical experimental set up for cancer treatment using magnetic fields (a) In vitro and in vivo treatment (b) Clinical trials.
Fig. 2.
Summary of effects of magnetic fields on cancer (adapted from Verginadis et al. [39] under the terms of the Creative Commons Attribution 3.0 License).
Effect of static magnetic fields (SMF) on cancer
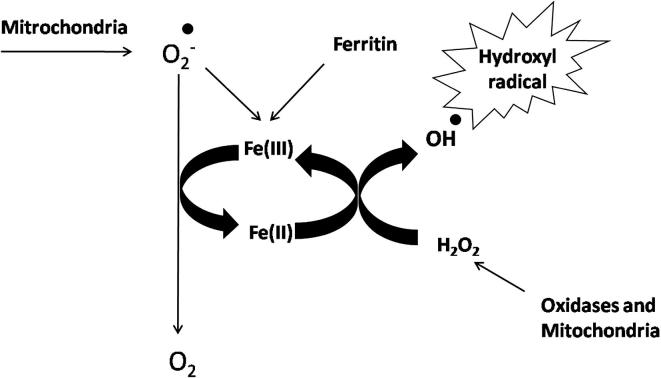
Static magnetic fields of varying strengths have been used, both in vitro and in vivo, to study their influence on cancer cell inhibition and tumor progression, with/without NPs and drugs [40]. The interaction between SMF and cancer cells primarily depends on ROS modulation (generation or reduction) due to enzymatic reactions [33]. Change in the radical pair recombination rates of oxygen inside the cell generally initiates membrane damage followed by cell lysis. The production of ROS directs DNA damage in cancer cells through Fenton reaction. The Fenton reaction is a process that is catalyzed by iron in which hydrogen peroxide (a product of oxidative respiration in the mitochondria) is converted into hydroxyl free radicals that are very potent and cytotoxic molecules. Schematic diagram showing the production of ROS through Fenton reaction is presented in Fig. 3.
Fig. 3.
Typical ROS production via Fenton reaction.
Vergallo et al. [41] used NdFeB permanent magnets to create SMF and studied its effect on neuroblastoma cells in vitro. In this work, SH-SY5Y cells (Human neuroblastoma) were treated with 200 mT SMF along with 0.1 mE cis-Pt (Cis-DichloroDiammine Platinum II). After 2 h of SMF treatment the cell viability decreased by 30% due to over expression of caspase-3 protein (46%), which plays a central role in cellular apoptosis. After 24 h of SMF exposure the production of ROS also increased by 23%. In another study [42], human hepatoma cell lines (BEL-7402 and HepG2) were treated with 200 mT SMF (30 min/24 h at 250 Hz, 400 Hz, and 500 Hz) for 3 and 6 days. After 6 days, significant apoptosis was induced in BEL-7402 with 400 Hz and 250 Hz treatment. In contrast, these treatment conditions had no measurable influence on HepG2 cells suggesting tailorability of magnetic treatment to target specific cancer cells. This treatment reduced the expression of Bcl-2 and Caspase 8 in treated BEL-7402 cells, while the Caspase 3 and Caspase 9 were significantly up regulated [42]. From these studies it is understandable that the use of SMF between 200 and 2000 mT on various cancer cells expresses apoptotic protein and increases apoptotic rate via altering gene expression of bcl-2, bax, p53 and hsp70 in freshly isolated human lymphocytes. These altered gene expressions controls the influx of Ca2+ towards cellular compartment by altering membrane permeability [43]. Moderate intensity of SMF (8.8 mT exposed for 12 h) found to affect metabolic activity (with or without 25 ng/mL Adriamycin) of cells, cell cycle distribution, DNA damage, cellular structure, and P-glycoprotein (P-gp) expression in K562 cells (human chronic myelogenous leukemia) [44]. These experiments also revealed that the use of SMF along with drugs changes cell membrane characteristics and enlarge vacuoles inside the cytoplasm. Analysis of cell cycle demonstrated that the ratio of G2/M phase increases, while cell concentration in S phase significantly decreases. This study demonstrated that 8.8 mT SMF enhances cytotoxic potency of Adriamycin on K562 cells due to decrease in the P-gp expression [44].
A-Mel3 tumors grown in dorsal skin fold chamber of hamsters and exposed to SMF (< 600 mT) showed significant reduction in capillary red blood cells velocities (vRBC) and segmental blood flow in the tumor micro vessels [45]. These changes are believed to be responsible for the observed reduction in tumor size as a result of insufficient nutrient flow. However, long-time exposure (64 h) of HTB-63 (melanoma), HTB 77 IP3 (ovarian carcinoma) and CCL 86 (lymphoma: Raji cells) cells to strong SMF (7 T) revealed relatively higher cell cycle arrest and cellular inhibition in HTB-63 than other cell lines [46]. Detailed pulsed-field electrophoretic analysis revealed no DNA fragmentation and therefore it appears that prolonged exposure to very strong magnetic fields can also inhibit in vitro growth of these human tumor cell lines [46].
Electromagnetic fields (EMF) and alternating magnetic fields (AMF) for cancer treatment
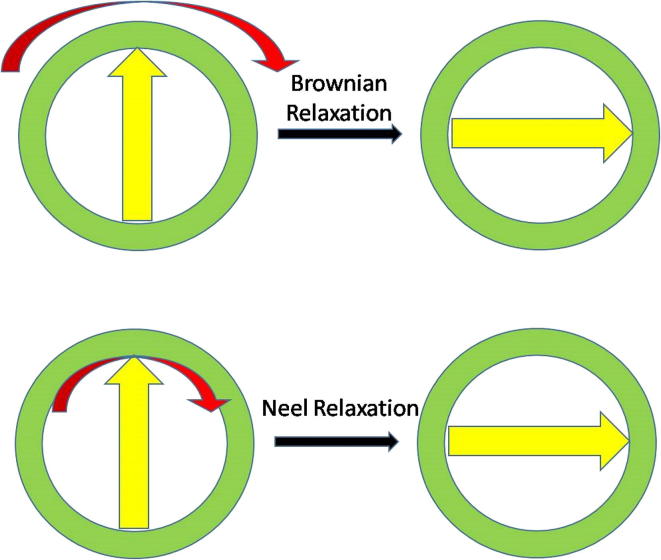
Hyperthermia based cancer treatments uses electromagnetic fields (EMF, generated using electromagnets instead of permanent magnets as in the case of SMF) with or without high frequency alternating or pulsed magnetic fields. In this treatment, hyperthermia with high heat is generated in the presence of magnetic NPs (typically iron oxide) due to Brownian and Néel relaxation [47], [48]. The origin of heat generation is primarily due to the production of eddy currents, hysteresis losses, relaxation losses and frictional losses. The Brownian relaxation (due to whole particle oscillation or rotation) and Néel relaxation (due to internal magnetic domain rotation) are responsible for heat generation in this cancer treatment (Fig. 4). However, hyperthermia created via Brownian relaxation appears to be more effective in inhibiting tumor growth due to its high heat generation capacity compared to Néel relaxation. Further, Hajiaghajani et al. [49] evaluated the importance of design and shaping of magnetic fields in enhancing the efficiency of these treatments, while simultaneously improving the immunity of healthy cells toward chemotherapeutic drugs. They proposed triangular magnetic fields which exhibited up to 90% target efficiency in axillary artery of breast tissues.
Fig. 4.
Brownian and Néel relaxation of MNPs exposed to AMF or PMF.
Earlier studies demonstrated that AMF/PMF therapies, in the presence of magnetic NPs, induce apoptosis in several tumor tissues and cancer cells (osteosarcoma, breast cancer, gastric cancer, colon cancer, and melanoma) [50], [51], [52]. These therapies have been extensively studied in vitro using various human cancer cell lines namely pheochromocytoma-derived (PC12), breast cancer (MCF7, MDA-MB-231 and T47D), and colon cancer (SW-480 and HCT-116) [53], [54], [55], [56]. An interesting in vitro study reported by Crocetti et al. [52] evaluated selective targeting of human breast adenocarcinoma cells (MCF7) using ultra-low intensity and frequency pulsed electromagnetic fields (PEMF). MCF7 cells along with normal breast epithelial cells (MCF10) were treated with 20 Hz PMF having 3 mT intensity for 30, 60, and 90 min/day up to 3 days. In vitro analysis in terms of apoptosis and cell electrical properties showed that MCF7 cells are highly reactive to 3 mT flux density and normal cells (MCF10) are unaffected. This investigation demonstrates that treatment parameters such as frequency, magnitude and treatment time can be tailored to selectively target malignant cells without harming healthy cells.
In vivo study on S-180 sarcoma (Mus musculus sarcoma) in mice using AMF/PMF of 0.8 T (22 ms, 1 Hz) suppressed the growth of sarcoma but enhanced the host immune cells. The heat generated (42–46 °C) with AMF/PMF application caused hyperthermic shock to tumor cells (cellular inactivation) leading to necrosis and apoptosis [57]. In addition, the exposure of AMF/PMF found to change environmental pH inside the tumor tissues along with perfusion and oxygenation of tumor microenvironment [58]. It was also revealed in Kunming mice (36–40 g) that the PMF can block the development of neo-vascularization required for tumor growth [29]. In this investigation, the mice were treated for 15 min/day with PMF of 0.6–2.0 T having a pulse width of 20–200 ms and frequency of 0.16–1.34 Hz. Post-treatment analysis revealed swallowed endothelial blood vessel cells, which occluded the blood vessels and stopped oxygen and nutrition supplies inside the tumor [29]. Although promising, similar studies on the effect of SMF and PMF on neo-vascularization under in vitro conditions would enable assessment of these treatments in treating large variety of cancers before expensive and time consuming in vivo trials.
Poor responsiveness of Glioblastoma multiforme (GBM, a malignant brain cancer) to surgery, chemotherapy and radiation therapy has also been effectively addressed using PEMF in conjunction with chemotherapeutic drugs. Combined use of 100 μM Temozolomide (TMZ) and EMF (100 Hz, 100 G) on U87 and T98G (human brain cancer cells) found to enhance cellular apoptosis synergistically by upregulation of p53, Bax, Caspase-3 and downregulation of Bcl-2 and Cyclin-D1 [58]. EMF treatment enhanced the efficiency of TMZ by increasing ROS production in both cell lines and induced pre and pro apoptotic gene expression [59]. In another study, U87 cells were treated with varying EMF (10–50 Hz, 10–100 G) for durations up to 24 h [60]. Depending on the EMF frequency and intensity the cell proliferation and apoptosis were found to vary, which suggest that the cancer cell inhibition can occur only under specific treatment conditions. Therefore, the treatment conditions must be tailored to suit specific cancer type and the conditions may differ under in vitro and in vivo conditions.
