Abstract
AIM
To evaluate the long-term results of different orbital decompression techniques performed in patients with Graves' ophthalmopathy (GO).
METHODS
Totally 170 cases with GO underwent orbital decompression between 1994 and 2014. Patients were divided into 4 groups as medial-inferior, medial-lateral (balanced), medial-lateral-inferior, and lateral only according to the applied surgical technique. Surgical indications, regression degrees on Hertel exophthalmometer, new-onset diplopia in the primary gaze and new-onset gaze-evoked diplopia after surgery and visual acuity in cases with dysthyroid optic neuropathy (DON) were compared between different surgical techniques.
RESULTS
The study included 248 eyes of 149 patients. The mean age for surgery was 42.3±13.2y. DON was the surgical indication in 36.6% of cases, and three-wall decompression was the most preferred technique in these cases. All types of surgery significantly decrease the Hertel values (P<0.005). Balanced medial-lateral, and only lateral wall decompression caused the lowest rate of postoperative new-onset diplopia in primary gaze. The improvement of visual acuity in patients with DON did not significantly differ between the groups (P=0.181).
CONCLUSION
The study show that orbital decompression surgery has safe and effective long term results for functional and cosmetic rehabilitation of GO. It significantly reduces Hertel measurements in disfiguring proptosis and improves visual functions especially in DON cases.
Keywords: orbital decompression, Graves' ophthalmopathy, dysthyroid optic neuropathy
INTRODUCTION
Graves' ophthalmopathy (GO) is the most frequently occurring and most complicated extrathyroidal symptom of Graves' disease (GD)[1]–[2]. The rate of GO in GD was reported as 25%, but it can be detected in up to 80% of the cases with detailed clinical and radiological evaluations[3]–[4]. Upper eyelid retraction is the most common feature of GO, but the clinical spectrum may also involve chemosis, exposure keratopathy, extraocular muscle dysfunction, exophthalmos, and dysthyroid optic neuropathy (DON)[5]–[10]. Most of the clinical features of GO can be explained by the inflammatory processes taking place in the restricted area of the bony orbit, which usually increase the intraorbital volume and retrobulbar pressure. In selected cases, orbital decompression surgery may be necessary to expand the bony orbit and/or excise orbital fatty tissue to avoid irreversible damage to the visual function.
Orbital decompression surgery was first described in 1911[11] to provide more space for the orbital soft tissue that can provide a decrease in exophthalmos[12]–[13]. The aim of this study was to evaluate the surgical results of the different orbital decompression techniques and their long-term outcomes.
SUBJECTS AND METHODS
Orbital decompression surgery was performed in 170 GO cases in the Oculoplastic and Orbital Surgery Unit of the Department of Ocular Diseases at Gazi University in Turkey between 1994 and 2014. The data from these patients was retrospectively reviewed. Ethical approval was obtained from the Ethical Committee of Clinical Research at Gazi University (approval number: 363, approval date: July 14, 2014). A consent form was obtained from each patient for the publication of their photographs.
Those patients that followed up for at least two years after the surgery were included in the study. Nine patients died and 12 were lost to follow-up. Finally, the data from 149 cases was recorded, including the age, gender, family history, smoking status, thyroid function, diagnostic age for GO, time elapsed from the first ocular symptoms to orbital surgery, presence of DON, orbital decompression technique, follow-up duration after orbital surgery, preoperative and postoperative new-onset diplopia, related complications, and additional medical and/or surgical interventions.
The patients were divided into 4 groups according to the surgical technique applied: inferomedial, balanced (medial and lateral), three-wall (medial, lateral, and inferior), and lateral wall only.
All of the patients underwent full ophthalmic examinations, including pupillary responses, visual acuity (VA), refraction, color vision, biomicroscopy, intraocular pressure, fundoscopy, and visual field analyses, and the axial proptosis was measured via Hertel exophthalmometry both preoperatively and postoperatively. Nasal endoscopic evaluations were performed in all cases. In each patient, the paranasal sinuses and bony orbit were evaluated via orbital computed tomography (CT) and/or magnetic resonance imaging (MRI) before and after the operation. The orbital images were evaluated for the involvement of the extraocular muscles, orbital fat compartment, optic nerve appearance, and apical crowding. The ocular motility and the degree of strabismus were assessed using an orthoptic examination, Hess chart, and prisms. The diagnosis of DON was based on the presence of any combination of visual deficits, including the VA, visual field, and color vision. It was also supported by at least one of the following: apical crowding, optic disc edema, and afferent pupillary defect[14]–[15].
