Abstract
AIM
To explore the relationship between different parameters of Ocular Response Analyzer (ORA) and Corvis ST (CST) in a sample of healthy Iranian school-aged children and the relationship between parameters of these 2 instruments against intraocular pressure (IOP), measured by the Goldmann applanation tonometer (GAT-IOP), age and gender, and find possible correlation between ORA and CST with GAT.
METHODS
This cross-sectional study included 90 healthy children. A general interview and complete eye examination were performed. Following successful GAT-IOP measurement, ORA and CST were conducted. The CST parameters were A 1/2 length (A1L, A2L), A 1/2 velocity (A1V, A2V), highest concavity deformation amplitude (HCDA), radius of curvature (RoC), peak distance (PD), central corneal thickness (CCT) and IOP. The ORA parameters were corneal hysteresis (CH), corneal resistance factor (CRF), Goldmann-correlated IOP (IOP-G) and corneal compensated IOP (IOP-CC). Extracted data was analyzed using the Statistical Package for Social Science software.
RESULTS
Totally 39 males with age of 9.08±1.60 (6-12)y and 51 females with age of 8.96±1.55 (6-13)y were included. Many CST parameters were significantly correlated with CH, CRF, IOP-G and IOP-CC. Some CST parameters had a significant correlation with GAT-IOP, including IOP-CST in both eyes and HCDA, A2L, PD, and RoC in the left eye, but none with age, except A2L in the right eye. The CRF measurement showed a significant correlation with GAT-IOP in both eyes and CH in the right eye, yet, none with age. Among all CST and ORA parameters, CCT-CST in both eyes and A1L in right eye had a significant correlation with gender, although this was a negligible negative correlation. Comparison of mean IOP values by different devices showed a significantly highest IOP overestimation by CST and lowest by IOP-CC compared with GAT. Also, IOP-G versus IOP-CST significantly had the lowest IOP overestimation among others. Overall, either low positive correlation or negligible correlation was found between IOP measurements by 3 instruments.
CONCLUSION
The study finds the highest IOP overestimation by CST and lowest by IOP-CC compared with GAT. Overall, either low positive correlation or negligible correlation is found between IOP measurements by the 3 instruments.
Keywords: Goldmann applanation tonometer, Ocular Response Analyzer, Corvis ST, intraocular pressure, children
INTRODUCTION
Measurement of intraocular pressure (IOP) is considered as an essential step in diagnosis and treatment of congenital and pediatric glaucoma, the accuracy of which could be affected by central corneal thickness (CCT)[1]–[2]. The most trusted method for measuring IOP is considered the Goldmann applanation tonometer (GAT), and is currently considered as the gold standard[3]. In spite of this, measurement of IOP with this contact method in children has problems that are not found in adults. In addition to the problem of lack of attention in children, the lack of understanding of rational of ocular examination and their cooperation makes it difficult to measure the IOP with this method and reduces the accuracy of the measurement[4]. It is obvious clear that non-contact methods are more expensive as compare as GAT however it would be interesting, particularly from the standpoint of convenience in pediatric age group to consider non-contact methods in measurement of IOP.
A recently introduced non-contact tonometry method, which may overcome the problem of children's lack of collaboration in the contact method IOP measurement, named Ocular Response Analyzer (ORA; Reichert, Buffalo, New York, USA) and measures the biomechanical response of the eye to a rapid air jet-induced deformation at the cornea[5]. This device measures 2 corneal characteristics, which include corneal hysteresis (CH) and corneal resistance factor (CRF)[6]. Besides, this device has 2 types of IOPs, including the Goldmann-correlated IOP (IOP-G) which is mean of P1 and P2, and the corneal compensated IOP (IOP-CC). The IOP-CC is designed to be less affected by corneal features than the IOP measured by GAT[7]. It has been applied for measurement of IOP and corneal properties among healthy emmetropic and ametropic children[8].
The Corvis ST (CST; Oculus, Wetzlar, Germany) is also a newly designed non-contact device that examines corneal deformation properties, besides IOP measurements. The device is equipped with a high-speed Scheimpflug camera that can record cornea movements in response to an air pulse[9]. It reports the following parameters: A 1/2 length (A1L, A2L), A 1/2 velocity (A1V, A2V), highest concavity deformation amplitude (HCDA), radius of curvature (RoC) as well as peak distance (PD), besides measuring CCT and IOP[10]. These parameters were successfully measured using the Corvis ST in healthy children[11].