Clinical trials involving the use of PMF/AMF to treat variety of cancers in different stages are also very limited. First pilot study by Ronchetto et al. [61] reported the effect of extremely low frequency-modulated SMF on 11 patients with stage IV cancers (adenocarcinoma, squamous cell carcinoma, etc.). The treatment (20–70 min/day over 4 weeks) found to be safe and tolerable for humans. Recently, one study examined 1524 frequencies (0.1–114 kHz) and identified tumor-specific frequency to treat 163 patients with different advanced cancers such as brain, pancreatic, ovarian, breast, prostate, lung, bladder [62]. During treatment of 28 patients for 278.4 months (60 min treatment, 3 times/day) none of them had significant side effects leading to treatment discontinuation. Patients with advanced hepatocellular carcinoma (HCC) have severely impaired liver function and therefore cannot tolerate standard chemotherapy or intrahepatic treatments. Therefore, recently Phase I/II clinical study involving PEMF treatment of forty-one advanced HCC patients has been carried out by Costa et al. [63]. In this study, the patients were treated with low levels of pulsed electromagnetic fields (100 Hz – 21 kHz) for 60 min (three times/day). Majority of the patients exhibited complete disappearance (5 patients) or immediate reduction in pain (2 patients) due to this treatment and no toxicities were observed. This study clearly showed stable disease (39%) for more than 12 weeks and therefore potentially provides safe and well tolerable treatment for HCC. The above clinical studies also demonstrate that tumor-specific frequencies can be effectively and safely used to treat variety of cancers, in different stages, and further studies prove to be highly beneficial for successful cancer treatment [64]. A brief summary of in vitro, in vivo and clinical observations made during PMF/AMF based cancer treatments is presented in Table 3.
Table 3.
Summary of PMF/AMF based cancer treatment observations (adapted from [64] with permission from John Wiley and Sons).
| Cancer cell line | Treatment | Observations | Ref. |
|---|---|---|---|
| In vitro studies | |||
| Human Breast cancer (MDA-MB-231) | PMF (50 Hz; 10 mT) for 24,48, and 72 h | Increased apoptosis of 20% and 50% after 24 and 72 h culture, respectively | [65] |
| Colon cancer (SW-480 and HCT116) | PMF (50 Hz; 10 mT) for 24,48, and 72 h | 11% and 6% increase in the apoptosis after 24 and 72 h culture, respectively | |
| Undifferentiated PC12 pheochromocytoma cells and differentiated PC12 cells | Short PMF (50 Hz, 0.1–1 mT) for 30 min | Undifferentiated PC12, increased ROS level and decreased Calalase activity. No change in Ca+ | [66] |
| Long PMF (50 Hz, 0.1–1 mT) for 7 days | Undifferentiated PC12, increased intracellular Ca+ concentration and Catalase activity. No significant finding in differentiated PC12 | ||
| Animal Type | Treatment | Method and observations | Ref. |
| In-vivo studies | |||
| T cell immunodeficient female nude mice (12 nos. in 4 grp, n = 3) | Breast tumor cell line [EpH4-MEK Bcl213 cells (1 * 106)] injected by IV route | Grp 1, 2, 3 were exposed to PMF (1 Hz, 100 mT) daily for 60,180, and 360 min for 4 weeks and Grp 4 no treatment Mice exposed to 60,180 min treatment showed 30–70% reduction in the reduce tumor |
[67] |
| Rats (60 Nos. strain not reported; divided into 6 grps) | Intraperitoneal injection of DEN (carcinogen) | Grp 1&4 PMF (2–3 Hz; 0.004 T) for 30 min/day till 6 days/week for 4 week. Grp 2&5 PMF (≤1 Hz, 0.6 T) for 15 min/day for 6 days/week for 4 week. Grp 3 and 6 remains untreated Significant decrease in serum AFP level and improvement in dielectric properties of liver |
[68] |
| SKH-1 immunocompetent albino mice (Nos. 23) | Sun-cutaneous injection of B-16 murine melanoma cells (1 * 105) | PMF (0.5 Hz, 0.2 T) 3 times a day for 6 days Exhibited significant pyknosis, reduction of cell nuclei by 54% within few minute and 68% reduction in 3 h. Reduction of blood flow in 15 min of treatment |
[69] |
| Female nude mice (Nos. 4) | Sub-cutaneous injection of melanoma cell (B16-F10-cGFP, 1 * 105) on mouse skin | PMF (5–7 Hz, 0.2 T) for 6 min till 10 days Melanoma reduced, pyknosis observed in 24 h |
[19] |
| Type | Pathology/Treatment | Observations | Ref. |
| Clinical trials | |||
| Companionate and investigative (28 Nos. patient) | Galioblastoma, Mesothalioma, Oligodendroglioma, Sarcoma, HCC and Breast, Neuroendocrine, Ovarian, Pancreatic, Prostate, Thyroid Cancer PMF (0.1–114 Hz for 60 min) 3 times a day till 278.4 month |
1 patient for thyroid cancer stable after 3 yrs 1 patient for meso-thelio metastasis to abdomen stable after 6 months 1 patient for non-small cell lung cancer stable after 5 months 1 patient for pancreatic metastasis stable after 4 months |
[62] |
| Open level single group Clinical trial phase I/II (41 nos. patient) | Advanced HCC observed. PMF (100 Hz–21 kHz, 1.5 T) for 60 min 3 times/day till 6 month |
Complete disappearance of VEGF structure in - 5 nos. Decrease in pain- 2 nos. Well responded- 4 nos. No change- 16 nos. |
[63] |
Use of magnetic fields with anticancer drug and drug delivery systems (DDS)
SMF and AMF/PMF have also been used to improve the efficiency of drugs and drug delivery systems (DDS) for potential cancer treatment. However, studies related to their in vivo use and clinical trials appear to be very limited. Some recent studies revealed that SMF also have strong influence on the reactivity of chemotherapeutic drugs, which can minimize drug dosage and its side effects [44]. Similarly the positive effect of 8.8 mT SMF on Cisplatin potency in inhibiting chronic myelogenous leukemia (K562) cells was reported by Chen et al. [70]. In this study, Authors treated four groups of cells: Grp 1: control group, Grp 2: SMF exposed group for 12 h, Grp 3: Cisplatin treated at 5, 10, and 20 µg/mL for 12 h and Grp 4: SMF + Cisplatin (5, 10, and 20 µg/mL) [70]. Maximum cell inhibition was found with SMF + Cisplatin treatment and the cells were found to halt in S phase. SMF is believed to change motion of Cisplatin molecules within and between the cells leading to increased intracellular drug levels. The synergistic effects of SMF and Cisplatin enhanced the DNA-Cisplatin interactions i.e. increased DNA damage associated with absorbability of drug MDR (Multidrug resistance-associated protein) expression and transport. Since Cisplatin is a radiosensitizer, Babincová et al. [71] studied the influence of combination treatment involving radiation, chemotherapy (Cisplatin) and PMF induced hyperthermia on lung carcinoma cell line. Two cell lines H460 and A549 (which are Cisplatin sensitive and resistant, respectively) were treated with combination treatment (15 min AMF, 1.5 Gy radiation, 2.1 µM and 59 µM Cisplatin for H460 and A549 cells, respectively). Both cells showed up to 90% inhibition indicating positive influence of this combination treatment on cancer cell inhibition. But no detailed mechanism of action could be identified [71].
In another study, the effect of different drugs on K562 cell (erythroleukemia type cell) activities under the influence of 9 mT SMF has been evaluated [72]. The drugs evaluated in this investigation were Taxol (10 ng/mL), Doxorubicin (25 ng/mL), Cisplatin (10 g/mL) and Cyclophosphamide (0.4 mg/mL). It was observed that after Taxol + SMF treatment (24 h) the cell surfaces exhibited 0.1–0.5 μm long pore-like structures. In addition to large apophyses (0.3–1.3 μm) with large holes of 0.47 μm diameter and irregular apophyses (1.85 and 2.04 μm in diameter) were also observed on the treated cells. These holes are believed to help in easy uptake of anticancer drug thus enhancing the drug’s potency. Similar changes in the cell surface were observed with Doxorubicin + SMF treatment. The use of other drugs (Cisplatin and Cyclophosphamide) also revealed pores of varying sizes on these cells [72]. From these results it can be concluded that the SMF induced alteration of membrane permeability increases drug internalization by the cancer cells and thus strengthen the effects of anticancer drugs. But very little information is available on clinical effects of SMF in the presence of chemotherapy drugs. However, Salvatore et al. [73] performed Phase I clinical trials on patients with advanced malignancy using SMF (3–28 mT, 15–30 min/day up to 14 days) and antineoplastic chemotherapy. The data of 10 patients, in terms of white blood cell and platelet count, showed no difference between treatment and control groups suggesting that this combination treatment is safe.
Several PMF studies also recorded significant improvement in the potency of anticancer drugs. For example, Dunn osteosarcoma cells exposed to PFM (0.3–0.4 mT, 10 Hz with 25 µs pulses) in the presence of 0.01 mg/mL Adriamycin showed over expression of P-glycoprotein in ADR-resistant osteosarcoma cells due to changes in their membrane functions [74]. On the other hand, PMF exposure promoted non-resistant cells growth in an ADR-free medium, while simultaneously suppressing the growth of more differentiation resistant cells [74]. Prompted by these in vitro results, in vivo trials using PMF (200 Hz, 4 mT) reportedly increased the life span of rat by 17.6% when treated along with Mitomycin C for 90 days [75]. Another important application of magnetic fields for cancer therapy involves the use of magnetic fluids to which biomolecules are chemically bound. These fluids are typically directed within the tumor using high energy magnetic fields. Preliminary experiments with malignant adeno carcinoma of colon or hypernephroma exposed to 0.2–0.5 T magnetic field in the presence of Epirubicin containing ferrofluid revealed excellent tumor responses [38]. Although the above studies demonstrated consistent evidence on drug potency enhancement with SMF and PMF applications, the effect of these magnetic fields on the drugs is not yet known.
Biological effects of ultrasound
Currently ultrasound (US) is being widely used in screening diseases, assessing tissue conditions and also in the treatment of diseases/conditions. US is the most popular and efficient non-invasive technique for diagnostics and treatment of different parts of human body without harmful effects. Generally, sound waves with a frequency between 0.7 and 3.3 MHz are used by placing a transducer or applicator on patient's skin and the penetration depth can be easily tailorable. Earlier, US has been mainly used for relaxation of connective tissues like ligaments, tendons, and fascia. Later developments showed that US can also be effectively used to treat muscle strains, joint inflammation, metatarsalgia, impingement syndrome, rheumatoid arthritis, osteoarthritis, and scar tissue adhesion [76]. High intensity focused ultrasound (HIFU) pulses have also been used to dissolve kidney stones and gallstones – a treatment widely known as Lithotripsy. Focused US generated microbubbles can act as effective non-invasive delivery medium of drugs across the blood-brain barrier. Another version of US is low intensity pulsed ultrasound (LIPUS) which is very popular for tooth/bone stimulation and regeneration/growth [77]. Recently, transcranial US has been used to aid tissue plasminogen activator treatment in stroke sufferers by US enhanced systemic thrombolysis [78]. US can also be applied for long durations to increase local circulation and accelerate musculoskeletal tissues healing after an injury. The diagnostic US uses frequencies in the range of 1–20 MHz, but for cancer treatment a frequency between 0.8 and 3.5 MHz is most effective. Application of various US in medicine is summarized in Table 4. The severity of known thermal and mechanical effects of US depends on US parameters (frequency, focusing, pulse repetition frequency, pulse duration, exposure time, intensity, etc.) and more importantly on the attenuation coefficient and acoustic impedance of biological tissues. Therefore, the thermal and mechanical effects required for cancer treatment might interfere with healthy tissues leading to adverse biological effects. As a result, apart from its known cellular effects the genetic, fetal, neural and pulmonary effects of US must be considered for effective and safe use of US for cancer treatment [79].