Statistical Analysis
The data were analyzed with the Statistical Package for the Social Sciences (SPSS, version 11.5, USA) for Windows. The Kolmogorov-Smirnov test was used to determine whether the continuous and categorical variables were normally distributed, and Levene's test was used to determine whether the variances were homogenous. The one-way analysis of variance (ANOVA), Mann-Whitney U, Kruskal-Wallis, Pearson's Chi-squared, likelihood ratio, Wilcoxon signed rank, McNemar's test, and paired samples t tests were employed when necessary. A P value of <0.05 was considered to be significant; however, the Bonferroni correction was performed to keep type I errors under control in all the multiple comparisons.
Surgical Technique
The lateral wall decompression was performed via an extended upper eyelid crease incision. Following the incision, the superior and lateral aspects of the orbital rim were exposed. A high-speed micromotor burr was used to sculpt the bone. The frontal, zygomatic, and frontalis bones were thinned from the frontozygomatic fissure to the superior orbital fissure. The zygomatic bone corpus was harvested, a thin orbital rim was left in the lateral site, and the thick bone of the greater wing of the sphenoid was excised by protecting the dura mater. The zygomatic and maxillary bones around the inferior orbital fissure were also excised. Incisions were made in the periorbital tissue, creating herniation of the orbital fat tissue and lacrimal gland into the newly created spaces.
If floor decompression was planned, a lateral canthotomy and cantholysis of the lower limb of the lateral canthal tendon was performed, and a transconjunctival incision was made just below the lower tarsal border, creating a swinging lower eyelid flap. The periosteal incision was made at the inferior orbital rim, and the periorbital fascia was reflected off the floor. A mallet and chisel were used to puncture the floor bone, and the pieces of bone were removed. Care was taken when removing the bony canal of the infraorbital nerve. The orbital floor was removed to the posterior wall of the maxillary sinus, and the overlying periorbital tissue was excised. In all cases, the inferomedial orbital strut was preserved to prevent globe luxation and decrease the risk of postoperative new-onset diplopia.
In all of the cases that underwent lateral wall decompression, the inferolateral fat pocket was removed by using a radiofrequency unit (Ellman International Inc., Hicksville, NY, USA) with a Colorado microneedle tip (Colorado Biomedical, Inc., Evergreen, CO, USA). This excision was performed under direct visualization to avoid injury to the dilated vessels and rectus muscles. An average of 3.5 cubic centimetre of fat was removed from this region.
The transcaruncular approach was used for the medial wall decompression. First, the eyeball and the orbital contents were deviated laterally with a malleable retractor. An incision was made in the periosteum parallel to the posterior lacrimal crest, and the subperiosteal dissection was done with a Freer elevator. The orbital medial wall was excised with Takahagi forceps. The bony opening was enlarged to the anterior ethmoidal foramen superiorly and the maxillary strut inferiorly. Finally, incisions were made to the periorbital fascia for the herniation of the orbital fat.
RESULTS
All of the patients were diagnosed as GD and they were euthyroid and taking antithyroid medications, with clinical and laboratory examinations being conducted at least 4mo before surgery [the free triiodothyronine (T3) and free thyroxine (T4) were within the normal ranges and the thyroid-stimulating hormone was low or within the normal range]. Family history for thyroid dysfunction was present in 41.6% of the cases. Smoking habit was present in 78.5% of the patients where 24.8% were current smokers. The demographic characteristics of the patients and the types of surgery are shown in Tables 1 and 2, respectively.