To the best of the author's knowledge, despite the growing number of publications on healthy and non-healthy adults, which assessed the relationship between parameters of ORA and CST against IOP measured by GAT (GAT-IOP)[10],[12]–[17], this association has not been studied in children. Therefore, the aim of the current study was to explore relationships between different parameters of ORA and CST in a sample of healthy Iranian school-aged children. Furthermore, another objective was to investigate the relationship between parameters of these 2 instruments against GAT-IOP, age and gender, and find possible correlation between ORA and CST with GAT.
SUBJECTS AND METHODS
Compliance with Ethics Guidelines
This cross-sectional study, as part of the Shiraz Pediatric Eye Study (SPES), received ethical approval at the departmental level performed at Shiraz City which is the fifth-most-populous city of Iran and the capital of Fars Province located in southwest of Iran. Informed consent was signed by all parents of children participating in the study. All examinations and paraclinical investigations were in accordance with the tenets of the Declaration of Helsinki performed free of charge to subjects.
Subjects
The SPES is a population-based study including 2400 school-aged children, aged 6 to 12 years old from 4 educational districts of Shiraz, using stratified random sampling.
Inclusion and Exclusion
All enrolled children were Persian, meaning that they were from 2 consecutive generations of Iranian parents and were living in Shiraz, Iran.
Based on structural questioner all cases with systemic and ocular abnormalities and those subjects with history of using medications were excluded. A general interview and complete eye examination were conducted for each case at Salouti Eye Clinic, including uncorrected near and distance visual acuity, best corrected distance visual acuity, external eye examination (strabismus assessment and eyelids evaluation), slit lamp examination, GAT-IOP measurement (in uncooperative subjects, IOP was measured by non-contact air-puff tonometer), Ishihara color vision test and stereoacuity measurement.
Study Procedures
Ninety pure healthy children, who had successful GAT-IOP measurement, were selected for further evaluation using ORA and CST. To compensate for the effect of diurnal variation, all measurement with GAT (Haag Streit, Könitz, Switzerland), CST (CST, Oculus, Wetzlar, Germany), and ORA (ORA, Reichert, Buffalo, New York, USA) were performed by a single examiner (Gharebaghi R) between 09:00 a.m. and 02:00 p.m. The GAT was set at 10 mm Hg prior to each measurement and IOP was taken following instillation of Tetracaine topical anesthetic eye drops (i.e. Tetracaine 0.50%, Sina Daru Co, Tehran, Iran), with fluorescein staining.
The order of ORA or CST was randomly selected per case. Patients were given around a 1-hour interval after GAT or ORA measurements. The CST measurements were done 3 times and the following parameters were extracted from print out of CST: A1L, A2L, A1V, A2V, HCDA, RoC, PD, CCT, and IOP. Test quality was proved based on “OK” quality index, presented on the CST monitor.
Patients were given around a 1-hour rest after GAT or CST measurements. The ORA measurements were done 3 times and the following parameters were extracted from print out of CST: CH, CRF, IOP-G, and IOP-CC. Test quality was proved based on quality index score of above 7.5 for each test.
Data Analyses
Sample size calculation was performed using a single mean formula with the level of significance set at 0.05. Extracted data were analyzed using the Statistical Package for Social Science software (ver. 22.0, SPSS, Inc., Chicago, IL, USA). Data are shown as mean±SD. Correlation coefficients between both eye parameters were conducted. Also, correlation coefficients between all CST parameters (A1L, A2L, A1V, A2V, HCDA, RoC, PD, CCT and IOP) and GAT-IOP, age and gender were calculated. The same calculation was conducted between ORA parameters (CH, CRF, IOP-CC and IOP-G) and GAT-IOP, age and gender as well as between all CST parameters and ORA parameters. The agreement between the methods of IOP measurement was investigated through Bland-Altman plots. P values less than 0.05 were considered statistically significant.
RESULTS
This study included 90 healthy school-aged children, 39 males with a mean age of 9.08±1.60 (6-12)y and 51 females with a mean age of 8.96±1.83 (6-13)y. Table 1 demonstrates a summary of subjects' characteristics. Mean±SD of GAT-IOP in the right and left eye of males and females was 13.18±2.42, 14.26±1.77, 13.33±2.25 and 14.02±1.81 mm Hg respectively.