Table 4.
Summary of FDA approved US therapies (adapted from [16] with permission from John Wiley and Sons).
| Type of ultrasound | Treatment | Mechanism | Frequency (MHz) | Ref. |
|---|---|---|---|---|
| Unfocused Beam | Tissue Warming | Heating by portable hand held machine | 1–3 | [80] |
| Hyperthermia | Cancer Therapy | Regional Heating | 1–1.3 | [81] |
| HIFU (High intensity focused ultrasound) | Uterine fibroid ablation | Thermal Lesion | 0.5–2 | [82] |
| HIFU | Glucoma Relief | Permiabilization with fixed probe | 4.6 | [83] |
| HIFU | Laproscopic tissue ablation | Thermal lesion with hand held machine | 4 | [84] |
| HIFU | Laproscopic open surgery | Thermal lesion | 3.8–6.4 | [85] |
| Focused Ultrasound | Skin Tissue Tightening | Thermal Lesion with hand held machine for both imaging and treatment | 4.4–7.5 | [86] |
| Extracorporeal Lithotripsy | Kidney stone | Mechanical stress, Cavitation with image guidance | ≈150 kHz | [87] |
| Intracorporeal Lithotripsy | Kidney Stone | Mechanical stress, Cavitation by percutaneous probe | 25 kHz | [88] |
| Extracorporeal Shockwave Therapy | Plantar fasciitis epicondylitis | Mechanism unknown | ≈150 kHz | [89] |
| Phacoemulsification | Lens removal | Vibration &cavitation generate with probe | 40 kHz | [90] |
| Liposuction | Adipose tissue removal | Fat liquification % cavitations generate with probe | 20–30 kHz | [91] |
| Tissue cutting and vessel sealing | Laproscopic or open surgery | Thermal lesion and vibration with hand held machine | 55 kHz | [92] |
| Intravascular US | Thrombus dissolution | Gas body cavitations by intravascular catheter | 2.2 | [93] |
| Skin permiabilization | Transdermal drug delivery | Unknown | 55 kHz | [94] |
| LIPUS | Bone fracture healing | Unknown | 1.5 | [95] |
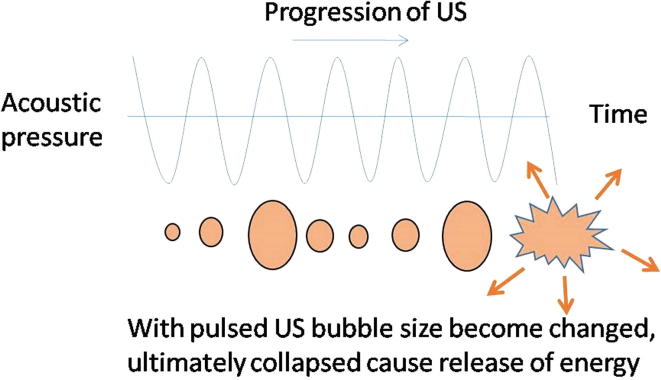
Therapeutic strategies based on US depend on the interaction of acoustic waves with biological soft tissues through thermal and non-thermal physical mechanisms producing wide range of biological effects. Thermal bio-effects originate from temperature increase due conversion of acoustic energy into heat. When US interacts with biological tissue it oscillates the tissue and rises its temperature, typically between 65 and 100 °C, depending on the US parameters and type of tissue being exposed. Through compression and rarefaction wave characteristics of US, the tissues oscillate about a fixed point of tissue rather than moving with the wave itself causing oscillation in the cells. These molecular vibrations in the tissue results in heat generation and the temperature rise can be tailored to achieve hyperthermia to treat cancer. HIFU is one such approach based on thermal effects induced by US. Non-thermal effects of US include mechanical effects, radiation force, acoustic streaming which act on tissues as physical stimuli [80]. US also create non-inertial cavitation in biological tissues which is responsible for slow growth of oscillating bubbles inside the cells. The repeated oscillation and collapse of these microbubbles, known as microstreaming, generates strong radiation forces within the tissue. The negative pressure created by bubble collapse and their harmonic oscillations generate microstreaming creating small sized pores in the cell plasma membrane. These US generated pores enable easy entry of extracellular agents such as markers, genes, anticancer drugs, in to cells via sonoporation (acoustic cavitations) mechanisms [96]. Therefore, various US have been used and shown to have positive influence on cancer treatment, which are discussed in the following sections. Generalized experimental set up for US mediated cancer treatment is shown in Fig. 5.
Fig. 5.
Generalized experimental set up for US mediated cancer treatment (a) In vitro and in vivo treatment (b) Clinical trials.
Cancer treatment using high intensity focused ultrasound (HIFU)
Usually surgery based cancer treatment is aimed at removing the tumor with an adequate normal tissue margin. But if there is a possibility to minimize the normal tissue damage by applying non-invasive technique, which can destroy the required tissue volume and results in disease free survival of the patient, then it will be a remarkable achievement in cancer therapeutics. For this purpose, US with frequencies between 0.8 and 3.5 MHz, with much higher energy levels than standard diagnostic US, have been used [97]. Cancer treatment using these US depends on heat generated due to conversion of mechanical energy into heat energy through ‘inertial cavitations’ as shown in Fig. 6. In this process the US progresses through tissues and causes alternating cycles of increased and reduced pressure (compression and rarefaction, respectively). This pressure oscillation inside the microbubbles collapses the bubbles releasing energy in the form of heat and mechanical/pressure energy. HIFU is one of the most popular US currently being used for cancer treatment using this principle. In several centers worldwide, it is now being used clinically to treat solid tumors (both malignant and benign), including those of the prostate, liver, breast, kidney, bone and pancreas, and soft-tissue sarcoma [97]. Further, the majority of cancer patients suffer from severe pain due to malignancies, which not only affects quality of life but also decreases treatment outcome. Current pain relief medications often result in systemic toxicity and other side effects. Very recently it has been found that HIFU can be effectively used to relieve pain by changing pain origin pathways influenced by neuromodulation, tissue denervation and tumor mass reduction [98].
Fig. 6.
Schematic showing the principle of high intensity focused ultrasound to produce energy via microbubbles inside tissues.
In vitro experiments on prostate cancer cells treated with HIFU resulted in rapid increase in apoptosis as evidenced by over expression of Chk2 [99]. Further, HIFU exposed area exhibited rapid increase in temperature up to 80 °C leading to cell destruction. Human prostate cancer cell lines LNCaP, PC-3, and DU-145, have also been treated for 15, 30, 60, 120 or 180 min in vitro. Out of 14 samples, Chk2 activation was detected in 8 cases. After HIFU treatment, Chk2 activation was observed in prostatic glands which were surrounded with areas of coagulative necrosis, but before HIFU treatment the Phosphorylated Chk2 staining was very weak. HIFU induced Chk2 activation caused DNA damage and resulted in cell cycle arrest or apoptosis and finally cell death [99]. Because of low duty cycle, pulsed HIFU can minimize heat generation and eliminate normal tissue destruction, but can selectively affect cancer cells via non-thermal mechanisms. In another study, murine squamous cell carcinoma (SCC) model (SCC7) [100] exposed to pulsed HIFU enhanced the inhibition of tumor growth when injected with tumor necrosis factor-α plasmid deoxyribonucleic acid. In this in vitro investigation, SCC7 cells were exposed to 10−8 or 10−7 mmol/L bortezomib (BTZ). Then the murine SCC7 cells were inoculated subcutaneously in the right flank of 33 immuno-competent syngeneic C3H mice. When the tumors reached a size of 100 mm3, the mice were individually randomized in one of three BTZ dose groups and exposed to HIFU (1 MHz) on 1st day of treatment. It was observed that the combination of HIFU and 1.0 mg/kg of BTZ can significantly slow down tumor growth. The treatment also enhanced apoptosis on 1st day, after treatment initiation, compared to control samples. This study demonstrated that pre-exposure of murine SCC xenografts to pulsed HIFU results in tumor growth inhibition and early induction of apoptosis at lower dose of BTZ than that of BTZ treatment alone. It is argued that HIFU exposure produce local temperature elevations of 4–5 °C in the targeted tissue, where the temperature sensitive liposomes activation enhances the uptake of BTZ. The local hyperthermia also increases blood flow to the targeted tumor because of increased vasodilation and hence BTZ rapidly cleared from circulatory system. Therefore, the pulsed HIFU appears to play a role in improving drug extravasation as well [100].
The success of HIFU in in vitro and in vivo experiments resulted in numerous clinical investigations to treat variety of cancers, including prostate, breast, liver, kidney, pancreas, and bone malignancy [101]. First clinical assessment (Phase I/II trials) of HIFU treatment efficiency and safety for prostate cancer treatment was reported by Gelet et al. [102]. Among 50 patients studied, 56% patients showed no residual cancer and in 80% of the patients local control of localized prostate cancer was observed. HIFU has also been clinically evaluated for advanced-stage pancreatic cancer [103]. The tumors appear to shrink in size due to the absence of blood supply, but the median survival time of patients was 11.25 months. Later studies on advanced pancreatic cancer (stage III or IV) treatment demonstrated good survival rates i.e., 52% for 6 months, 30% for 12 months, and 22% for 18 months [104]. Further increase in survival rates (up to 82%) with significant pain relief (79%) has also been reported by Gao et al. [105]. However, recent study of 224 advanced pancreatic cancer cases demonstrated that HIFU treatment may not be safe for all patients unless careful preoperative preparation is followed [106]. Similarly, controlling the depth of ablation by HIFU is an important factor for clinical success of this non-invasive treatment. A single-center study by Ge et al. [107] revealed that ablation decreases by 30% with 1 cm increase in the tumor depth (a critical parameter for treatment procedure). This means that the efficiency of HIFU decreases significantly with increase in the tumor depth. It was concluded that posterior tumor depths < 7 cm can be effectively treated with HIFU with minimal adverse effects.
