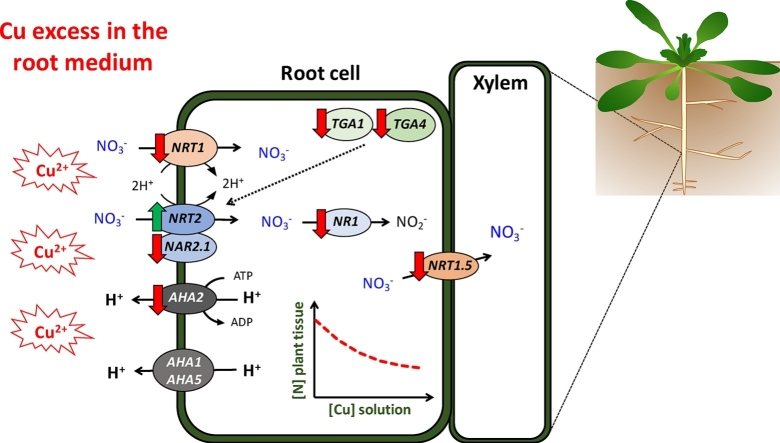
Graphical abstract
Abbreviations: HAT, high-affinity transporters; LAT, low-affinity transporters; LOX, lipoxygenase; ROS, reactive oxygen species
Keywords: Nitrate transporter, Proton pump, Metal toxicity, Nutrient uptake
Abstract
Nitrate uptake by plants is mediated by specific transport proteins in roots (NRTs), which are also dependent on the activity of proton pumps that energize the reaction. Nitrogen (N) metabolism in plants is sensitive to copper (Cu) toxicity conditions. To understand how Cu affects the uptake and assimilation processes, this study assesses the inhibitory effects of elevated Cu levels on the expression of genes related to N absorption, transport and assimilation in roots of Arabidopsis. Plants were grown hydroponically for 45 days, being exposed to a range of Cu concentrations in the last 72 h or alternatively exposed to 5.0 μM Cu for the last 15 days. High Cu levels decreased the uptake and accumulation of N in plants. It down-regulated the expression of genes encoding nitrate reductase (NR1), low-affinity nitrate transporters (NRT1 family) and bZIP transcription factors (TGA1 and TGA4) that regulate the expression of nitrate transporters. Cu toxicity also specifically down-regulated the plasma membrane proton pump, AHA2, whilst having little effect on AHA1 and AHA5. In contrast, there was an up-regulation of high-affinity nitrate transporters from the NRT2 family when exposed to medium level of Cu excess, but this was insufficient for restoring N absorption by roots to control levels. These results demonstrate that plants display specific responses to Cu toxicity, modulating the expression of particular genes related to nitrate uptake, such as low-affinity nitrate transporters and proton pumps.
1. Introduction
Nitrogen (N) is a key element for plants and its availability is a major factor determining plant growth and crop production. Specific transporters are important in N acquisition by plant roots and contribute to N use efficiency (Williams and Miller, 2001; Araki and Hasegawa, 2006). Among the inorganic N-forms, nitrate is the main source in aerobic soils (Crawford and Glass, 1998). Nitrate uptake across the plasma membrane of root cells occurs through specific transporters categorized as the NRT1/PTR family (NPF), “low-affinity” transporters (LAT), and the NRT2, “high-affinity” transporters (HAT) (Wang et al., 2012). The LAT genes are highly expressed in high (>0.5 mM) external nitrate concentration, while HAT genes are expressed when external nitrate concentration in the medium is low (≤0.5 mM) (Wang et al., 2012; Fan et al., 2017). In Arabidopsis, fifty-three genes were characterised that encode LAT genes and at least seven genes encoding the HATs (Wang et al., 2012).
The activity and transcription of genes that encodes nitrate transporters are dependent also on other genes. NAR2.1 (nitrate assimilation related protein; also known as NRT3.1) interacts with NRT2 and works together as a HAT of nitrate (Kotur et al., 2012). In Arabidopsis, six of the seven NRT2 family members require NAR2.1 for transporting nitrate across the plasma membrane. The exception is NRT2.7, which controls nitrate concentration in seeds, and is thought not to require NAR2.1 (Kotur et al., 2012). TGA1 and TGA4 encode bZIP-transcription factors that are candidate regulatory factors mediating nitrate responses (Alvarez et al., 2014). TGA1 and its close homolog TGA4 were shown to participate in plant defence responses against pathogen and chemical stress (Gatz, 2013), and are induced in response to nitrate treatments in Arabidopsis roots (Alvarez et al., 2014). They are also important in pathways leading to root hair development in response to nitrate (Canales et al., 2017).
Plasma membrane (PM) H+-ATPases also play a role in the absorption of N by energizing transport and creating a proton gradient (H+) between the cytosol and apoplast (Camacho-Cristóbal and González-Fontes, 2007; Sperandio et al., 2011). The absorption of nitrate depends on H+ pumping, which requires the entry of 2H+ for each nitrate actively transported across the PM (Chapman and Miller, 2011). In Arabidopsis there are eleven members of AHA family (AHA1-AHA11; Młodzińska et al., 2015). AHA1 and AHA2 are genes expressed at high levels in Arabidopsis by a range of stimuli (Haruta et al., 2010). AHA2 is the predominant proton pump in roots and it is upregulated after nitrate supply (Młodzińska et al., 2015). In rice (Oryza sativa), the expression of the genes OsA1, OsA2 and OsA5 were reduced in roots by nitrate-starvation in the root medium (Sperandio et al., 2011).
Copper (Cu) is also an essential element for plant growth, required in trace amounts (Hall and Williams, 2003; Mikkelsen et al., 2012; Yruela, 2013). In excess it causes oxidative stress, with increased production of reactive oxygen species (ROS) via the Haber-Weiss reaction and consequent peroxidation of lipid components of cell membranes (Hall, 2002; Ravet and Pilon, 2013). Excess Cu increases the activity of lipoxygenase (LOX) enzyme that also catalyse the peroxidation of lipids, mainly using free fatty acids as substrates (Newcomer and Brash, 2015; Cuypers et al., 2016). High Cu levels also inhibit the synthesis of proteins and their function with irreversible linkages established between the metal and sulfhydryl groups. As an overall process, photosynthesis rate decreases (Drzewiecka et al., 2017; Hippler et al., 2018) and plant growth is affected (Mattos Jr et al., 2010). Nutritional disorders caused by Cu excess include an interference in the reduction of nitrate to ammonium, primarily by inhibiting the activity of nitrate reductase. In Citrus this subsequently impairs the incorporation of N into proteins, leading to the accumulation of nitrate in plant tissues (Hippler et al., 2016). In grape vine (Vitis vinifera) excess Cu also impairs the absorption of nitrate by roots, but accumulation of nitrate did not occur in this case despite a reduced nitrate reductase activity (Llorens et al., 2000). Elevated Cu also causes inhibition of the PM H+-ATPase in tomato (Solanum lycopersicum; Zhang et al., 2009) but the underlying mechanisms involved are not certain. The intensity of metal stress effects on H+-ATPase activity depends on the type and concentration of the heavy metal and the length of the exposure time of roots (Janicka-Russak et al., 2012).