Table 1. Patient characteristics.
| Characteristics | Value (149 cases) |
| Surgery age (y) | 42.3±13.2 (14-81) |
| Diagnostic age (y) | 39.0±13.3 (5-80) |
| Sex | |
| Male | 60 (40.3) |
| Female | 89 (59.7) |
| Surgical technique | 248 eyes |
| Inferomedial wall | 15 (6) |
| Balanced (medial-lateral)+fat | 181 (73) |
| Three wall (medial-lateral-inferior)+fat | 39 (15.75) |
| Lateral+fat | 13 (5.25) |
| DON | 248 eyes |
| Negative | 157 (63.4) |
| Positive | 91 (36.6) |
| Final patient situation | 170 patients |
| In follow-up | 149 (87.7) |
| Deceased | 9 (5.3) |
| Lost to follow-up | 12 (7) |
DON: Dysthyroid optic neuropathy.
n (%)
Table 2. Demographic characteristics according to the operation type.
| Variable | Medial-inferior (15 eyes) | Medial-lateral (181 eyes) | Medial-lateral-inferior (39 eyes) | Lateral (13 eyes) | P |
| Surgical age (y) | 42.1±12.1 | 40.5±13.2 | 48.4±10.5 | 45.6±17.9 | 0.082a |
| Diagnostic age (y) | 39.7±10.8 | 37.4±13.6 | 43.9±9.7 | 40.9±20.1 | 0.237a |
| Sex, n (%) | 0.451b | ||||
| Male | 3 (30.0) | 42 (39.6) | 12 (50) | 3 (33.3) | |
| Female | 7 (70.0) | 64 (60.3) | 12 (50) | 6 (66.6) | |
| Follow-up duration (y) | 4 (2-20) | 6 (2-17) | 6.5 (2-16) | 6 (3-10) | 0.158c |
aOne-way ANOVA; bPearson Chi-squared test; cKruskal-Wallis test.
The preference for three-wall decompression surgery in the DON cases was significantly higher (P<0.001), whereas the balanced medial and lateral or lateral wall only decompressions were preferred for the cosmetic cases (Table 3).
Table 3. DON frequency according to the type of procedure.
| Variable | Medial-inferior (15 eyes) | Medial-lateral (181 eyes) | Medial-lateral-inferior (39 eyes) | Lateral (13 eyes) |
| DON | 5 (33.3%)a,b,d | 59 (32.6%)a,c,f | 25 (64.1%)c,d,e | 2 (15.3%)b,e,f |
DON: Dysthyroid optic neuropathy. Pearson's Chi-squared test. aP=0.942, medial-inferior vs medial-lateral groups; bP=0.274, medial-inferior vs lateral groups was statistically insignificant; cP<0.001, medial-lateral vs medial-lateral-inferior groups; dP<0.001, medial-inferior vs medial-lateral-inferior groups; eP=0.002, medial-lateral-inferior vs lateral groups; fP=0.201, medial-lateral vs lateral groups.
Both the intragroup and intergroup comparisons of the preoperative and postoperative Hertel values were compared regarding the types of surgery. The postoperative Hertel measurements were significantly lower in all the groups, and Table 4 summarizes the mean preoperative and postoperative Hertel values with the mean decreases.
Table 4. Mean preoperative and postoperative Hertel values.
| Hertel value | No. of eyes | Preoperative | Postoperative | gP | Difference |
| Medial-inferior | 15 | 26.8±3.3 (22-32) | 19±3.4 (13-25) | <0.001 | 7.8±1.9 (4-11)a,b,f |
| Medial-lateral | 181 | 25.9±3.3 (19-35) | 20.1±2.6 (14-27) | <0.001 | 5.7±2.7 (1-13)a,c,d |
| Medial-lateral-inferior | 39 | 27±3.3 (20-35) | 20.1±3.8 (13-28) | <0.001 | 6.9±2.7 (3-14)c,e,f |
| Lateral | 13 | 23.6 ±1.8 (21-27) | 19.9±2.1 (16-23) | 0.002 | 3.7±1.6 (2-6)b,d,e |
According to the Bonferroni adjustment, P<0.0125 was significant. Intergroup comparisons of preoperative and postoperative Hertel values (Kruskal-Wallis test). aP=0.001, medial-inferior vs medial-lateral groups; bP=0.001, medial-inferior vs lateral groups; cP=0.030, medial-lateral vs medial-lateral-inferior groups; dP=0.003, medial-lateral vs lateral groups; eP<0.001, medial-lateral-inferior vs lateral groups; fP=0.09, medial-inferior vs medial-lateral-inferior groups. gIntragroup comparisons of preoperative and postoperative Hertel values (Wilcoxon signed rank test).