Table 1. Demographics of the study subjects.
| Variables | Values | P |
| Age (y), mean±SD | 9.01±1.56 (6-13) | |
| M | 9.08±1.60 (6-12) | 0.729 |
| F | 8.96±1.55 (6-13) | |
| Gender, n (%) | ||
| M | 39 (43.33) | |
| F | 51 (56.66) | |
| OD/OS | 90/90 | |
| GAT-IOP (mean±SD, mm Hg) | ||
| OD (F/M) | 13.18±2.42/14.26±1.77 | 0.022a |
| OS (F/M) | 13.33±2.25/14.02±1.81 | 0.115 |
| CCT (mean±SD, µm) | 0.058 | |
| OD (F/M) | 542.69±27.41/516.25±82.33 | |
| OS (F/M) | 543.97±26.85/526.63±38.32 | 0.015a |
SD: Standard deviation; GAT: Goldmann applanation tonometry; IOP: Intraocular pressure; CCT: Central corneal thickness. CCT measured by Corvis ST; OD: Right eye; OS: Left eye; aP values less than 0.05.
Table 2 shows the correlation between CST and ORA parameters against GAT-IOP, age and gender. Corneal resistance factor, IOP-G and IOP-CST were significantly related to GAT-IOP in the both eyes; CH was significantly related to GAT-IOP in the right eye and IOP-CC, HCDA, A2L, PD and RoC were significantly related to GAT-IOP in the left eye. However, other parameters were not significantly correlated. Also, except for A2L (r=-0.212, P=0.045) in the right eye, age had no significant relationship with other CST and ORA parameters. Interestingly, CCT-CST in both eyes and A1L in right eye had a significant correlation with gender, although this was a negligible negative correlation.
Table 2. Correlation coefficients (with significance levels) between ORA/CST parameters against IOP measured by the Goldmann applanation tonometer, age and gender.
| Parameters | GAT-IOP (mm Hg) | Patients' age | Patient's gender |
| OD CH | 0.257 (0.014)a | -0.103 (0.334) | 0.093 (0.382) |
| OD CRF | 0.381 (<0.001) a | -0.060 (0.573) | 0.087 (0.416) |
| OD IOP-G | 0.379 (<0.001) a | 0.040 (0.705) | 0.004 (0.971) |
| OD IOP-CC | 0.193 (0.068) | 0.107 (0.314) | 0.006 (0.955) |
| OD IOP-CST | 0.243 (0.021) a | 0.145 (0.172) | -0.024(0.821) |
| OD CCT-CST | -0.040 (0.705) | -0.066 (0.536) | -0.219 (0.038) a |
| OD HCDA | -0.150 (0.159) | 0.060 (0.577) | 0.019 (0.859) |
| OD A1L | -0.064 (0.549) | 0.024 (0.824) | -0.216 (0.041) a |
| OD A2L | 0.084 (0.430) | -0.212(0.045) a | 0.181 (0.087) |
| OD A1V | -0.189 (0.074) | -0.116 (0.275) | 0.171 (0.108) |
| OD A2V | -0.027 (0.808) | -0.008 (0.940) | 0.162 (0.141) |
| OD PD | -0.032 (0.764) | 0.145 (0.173) | 0.033 (0.759) |
| OD RoC | 0.041 (0.701) | 0.043 (0.686) | -0.187 (0.077) |
| OS CH | 0.062 (0.560) | -0.006 (0.958) | -0.051 (0.636) |
| OS CRF | 0.216 (0.041) a | 0.043 (0.686) | -0.032 (0.762) |
| OS IOP-G | 0.335 (0.001) a | 0.098 (0.357) | -0.078 (0.464) |
| OS IOP-CC | 0.265 (0.012) a | 0.095 (0.372) | -0.060 (0.577) |
| OS IOP-CST | 0.361 (<0.001) a | 0.001 (0.991) | 0.088 (0.407) |
| OS CCT-CST | 0.111 (0.301) | 0.039 (0.714) | -0.240 (0.023) a |
| OS HCDA | -0.426 (<0.001) a | 0.065 (0.543) | -0.107 (0.316) |
| OS A1L | 0.119 (0.262) | -0.041 (0.703) | -0.141 (0.185) |
| OS A2L | 0.214 (0.043) a | -0.069 (0.519) | -0.105 (0.325) |
| OS A1V | -0.072 (0.499) | 0.062 (0.562) | 0.122 (0.253) |
| OS A2V | 0.057 (0.602) | -0.002 (0.989) | 0.080 (0.463) |
| OS PD | -0.235 (0.026) a | -0.053 (0.618) | -0.023 (0.827) |
| OS RoC | 0.266 (0.011) a | -0.077 (0.471) | -0.013 (0.900) |
CST: Corvis ST; ORA: Ocular Response Analyzer; GAT-IOP: IOP measured by Goldmann applanation tonometry; OD: Right eye; OS: Left eye; CH: Corneal hysteresis; CRF: Corneal resistance factor; IOP-G: Goldmann-correlated IOP; IOP-CC: Corneal compensated IOP; IOP-CST: Corrected IOP measured by CST; CCT-CST: Central corneal thickness measured by CST; HCDA: Highest concavity deformation amplitude; A1L: A1 length; A2L: A2 length; A1V: A1 velocity; A2V: A2 velocity; RoC: Radius of curvature; PD: Peak distance. aP values less than 0.05.