Cu-contaminated soils occur world-wide due to the intensive applications of cupric based fungicides; therefore it is important to assess how Cu excess affects plant responses, in particular the acquisition and assimilation of nitrate, the main source of inorganic-N to plant roots. In this study, we investigated the effects of elevated Cu on the expression of genes related to nitrate uptake, transport and assimilation in roots of Arabidopsis and demonstrate specific responses to Cu toxicity.
2. Materials and methods
2.1. Plant growth
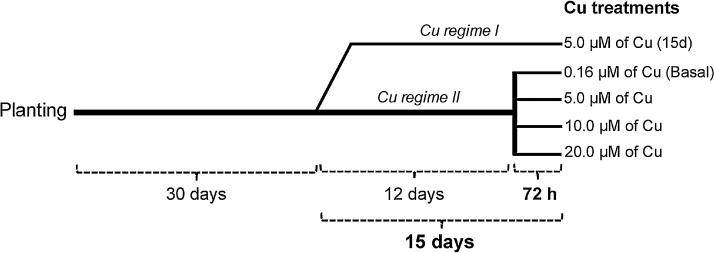
Sterilised seeds of Arabidopsis thaliana (L.) Heynh Columbia (Col-0) were placed individually in plastic tubes (0.5 mL) containing 0.5% (w/v) agar, stored in the dark at 4 °C for 48 h and then transferred into a controlled-environment growth room (23 °C, 8 h light: 120 μmol photons m−2 s–1/ 23 °C, 16 h dark cycle). Seedlings were grown in hydroponic conditions with half-strength concentration of a control nutrient solution for 15 days and, subsequently with full-strength concentration. The control nutrient solution was modified from Hoagland, and contained 1.25 mM KNO3, 0.5 mM Ca(NO3)2·4H2O, 0.5 mM MgSO4·7H2O, 0.625 mM KH2PO4, 2.0 mM NaCl; 42.5 μM FeNa-EDTA, 0.38 μM ZnSO4·7H2O, 1.8 μM MnSO4·7H2O, 45.0 μM H3BO3, 0.015 μM (NH4)6Mo7O24·4H2O, 0.01 μM CoCl2 and 0.16 μM CuSO4·5H2O, adjusted to pH 5.6 using 0.5 M KOH (Nazri et al., 2017). Thirty days after planting, a set of plants were grown as indicated in Fig. 1 under Cu regime I. This was with the same control nutrient solution but containing 5.0 μM Cu, for a further 15 days (Fig. 1). Another set of plants, continuously growing with the control nutrient solution (42 days with full-strength concentration), were grown under Cu regime II with varying Cu concentrations (5.0, 10.0 or 20.0 μM Cu), for 72 h (Fig. 1). In all growing conditions, nutrient solutions were changed weekly. Additionally, control plants were also grown with the nutrient solution containing 0.16 μM Cu (Basal; Fig. 1). Each Cu treatment had 15 plants and was replicated three times. Root samples were collected at 6, 24 and 72 h after initiation of the Cu treatments in liquid N and stored at −80 °C for gene expression analysis.
Fig. 1.
Schematic time line showing experimental treatments for Arabidopsis thaliana grown in nutrient solution with varying copper (Cu) concentrations up to 72 h or 15 days (15d). During the first 30 days of the experiment, plants received 0.16 μM Cu as a basal condition.
2.2. RNA extraction and Real-time PCR
Total RNA was extracted using TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), and reverse transcription using the SuperScript system (Invitrogen) and oligo(dT) primer was carried out according to the manufacturer’s instructions. Real-time PCR was performed to measure gene expression was carried out as previously described (Jaffé et al., 2012; Nazri et al., 2017) using SYBR Green (Finnzymes) and an Opticon DNA Engine Continuous Fluorescence Detector (GRI Ltd.). PCR was performed using specific forward and reverse primers for each gene (Supporting information Table S1) at 95 °C for 10 min followed by 35 cycles of 95 °C for 15 s and 60 °C for 1 min. All gene expression analysis was performed with at least three independent biological replicates and the reactions were set up in triplicate for each sample. All data were standardized by normalizing to SAND or Yellow-Leaf-Specific gene8 (YLS8) expression (Remans et al., 2008; Jaffé et al., 2012) and analysed using Opticon Monitor III software (Biorad). Quantification of the relative transcript levels was performed using the comparative Ct (threshold cycle) method.
2.3. Plants growth and nutritional status
Plants were collected and separated into shoots and roots and dried at 58–60 °C to constant dry weight. Samples were ground and nutrient content were measured by nitro-perchloric digestion according to Bataglia et al. (1983) by plasma emission spectrometry (ICP-OES, Perkin-Elmer 5100 PC, Norwalk, CT, USA).
2.4. Statistical analyses
Analysis of variance (ANOVA) was used to evaluate the results with a level of significance of α = 0.05. Effects of treatments were compared using Tukey test at 5%.
3. Results
3.1. Biomass production and nutritional status of plants
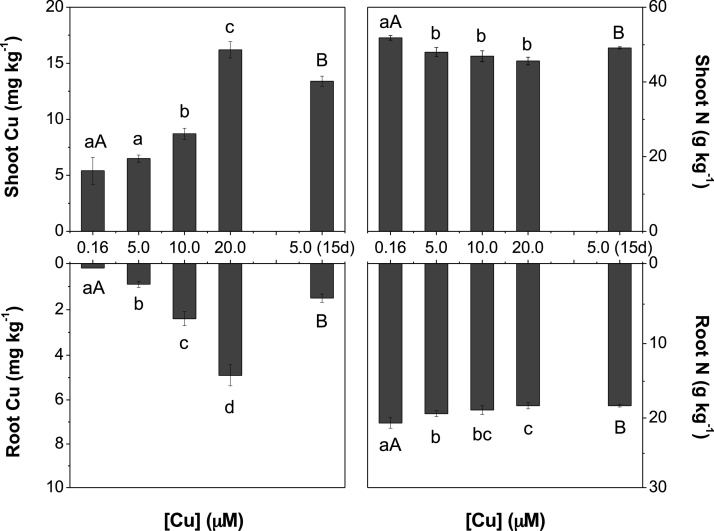
The effect of two Cu regimes was determined in Arabidopsis. Forty-five day-old plants were assessed following exposure to varying Cu levels for the final 72 h or to 5.0 μM Cu for the final fifteen days (Fig. 1). No marked visual symptoms of Cu toxicity were observed in these plants at the concentrations tested (Fig. 2); however, root biomass decreased in the plants exposed to 10.0 and 20.0 μM Cu for 72 h and there was a reduction in shoot and root fresh weight for plants exposed to 5.0 μM Cu after 15 days (Fig. 2). Here we show that when exposed to Cu concentrations in the nutrient solution at ≥5.0 μM Cu, the concentration of this metal increased whereas N levels decreased both in shoot and root tissues (Fig. 3). Furthermore, the elevated levels of Cu in the nutrient solution also reduced the concentration of phosphorous (P), calcium (Ca), iron (Fe), manganese (Mn) and boron (B) in the shoots and Mn in the roots (Table 1). However, Fe concentration in roots increased with the higher Cu levels in the nutrient solution (Table 1). No changes were observed in concentrations of the potassium (K), magnesium (Mg), sulphur (S) and zinc (Zn) after Cu treatments (data not shown).