In many reports HIFU has been successively used for breast cancer treatment. HIFU beam power between 150 W and 400 W (intensity was 5000–20,000 W/cm2) was used in 25 patients to ablate breast cancer [108]. In 12 month follow-up no metastatic lesion was detected in these patients. HIFU treatment destroyed tumor capillary ultra structure along with disintegrated capillary endothelium and cavitated peritubular cells. Multiple irradiations were required for complete tumor eradication but small tumors of size < 1 cm3 could be completely eradicated by single pulse of irradiation. Hematoxylin-eosin (HE) staining results showed immediate cell damage followed by cell pyknosis, significant widened cell gaps, intact cell contours, and tumor vascular thrombosis. However, some mild complications like edema, mild fever, pain were also observed, which were controlled by symptomatic treatment [108].
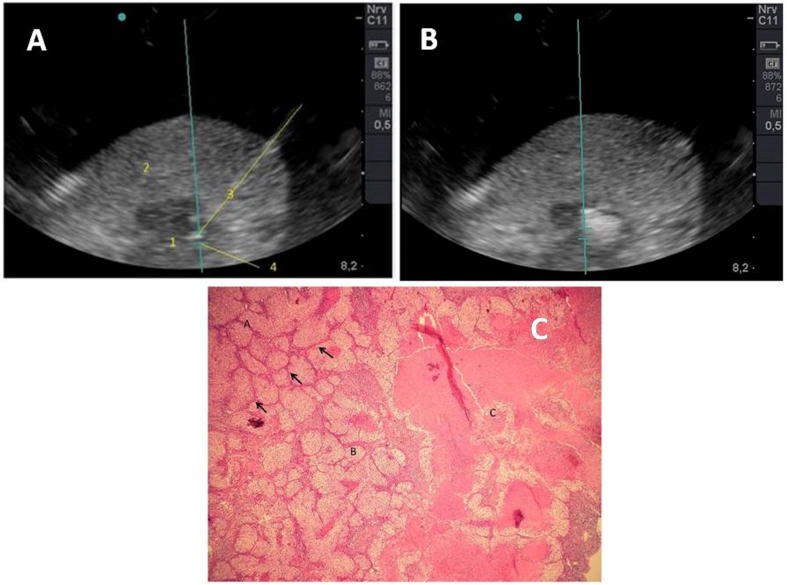
Initial trials of lung cancer treatment using HIFU have been unsuccessful as ventilated lung is a total acoustic absorber and reflector. However, the problem has recently been addressed by lung flooding [109]. This study used ex vivo human lung cancer model and simulated tumors in vivo in pigs. It has been shown that HIFU treatment increases temperature by 52.1 K after ten seconds of exposure, which results in coagulation necrosis of cancer tissue. Treated cancer tissue became strongly hyperechoic after HIFU exposure as shown in Fig. 7a and b. Coagulative necrosis and cellular membranes alteration was observed with HE staining, Fig. 7c. This study revealed that in combination with lung flooding, HIFU treatment produces thermal effect that has potential for lung cancer treatment [109]. A review of clinical outcome on breast cancer treatment using HIFU in China and Europe indicated its safety and feasibility for small tumors (<2 cm) with very high success rates up to 100% [110]. However, randomized clinical trials and comparison with standard surgery are yet to be performed.
Fig. 7.
(A) Adenocarcinoma of lung (B) After single HIFU treatment (C) Strong hyperechoic sonolesion observed in the tumor (adapted from [109] with permission under the terms of the Creative Commons Attribution 2.0 License).
All these studies show that the focused HIFU cause thermal ablation of cancer tissues without effecting adjacent tissues. Typical stages of cancer tumor ablation consists of (i) cellular homeostasis at ∼40 °C, (ii) between 40 °C and 45 °C hyper thermic shock of tumor tissues, (iii) slow rate of cellular damage in the temperature range of 46–52 °C and (iv) at 60–100 °C destruction of infected tissues by necrosis. Finally at 105 °C vaporization and carbonization of cellular content can also takes place. HIFU appears to cause acoustic cavitation as well, which enhances the heating effects as a result of absorption of broadband acoustic emissions generated by inertial cavitation [111]. Initially tiny gas bubbles distributed in the cells create large frictional pressure at infectious nuclear site and when this frictional pressure exceeds certain threshold, the inner lining of blood vessel damages leading to rupture of blood vessel and cellular membrane. Currently, HIFU treatment of pancreatic cancer is available in China, South Korea, and Europe [112].
Cancer treatment using low intensity ultrasound (LIU)
Recently, the use of LIU for cancer treatment is gaining importance. LIU directly affect cancer cells and their components by enhancing the activity of chemotherapeutic drugs via sonoporation. LIU induced cavitation produces free radicals that can kill rapidly dividing cancer cells. Hematoporphyrin and its derivatives up-taken and retained in the tumors can be facilitated by LIU. Therefore LIU treatment damages cancer cells with minimal bio-effects. These hematoporphyrin, like all other sonosensitizers, are initially injected intravenously prior to insonation to enable uniform distribution inside the tumor. The sonication parameters (typically 1.0–2.0 MHz with an intensity of 0.5–3.0 W cm−2) generate inertial cavitation inside the tumor. The rapid production and collapse of microbubbles produce mechanical shock waves, free radicals and apoptotic initiators, which inhibit cancer cell growth [113]. LIU mediated in vivo delivery of Cisplatin revealed enhanced effectiveness of the drug and reduced its harmful side effects [114]. Collapsing and cavitating microbubbles (induced by LIU) generate sufficient pressure to permealize cancer cellular membrane enabling easy entry of exogenous drug molecules inside the cells followed by endocytosis of therapeutic compound. Significant increase in the apoptosis of murine colon carcinoma and murine mammary carcinoma cells was observed with the use of LIU (1.5 MHz, 0.03 W cm−2, 0.1 MPa) in the presence of anticancer drug [115].
Four major areas of cancer treatment namely sonodynamic therapy, US assisted chemotherapy, US mediated gene delivery and US based anti-vascular therapy have been reportedly used LIU [116]. Typically the intensity of US for these treatments was <5.0 W/cm2 with a pressure of 0.3 MPa. In sonodynamic therapy, LIU (0.5–3.0 W/cm2, 1.0–2.0 MHz) produces cavitation microbubbles that collapse and create shockwaves creating free radical and other molecular events that activate sonosensitizers leading cancer cell death. Other effects of LIU such as thermal and anti-vascular have also been reported to have influence on observed apoptosis and ROS production [117], [118]. In addition to popular sonosensitizers (Hematoporphyrin and Protoporphyrin IX), anticancer drugs have also been used as sonosensitizers in LIU treatment. In vitro studies on several cell lines (Hepatic, Glioma, Human breast, Ovarian, Human leukemia, Human melanoma, Murine sarcoma 180, etc.) and in vivo trials with variety of tumors (Murine sarcoma 180, Colon, Hepatic, Gastric, Galioma, Breast, osteosarcoma, etc.) have reported positive effects of sonodynamic therapy on cancer treatment [118]. However, trials on large animals and humans are not yet reported.
LIU application in the presence of chemotherapeutic drugs demonstrated to enhance their internalization and delivery to the cancer cells. Its use minimizes the toxic effect of drugs on nearby healthy cells. This approach has been used in variety of treatment combinations such as LIU + drugs, LIU + drugs + microbubbles and LIU + drug loaded microbubbles [118], [121]. Here again several in vitro and in vivo trials have been reported to have significant benefits of using LIU-mediated drug delivery for targeted cancer treatment. In vitro studies revealed increased cell uptake of drugs due to LIU generated microjets (assisted by cavitation) which destabilizes cancer cell membranes [119]. Yoshida et al. [120] observed enhanced inhibition and apoptosis of U937 (human histiocytic lymphoma) cells due to increased formation of Hydroxyl radicals when LIU (at ≥0.3 W/cm2) was used with doxorubicin (DOX). Increasing DOX concentration and treatment time resulted in significant changes in cell membrane. Therefore, it seems that the enhanced drug intake is due to easy cavitation and sonoporation of cell membrane as a result of DOX induced weakening of cells. Tumors treated with LIU in the presence drugs also showed uniform distribution of drug throughout the tumor leading to decrease in vascularization and tumor growth [121].
Use of ultrasound with anticancer drug and DDS
Ultrasound mediated targeted drug delivery has been evaluated in several cancer cell lines with minimal lysis. The US irradiation found to increase cancer cell inhibition or death in the presence of drugs and DDS. It has also been observed that malignant cells are more sensitive to US irradiation due to their unique cell membrane properties compared to normal cells. Therefore, US can be used to selectively alter the membranes of diseased cells. Further, it has been recently demonstrated that the use of MNPs as DDS can significantly enhance ultrasonic hyperthermia [122]. The enhancement in thermal effects of US is primarily attributed to the increase in US absorption in the tissue-mimicking phantoms with MNPs. Reported temperature change [122] was 19 mK/s, 42 mK/s, and 91 mK/s for magnetic hyperthermia, US hyperthermia, and magnetic + US hyperthermia, respectively. Similar enhancement in US heating has been reported by using multifunctional MNPs (γ-Fe2O3) along with low-power US frequencies (1 and 3.5 MHz) [123]. Detailed experimental and numerical modeling studies showed a temperature increase between 28 °C and 31 °C with 3 min exposure of 3.5 MHz US in the presence of 0.26–0.35% (w/w) MNPs. Such an enhancement in elevated cytotoxic temperature, using US + MNPs, enable achieving desired therapeutic goal with lower intensity and duration of US treatment. Moreover, the presence of MNPs alone can effectively induce hyperthermia with AMF, as discussed earlier. Recently, Shakeri-Zadeh et al. [124] reported that the temperature changes within and surrounding the tumor can be controlled with US-assisted magnetic drug delivery. They used colon tumor (CT26) in BALB/c mice administered with 5-Fu-loaded MNPs and treated with 3-MHz US at 0.1, 0.3, 0.5, and 1 W/cm2 for 10 min. It was observed that by changing the US intensity tumor temperature can be tailored [124].