r (P)
Table 3 shows the relationship between CST and ORA parameters. CH was significantly correlated with A2L in the right eye, and CCT and PD in the left eye. Also, a significant relationship was found between CRF and IOP-CST, CCT-CST, and A2L in both eyes; A1V in the right eye and HCDA, A2V and RoC in the left eye. However, other parameters were not significantly correlated. Moreover, IOP-G was significantly correlated with IOP-CST, CCT-CST, HCDA and RoC in both eyes; A1V and PD in right eye; and A1L and A2V in left eye. Finally, IOP-CC was significantly correlated with IOP-CST, HCDA and RoC in both eyes, A1V and PD in right eye and A2V in left eye.
Table 3. Correlation coefficient (with significance levels) between CST parameters and ORA parameters.
| Parameters | CH | CRF | IOP-G | IOP-CC |
| OD IOP-CST | 0.052 (0.628) | 0.253 (0.016) a | 0.471 (<0.001)a | 0.403 (<0.001) a |
| OD CCT-CST | 0.170 (0.109) | 0.232 (0.028) a | 0.219 (0.038) a | 0.092 (0.388) |
| OD HCDA | -0.032 (0.766) | -0.144 (0.174) | -0.257 (0.014) a | -0.223 (0.035) a |
| OD A1L | 0.140 (0.187) | 0.188 (0.076) | 0.176 (0.097) | 0.075 (0.480) |
| OD A2L | 0.278 (0.008) a | 0.294 (0.005) a | 0.182 (0.086) | -0.025 (0.814) |
| OD A1V | -0.159 (0.135) | -0.328 (0.002) a | -0.460 (<0.001) a | -0.322 (0.002) a |
| OD A2V | 0.110 (0.319) | 0.109 (0.325) | 0.052 (0.636) | -0.027 (0.810) |
| OD PD | 0.126 (0.237) | -0.004 (0.973) | -0.212 (0.044) a | -0.291 (0.005) a |
| OD RoC | -0.021 (0.842) | 0.120 (0.259) | 0.296 (0.005) a | 0.296 (0.005) a |
| OS IOP-CST | -0.020 (0.849) | 0.216 (0.041) a | 0.455 (<0.001) a | 0.433 (<0.001) a |
| OS CCT-CST | 0.332 (0.001) a | 0.498 (<0.001) a | 0.491 (<0.001) a | 0.242 (0.022) |
| OS HCDA | 0.043 (0.689) | -0.261 (0.013) a | -0.579 (<0.001) a | -0.562 (<0.001) a |
| OS A1L | 0.105 (0.326) | 0.189 (0.074) | 0.219 (0.038) a | 0.131 (0.219) |
| OS A2L | 0.177 (0.096) | 0.251 (0.017) a | 0.223 (0.035) | 0.092 (0.388) |
| OS A1V | 0.025 (0.815) | 0.023 (0.829) | 0.007 (0.947) | -0.010 (0.926) |
| OS A2V | 0.155 (0.151) | 0.308 (0.004) a | 0.369 (<0.001) a | 0.252 (0.019) a |
| OS PD | -0.228 (0.031) a | -0.203 (0.055) | -0.060 (0.574) | 0.089 (0.42) |
| OS RoC | 0.185 (0.081) | 0.371 (<0.001) a | 0.454 (<0.001) a | 0.301 (0.004) a |
CST: Corvis ST; ORA: Ocular Response Analyzer; OD: Right eye; OS: Left eye; CH: Corneal hysteresis; CRF: Corneal resistance factor; IOP-G: Goldmann-correlated IOP; IOP-CC: Corneal compensated IOP; IOP-CST: Corrected IOP measured by CST; CCT-CST: Central corneal thickness measured by CST; HCDA: Highest concavity deformation amplitude; A1L: A1 length; A2L: A2 length; A1V: A1 velocity; A2V: A2 velocity; RoC: Radius of curvature; PD: Peak distance. aP values less than 0.05.