Fig. 2.
Elevated copper (Cu) negatively impacts fresh weight of shoots and roots of Arabidopsis thaliana. Plants were grown under hydroponic conditions for up to 72 h or 15 days (15d). Mean (+/– SEM) are shown (n = 3). For the Cu treatments for 72 h different lowercase letters indicate mean values are significantly different among the [Cu] (0.16, 5.0, 10.0 and 20.0 μM) by Tukey’s test (p < 0.05). For the Cu treatments for 15 days different uppercase letters indicate mean values are significantly different between the [Cu] (0.16 and 5.0 μM) by Tukey’s test (p < 0.05). White line in the image represent 2 cm.
Fig. 3.
Elevated copper in the media (Cu) negatively affected nitrogen (N) and Cu concentrations in shoot and root of Arabidopsis thaliana. Plants were supplied with different Cu levels in the nutrient solution for 72 h or 15 days (15d). Mean (+/– SEM) are shown (n = 3). For the Cu treatments for 72 h different lowercase letters indicate mean values are significantly different among the [Cu] (0.16, 5.0, 10.0 and 20.0 μM) by Tukey’s test (p < 0.05). For the Cu treatments for 15 days different uppercase letters indicate mean values are significantly different between the [Cu] (0.16 and 5.0 μM) by Tukey’s test (p < 0.05).
Table 1.
Elevated copper (Cu) affected the concentration of phosphorus (P), calcium (Ca), iron (Fe), manganese (Mn) and boron (B) in the shoots and Fe and Mn in the root of Arabidopsis thaliana supplied with different Cu levels in the nutrient solution for 72 h or 15 days (15d).
| Cu concentrations | Shoot |
Root |
||||||
|---|---|---|---|---|---|---|---|---|
| P | Ca | Fe | Mn | Zn | B | Fe | Mn | |
| μM | g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 |
| 0.16 | 6.7 aaAb | 30 aA | 114 aA | 115 aA | 31 aA | 31 aA | 19 aA | 1.6 aA |
| 5.0 | 6.7 a | 26 ab | 95 b | 113 a | 30 a | 28 a | 31 b | 1.3 ab |
| 10.0 | 6.5 a | 24 b | 94 b | 102 ab | 29 a | 29 a | 29 b | 0.7 bc |
| 20.0 | 5.5 b | 23 b | 95 b | 85 b | 23 b | 22 b | 32 b | 0.4 c |
| 5.0 (15d) | 6.3 A | 25 B | 84 B | 90 B | 30 A | 31 A | 29 B | 0.2 B |
For the Cu treatments for 72 h different lowercase letters indicate mean values are significantly different among the [Cu] (0.16, 5.0, 10.0 and 20.0 μM) by Tukey’s test (p < 0.05).
For the Cu treatments for 15 days different uppercase letters indicate mean values are significantly different between the [Cu] (0.16 and 5.0 μM) by Tukey’s test (p < 0.05).
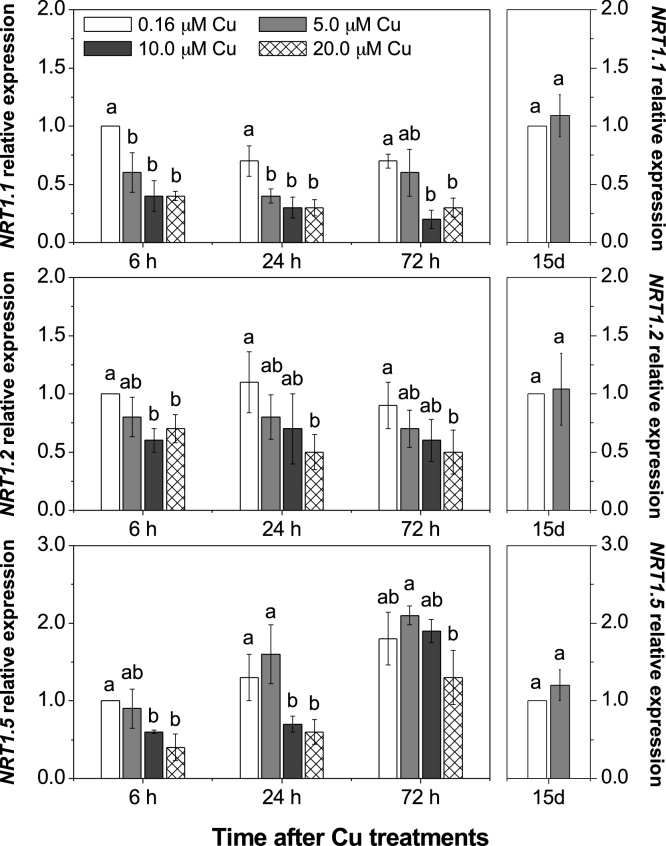
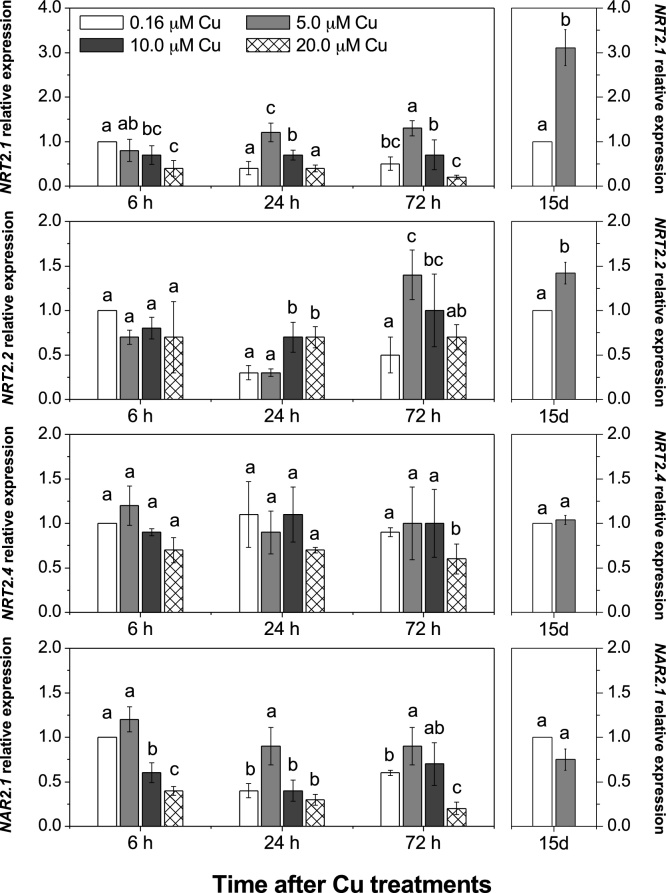
3.2. Excess Cu alters the expression levels of nitrate transporter genes in roots
The expression levels of genes encoding low and high-affinity transporters of nitrate in roots were analysed in response to Cu treatment. A decrease in NRT1.1 (also named NPF6.3 or CHL1; Fan et al., 2017) gene expression was observed when Cu increased ≥5.0 μM in the nutrient solution, whereas the expression of NRT1.2 gene decreased for 10.0 up to 6 h or 20.0 μM Cu up to 72 h (Fig. 4). Furthermore, increased Cu concentration resulted in down-regulation of NRT1.5 (Fig. 4), responsible for the transport of nitrate from the roots to the xylem vessels and consequently to the shoots. In contrast, for the HAT genes, NRT2.1 and NRT2.2, there was an up-regulation in plants with 5.0 or 10.0 μM Cu after 24 h for NRT2.1 and after 72 h for NRT2.2, both compared to basal Cu concentration in the nutrient solution (Fig. 5). NRT2.1 and NRT2.2 also showed an up-regulation following the 15 days Cu treatment at 5.0 μM Cu. NRT2.4 is also classified as a HAT gene, which is reportedly up-regulated before NRT2.1 and NRT2.2 when plants are grown under nitrate deficiency (Wang et al., 2012). In this study, plants grown with 20.0 μM Cu exhibited a down-regulated expression after 72 h, while no change was observed for other Cu levels tested (Fig. 5). NAR2.1 (also known as NRT3.1) encodes a protein required for the activation of NRT2 members (Fig. 5). This showed a similar level of expression to NRT2.1 apart from the 15 days Cu regime where in contrast to NRT2.1 it was not markedly upregulated.