The groups were also evaluated in terms of new-onset diplopia in the primary gaze and new-onset gaze-evoked diplopia after surgery (Tables 5 and 6). Gaze-evoked diplopia emerged postoperatively in different gaze positions defined as new onset gaze-evoked diplopia. The patients who underwent different types of surgery for each of their eyes were not included in the evaluation. Of the patients with new-onset diplopia in the primary gaze, one having medial-inferior wall decompression, four having medial-lateral wall decompression, and two having medial-lateral-inferior wall decompression underwent strabismus surgery, and one having medial-lateral wall decompression and two having medial-lateral-inferior wall decompression were treated with prismatic glasses. The preoperative diplopia in the primary gaze regressed in one patient who underwent medial-inferior wall decompression, one patient who underwent medial-lateral-inferior wall decompression and two patients who underwent medial-lateral wall decompression. Since the numbers of these patients were limited, no statistical evaluation was conducted.
Table 5. Comparison of the new-onset diplopia in the primary gaze rates.
| Surgical technique | Patients without preoperative diplopia in primary gaze | New-onset diplopia in primary gaze |
| Medial-inferior | 6 | 1/6 (16.7%)b,c |
| Medial-lateral | 85 | 5/85 (5.8%)a,c |
| Medial-lateral-inferior | 16 | 4/16 (25%)a,b |
| Lateral | 6 | 0 |
Intergroup comparisons of new-onset diplopia in the primary gaze, Likelihood ratio test (Chi-squared). aP=0.031, medial-lateral vs medial-lateral-inferior groups; bP=0.6, medial-inferior vs medial-lateral-inferior groups; cP=0.37, medial-inferior vs medial-lateral groups.
Table 6. Comparison of new-onset gaze-evoked diplopia rates.
| Surgical technique | Patients without preoperative gaze-evoked diplopia | New-onset gaze-evoked diplopia |
| Medial-inferior | 6 | 1/6 (16.6%)b,c |
| Medial-lateral | 83 | 13/83 (15.6%)a,c |
| Medial-lateral-inferior | 15 | 3/15 (20%)a,b |
Intergroup comparisons of new-onset diplopia in the primary gaze. Likelihood ratio test (Chi-squared). aP=0.44, medial-lateral and medial-lateral-inferior groups; bP=0.73, medial-inferior and medial-lateral-inferior groups; cP=0.93, medial-inferior and medial-lateral groups.
The effects of the different types of surgery on the VA in the DON cases are shown in Table 7. The VA improvement did not significantly differ between the groups. Of the two eyes having only light sensation, a VA of finger counting and 0.6 (Snellen chart) were achieved after the three-wall decompression surgery (Figure 1). Of the two patients without light sensation, finger counting levels were achieved after the three-wall decompression surgery (Figure 2). The relative afferent pupillary defect (RAPD) did not improve and color vision remained at 0/20 in all but one case having a VA of 0.6 (Snellen chart) after the operation.
Table 7. Preoperative and postoperative VA values (logMAR) of the DON patients.
| Group | No. of eyes | Preoperative | Postoperative | P | Difference |
| 0.181a | |||||
| Medial-inferior | 5 | 0.74±1.30 | 0.08±0.18 | 0.260b | -0.66±1.13 |
| Medial-lateral | 59 | 0.52±0.70 | 0.19±0.38 | <0.001c | -0.33±0.53 |
| Medial-lateral-inferior | 25 | 0.78±0.79 | 0.18±0.42 | <0.001c | -0.60±0.60 |
| Lateral | 2 | 0.18±0.02 | 0.00 | 0.310b | -0.17±0.01 |
VA: Visual acuity; DON: Dysthyroid optic neuropathy. aIntergroup comparisons of VA improvement, one-way ANOVA. According to the Bonferroni adjustment, P<0.025 was significant; bWilcoxon Signed Ranks test; cIntragroup comparisons of preoperative and postoperative VA values (logMAR), Paired samples t-test. According to the Bonferroni adjustment, P<0.00625 was significant.
Figure 1. Patient with GO.