r (P)
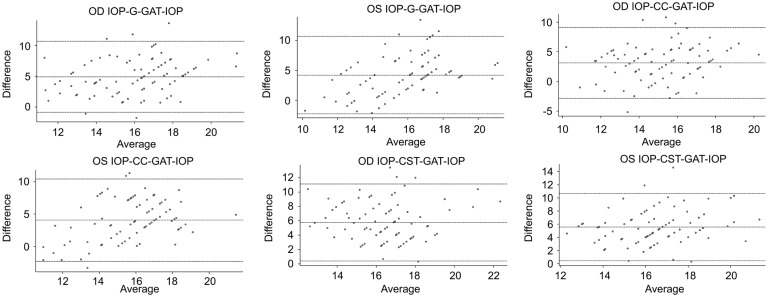
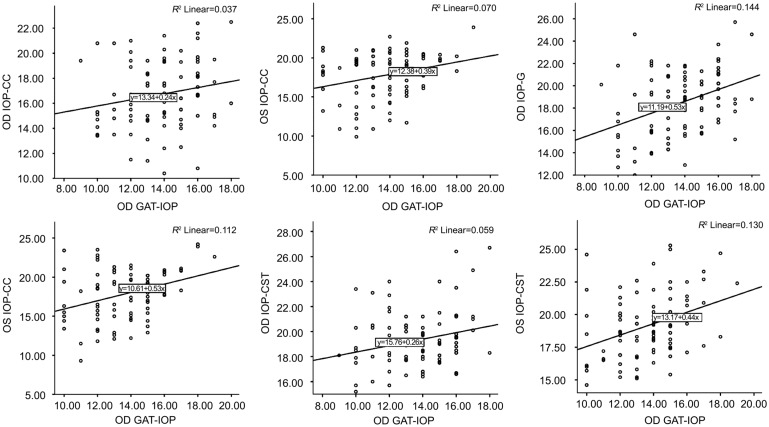
Table 4 shows comparisons of mean IOP values found by the different instruments. The CST showed a significantly highest IOP overestimation compared with GAT (+5.57 mm Hg, P<0.0001 OD, and +5.47, P<0.0001 OS), and IOP-CC showed a significantly lowest IOP overestimation compared with GAT (-2.94 mm Hg, P<0.0001 OD, and -4.06 mm Hg, P<0.0001 OS). Also, ORA (IOP-G) versus IOP-CST showed a significantly lowest IOP overestimation (+0.86 mm Hg, P=0.004 OD, and +1.30 mm Hg, P<0.0001 OS), among others. Figure 1 reveals agreement on Bland-Altman analysis between mean GAT-IOP and mean IOP-G, IOP-CC, and IOP-CST in both eyes. Figure 2 shows the results of linear regression between IOPs, measured by different instruments. Coefficient of correlation or r value between IOP-G, IOP-CC, and IOP-CST with GAT-IOP were 0.379 (P<0.001), 0.193 (P=0.068), and 0.243 (P=0.021) for the right eye, and 0.335 (P=0.001), 0.265 (P=0.012), and 0.361 (P<0.001) for the left eye. These findings could be interpreted as[18] significantly low positive correlation between IOP-G and GAT-IOP and negligible correlation between IOP-CC and GAT-IOP in both eyes, negligible correlation between IOP-CST and GAT-IOP in the right eye, and low positive correlation between IOP-CST and GAT-IOP in the left eye.
Table 4. Mean and significance of instrument comparisons.
| Differences | OD (mean) | OS (mean) | P |
| GAT vs ORA (IOP-G) | -4.71 | -4.17 | <0.0001a |
| GAT vs ORA (IOP-CC) | -2.94 | -4.06 | <0.0001 a |
| GAT vs CST | -5.57 | -5.47 | <0.0001 a |
| ORA (IOP-G) vs CST | -0.86 | -1.30 | 0.004 a |
| ORA (IOP-CC) vs CST | -2.63 | -1.41 | <0.0001 a |
CST: Corvis ST; ORA: Ocular Response Analyzer; GAT: Goldmann applanation tonometry; OD: Right eye; OS: Left eye. aP values less than 0.05.
mm Hg
Figure 1. The differences between tonometry and GAT (IOP-G), ORA (IOP-CC), IOP-CST and GAT compared with their means in the right and left eye.