Fig. 4.
Elevated Cu negatively affected expression of low-affinity nitrate transporter (NRT1 family).
Roots of Arabidopsis thaliana were supplied with different Cu levels in the nutrient solution for 72 h or 15 days (15d). Mean (+/– SEM) are shown (n = 3). Different letters in the same period are significantly different among the [Cu] (Tukey’s test, p < 0.05).
Fig. 5.
Elevated Cu up-regulated the high-affinity nitrate transporter (NRT2 family and NAR2.1).
Roots of Arabidopsis thaliana were supplied with different Cu levels in the nutrient solution for 72 h or 15 days (15d). Mean (+/– SEM) are shown (n = 3). Different letters in the same period are significantly different among the [Cu] (Tukey’s test, p < 0.05).
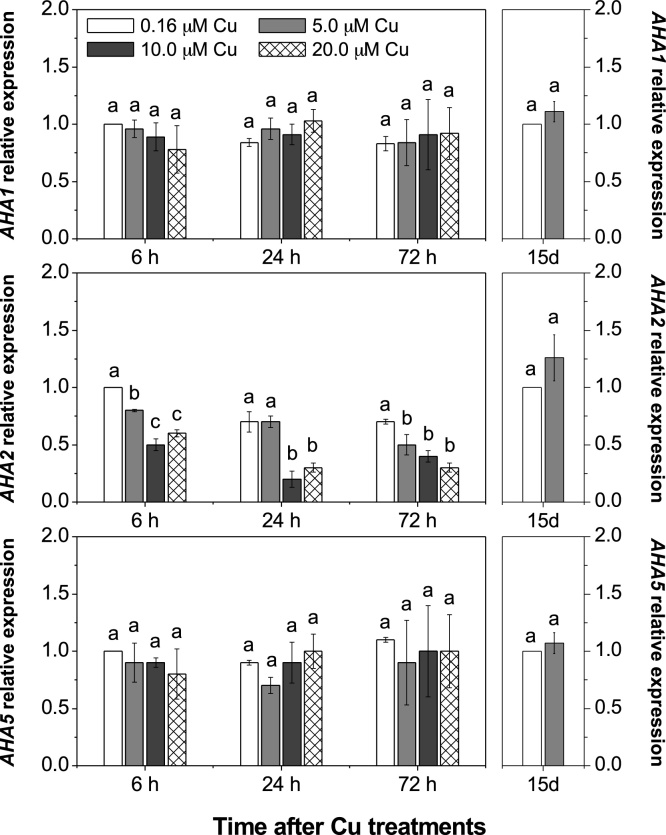
3.3. Specific effects of copper excess on transcript levels of H+-ATPase genes
Nitrate taken up by roots does not depend only on the NRT transporters, but also on H+-ATPases at the plasma membrane to energize the process and maintain the equilibrium of protons and anions in the cell. Here we have evaluated the expression of AHA1, AHA2 and AHA5, after plants were exposed to different Cu concentrations, since these are considered among the most important genes transcribed in relation to nitrate uptake (Sperandio et al., 2011). In this study, only AHA2 expression was specifically affected and this was seen to be down-regulated by Cu excess throughout the shorter time course regime (Fig. 6).
Fig. 6.
Effect of elevated Cu on H+-ATPase genes (AHA family).
Roots of Arabidopsis thaliana were supplied with different Cu levels in the nutrient solution for 72 h or 15 days (15d). Mean (+/– SEM) are shown (n = 3). Different letters in the same period are significantly different among the [Cu] (Tukey’s test, p < 0.05).
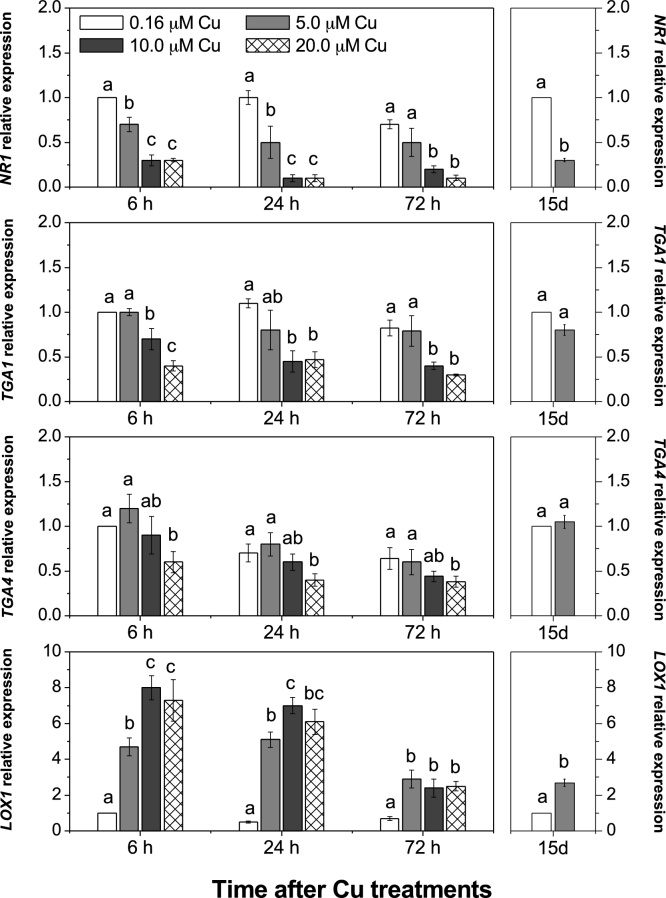
3.4. Nitrate reductase, transcription factors and lipoxygenase
Nitrate reductase gene expression (NR1) was also highly affected by increasing Cu concentrations in the nutrient solution (Fig. 7). A decrease in NR1 gene expression was observed under both Cu regimes. The bZIP transcription factors, TGA1 and TGA4, which are reported to influence the expression of NRT2.1 and NRT2.2 in response to nitrate treatments (Alvarez et al., 2014) were down-regulated in roots of plants exposed to Cu excess (Fig. 7). TGA1 gene expression was reduced when plants were exposed to 10.0 and 20.0 μM Cu, whereas TGA4 occurred only for 20.0 μM, both after 6 h, 24 h and 72 h of metal exposure (Fig. 7).