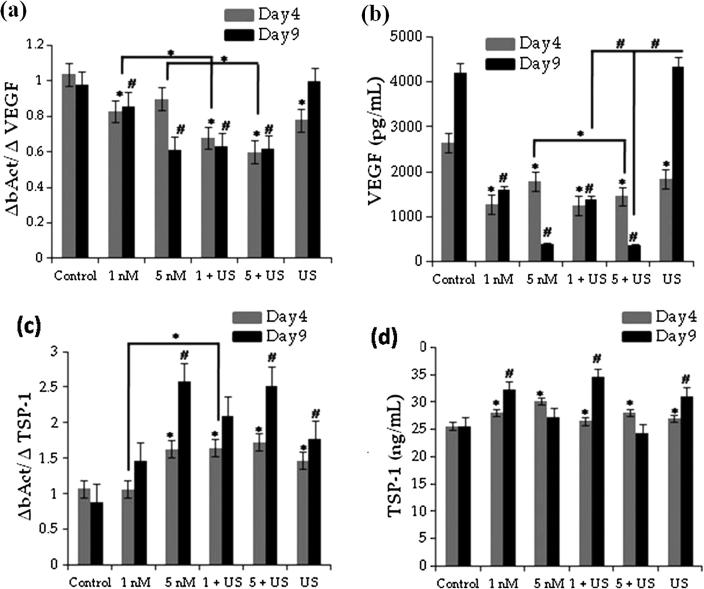
Metronomic chemotherapy is a new approach (frequent dosing of small amounts of anticancer 5-FU derivative (5′-DFUR)) that is being proposed to lower the immune competence of patients showing no response to US as adjuvant treatment [125]. LIU (1 MHz, 2 W/cm2, 50% duty cycle, 60 s) has been used to treat human uterine sarcoma cell line (FU-MMT-3) in the presence of Irinotecan (CPT-11) and SN38 [125]. It was found that the treatment significantly decreased VEGF expression during early time period of 4 days, Fig. 8a and b. Further, the TSP-1 (Thrombospondin-1) mRNA expression decreased significantly in FU-MMT-3 cells exposed to 5 nM SN38 as shown in Fig. 8c. However, 5 nM SN38 showed a significant anti-proliferative effect compared to control on day 4 (Fig. 8d).
Fig. 8.
(a) Inhibition of VEGF mRNA expression with US treatment along with SN38, (b) The concentration of VEGF secreted from the cells, (c) TSP-1 expression in the treated cells, and (d) The concentration of TSP-1 secreted from cell [125] (With permission from John Wiley and Sons).
This combination treatment clearly showed significant reduction in tumor volume and extended the survival of mice compared to treatment alone. It appears that the effect of chemotherapy drug has been accelerated by US for human uterine sarcoma treatment. Similarly, the cytotoxicity of Doxorubicin (DOX) on human primary liver cancer (PLC) cells has been enhanced with LIU [126]. In this study, US with 1.0 MHz frequency and 100 Hz pulse repetition frequency with 0.2–0.5 W/cm2 was used. US treatment at 0.5 W/cm2 in the presence of DOX significantly enhanced cell killing and apoptosis. When the intensity was 0.3 W/cm2, DOX induced inhibition was high after 60 min of incubation with only 10 μM of drug [126]. Another important effect of such combinational therapy is hyperthermia in human lymphoma cells, where 50% of cells were in apoptotic region. The histology of animal tissues treated with drug or DDS along with US revealed mitochondrial inflammation with chromatin condensation and rupture of cellular membrane, which are clear indicators of early apoptosis in these tumors [127]. A summary of different studies performed to assess the influence of US on in vitro and in vivo cancer cell inhibition in the presence of drugs or DDS is presented in Table 5.
Table 5.
Summary of in vitro and in vivo US therapy in the presence of drug or DDS (adapted from [116] with permission from Elsevier).
|
In vitro studies | ||||
|---|---|---|---|---|
| Cancer cells | Drug/DDS | US parameters |
References | |
| MHz | W cm−2 | |||
| Murine Sarcoma 180 | Hematoporphyrin | 1.6–1.92 | 1.0–6.0 | [130], [131], [132] |
| Protoporphytin IX | 1.0–2.2 | 0.64–5.0 | [133] | |
| Hepatic | Titanium NPs | 0.5–1.0 | 0.1–0.8 | [128], [129] |
| Human Breast | Chlorine6 + adriamycin | 1.0 | 0.5–2 | [130] |
| Ovarian | Cisplatin | 1.0 | 2.0 | [131] |
| Colon | ProtoporphyrinIX + NPs | 1.1 | 2.0 | [132] |
| Osteosarcoma (rat) | Hematoporphyrin | 10.5 | 0.8 | [133] |
| In vivo studies | ||||
| Tumor (animal model) | Drug/DDS | US parameters | Reference | |
| MHz | W cm−2 | |||
| Murine Sarcoma 180 | Hematoporphyrin | 1.92 | 1.7 | [134] |
| Pheobromide-a | 1.92 | 3.0 | [135] | |
| Sinoporphyrin sodium | 1.9 | 2.0–6.0 | [136] | |
| ProtoporphyrinIX | 2.2 | 5 | [137] | |
| Hepatic | Titanium NPs | 1.0 | 1 | [128] |
| Hematoporphyrin microbubles | 1.0 | 2.0 | [138] | |
| Gastric | Antibody/Ga-porphyrin | 1.0 | 2 | [139] |
| Porphyrin derivatives | 1.0 | 2 | [140] | |
| Colon | ATX S10 | 2.0 | 3.0 | [141] |
| Protoporphyrin + NPs | 1.1 | 2 | [132] | |
Conclusions and future perspectives
Magnetic fields of various strengths, ranging from mT to T, have been found to influence variety of cancer cellular activities and particularly significant inhibitory effect was observed on cancer cell growth. The use of static magnetic fields generates free radicals in the form of ROS/RNS and induces apoptosis in cancer cells. Hyperthermia generated by alternating magnetic fields inhibits cancer cell proliferation and enhances the treatment efficiency by easy internalization of drugs. Among different magnetic field-assisted cancer therapies, pulsed electromagnetic field (PEMF) treatment appears to have strong application potential due to reasonably good understanding of tumor-specific frequencies. Although the reports on PEMF treatment effectiveness in humans have been limited, further exploration of these tumor-specific frequencies can lead to successful application of PEMF for cancer treatment.
The use of ultrasound (US) for cancer treatment rely on both thermal and non-thermal effects i.e., hyperthermia and acoustic streaming/radiation force, respectively. Among different US, high intensity focused ultrasound (HIFU) has been investigated extensively and found to be highly effective in treating different cancers via hyperthermia. The US has also been very effective in creating stroma in the cell membrane thus enhancing the drug intake. The tumor cells demonstrated to acquire thermo-resistance due to repeated hyperthermia and the efficiency of hyperthermia based treatments decreases with repeated use. Therefore, among magnetic and US based treatments, the later demonstrated to have more effectiveness and strong application potential. However, US treatments also have some limitations in terms of tumor accessibility which require special transducers.
Although the studies discussed in this review provide good understanding on the effects of magnetic fields and ultrasound on cancer cells and tissues, further studies are required to establish their efficiency in clinical environment. For example, comparison of results of different literature appears to be very difficult due to large variations in US treatment conditions. This is equally applicable to magnetic field-based treatments as the treatment outcome strongly depends on magnetic field parameters. Majority of the studied reviewed here, except few, could not provide information on the influence of these treatments on drug resistant cancer cells, as these treatments found to have opposite effects on these cells. Therefore, the selectivity of treatments and/or treatment parameters based on cell resistance towards drugs may be studied. Further, the influence of US and magnetic fields on anticancer drugs and sonosensitizers is largely unknown. Therefore, more investigations in these lines are also required.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
Authors thank the financial support to Mrs. Somoshree Sengupta from Department of Science and Technology, New Delhi, India through ‘Disha Programme for Women in Science’ (No. SR/WOS-A/LS-46/2017 (G)).
Biographies

Somoshree Sengupta is a PhD candidate in science at the Academy of Scientific and Innovative Research (AcSIR). She received her B.Sc degree in Microbiology from Pune University (India) and M.Sc degree from Amity University (Delhi, India) in the year 2009. She authored 9 SCI(E) journal publications and received CSIR-SRF fellowship in the year 2012. Her area of expertise includes molecular biology, drug delivery and nanotechnology.

Vamsi Krishna Balla is a Senior Principal Scientist at CSIR-CGCRI, India and a Professor of Engineering Sciences at AcSIR. He received his PhD in Engineering from Indian Institute of Technology Madras, India. He was a postdoctoral researcher and Assistant Research Professor at Washington State University, USA. Dr. Balla publications include over 120 peer reviewed journal articles and 6 book chapters, which have been cited over 4000 times. His research interests focus on biomaterials, orthopaedic implants design and development, drug delivery systems, 3D printing, and laser processing.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.American Cancer Society. Cancer Facts & Figures 2018. 2018. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf [accessed 19 June 2018].
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Stewart BW, Wild CP. World cancer report; 2014. p. 1–2.