Figure 2. Linear regression between GAT-IOP and ORA (IOP-G), ORA (IOP-CC) and IOP-CST in the right and left eye.
DISCUSSION
In this cross sectional study, GAT, CST and ORA measurements were carried out in 90 healthy school-aged children. Some CST parameters had a significant correlation with GAT-IOP, including IOP-CST in both eyes, and HCDA, A2L, PD, and RoC in the left eye, yet none with age, except A2L in the right eye. The CRF measurement showed a significant correlation with GAT-IOP in both eyes and CH in the right eye, yet none with age. Among all CST and ORA parameters, CCT-CST in both eyes and A1L in right eye had a significant correlation with gender, although this was a negligible negative correlation. Many CST parameters were significantly correlated with CH, CRF, IOP-G, and IOP-CC. Comparisons of mean IOP values by different devices showed a significantly highest IOP overestimation by CST and lowest by IOP-CC compared with GAT. Also, IOP-G versus IOP-CST had a significantly lower IOP overestimation, among others. Overall, either low positive or negligible correlation was found between the IOP measurements by 3 instruments.
In the current study, CRF was significantly related to GAT-IOP in both eyes, similar to previous studies on adult subjects[5],[19]–[23] and one study on nanophthalmos children[24]. However, CH had a negligible correlation with GAT-IOP in the right eye and no correlation in the left eye, which is in agreement with previous reports among adult subjects[20]–[23], and one study on nanophthalmos children[24]. Although reduction in these 2 parameters was shown with age among adult subjects[22],[25], age was not significantly correlated with CH or CRF in the current study. This may be due to the narrow age range of the subjects or natural differences in values of ocular parameters among children compared with their adult counterparts.
Previous studies revealed the correlation of A1 time and A1V with GAT-IOP, both in healthy[26] and glaucomatous[10] adult subjects. In contrast with the mentioned studies, the current research did not find a significant correlation between A1V and GAT-IOP in both eyes. However, some CST parameters had a significant correlation with GAT-IOP, including IOP-CST in both eyes, and HCDA, A2L, PD, and RoC in the left eye. The possible justification for this difference could be the difference in the age or racial background of the current study subjects with previous studies as indicated by a recent Meta-analysis regarding CCT and IOP among healthy children, published by the current authors[27]. For instance, there are various hypothesis that race could influence the biometry of the eyes[28]. However, further studies with more samples on multiple age groups as well as different races are needed to reliably disclose the underlying cause. One possible explanation for negligible or low interocular difference could arise from some physiologic differences between two eyes. However, to elaborate exact etiology of those differences, further well-designed cohort studies with long follow-up period and high sample size seems to be necessary.
The current study compared non-contact IOP measuring tonometer, including ORA and CST with a contact tonometer, GAT, which is a routine instrument for IOP measurement, universally. It was firmly evident that due to significantly low positive or negligible correlation, none of these 2 non-contact tonometers can replace the GAT. Also, IOP-CST showed a low positive correlation with both IOP-G and IOP-CC, which indicates that these 2 tonometers were different from each other[16].
Although a range of environmental and genetic factors are contributed to ocular parameters, we assume that socioeconomic status of the family and environmental factors which may be related to level of malnutrition should be related too. This may require additional investigations as longitudinal studies to classify subjects based on their socioeconomic status in measurement effect of environmental factors on ocular anatomy and physiology[2],[27]–[28].
A possible limitation of the current study was the cross sectional design. Also, this study did not allocate subjects to different age groups due to narrow age range of enrolled cases. Finally, a future study should be performed to evaluate reproducibility of the CST and ORA parameters among children and cost-effectiveness of these procedures. As stated in methods, Shiraz City is located in southwest of country therefore our results are related to this specific geographical region could not be generalized to county population or Middle East. The current study could contribute to better knowledge of 3 tonometers and their correlation among children, which was the first investigation on this age group. Importantly, these results suggest that they have either no or negligible correlation with each other, which should be considered in clinical practice, particularly for the management and evaluation of glaucomatous children.