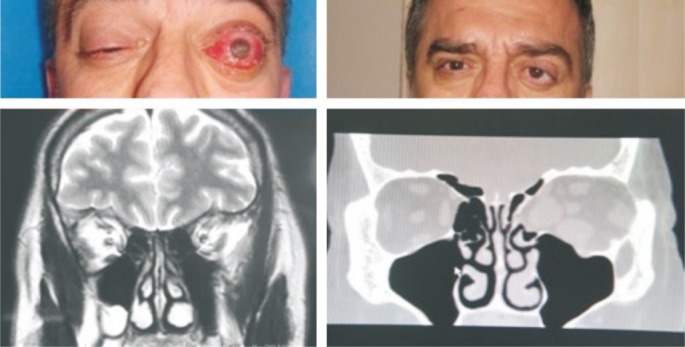
Right eye VA=10/10, left eye VA=light perception (+) (Snellen chart), left unilateral exposure keratopathy with DON. Left eye VA=6/10 (Snellen chart) after medial-inferior-lateral decompression surgery.
Figure 2. Patient with GO.
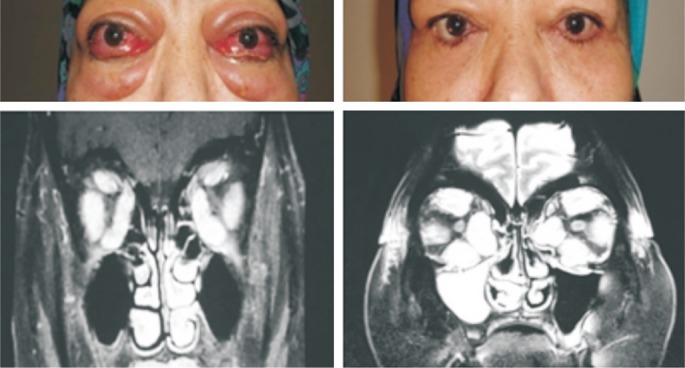
Right eye VA= finger counting, left eye VA=light perception (-), bilateral DON (+). Right eye VA=6/10 (Snellen chart), left eye VA=finger counting after bilateral medial-inferior-lateral decompression surgery.
Only one patient who underwent medial-lateral-inferior wall decompression showed intraorbital vision-threatening hemorrhaging on the first day after surgery. Surgical drainage was performed and the patient did not have any other surgery-related complications. In the DON cases, systemic steroid treatment was necessary in 5 eyes after medial-inferior-lateral wall decompression and in 4 eyes after 2-wall decompression due to the features of persistent optic neuropathy. Six eyes responded to additional intravenous steroid treatment. The three eyes that underwent medial and lateral wall decompression necessitated further decompression within 3-9mo after initial surgery to improve optic nerve functions and underwent inferior wall decompression with additional orbital fat removal. Visual improvement was achieved in all these cases after second surgery and remained stable during the follow-up period.
DISCUSSION
In the current study, the indication for surgery was DON in 91 of 248 eyes (36.6%). DON was determined in 25 of 39 eyes that underwent medial, lateral, and inferior wall decompression (64.1%). This rate was 32.6% (59 of 181 eyes), 33.3% (5 of 15 eyes), and 15.3% (2 of 13 eyes) in medial-lateral, medial-inferior, and lateral only group, respectively. Although our findings were partly consistent with the literature[16]–[17], it appeared that the medial-lateral-inferior wall decompression was performed more frequently in the presence of DON in our practice. Medial and lateral wall decompression was performed for cosmetic reasons in 122 of 181 eyes (67.4%), and this rate was 84.6% (11 of 13 eyes) in the lateral wall group. In parallel with the recent tendency, a large number of cases in the present study underwent decompression surgery for cosmetic reasons[16]–[17]. Balanced medial and lateral wall or only lateral wall decompression were the preferred techniques of orbital decompression for cosmesis.
In the present study, four different types of surgery caused significant decrease in the Hertel values. The medial-inferior wall decompression and medial-lateral-inferior wall decompression exhibited similar decreases in the Hertel values (P=0.09). Overall, the mean decreases in the Hertel values were 7.8 mm after the medial-inferior wall decompression, 6.9 mm after the medial-lateral-inferior wall decompression, 5.7 mm after the medial-lateral wall decompression and 3.7 mm after the lateral wall decompression alone. According to retrospective studies, the decompression of the lateral wall only, inferior and medial walls, lateral and medial walls, and three walls brought about regressions in proptosis of 2-3 mm, 4-6 mm, 4-5 mm, and 8 mm, respectively[17]–[21]. The decompression of four walls can achieve a regression of 12-14 mm[18]–[19]. The degree of retroplacement achieved by medial-lateral wall decompression was similar to that reported in the literature[17],[20]–[21]. However, the medial-inferior wall decompression yielded a slightly higher degree of retroplacement when compared to that reported in the literature[18]–[19],[22]–[27]. The reason of this difference may be the limited number of eyes included in this group. In the present study, the degree of retroplacement was slightly lower than in the literature in the three-wall decompression surgery due to its more frequent use in the presence of DON and more marked fibrotic process in the orbit in patients with DON[14],[28]. Consistent with this finding, it has been reported that the mean amount of retroplacement was 6.4 mm after a three-wall orbital decompression in GO patients with optic nerve involvement[29]. Lateral wall decompression alone produced a mean retroplacement of 3.7 mm in the present study, which is compatible with that in the literature[30]–[34].