- 4.Haanen J.B.A.G., Carbonnel F., Robert C., Kerr K.M., Peters S., Larkin J. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 5.Wilson M.R., Lightbody J.H., Donaldson K., Sales J., Stone V. Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicol Appl Pharmacol. 2002;184(3):172–179. doi: 10.1006/taap.2002.9501. [DOI] [PubMed] [Google Scholar]
- 6.Croy S.R., Kwon G.S. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12(36):4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 7.Madaan K., Kumar S., Poonia N., Lather V., Pandita D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6(3):139–150. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naseri N., Valizadeh H., Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–313. doi: 10.15171/apb.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samad A., Sultana Y., Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4(4):297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- 10.Ghaderi S., Ramesh B., Seifalian A.M. Fluorescence nanoparticles “quantum dots” as drug delivery system and their toxicity: a review. J Drug Target. 2011;19(7):475–486. doi: 10.3109/1061186X.2010.526227. [DOI] [PubMed] [Google Scholar]
- 11.Ladewig K., Xu Z.P., Lu G.Q. Layered double hydroxide nanoparticles in gene and drug delivery. Expert Opin Drug Deliv. 2009;6(9):907–922. doi: 10.1517/17425240903130585. [DOI] [PubMed] [Google Scholar]
- 12.Arvizo R., Bhattacharya R., Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opin Drug Deliv. 2010;7(6):753–763. doi: 10.1517/17425241003777010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wahajuddin Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomed. 2012;7(6):3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharti C., Nagaich U., Pal A.K., Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5(3):124–133. doi: 10.4103/2230-973X.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao J., Feng J., Chen J. External-stimuli responsive systems for cancer theranostic. Asian J Pharm Sci. 2016;11(5):585–595. [Google Scholar]
- 16.Miller D.L., Smith N.B., Bailey M.R., Czarnota G.J., Hynynen K., Makin I.R.S. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31(4):623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varshney A., Kumar G. Effects of magnetic field on cancer cell line. J Exp Biol Agric Sci. 2013;1(2):91–96. [Google Scholar]
- 18.You D.G., Deepagan V.G., Um W., Jeon S., Son S., Chang H. ROS-generating TiO2 nanoparticles for non-invasive sonodynamic therapy of cancer. Sci Rep. 2016;6:23200. doi: 10.1038/srep23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuccitelli R., Tran K., Sheikh S., Athos B., Kreis M., Nuccitelli P. Optimized nanosecond pulsed electric field therapy can cause murine malignant melanomas to self-destruct with a single treatment. Int J Cancer. 2010;127(7):1727–1736. doi: 10.1002/ijc.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Öcal I., Kalkan T., Günay I. Effects of alternating magnetic field on the metabolism of the healthy and diabetic organisms. Braz Arch Biol Technol. 2008;51(3):523–530. [Google Scholar]
- 21.Ross C.L., Siriwardane M., Almeida-Porada G., Porada C.D., Brink P., Christ G.J. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015;15(1):96–108. doi: 10.1016/j.scr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris C.E., Skalak T.C. Acute exposure to a moderate strength static magnetic field reduces edema formation in rats. Am J Physiol - Hear Circ Physiol. 2008;294(1) doi: 10.1152/ajpheart.00529.2007. H50 LP-H57. [DOI] [PubMed] [Google Scholar]
- 23.Vergallo C., Dini L., Szamosvölgyi Z., Tenuzzo B.A., Carata E., Panzarini E. In Vitro analysis of the anti-inflammatory effect of inhomogeneous static magnetic field-exposure on human macrophages and lymphocytes. PLoS One. 2013;8:e72374. doi: 10.1371/journal.pone.0072374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwolińska J., Gąsior M., Śnieżek E., Kwolek A. The use of magnetic fields in treatment of patients with rheumatoid arthritis. Review of the literature. Reumatologia. 2016;54(4):201–206. doi: 10.5114/reum.2016.62475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon R.T., Gordon D. Selective resolution of plaques and treatment of atherosclerosis by biophysical alteration of “cellular” and “intracellular” properties. Med Hypotheses. 1981;7(2):217–229. doi: 10.1016/0306-9877(81)90118-3. [DOI] [PubMed] [Google Scholar]
- 26.Suslick K.S. Sonochemistry. Science. 1990;247(4949):1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 27.Creixell M., Bohorquez A.C., Torres-Lugo M., Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano. 2011;5(9):7124–7129. doi: 10.1021/nn201822b. [DOI] [PubMed] [Google Scholar]
- 28.Evangelou A., Toliopoulos I., Giotis C., Metsios A., Verginadis I., Simos Y. Functionality of natural killer cells from end-stage cancer patients exposed to coherent electromagnetic fields. Electromagn Biol Med. 2011;30(1):46–56. doi: 10.3109/15368378.2011.566776. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X. Extremely low frequency (elf) pulsed-gradient magnetic fields inhibit malignant tumour growth at different biological levels. Cell Biol Int. 2002;26(7):599–603. doi: 10.1006/cbir.2002.0883. [DOI] [PubMed] [Google Scholar]
- 30.Montalibet A., Jossinet J., Matias A., Cathignol D. Electric current generated by ultrasonically induced Lorentz force in biological media. Med Biol Eng Comput. 2001;39(1):15–20. doi: 10.1007/BF02345261. [DOI] [PubMed] [Google Scholar]
- 31.Knorr D., Bachanova V., Verneris M.R., Miller J.S. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26(2):161–172. doi: 10.1016/j.smim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kut C., Mac Gabhann F., Popel A.S. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. 2007;97(7):978–985. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghodbane S., Lahbib A., Sakly M., Abdelmelek H. Bioeffects of static magnetic fields: Oxidative stress, genotoxic effects, and cancer studies. Biomed Res Int. 2013;2013:602987. doi: 10.1155/2013/602987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nathan C. Nitric oxide as a secretory product of mammalian cells. Faseb J. 1992;6(12):3051–3064. [PubMed] [Google Scholar]
- 35.van den Tempel N., Horsman M.R., Kanaar R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int J Hyperth. 2016;32(4):446–454. doi: 10.3109/02656736.2016.1157216. [DOI] [PubMed] [Google Scholar]
- 36.Lepock J.R. Role of nuclear protein denaturation and aggregation in thermal radiosensitization. Int J Hyperther. 2004;20(2):115–130. doi: 10.1080/02656730310001637334. [DOI] [PubMed] [Google Scholar]
- 37.Balogh G., Horváth I., Nagy E., Hoyk Z., Benkõ S., Bensaude O. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. Febs J. 2005;272(23):6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 38.Lübbe A.S., Bergemann C., Huhnt W., Fricke T., Riess H., Brock J.W. Preclinical experiences with magnetic drug targeting: tolerance and efficacy. Cancer Res. 1996;56(20):4694–4701. [PubMed] [Google Scholar]
- 39.Verginadis I., Velalopoulou A., Karagounis I., Simos Y., Peschos D., Karkabounas S. Electromagnetic radiation. In: Bashir S.O., editor. Beneficial Effects of Electromagnetic Radiation in Cancer. University of Ioannina; Ioannina: 2012. pp. 249–268. [Google Scholar]
- 40.Ma M., Zhang Y., Shen X., Xie J., Li Y., Gu N. Targeted inductive heating of nanomagnets by a combination of alternating current (AC) and static magnetic fields. Nano Res. 2015;8(2):600–610. [Google Scholar]
- 41.Vergallo C., Ahmadi M., Mobasheri H., Dini L. Impact of inhomogeneous static magnetic field (31.7-232.0 mT) exposure on human neuroblastoma SH-SY5Y cells during cisplatin administration. PLoS One. 2014;9(11):e113530. doi: 10.1371/journal.pone.0113530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J., Ma Y., Li N., Cao Y., Zhu Y. Natural static magnetic field-induced apoptosis in liver cancer cell. Electromagn Biol Med. 2014;33(1):47–50. doi: 10.3109/15368378.2013.783850. [DOI] [PubMed] [Google Scholar]
- 43.Tenuzzo B., Vergallo C., Dini L. Effect of 6 mT static magnetic field on the bcl-2, bax, p53 and hsp70 expression in freshly isolated and in vitro aged human lymphocytes. Tissue Cell. 2009;41(1):169–179. doi: 10.1016/j.tice.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Hao Q., Wenfang C., Xia A., Qiang W., Ying L., Kun Z. Effects of a moderate-intensity static magnetic field and adriamycin on K562 cells. Bioelectromagnetics. 2011;32(3):191–199. doi: 10.1002/bem.20625. [DOI] [PubMed] [Google Scholar]
- 45.Strieth S., Strelczyk D., Eichhorn M.E., Dellian M., Luedemann S., Griebel J. Static magnetic fields induce blood flow decrease and platelet adherence in tumor microvessels. Cancer Biol Ther. 2008;7(6):814–819. doi: 10.4161/cbt.7.6.5837. [DOI] [PubMed] [Google Scholar]
- 46.Raylman R.R., Clavo A.C., Wahl R.L. Exposure to strong static magnetic field slows the growth of human cancer cells in vitro. Bioelectromagnetics. 1996;17(5):358–363. doi: 10.1002/(SICI)1521-186X(1996)17:5<358::AID-BEM2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 47.Nedelcu G. Magnetic nanoparticles impact on tumoral cells in the treatment by magnetic fluid hyperthermia. Dig J Nanomater Biostruct. 2008;3(3):103–107. [Google Scholar]
- 48.de Châtel P.F., Nándori I., Hakl J., Mészáros S., Vad K. Magnetic particle hyperthermia: Néel relaxation in magnetic nanoparticles under circularly polarized field. J Phys Condens Matter. 2009;21:124202. doi: 10.1088/0953-8984/21/12/124202. [DOI] [PubMed] [Google Scholar]
- 49.Hajiaghajani A., Abdolali A. Magnetic field pattern synthesis and its application in targeted drug delivery: design and implementation. Bioelectromagnetics. 2018;39(4):325–338. doi: 10.1002/bem.22107. [DOI] [PubMed] [Google Scholar]
- 50.Miao X., Yin S., Shao Z., Zhang Y., Chen X. Nanosecond pulsed electric field inhibits proliferation and induces apoptosis in human osteosarcoma. J Orthop Surg Res. 2015;10:104. doi: 10.1186/s13018-015-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao C., Mi Y., Hu X., Li C., Sun C., Tang J. Experiment and mechanism research of SKOV3 cancer cell apoptosis induced by nanosecond pulsed electric field. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1044–1047. doi: 10.1109/IEMBS.2008.4649338. [DOI] [PubMed] [Google Scholar]
- 52.Crocetti S., Beyer C., Schade G., Egli M., Fröhlich J., Franco-Obregón A. Low intensity and frequency pulsed electromagnetic fields selectively impair breast cancer cell viability. PLoS One. 2013;8(9):e72944. doi: 10.1371/journal.pone.0072944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morabito C., Guarnieri S., Fanò G., Mariggiò M.A.M.A. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell Physiol Biochem. 2011;26(6):947–958. doi: 10.1159/000324003. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y.C., Chen C.C., Tu W., Cheng Y.T., Tseng F.G. Design and fabrication of a microplatform for the proximity effect study of localized ELF-EMF on the growth of in vitro HeLa and PC-12 cells. J Micromech Microeng. 2010;20:125023. [Google Scholar]