In conclusion, results of the current study revealed significantly highest IOP overestimation by CST and lowest by IOP-CC compared with GAT. In addition, IOP-G versus IOP-CST showed a significantly lower IOP overestimation, among others. Overall, either low positive correlation or negligible correlation was found between IOP measurements by 3 instruments. The CST and ORA parameters had no significant correlation with age, and CCT-CST in both eyes and A1L in right eye had a significant negligible negative correlation with gender. However, many CST parameters were significantly correlated with CH, CRF, IOP-G, and IOP-CC.
Acknowledgments
Foundation: Supported by deputy dean in research of School of Medicine according to study project and deputy vice chancellor of Shiraz University of Medical Sciences.
Conflicts of Interest: Salouti R, None; Alishiri AA, None; Gharebaghi R, None; Naderi M, None; Jadidi K, None; Shojaei-Baghini A, None; Talebnejad MR, None; Nasiri Z, None; Hosseini SM, None; Heidary F, None.
REFERENCES
- 1.Biglan AW. Glaucoma in children: are we making progress? J AAPOS. 2006;10(1):7–21. doi: 10.1016/j.jaapos.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Nejabat M, Heidary F, Talebnejad MR, et al. Correlation between intraocular pressure and central corneal thickness in persian Children. Ophthalmol Ther. 2016;5(2):235–243. doi: 10.1007/s40123-016-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbonaro F, Andrew T, Mackey DA, Spector TD, Hammond CJ. Comparison of three methods of intraocular pressure measurement and their relation to central corneal thickness. Eye (Lond) 2010;24(7):1165–1170. doi: 10.1038/eye.2010.11. [DOI] [PubMed] [Google Scholar]
- 4.Krzyżanowska-Berkowska P, Asejczyk-Widlicka M, Pierscionek B. Intraocular pressure in a cohort of healthy eastern European schoolchildren: variations in method and corneal thickness. BMC Ophthalmol. 2012;12:61. doi: 10.1186/1471-2415-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31(1):156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J Glaucoma. 2006;15(5):364–370. doi: 10.1097/01.ijg.0000212268.42606.97. [DOI] [PubMed] [Google Scholar]
- 7.Huseynova T, Waring GO, 4th, Roberts C, Krueger RR, Tomita M. Corneal biomechanics as a function of intraocular pressure and pachymetry by dynamic infrared signal and Scheimpflug imaging analysis in normal eyes. Am J Ophthalmol. 2014;157(4):885–893. doi: 10.1016/j.ajo.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Bueno-Gimeno I, España-Gregori E, Gene-Sampedro A, Lanzagorta-Aresti A, Piñero-Llorens DP. Relationship among corneal biomechanics, refractive error, and axial length. Optom Vis Sci. 2014;91(5):507–513. doi: 10.1097/OPX.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 9.Hong J, Xu J, Wei A, Deng SX, Cui X, Yu X, Sun X. A new tonometer-the Corvis ST tonometer: clinical comparison with noncontact and Goldmann applanation tonometers. Invest Ophthalmol Vis Sci. 2013;54(1):659–665. doi: 10.1167/iovs.12-10984. [DOI] [PubMed] [Google Scholar]
- 10.Matsuura M, Hirasawa K, Murata H, Yanagisawa M, Nakao Y, Nakakura S, Kiuchi Y, Asaoka R. The relationship between Corvis ST tonometry and Ocular Response Analyzer measurements in eyes with glaucoma. PLoS One. 2016;11(8):e0161742. doi: 10.1371/journal.pone.0161742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He M, Ding H, He H, Zhang C, Liu L, Zhong X. Corneal biomechanical properties in healthy children measured by corneal visualization scheimpflug technology. BMC Ophthalmol. 2017;17(1):70. doi: 10.1186/s12886-017-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanza M, Iaccarino S, Mele L, Carnevale UA, Irregolare C, Lanza A, Femiano F, Bifani M. Intraocular pressure evaluation in healthy eyes and diseased ones using contact and non contact devices. Cont Lens Anterior Eye. 2016;39(2):154–159. doi: 10.1016/j.clae.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Lanza M, Iaccarino S, Cennamo M, Irregolare C, Romano V, Carnevale UA. Comparison between Corvis and other tonometers in healthy eyes. Cont Lens Anterior Eye. 2015;38(2):94–98. doi: 10.1016/j.clae.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Mollan SP, Iaccarino S, Cennamo M, Irregolare C, Romano V, Carnevale UA. Accuracy of Goldmann, Ocular Response Analyser, Pascal and TonoPen XL tonometry in keratoconic and normal eyes. Br J Ophthalmol. 2008;92(12):1661–1665. doi: 10.1136/bjo.2007.136473. [DOI] [PubMed] [Google Scholar]