The rate of diplopia in the primary gaze after medial-inferior wall decompression surgery was 16.7%, which is similar to that reported in the literature[23]–[24]. Cruz et al[27] reported that the rate of new-onset diplopia after transconjunctival inferomedial wall decompression, without the protection of inferomedial orbital support (orbital strut), was 13%. Eing et al[35] reported that the rate of diplopia appearing after transconjunctival inferomedial wall decompression was 14%. In fact the rate of diplopia appearing after inferomedial wall decompression ranges from 13% to 84%[22],[27],[35]–[36]. However, the rate of newly emerging diplopia after transconjunctival inferomedial wall decompression combined with lateral wall decompression was reported to be 1.8%-12.5% in the studies by Paridaens et al[25] and Bailey et al[26]. Both studies emphasized that the preservation of the ethmoid and maxillary junction, considered to be inferomedial orbital support (orbital strut), caused a decrease in the diplopia rate[25]–[26]. This bony structure prevents the inferomedial displacement of the globe and decreases both the hypoglobus rate and iatrogenic diplopia[25]–[26].
Although we protected the orbital strut during surgery, our rate of diplopia in the primary gaze after medial-lateral-inferior decompression surgery was 25%, which was higher than in the literature. This high rate of diplopia might have been due to the preference for three-wall decompression, especially in the presence of DON, since it is known that ocular motility problems frequently appear in patients with DON[14]–[15]. Mainville et al[37] reported higher rates of new-onset diplopia in patients with open periorbital tissue, another cause of our high rates could be the opening of the periorbital tissue in all the eyes. A different surgical method called the orbital sling procedure was defined in 2002[38]. In this procedure, the periorbital section supporting the medial rectus muscle was kept intact, and the rate of diplopia appearing after this procedure was reported from 0 to 7.7%[38]–[39]. In the present study, the orbital sling procedure was not applied in any of our patients.
Balanced medial-lateral wall decompression is directed to prevent the extraorbital muscle imbalance and diplopia caused by a single or asymmetrical wall excision[17]. There have been several studies emphasizing the low incidence of postoperative diplopia with balanced orbital decompression[17],[20]–[21]. The rate of diplopia after medial-lateral wall decompression has been reported to range from 0 to 33%, and the study revealing the rate of 33% included nine patients[20]–[21],[40]–[44]. In the present study, 5.8% of the cases undergoing medial-lateral wall decompression developed diplopia in the primary gaze. When compared to the medial-inferior and medial-lateral-inferior wall decompression, the medial-lateral wall decompression was found to cause a lower rate of diplopia.
Even though it was not statistically significant, the rate of new-onset gaze-evoked diplopia was lower in the medial-lateral wall decompression when compared with the other surgical techniques (medial-inferior and medial-lateral-inferior decompression).
Decompression surgery in the presence of DON achieved a rapid resolution in addition to its acceptable side-effect profile. It is thought that decompression surgery exerts its effects by eliminating persistent congestion by creating newly space for congested and inflamed orbital soft tissue.
It has been shown in the literature that decompression surgery using different techniques can provide an improvement in visual function by 70%-95% in patients with DON[22]–[24],[28],[30],[45]–[48]. However, it is emphasized that relapses in optic neuropathy can appear after decompression, and that additional medical and/or surgical management may be needed[45]. Similarly, in the current study, three eyes required further decompression within 3-9mo after initial surgery to improve optic nerve functions and 9 eyes required additional steroid therapy.