Fig. 7.
Effect of elevated Cu on the expression of genes encoding nitrate reductase (NR1), transcription factors (TGA1 and TGA4) and lypoxygenase (LOX1).
Roots of Arabidopsis thaliana supplied with different Cu levels in the nutrient solution for 72 h or 15 days (15d). Mean (+/- SEM) are shown (n = 3). Different letters in the same period are significantly different among the [Cu] (Tukey’s test, p < 0.05).
LOX1, codes for an enzyme identified as lipoxygenase (LOX). Consistent with previous investigations (Cuypers et al., 2011), LOX1 was markedly up-regulated by elevated Cu. This was observed under both Cu regimes used in this study (Fig. 7).
4. Discussion
Soil contamination with heavy metals and plant responses under abiotic stress conditions have been intensively studied. Accumulation of Cu in soil is a problem after intensive use of cupric fungicides that are used to preventively control diseases in many crops such as tomato (Rooney et al., 2006), vines (Ruyters et al., 2013) and citrus (Hippler et al., 2016). Excess Cu has previously been reported to reduce plant development and growth due to deleterious effects of this metal on protein and enzyme functions, as well as increasing reactive oxygen species (ROS) that impair physiological and biochemical processes such as photosynthesis (Drzewiecka et al., 2017). An increase in Cu availability in soils up to toxic levels is also followed by nutritional disorders affecting uptake and assimilation processes. N is often one of the most affected nutrients in plants and this can have implications on yield (Mattos Jr et al., 2010; Drzewiecka et al., 2017). Therefore, an understanding of the mechanism by which nutritional disorders occur in response to Cu toxicity is important.
In this study, we have investigated the effects of Cu toxicity in relation to N uptake and assimilation in more detail in roots of Arabidopsis. The threshold of Cu concentration that better demonstrated the specific effects of the metal excess on N absorption and assimilation processes were observed for those plants exposed to 5.0 μM and 10.0 μM Cu (Fig. 3; Supplementary File Fig. S1). Under 20.0 μM Cu, metal concentration increased in plants up to 3-fold in shoot and 25-fold in root (Fig. 3), both when compared to basal level, which resulted in plant growth inhibition (Fig. 2; Supplementary File, Fig. S2) and down-regulation of genes related to N absorption and assimilation, evaluated in this study (Fig. 4, Fig. 5, Fig. 6, Fig. 7).
In the first 72 h exposed to Cu excess, plants exhibited a reduction in the expression of NRT1.1 and NRT1.2, representing the LAT of nitrate in roots (Fig. 3). These genes are important transporters responsible for nitrate uptake in roots when the nitrate concentration in the solution is adequate (≥0.5 mM; Wang et al., 2012), which was the case in this study. Increased Cu concentrations also resulted in down-regulation of NRT1.5 (Fig. 4), which regulates long distances transport of nitrate into the xylem vessels and consequently to the shoots (Lin et al., 2008; Tegeder and Masclaux-Daubresse, 2017). Down-regulation of NRT1.5 in Arabidopsis has been suggested as a tolerance mechanism for abiotic stress, in which nitrate is reallocated to plant roots (Chen et al., 2012; Goel and Singh, 2015). The nrt1.5 Arabidopsis mutant exhibited higher tolerance to either Cd, salt or drought stresses compared to wild type plants, which suggests that nitrate reallocation in roots contributes essentially to stress tolerance (Chen et al., 2012). Regardless of the down-regulation of the LAT genes (Fig. 4), plants exhibited an up-regulation of NRT2.1 and NRT2.2 after exposure to 5.0 and 10.0 μM Cu after 72 h and after 15 days of metal exposure for 5.0 μM Cu (Fig. 5). Under nitrate deficiency it has previously been reported that NRT1 genes are down-regulated while NRT2 genes are up-regulated to recover the nitrate from the solution (Araki and Hasegawa, 2006; Sperandio et al., 2011; Lupini et al., 2016). These results demonstrate that plants under medium Cu excess (not observed for 20.0 μM Cu) up-regulated HAT genes (Fig. 5) as an attempt to recover N uptake capacity, although this was not sufficient to restore the N content of plants (Fig. 3).
An accumulation of auxins and cytokinins in roots was observed in Arabidopsis exposed to Cu toxicity, which also influenced root architecture, resulting in low growth of primary roots and death of the root apical meristem (Lequeux et al., 2010). Changes in the expression of the NRT transporters could also affect hormonal signalling and transport (Chiba et al., 2015). Further understanding on the effect of Cu toxicity inhibiting the transport of some hormones due the down-regulation of NRTs genes is still required. NRT1.1 expression modulates auxin levels in meristematic regions of the roots and consequently affects the root architecture (Mounier et al., 2014), mainly lateral root growth, which is critical for water and nutrient uptake (Forde, 2014). For instance, Arabidopsis with 25.0 μM Cu in the nutrient solution exhibited moderate reduction in the primary root growth and absence of lateral root growth (Lequeux et al., 2010). However, with 50.0 μM Cu in solution, plants exhibited high density of lateral roots, but with drastic reduction in the elongation of both primary and lateral roots (Lequeux et al., 2010). Similarly, Brassica also exhibited reduced lateral root growth when exposed to Cu excess in the root medium (Feigl et al., 2013). Moreover, lateral roots are the location of NRT1.2 (Wang et al., 2012), and a down-regulation of this gene was observed under Cu excess (Fig. 4).
NRT2.4 is classified as a HAT gene like NRT2.1 and NRT2.2, but no difference in gene expression was observed under the varying Cu levels in this study, with the exception of plants after 72 h with 20.0 μM Cu, where expression was decreased (Fig. 5). NRT2.4 is expressed mainly in the root epidermis, whereas NRT2.1 is expressed both in root cortex and epidermis (Kiba et al., 2012). In Arabidopsis under nitrate starvation, the expression of NRT2.4 was observed after 3 days, while the enhancement of NRT2.1 expression was observed after just a few hours (Wang et al., 2012; Fan et al., 2017). Under Cu excess we are also seeing a different response in these genes with NRT2.1 and NRT2.2 showing an up-regulation but NRT2.4 responding later and showing a down-regulation (Fig. 5).
NAR2.1 encodes a protein that does not play a role as a transporter of nitrate, but is crucial for the activation of the NRT2 transporters (Orsel et al., 2006; Krapp et al., 2014). Both genes operate interdependently and their expression occurs simultaneously (Okamoto et al., 2006; Fan et al., 2017). Furthermore, with 5.0 and 10.0 μM Cu, NRT2.1 and NAR2.1 genes were up-regulated, whereas at the highest concentration of Cu both genes were down-regulated (Fig. 5). Indeed, the lower efficiency of the HATs to promote N uptake recovery by plants under Cu excess, as demonstrated by a lower N concentration in the plant tissue (Fig. 3), could be in part explained by the reduced expression of NAR2.1, which did not follow the up-regulation of the NRT2.1 after 15 days (Fig. 7). The nitrate absorption by HATs is totally dependent on NAR2.1 expression, which encodes a protein that binds with NRT2 family members, with the exception of NRT2.7 (Fan et al., 2017). The Arabidopsis nar2.1 mutant exhibited reduced NRT2.1 protein amounts in the membrane fraction, as well as lower efficiency of nitrate absorption by the HAT when grown in nutrient solution with low concentrations of nitrate (Orsel et al., 2006).