- 15.Tejwani S, Devi S, Dinakaran S, Shetty R, Meshram P, Francis M, Sinha Roy A. Diagnostic efficacy of normalization of corneal deformation variables by the intraocular pressure in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2016;57(3):1082–1086. doi: 10.1167/iovs.15-18569. [DOI] [PubMed] [Google Scholar]
- 16.Erratum: a cross-sectional study to compare intraocular pressure measurement by sequential use of Goldman applanation tonometry, dynamic contour tonometry, ocular response analyzer, and Corvis ST. Indian J Ophthalmol. 2017;65(9):904. doi: 10.4103/0301-4738.214668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao F, Huang Z, Huang J, Wang J, Deng M, Li L, Yu A, Wang Q, Elsheikh A. Clinical evaluation of methods to correct intraocular pressure measurements by the Goldmann applanation tonometer, ocular response analyzer, and Corvis ST tonometer for the effects of corneal stiffness parameters. J Glaucoma. 2016;25(6):510–519. doi: 10.1097/IJG.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 18.Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Kotecha A, Russell RA, Sinapis A, Pourjavan S, Sinapis D, Garway-Heath DF. Biomechanical parameters of the cornea measured with the Ocular Response Analyzer in normal eyes. BMC Ophthalmol. 2014;14(1) doi: 10.1186/1471-2415-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayanaswamy A, Chung RS, Wu RY, Park J, Wong WL, Saw SM, Wong TY, Aung T. Determinants of corneal biomechanical properties in an adult Chinese population. Ophthalmology. 2011;118(7):1253–1259. doi: 10.1016/j.ophtha.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Kaushik S, Pandav SS, Banger A, Aggarwal K, Gupta A. Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma. Am J Ophthalmol. 2012;153(5):840–849.e2. doi: 10.1016/j.ajo.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 22.Kotecha A, Elsheikh A, Roberts CR, Zhu H, Garway-Heath DF. Corneal thickness- and age-related biomechanical properties of the cornea measured with the Ocular Response Analyzer. Invest Ophthalmol Vis Sci. 2006;47(12):5337–5347. doi: 10.1167/iovs.06-0557. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan-Mee M, Billingsley SC, Patel AD, Halverson KD, Alldredge BR, Qualls C. Ocular Response Analyzer in subjects with and without glaucoma. Optom Vis Sci. 2008;85(6):463–470. doi: 10.1097/OPX.0b013e3181784673. [DOI] [PubMed] [Google Scholar]
- 24.Altan C, Kara N, Baz O, Şatana B, Demirok A, Yilmaz OF. Corneal biomechanical properties and intraocular pressure measurement in patients with nanophthalmos. Br J Ophthalmol. 2012;96(6):806–810. doi: 10.1136/bjophthalmol-2011-300557. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya K, Shimizu K, Ohmoto F. Effect of aging on corneal biomechanical parameters using the Ocular Response Analyzer. J Refract Surg. 2009;25(10):888–893. doi: 10.3928/1081597X-20090917-10. [DOI] [PubMed] [Google Scholar]
- 26.Asaoka R, Nakakura S, Tabuchi H, Murata H, Nakao Y, Ihara N, Rimayanti U, Aihara M, Kiuchi Y. The relationship between Corvis ST tonometry measured corneal parameters and intraocular pressure, corneal thickness and corneal curvature. PLoS One. 2015;10(10):e0140385. doi: 10.1371/journal.pone.0140385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farvardin M, Heidary F, Sayehmiri K, Gharebaghi R, Jabbarvand Behrooz M. A comprehensive Meta-analysis on intraocular pressure and central corneal thickness in healthy children. Iran J Public Health. 2017;46(6):724–732. [PMC free article] [PubMed] [Google Scholar]
- 28.Heidary F, Gharebaghi R, Wan Hitam WH, Shatriah I. Nerve fiber layer thickness. Ophthalmology. 2010;117(9):1861–1862. doi: 10.1016/j.ophtha.2010.05.024. [DOI] [PubMed] [Google Scholar]