- 55.Sadeghipour R., Ahmadian S., Bolouri B., Pazhang Y., Shafiezadeh M. Effects of extremely low-frequency pulsed electromagnetic fields on morphological and biochemical properties of human breast carcinoma cells (T47D) Electromagn Biol Med. 2012;31(12):425–435. doi: 10.3109/15368378.2012.683844. [DOI] [PubMed] [Google Scholar]
- 56.Loja T., Stehlikova O., Palko L., Vrba K., Rampl I., Klabusay M. Influence of pulsed electromagnetic and pulsed vector magnetic potential field on the growth of tumor cells. Electromagn Biol Med. 2014;33(3):190–197. doi: 10.3109/15368378.2013.800104. [DOI] [PubMed] [Google Scholar]
- 57.Skitzki J.J., Repasky E.A., Evans S.S. Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs. 2009;10(6):550–558. [PMC free article] [PubMed] [Google Scholar]
- 58.Wilhelm C., Fortin J.-P., Gazeau F. Tumour cell toxicity of intracellular hyperthermia mediated by magnetic nanoparticles. J Nanosci Nanotechnol. 2007;7(8):2933–2937. doi: 10.1166/jnn.2007.668. [DOI] [PubMed] [Google Scholar]
- 59.Akbarnejad Z., Eskandary H., Vergallo C., Nematollahi-Mahani S.N., Dini L., Darvishzadeh-Mahani F. Effects of extremely low-frequency pulsed electromagnetic fields (ELF-PEMFs) on glioblastoma cells (U87) Electromagn Biol Med. 2017;36(3):238–247. doi: 10.1080/15368378.2016.1251452. [DOI] [PubMed] [Google Scholar]
- 60.Akbarnejad Z., Eskandary H., Dini L., Vergallo C., Nematollahi-Mahani S.N., Farsinejad A. Cytotoxicity of temozolomide on human glioblastoma cells is enhanced by the concomitant exposure to an extremely low-frequency electromagnetic field (100Hz, 100G) Biomed Pharmacother. 2017;92:254–264. doi: 10.1016/j.biopha.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 61.Ronchetto F., Barone D., Cintorino M., Berardelli M., Lissolo S., Orlassino R. Extremely low frequency-modulated static magnetic fields to treat cancer: a pilot study on patients with advanced neoplasm to assess safety and acute toxicity. Bioelectromagnetics. 2004;25(8):563–571. doi: 10.1002/bem.20029. [DOI] [PubMed] [Google Scholar]
- 62.Barbault A., Costa F.P., Bottger B., Munden R.F., Bomholt F., Kuster N. Amplitude-modulated electromagnetic fields for the treatment of cancer: Discovery of tumor-specific frequencies and assessment of a novel therapeutic approach. J Exp Clin Cancer Res. 2009;28:51. doi: 10.1186/1756-9966-28-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costa F., de Oliveira A.C., Meirelles R., Zanesco T., Surjan R., Chammas M. A phase II study of amplitude-modulated electromagnetic fields in the treatment of advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2007;25(18) doi: 10.1038/bjc.2011.292. 15155-15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vadalà M., Morales-Medina J.C., Vallelunga A., Palmieri B., Laurino C., Iannitti T. Mechanisms and therapeutic effectiveness of pulsed electromagnetic field therapy in oncology. Cancer Med. 2016;5(11):3128–3139. doi: 10.1002/cam4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filipovic N., Djukic T., Radovic M., Cvetkovic D., Curcic M., Markovic S. Electromagnetic field investigation on different cancer cell lines. Cancer Cell Int. 2014;14:84. [Google Scholar]
- 66.Morabito C., Guarnieri S., Fano G., Mariggio M.A. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell Physiol Biochem. 2010;26(1):947–958. doi: 10.1159/000324003. [DOI] [PubMed] [Google Scholar]
- 67.Tatarov I., Panda A., Petkov D., Kolappaswamy K., Thompson K., Kavirayani A. Effect of magnetic fields on tumor growth and viability. Comp Med. 2011;61(4):339–345. [PMC free article] [PubMed] [Google Scholar]
- 68.Emara S.O., EL-Kholy S.M., Kazem A.H., Hussein N.A., Al-dein R.S.S. Therapeutic effects of low frequency pulsed electromagnetic fields on rat liver cancer. Res Inven Int J Eng Sci. 2013;2(9):17–18. [Google Scholar]
- 69.Nuccitelli R., Pliquett U., Chen X., Ford W., James Swanson R., Beebe S.J. Nanosecond pulsed electric fields cause melanomas to self-destruct. Biochem Biophys Res Commun. 2006;343(2):351–360. doi: 10.1016/j.bbrc.2006.02.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen W.-F., Qi H., Sun R.-G., Liu Y., Zhang K., Liu J.-Q. Static magnetic fields enhanced the potency of cisplatin on K562 cells. Cancer Biother Radiopharm. 2010;25(4):401–408. doi: 10.1089/cbr.2009.0743. [DOI] [PubMed] [Google Scholar]
- 71.Babincová M., Kontrišová K., Durdík Š., Bergemann C., Sourivong P. Radiation enhanced efficiency of combined electromagnetic hyperthermia and chemotherapy of lung carcinoma using cisplatin functionalized magnetic nanoparticles. Pharmazie. 2014;699(2):128–131. [PubMed] [Google Scholar]
- 72.Liu Y., Qi H., Sun R., Chen W. An investigation into the combined effect of static magnetic fields and different anticancer drugs on K562 cell membranes. Tumori. 2011;97(3):386–392. doi: 10.1177/030089161109700322. [DOI] [PubMed] [Google Scholar]
- 73.Salvatore J.R., Harrington J., Kummet T. Phase I clinical study of a static magnetic field combined with anti-neoplastic chemotherapy in the treatment of human malignancy: initial safety and toxicity data. Bioelectromagnetics. 2003;24(7):524–527. doi: 10.1002/bem.10149. [DOI] [PubMed] [Google Scholar]
- 74.Miyagi N., Sato K., Rong Y., Yamamura S., Katagiri H., Kobayashi K. Effects of PEMF on a murine osteosarcoma cell line: drug-resistant (P-glycoprotein-positive) and non-resistant cells. Bioelectromagnetics. 2000;21(2):112–121. doi: 10.1002/(sici)1521-186x(200002)21:2<112::aid-bem6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 75.Omote Y., Hosokawa M., Komatsumoto M., Namieno T., Nakajima S., Kubo Y. Treatment of experimental tumors with a combination of a pulsing magnetic field and an antitumor drug. Jpn J Cancer Res. 1990;81(9):956–961. doi: 10.1111/j.1349-7006.1990.tb02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faltus J., Boggess B., Bruzga R. The use of diagnostic musculoskeletal ultrasound to document soft tissue treatment mobilization of a quadriceps femoris muscle tear: a case report. Int J Sports Phys Ther. 2012;7(3):342–349. [PMC free article] [PubMed] [Google Scholar]
- 77.Dedhia J.P.A. Low intensity pulsed ultrasound (LIPUS) - the future of dental therapeutics. J Dent Oral Biol. 2017;16(7):79–81. [Google Scholar]
- 78.Daffertshofer M., Gass A., Ringleb P., Sitzer M., Sliwka U., Els T. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia. Stroke. 2005;36(7) doi: 10.1161/01.STR.0000170707.86793.1a. 1441 LP-6. [DOI] [PubMed] [Google Scholar]
- 79.Shankar H., Pagel P.S. Potential adverse ultrasound-related biological effects. Anesthesiology. 2011;115(5):1109–1124. doi: 10.1097/ALN.0b013e31822fd1f1. [DOI] [PubMed] [Google Scholar]
- 80.Robertson V.J., Baker K.G. A review of therapeutic ultrasound: effectiveness studies. Phys Ther. 2001;81(7):1339–1350. [PubMed] [Google Scholar]
- 81.Samulski T.V., Grant W.J., Oleson J.R., Leopold K.A., Dewhirst M.W., Vallario P. Clinical experience with a multi-element ultrasonic hyperthermia system: analysis of treatment temperatures. Int J Hyperth. 1990;6(5):909–922. doi: 10.3109/02656739009140972. [DOI] [PubMed] [Google Scholar]
- 82.Tempany C.M.C., Stewart E.A., McDannold N., Quade B.J., Jolesz F.A., Hynynen K. MR Imaging–guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897–905. doi: 10.1148/radiol.2271020395. [DOI] [PubMed] [Google Scholar]
- 83.Burgess S.E.P., Silverman R.H., Coleman D.J., Yablonski M.E., Lizzi F.L., Driller J. Treatment of glaucoma with high-intensity focused ultrasound. Ophthalmology. 1986;93(6):831–838. doi: 10.1016/s0161-6420(86)33672-8. [DOI] [PubMed] [Google Scholar]
- 84.Klingler H.C., Susani M., Seip R., Mauermann J., Sanghvi N., Marberger M.J. A novel approach to energy ablative therapy of small renal tumours: laparoscopic high-intensity focused ultrasound. Eur Urol. 2008;53(4):810–818. doi: 10.1016/j.eururo.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 85.Ninet J., Roques X., Seitelberger R., Deville C., Pomar J.L., Robin J. Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: results of a multicenter trial. J Thorac Cardiovasc Surg. 2005;130(3) doi: 10.1016/j.jtcvs.2005.05.014. 803-e1. [DOI] [PubMed] [Google Scholar]
- 86.Alam M., White L.E., Martin N., Witherspoon J., Yoo S., West D.P. Ultrasound tightening of facial and neck skin: a rater-blinded prospective cohort study. J Am Acad Dermatol. 2010;62(2):262–269. doi: 10.1016/j.jaad.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 87.Weizer A.Z., Zhong P., Preminger G.M. New concepts in shock wave lithotripsy. Urol Clin North Am. 2007;34(3):375–382. doi: 10.1016/j.ucl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 88.Lowe G., Knudsen B.E. Ultrasonic, pneumatic and combination intracorporeal lithotripsy for percutaneous nephrolithotomy. J Endourol. 2009;23(10):1663–1668. doi: 10.1089/end.2009.1533. [DOI] [PubMed] [Google Scholar]
- 89.Haake M., Buch M., Schoellner C., Goebel F., Vogel M., Mueller I. Extracorporeal shock wave therapy for plantar fasciitis: randomised controlled multicentre trial. BMJ. 2003;327(7406):75. doi: 10.1136/bmj.327.7406.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Packer M., Fishkind W.J., Fine I.H., Seibel B.S., Hoffman R.S. The physics of phaco: a review. J Cataract Refract Surg. 2005;31(2):424–431. doi: 10.1016/j.jcrs.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 91.Mann M.W., Palm M.D., Sengelmann R.D. New advances in liposuction technology. Semin Cutan Med Surg. 2008;27(1):72–82. doi: 10.1016/j.sder.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 92.Koch C., Borys M., Fedtke T., Richter U., Pohl B. Determination of the acoustic output of a harmonic scalpel. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49(11):1522–1529. doi: 10.1109/tuffc.2002.1049734. [DOI] [PubMed] [Google Scholar]
- 93.Parikh S., Motarjeme A., McNamara T., Raabe R., Hagspiel K., Benenati J.F. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol. 2008;19(4):521–528. doi: 10.1016/j.jvir.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 94.Smith N.B. Applications of ultrasonic skin permeation in transdermal drug delivery. Expert Opin Drug Deliv. 2008;5(10):1107–1120. doi: 10.1517/17425247.5.10.1107. [DOI] [PubMed] [Google Scholar]
- 95.Gebauer D., Mayr E., Orthner E., Ryaby J.P. Low-intensity pulsed ultrasound: effects on nonunions. Ultrasound Med Biol. 2005;31(10):1391–1402. doi: 10.1016/j.ultrasmedbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 96.Zhou Y., Shi J., Cui J., Deng C.X. Effects of extracellular calcium on cell membrane resealing in sonoporation. J Control Release. 2008;126(1):34–43. doi: 10.1016/j.jconrel.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kennedy J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5(4):321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 98.Dababou S., Marrocchio C., Scipione R., Erasmus H.-P., Ghanouni P., Anzidei M. High-intensity focused ultrasound for pain management in patients with cancer. Radio Graphics. 2018;38(2):603–623. doi: 10.1148/rg.2018170129. [DOI] [PubMed] [Google Scholar]