Nitrate taken up by roots does not only depend on the NRT transporters, but also on H+-ATPase activity at the plasma membrane to maintain the equilibrium of protons and anions in the cell and energize the process (Camacho-Cristóbal and González-Fontes, 2007). Here we show that only AHA2 expression was affected by Cu toxicity (Fig. 6). This effect is unlikely to be due to a general increase in ROS production, since it would affect the transcription of other related H+-ATPase genes. Our evidence presented in this study indicates that Cu-excess effects in plants are different when compared to other heavy and non-nutrient metals in plants, such as Cd (Maksymiec and Krupa, 2006; Cuypers et al., 2011; Janicka-Russak et al., 2012). Differences in proton pump activity in membrane vesicles of cucumber (Cucumis sativus L.) exposed to Cu and Cd have been reported, with the former causing less damage to plants (Janicka-Russak et al., 2012). This would be possible considering that, even though excess Cu or Cd increases ROS accumulation and cellular damage, up to a certain level, Cu also enhances the activity of antioxidant enzymes, such as superoxide dismutase (Cu/Zn-SOD) and catalase, reducing superoxide radical and hydrogen peroxide in cells (Janicka-Russak et al., 2012; Hippler et al., 2016, Hippler et al., 2018).
Besides nitrate transporters, the expression of the gene encoding nitrate reductase (NR1) was very sensitive to Cu excess (Fig. 7). The activity of this enzyme has previously been reported to be affected by Cu toxicity in vines (Llorens et al., 2000) and citrus (Hippler et al., 2016, Hippler et al., 2018). In this study, the reduction of NR1 could be a direct effect of Cu excess by increasing either metal active effects or ROS accumulation in the cell, as well as by a reduction of nitrate uptake resulting after down-regulation of the LATs transporters expression (Fig. 5). Overall, this study demonstrates that under elevated Cu, a sequence of mechanisms operates regulating the uptake and assimilation of N into the roots. Although excess Cu reduced the expression of low-affinity transporters of nitrate (NRT1.1 and NRT1.2; Fig. 4) and NR1 (Fig. 7), we also observed an increase in the expression of the NRT2.1 gene up to 72 h (Fig. 5). The increase in the expression of HAT genes followed by the reduction of the NR1 could in fact result in accumulation of nitrate in the root tissue. However, such accumulation appears not to result in sufficient proportions of N assimilated into proteins (Martins et al., 2014; Tegeder and Masclaux-Daubresse, 2017).
The regulation of TGA1 and TGA4 by nitrate has been shown to depend on nitrate transport in plants (Alvarez et al., 2014). Therefore, the observed down-regulation of TGA1 and TGA4 (Fig. 7) could be explained by a direct effect of Cu excess or indirectly by Cu-dependent down-regulation of nitrate transporters (Alvarez et al., 2014). Even though the expression of TGA1 and TGA4 was reduced by the higher concentrations of Cu in the nutrient solution, it did not limit the up-regulation of NRT2.1 and NRT2.2 genes in those plants with 5.0 and 10.0 μM Cu (Fig. 5). Interestingly, TGA1 and TGA4 are transcription factors induced by nitrate supply that control the expression of the HATs NRT2.1 and NRT2.2, by direct binding to promoters such as the SLP9 (Alvarez et al., 2014). In addition to the specific deleterious effects that Cu excess causes on the expression of genes related to uptake, transport and assimilation processes of nitrate in roots, these effects could, in part, result from an increase in ROS production and consequently cellular damage.
With high levels of Cu in plant tissues, membrane lipid peroxidation is likely a response caused by the enzyme activity of lipoxygenase (LOX) (Cuypers et al., 2011), as well as by the hydroxyls radicals (OH−) produced by the Fenton reaction under Cu excess (Yruela, 2013). In addition, in Arabidopsis, it has been suggested that LOXs can give rise to oxylipins, in particular those belonging to the jasmonate family, which likely act as plant signalling of multiple defence responses under metal stress (Maksymiec and Krupa, 2006; Cuypers et al., 2016). In our study, a marked increase in LOX1 expression in roots was observed with increasing Cu concentrations in the nutrient solution, and levels were highest at 6 and 24 h after plant exposure to the metal, indicating that such oxylipins were in fact associated with Cu-induced responses observed in our study (Fig. 7).
The increase in membrane lipid peroxidation would lead to a decrease in expression of some genes related to nitrate uptake or proton pump functioning. Brassica juncea (L.) exhibited down-regulation of genes related to transport and assimilation of N, both nitrate and ammonium, after abiotic stress conditions including cold, heat, osmotic or salt (Goel and Singh, 2015). Notwithstanding, expression of BjNRT1.2 was specifically down-regulated by temperature, but not by osmotic or excess salt conditions, our data support that such down-regulation is also likely affected by excess Cu.
Elevated Cu generally reduced accumulation of a number of nutrients in the shoot, as seen for P, Ca, Mn, Zn and B (Table 1). However, Fe was increased in the roots. This has been reported for citrus (Hippler et al., 2016) and Arabidopsis (Andrés-Bordería et al., 2017). Therefore, excess Cu in the medium probably reduces the transport of Fe from root to shoot leading to an accumulation in the roots. (Table 1). Fe immobilisation in roots of rice and citrus under high levels of Cu, Zn or Cd in the root medium is likely a response of the plant homeostatic mechanism to reduce metal translocation from roots to shoots, by increasing the amount of organic chelators in the cells, such as histidine, glutathione and nicotianamine (Kendziorek et al., 2014; López-Climent et al., 2014; Gratão et al., 2015; Andrés-Bordería et al., 2017).
Therefore, we demonstrated that high levels of Cu in the root medium decreased the uptake and accumulation of N and other nutrients in plants. This resulted from specific effects on a subset of genes studied: nitrate reductase, the low-affinity transporters of nitrate, members of the proton pump family and bZIP transcription factors that regulate the expression of nitrate transporters. The increased expression of high-affinity transporters of nitrate (NRT2 family) under Cu excess that we observed was insufficient to regain nitrogen absorption by roots. In conclusion, the present study demonstrates the regulation of various N processes under Cu-excess and extends our understanding on the role of genes related to nitrate uptake and assimilation in plants. This will contribute to the selection of genotypes more tolerant to potential excesses of Cu in the environment resulting from intensive use of cupric-based pesticides for healthy management of crops.
Author contributions
FWRH, DMJ, RMB and LEW designed the study. FWRH performed the experiment. FWRH and LEW analysed the data. FWRH, DMJ and LEW wrote the manuscript. All authors revised the manuscript.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgements
FWRH was supported by a scholarship to work in the LEW laboratory from São Paulo Research Foundation (FAPESP; grant #2012/13917-7 and #2015/00720-9). Research in the LEW lab is supported by BBSRC (BB/L010313/1). DMJ also thanks the National Council for Scientific and Technological Development (CNPq) which granted his fellowship.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jplph.2018.06.005.
Contributor Information
Franz W.R. Hippler, Email: franz@ccsm.br.
Lorraine E. Williams, Email: lew@soton.ac.uk.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Alvarez J.M., Riveras E., Vidal E.A., Gras D.E., Contreras-López O., Tamayo K.P., Aceituno F., Gómez I., Ruffel S., Lejay L., Jordana X., Gutiérrez R.A. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J. 2014;80:1–13. doi: 10.1111/tpj.12618. [DOI] [PubMed] [Google Scholar]
- Andrés-Bordería A., Andrés F., Garcia-Molina A., Perea-García A., Domingo C., Puig S., Peãrrubia L. Copper ectopic expression of the Arabidopsis transport protein COPT1 alter iron homeostasis in rice (Oryza sativa L.) Plant Mol. Biol. 2017;95:17–32. doi: 10.1007/s11103-017-0622-8. [DOI] [PubMed] [Google Scholar]
- Araki R., Hasegawa H. Expression of rice (Oryza sativa L.) genes involved in high-affinity nitrate transport during the period of nitrate induction. Breed Sci. 2006;56:295–302. [Google Scholar]
- Bataglia O.C., Furlani A.M.C., Teixeira J.P.F., Furlani P.R., Gallo J.R. Instituto Agronômico, Campinas; 1983. Método de Análise Química de Plantas. (Boletim técnico n.78) (In Portuguese) [Google Scholar]
- Camacho-Cristóbal J.J., González-Fontes A. Boron deficiency decreases plasmalemma H++-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta. 2007;26:443–451. doi: 10.1007/s00425-007-0494-2. [DOI] [PubMed] [Google Scholar]
- Canales J., Contreras-López O., Álvarez J.M., Gutiérrez R.A. Nitrate induction of root hair density is mediated by TGA1/TGA4 and CPC transcription factors in Arabidopsis thaliana. Plant J. 2017;92:305–316. doi: 10.1111/tpj.13656. [DOI] [PubMed] [Google Scholar]
- Chapman N., Miller T. Nitrate transporters and root architecture. In: Geisler M., Venema K., editors. Transport and Pumps in Plant Signaling. Springer; Berlin: 2011. pp. 165–190. [Google Scholar]
- Chen C.Z., Lv X.F., Li Y.J., Yi H.Y., Gong J.M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012;159:1582–1590. doi: 10.1104/pp.112.199257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y., Shimizu T., Miyakawa S., Kanno Y., Koshiba T., Kamiya Y., Seo M. Identification of Arabidopsis thaliana NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones. J. Plant Res. 2015;128:679–686. doi: 10.1007/s10265-015-0710-2. [DOI] [PubMed] [Google Scholar]
- Crawford N.M., Glass A.D.M. Molecular and physiological aspects of nitrate uptake in plants. Trend. Plant Sci. 1998;3:389–395. [Google Scholar]
- Cuypers A., Smeets K., Ruytinx J., Opdenakker K., Keunen E., Remans T., Horemans N., Vanhoudt N., Van Sanden S., Van Belleghem F., Guisez Y., Colpaert J., Vangronsveld J. The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J. Plant Physiol. 2011;168:309–316. doi: 10.1016/j.jplph.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Cuypers A., Hendrix S., Reis R.A., Smet S., Deckers J., Gielen H., Jozefczak M., Loix C., Vercampt H., Vangronsveld J., Keunen E. Hydrogen peroxide, signalling in disguise during metal phytotoxicity. Front. Plant Sci. 2016;7:470. doi: 10.3389/fpls.2016.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecka K., Mlleczek M., Gasecka M., Magdziak Z., Budka A., Chadzinikolau T., Kaczmarek Z., Golinski P. Copper and nickel co-treatments alters metal uptake and stress parameters of Salix purpurea x viminalis. J. Plant Physiol. 2017;216:125–134. doi: 10.1016/j.jplph.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Fan X., Naz M., Fan X., Xuan W., Miller A.J., Xu G. Plant nitrate transporters: from gene function to application. J. Exp. Bot. 2017;68(10):2463–2475. doi: 10.1093/jxb/erx011. [DOI] [PubMed] [Google Scholar]
- Feigl G., Kumar D., Lehotai N., Tugyi N., Molnár A., Ordog A., Szepesi A., Gémes K., Laskay G., Erdei L., Kolbert Z. Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapessed (Brassica napus L.) to copper stress. Ecotoxicol. Environ. Saf. 2013;94:179–189. doi: 10.1016/j.ecoenv.2013.04.029. [DOI] [PubMed] [Google Scholar]
- Forde B.G. Nitrogen signalling pathways shaping root system architecture: an update. Curr. Opin. Plant Biol. 2014;21:30–36. doi: 10.1016/j.pbi.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Gatz C. From pioneers to team players: TGA transcription factors provide a molecular link between different stress pathways. Mol. Plant Microbe Interact. 2013;26(2):151–159. doi: 10.1094/MPMI-04-12-0078-IA. [DOI] [PubMed] [Google Scholar]
- Goel P., Singh A.K. Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation in Brassica juncea L. PLoS One. 2015;10(11):e0143645. doi: 10.1371/journal.pone.0143645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratão P.L., Monteiro C.C., Tezotto T., Carvalho R.F., Alves L.R., Peres L.E.P., Azevedo R.A. Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals. 2015;28:803–816. doi: 10.1007/s10534-015-9867-3. [DOI] [PubMed] [Google Scholar]
- Hall J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002;53:1–11. [PubMed] [Google Scholar]
- Hall J.L., Williams L.E. Transition metal transporters in plants. J. Exp. Bot. 2003;54:2601–2613. doi: 10.1093/jxb/erg303. [DOI] [PubMed] [Google Scholar]
- Haruta M., Burch H.L., Nelson R.B., Barrett-Wilt G., Kline K.G., Mohsin S.B., Young J.C., Otegui M.S., Sussman M.R. Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J. Biol. Chem. 2010;285:17918–17929. doi: 10.1074/jbc.M110.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippler F.W.R., Cipriano D.O., Boaretto R.M., Quaggio J.A., Gaziola S.A., Azevedo R.A., Mattos-Jr D. Citrus rootstocks regulate the nutritional status and antioxidant system of trees under copper stress. Environ. Exp. Bot. 2016;130:42–52. [Google Scholar]
- Hippler F.W.R., Dovis V.L., Boaretto R.M., Quaggio J.A., Azevedo R.A., Williams L.E., Mattos-Jr D. Photosynthesis is differently regulated during and after copper-induced nutritional stress in citrus trees. Physiol. Plant. 2018:s1. doi: 10.1111/ppl.12746. In press. [DOI] [PubMed] [Google Scholar]
- Jaffé F.W., Freschet G.E.C., Valdes B.M., Runions J., Terry M.J., Williams L.E. G protein–coupled receptor-type G proteins are required for light-dependent seedling growth and fertility in Arabidopsis. Plant Cell. 2012;24:3649–3668. doi: 10.1105/tpc.112.098681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicka-Russak M., Kabala K., Burzynski M. Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J. Exp. Bot. 2012;63:4133–4142. doi: 10.1093/jxb/ers097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendziorek M., Barabasz A., Rudzka J., Tracz K., Mills R.F., Williams L.E., Antosiewicz D.M. Approach to engineer tomato by expression of AtHMA4 to enhance Zn in the aerial parts. J. Plant Physiol. 2014;171:1413–1422. doi: 10.1016/j.jplph.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Kiba T., Feria-Bourrellier A.B., Lafouge F., Lezhneva L., Boutet-Mercey S., Orsel M., Bréhaut V., Miller A., Daniel-Vedele F., Sakakibara H., Krapp A. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell. 2012;24:245–248. doi: 10.1105/tpc.111.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotur Z., Mackenzie N., Ramesh S., Tyerman S.D., Kaiser B.N., Glass A.D.M. Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1. New Phytol. 2012;194:724–731. doi: 10.1111/j.1469-8137.2012.04094.x. [DOI] [PubMed] [Google Scholar]