- 99.Ide H., Nakagawa T., Terado Y., Yasuda M., Kamiyama Y., Muto S. DNA damage response in prostate cancer cells after high-intensity focused ultrasound (HIFU) treatment. Anticancer Res. 2008;28(2A):639–643. [PubMed] [Google Scholar]
- 100.Poff J.A., Allen C.T., Traughber B., Colunga A., Xie J., Chen Z. Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology. 2008;248(2):485–491. doi: 10.1148/radiol.2482071674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsiao Y.-H., Kuo S.-J., Tsai H.-D., Chou M.-C., Yeh G.-P. Clinical application of high-intensity focused ultrasound in cancer therapy. J Cancer. 2016;7(3):225–231. doi: 10.7150/jca.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gelet A., Chapelon J.Y., Bouvier R., Pangaud C., Lasne Y. Local control of prostate cancer by transrectal high intensity focused ultrasound therapy: preliminary results. J Urol. 1999;161(1):156–162. [PubMed] [Google Scholar]
- 103.Wu F., Wang Z.B., Zhu H., Chen W.Z., Zou J.Z., Bai J. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236(3):1034–1040. doi: 10.1148/radiol.2362041105. [DOI] [PubMed] [Google Scholar]
- 104.Sung H.Y., Jung S.E., Cho S.H., Zhou K., Han J.Y., Han S.T. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40(7):1080–1086. doi: 10.1097/MPA.0b013e31821fde24. [DOI] [PubMed] [Google Scholar]
- 105.Gao H.-F., Wang K., Meng Z.-Q., Chen Z., Lin J.-H., Zhou Z.-H. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology. 2013;60(128):1906–1910. doi: 10.5754/hge13498. [DOI] [PubMed] [Google Scholar]
- 106.Wang K., Zhu H., Meng Z., Chen Z., Lin J., Shen Y. Safety evaluation of high-intensity focused ultrasound in patients with pancreatic cancer. Onkologie. 2013;36(3):88–92. doi: 10.1159/000348530. [DOI] [PubMed] [Google Scholar]
- 107.Ge H.Y., Miao L.Y., Xiong L.L., Yan F., Zheng C.S., Wang J.R. High-intensity focused ultrasound treatment of late-stage pancreatic body carcinoma: Optimal tumor depth for safe ablation. Ultrasound Med Biol. 2014;40(5):947–955. doi: 10.1016/j.ultrasmedbio.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 108.Guan L., Xu G. Damage effect of high-intensity focused ultrasound on breast cancer tissues and their vascularities. World J Surg Oncol. 2016;14(1):153. doi: 10.1186/s12957-016-0908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolfram F., Boltze C., Schubert H., Bischoff S., Lesser T.G. Effect of lung flooding and high-intensity focused ultrasound on lung tumours: an experimental study in an ex vivo human cancer model and simulated in vivo tumours in pigs. Eur J Med Res. 2014;199(1):1. doi: 10.1186/2047-783X-19-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Y. Noninvasive treatment of breast cancer using high-intensity focused ultrasound. J Med Imag Heal Inform. 2013;3(2) 141–156(16) [Google Scholar]
- 111.Coussios C.C., Farny C.H., Ter Haar G., Roy R.A. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU) Int J Hyperth. 2007;23(2):105–120. doi: 10.1080/02656730701194131. [DOI] [PubMed] [Google Scholar]
- 112.Ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperth. 2007;23(2):89–104. doi: 10.1080/02656730601186138. [DOI] [PubMed] [Google Scholar]
- 113.Misík V., Riesz P. Free radical intermediates in sonodynamic therapy. Ann N Y Acad Sci. 2000;899:335–348. doi: 10.1111/j.1749-6632.2000.tb06198.x. [DOI] [PubMed] [Google Scholar]
- 114.Heath C.H., Sorace A., Knowles J., Rosenthal E., Hoyt K. Microbubble therapy enhances anti-tumor properties of cisplatin and cetuximab In Vitro and In Vivo. Otolaryngol Neck Surg. 2012;146(6):938–945. doi: 10.1177/0194599812436648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watanabe Y., Aoi A., Horie S., Tomita N., Mori S., Morikawa H. Low-intensity ultrasound and microbubbles enhance the antitumor effect of cisplatin. Cancer Sci. 2008;99(12):2525–2531. doi: 10.1111/j.1349-7006.2008.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wood A.K.W., Sehgal C.M. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41(4):905–928. doi: 10.1016/j.ultrasmedbio.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X., Wang Y., Wang P., Cheng X., Liu Q. Sonodynamically induced anti-tumor effect with protoporphyrin IX on hepatoma-22 solid tumor. Ultrasonics. 2011;51(5):539–546. doi: 10.1016/j.ultras.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 118.Gao Z., Zheng J., Yang B., Wang Z., Fan H., Lv Y. Sonodynamic therapy inhibits angiogenesis and tumor growth in a xenograft mouse model. Cancer Lett. 2013;335(1):93–99. doi: 10.1016/j.canlet.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Feril L.B., Tachibana K. Use of ultrasound in drug delivery systems: Emphasis on experimental methodology and mechanisms. Int J Hyperth. 2012;28(4):282–289. doi: 10.3109/02656736.2012.668640. [DOI] [PubMed] [Google Scholar]
- 120.Yoshida T., Kondo T., Ogawa R., Feril L.B., Zhao Q.L., Watanabe A. Combination of doxorubicin and low-intensity ultrasound causes a synergistic enhancement in cell killing and an additive enhancement in apoptosis induction in human lymphoma U937 cells. Cancer Chemother Pharmacol. 2008;61(4):559–567. doi: 10.1007/s00280-007-0503-y. [DOI] [PubMed] [Google Scholar]
- 121.Nomikou N., Li Y.S., McHale A.P. Ultrasound-enhanced drug dispersion through solid tumours and its possible role in aiding ultrasound-targeted cancer chemotherapy. Cancer Lett. 2010;288(1):94–98. doi: 10.1016/j.canlet.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 122.Józefczak A., Kaczmarek K., Hornowski T., Kubovčíková M., Rozynek Z., Timko M. Magnetic nanoparticles for enhancing the effectiveness of ultrasonic hyperthermia. Appl Phys Lett. 2016;108(26):263701. [Google Scholar]
- 123.Kaczmarek K., Hornowski T., Kubovčíková M., Timko M., Koralewski M., Józefczak A. Heating induced by therapeutic ultrasound in the presence of magnetic nanoparticles. ACS Appl Mater Interfaces. 2018;10(14):11554–11564. doi: 10.1021/acsami.8b02496. [DOI] [PubMed] [Google Scholar]
- 124.Shakeri-Zadeh A., Khoei S., Khoee S., Sharifi A.M., Shiran M.-B. Combination of ultrasound and newly synthesized magnetic nanocapsules affects the temperature profile of CT26 tumors in BALB/c mice. J Med Ultrason. 2015;42(1):9–16. doi: 10.1007/s10396-014-0558-4. [DOI] [PubMed] [Google Scholar]
- 125.Choijamts B., Naganuma Y., Nakajima K., Kawarabayashi T., Miyamoto S., Tachibana K. Metronomic irinotecan chemotherapy combined with ultrasound irradiation for a human uterine sarcoma xenograft. Cancer Sci. 2011;102(2):452–459. doi: 10.1111/j.1349-7006.2010.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kondo T., Yoshida T., Ogawa R., Hassan M.A., Furusawa Y., Zhao Q.-L. Low-intensity ultrasound adjuvant therapy: enhancement of doxorubicin-induced cytotoxicity and the acoustic mechanisms involved. J Med Ultrason. 2009;36(2):61–68. doi: 10.1007/s10396-009-0212-8. [DOI] [PubMed] [Google Scholar]
- 127.Kerr J.F.R., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ninomiya K., Ogino C., Oshima S., Sonoke S., Kuroda S., Shimizu N. Targeted sonodynamic therapy using protein-modified TiO2 nanoparticles. Ultrason Sonochem. 2012;19(3):607–614. doi: 10.1016/j.ultsonch.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 129.Ninomiya K., Noda K., Ogino C., Kuroda S., Shimizu N. Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: its application to targeted sonodynamic therapy. Ultrason Sonochem. 2014;21(1):289–294. doi: 10.1016/j.ultsonch.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 130.Gao H., Zhang W., Wang X., Zheng R. Adriamycin enhances the sonodynamic effect of chlorin e6 against the proliferation of human breast cancer MDA-MB-231 cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30(10):2291–2294. [PubMed] [Google Scholar]
- 131.Bernard V., Fojt L., Skorpíková J., Mornstein V. Determination of free cisplatin in medium by differential pulse polarography after ultrasound and cisplatin treatment of a cancer cell culture. Indian J Biochem Biophys. 2011;48(1):59–62. [PubMed] [Google Scholar]
- 132.Sazgarnia A., Shanei A., Meibodi N.T., Eshghi H., Nassirli H. A novel nanosonosensitizer for sonodynamic therapy. J Ultrasound Med. 2011;30(20):1321–1329. doi: 10.7863/jum.2011.30.10.1321. [DOI] [PubMed] [Google Scholar]
- 133.Tian Z., Quan X., Xu C., Dan L., Guo H., Leung W. Hematoporphyrin monomethyl ether enhances the killing action of ultrasound on osteosarcoma in vivo. J Ultrasound Med. 2009;28(12):1695–1702. doi: 10.7863/jum.2009.28.12.1695. [DOI] [PubMed] [Google Scholar]
- 134.Yumita N., Nishigaki R., Umemura K., Umemura S. Synergistic effect of ultrasound and hematoporphyrin on sarcoma 180. Jpn J Cancer Res. 1990;81(3):304–308. doi: 10.1111/j.1349-7006.1990.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Umemura K., Yumita N., Nishigaki R., Umemura S. Sonodynamically induced antitumor effect of pheophorbide a. Cancer Lett. 1996;102(1–2):151–157. doi: 10.1016/0304-3835(96)04174-2. [DOI] [PubMed] [Google Scholar]
- 136.Li H., Fan H., Wang Z., Zheng J., Cao W. Potentiation of scutellarin on human tongue carcinoma xenograft by low-intensity ultrasound. PLoS One. 2013;8(3):e59473. doi: 10.1371/journal.pone.0059473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Q., Wang X., Wang P., Xiao L. Sonodynamic antitumor effect of protoporphyrin IX disodium salt on S180 Solid tumor. Chemotherapy. 2007;53(6):429–436. doi: 10.1159/000110008. [DOI] [PubMed] [Google Scholar]
- 138.Zheng Y., Zhang Y., Ao M., Zhang P., Zhang H., Li P. Hematoporphyrin encapsulated PLGA microbubble for contrast enhanced ultrasound imaging and sonodynamic therapy. J Microencapsul. 2012;29(5):437–444. doi: 10.3109/02652048.2012.655333. [DOI] [PubMed] [Google Scholar]
- 139.Abe H., Kuroki M., Tachibana K., Li T., Awasthi A., Ueno A. Targeted sonodynamic therapy of cancer using a photosensitizer conjugated with antibody against carcinoembryonic antigen. Anticancer Res. 2002;22(3):1575–1580. [PubMed] [Google Scholar]
- 140.Tsuru H., Shibaguchi H., Kuroki M., Yamashita Y., Kuroki M. Tumor growth inhibition by sonodynamic therapy using a novel sonosensitizer. Free Radic Biol Med. 2012;53(3):464–472. doi: 10.1016/j.freeradbiomed.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 141.Yumita N., Nishigaki R., Sakata I., Nakajima S., Umemura S. Sonodynamically Induced Antitumor Effect of 4-Formyloximethylidene-3-Hydroxy-2-vinyl-deuterio-porphynyl(IX)-6,7-diaspartic Acid (ATX-S10) Jpn J Cancer Res. 2000;91(2):255–260. doi: 10.1111/j.1349-7006.2000.tb00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]