- Krapp A., David L.C., Chardin C., Girin T., Marmagne A., Leprince A.S., Chaillou S., Ferrario-Méry S., Meyer C., Danel-Vedele F. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 2014;65:789–798. doi: 10.1093/jxb/eru001. [DOI] [PubMed] [Google Scholar]
- Lequeux H., Hermans C., Lutts S., Verbruggen N. Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Biochem. 2010;48:673–682. doi: 10.1016/j.plaphy.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Lin S.H., Kuo H.F., Canivenc G., Lepetit M., Hsu P.K., Tillard P., Lin H.L., Wang Y.Y., Tsa C.B., Gojon A., Tsay Y.F. Mutation of the Arabidopsis NRT1.5 nitrate trasnporter causes defective root-to-shoot nitrate transport. Plant Cell. 2008;20:2514–2528. doi: 10.1105/tpc.108.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens N., Arola L., Bladé C., Mas A. Effect of copper exposure upon nitrogen metabolism in tissue cultured Vitis vinifera. Plant Sci. 2000;160:159–163. doi: 10.1016/s0168-9452(00)00379-4. [DOI] [PubMed] [Google Scholar]
- López-Climent M.F., Arbona V., Pérez-Clemente R.M., Zandalinas I., Gómez-Cadenas A. Effect of cadmium and calcium treatments on phytochelatin and glutathione levels in citrus plants. Plant Biol. 2014;16:79–87. doi: 10.1111/plb.12006. [DOI] [PubMed] [Google Scholar]
- Lupini A., Mercati F., Araniti F., Miller A.J., Sunseri F., Abenavoli M.R. NAR2.1/NRT2.1 functional interaction with NO3− and H+ fluxes in high-affinity nitrate transport in maize root regions. Plant. Physiol. Biochem. 2016;102:107–114. doi: 10.1016/j.plaphy.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Maksymiec W., Krupa Z. The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ. Exp. Bot. 2006;57:187–194. [Google Scholar]
- Martins V., Teixeira A., Bassil E., Blumwald E., Gerós H. Metabolic changes of Vitis vinifera berries and leaves exposed to Bordeaux mixture. Plant Physiol. Biochem. 2014;82:270–278. doi: 10.1016/j.plaphy.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Mattos Jr D., Ramos U.M., Quaggio J.A., Furlani P.R. Nitrogênio e cobre na produção de mudas de citros em diferentes porta-enxertos. Bragantia. 2010;69:135–147. In Portuguese. [Google Scholar]
- Mikkelsen M.D., Pedas P., Schiller M., Vincze E., Mills R.F., Borg S., Moller A., Schjoerring J.K., Williams L.E., Baekgaard L., Holm P.B., Palmgren M.G. Barley HvHMA1 is a heavy metal pump involved in mobilizing organellar Zn and Cu and plays a role in metal loading into grains. Plos One. 2012;7:e49027. doi: 10.1371/journal.pone.0049027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Młodzińska E., Kłobus G., Christensen M.D., Fuglsang A.T. The plasma membrane H+‐ATPase AHA2 contributes to the root architecture in response to different nitrogen supply. Physiol. Plant. 2015;154:270–282. doi: 10.1111/ppl.12305. [DOI] [PubMed] [Google Scholar]
- Mounier E., Pervent M., Ljung K., Gojon A., Nacry P. Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell. Environ. 2014;37:162–174. doi: 10.1111/pce.12143. [DOI] [PubMed] [Google Scholar]
- Nazri A.Z., Griffin J.H.C., Peaston K.A., Alexander-Webber D.G.A., Williams L.E. F-group bZIPs in barley – a role in Zn deficiency. Plant Cell Environ. 2017;40:2754–2770. doi: 10.1111/pce.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer M.E., Brash A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015;24:298–309. doi: 10.1002/pro.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Kumar A., Li W., Wang Y., Siddiqi M.Y., Crawford N.M., Glass A.D. High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol. 2006;140:1036–1046. doi: 10.1104/pp.105.074385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsel M., Chopin F., Leleu O., Smith S.J., Krapp A., Daniel-Vedele F., Miller A.J. Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis physiology and protein–protein interaction. Plant Physiol. 2006;142:1304–1317. doi: 10.1104/pp.106.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravet K., Pilon M. Copper and iron homeostasis in plants: the challenges of oxidative stress. Antioxid. Redox Signal. 2013;19:919–932. doi: 10.1089/ars.2012.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T., Smeets K., Opdenakker K., Mathjsen D., Vangronsveld J., Cuypers A. Normalisation of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta. 2008;227:1343–1349. doi: 10.1007/s00425-008-0706-4. [DOI] [PubMed] [Google Scholar]
- Rooney C.P., Zhao F.J., McGrath S.P. Soil factors controlling the expression of copper toxicity to plants in a wide range of European soils. Environ. Toxicol. Chem. 2006;25:726–732. doi: 10.1897/04-602r.1. [DOI] [PubMed] [Google Scholar]
- Ruyters S., Salaets P., Oorts K., Smolders E. Copper toxicity in soils under established vineyards in Europe: a survey. Sci. Total Environ. 2013;443:470–477. doi: 10.1016/j.scitotenv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Sperandio M.V.L., Santos L.A., Bucher C.A., Fernandes M.S., Souza S.R. Isoforms of plasma membrane H+-ATPase in rice root and shoot are differentially induced by starvation and resupply of NO3− or NO4+ Plant Sci. 2011;180:251–258. doi: 10.1016/j.plantsci.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Tegeder M., Masclaux-Daubresse C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2017;217:35–53. doi: 10.1111/nph.14876. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Hsu P.K., Tsay Y.F. Uptake, allocation and signaling of nitrate. Trend Plant Sci. 2012;17:458–467. doi: 10.1016/j.tplants.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Williams L.E., Miller A.J. Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:659–688. doi: 10.1146/annurev.arplant.52.1.659. [DOI] [PubMed] [Google Scholar]
- Yruela I. Copper in plants: acquisition, transport and interactions. Funct. Plant Biol. 2013;36(5):409–430. doi: 10.1071/FP08288. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Han X., Chen X., Jin H., Cui X. Exogenous nitric oxide on antioxidative system and ATPase activities from tomato seedlings under copper stress. Sci. Hortic. 2009;123:217–223. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.