In the current study, medial-lateral wall decompression and medial-lateral-inferior wall decompression provided significant increases in the VA in DON cases. The VA also increased in those cases undergoing medial-inferior wall decompression and in the lateral group, although not significantly. This finding can be explained by the small number of patients in these groups. Although the VA showed similar improvement between the groups, it was clinically more marked in the medial-lateral-inferior wall decompression group. This implies that the three-wall decompression should be preferred, especially in cases with very low VA. Of the two cases having the sense of light upon admission, medial-lateral-inferior wall decompression provide a VA of 0.6 (Snellen chart) and counting finger levels after surgery.
Of the two patients without the sense of light before the medial-inferior-lateral decompression surgery, VA of counting finger levels was achieved after surgery. Therefore, it is suggested that those patients without the sense of light should also be given a chance for improvement. It has been stated in the literature that the presence of preoperative optic atrophy is not a predictor of the improvement in the VA[24], which seems to be supported by the findings of the present study.
The most important limitation of the present study was that it had a retrospective design. In addition, the heterogeneous distribution of the cases in the groups did not allow for a clear statistical evaluation of some of the findings. However, the results of the study show that medial-lateral wall decompression, medial-inferior wall decompression, and medial-lateral-inferior wall decompression are effective and reliable treatment options with regard to the regression of the Hertel values and correction of visual function in GO patients. Overall, medial-lateral wall decompression caused a lower rate of diplopia in the primary gaze. Nevertheless, prospective, randomized studies that will allow a more reliable comparison of the different surgical techniques with regard to the Hertel values, visual function, and postoperative incidence of diplopia are needed.
Acknowledgments
Conflicts of Interest: Cubuk MO, None; Konuk O, None; Unal M, None.
REFERENCES
- 1.Novaes P, Diniz Grisolia AB, Smith TJ. Update on thyroid-associated Ophthalmopathy with a special emphasis on the ocular surface. Clinical Diabetes Endocrinology. 2016;2:19. doi: 10.1186/s40842-016-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiler DL. Thyroid eye disease: a review. Clin Exp Optom. 2017;100(1):20–25. doi: 10.1111/cxo.12472. [DOI] [PubMed] [Google Scholar]
- 3.Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, Pariani N, Gallo D, Azzolini C, Ferrario M, Bartalena L. Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed Graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98(4):1443–1449. doi: 10.1210/jc.2012-3873. [DOI] [PubMed] [Google Scholar]
- 4.Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. The incidence of Graves' ophthalmopathy in Olmsted County, Minnesota. Am J Ophthalmol. 1995;120(4):511–517. doi: 10.1016/s0002-9394(14)72666-2. [DOI] [PubMed] [Google Scholar]
- 5.Li HX, Xiang N, Hu WK, Jiao XL. Relation between therapy options for Graves' disease and the course of Graves' ophthalmopathy: a systematic review and meta-analysis. J Endocrinol Invest. 2016;39(11):1225–1233. doi: 10.1007/s40618-016-0484-y. [DOI] [PubMed] [Google Scholar]
- 6.Benzimra JD, Quinn AG, Kersey T, McGrane D, Goss L, Vaidya B. Management of patients in a combined thyroid eye clinic in secondary care. Int Ophthalmol. 2014;34(1):1–6. doi: 10.1007/s10792-013-9768-9. [DOI] [PubMed] [Google Scholar]
- 7.Kendler DL, Lippa J, Rootman J. The initial clinical characteristics of Graves' orbitopathy vary with age and sex. Arch Ophthalmol. 1993;111(2):1997–2001. doi: 10.1001/archopht.1993.01090020051022. [DOI] [PubMed] [Google Scholar]
- 8.Migliori ME, Gladstone GJ. Determination of the normal range of exophthalmometric values for black and white adults. Am J Ophthalmol. 1984;98:438–442. doi: 10.1016/0002-9394(84)90127-2. [DOI] [PubMed] [Google Scholar]
- 9.Vargason CW, Chelnis JG, Barahimi BI, Mawn LA. Socioeconomic disparities in the presentation and treatment of Graves' disease and thyroid eye disease. Semin Ophthalmol. 2016;31(4):409–414. doi: 10.1080/08820538.2016.1185322. [DOI] [PubMed] [Google Scholar]
- 10.McAlinden C. An overview of thyroid eye disease. Eye Vis (Lond) 2014;1:9. doi: 10.1186/s40662-014-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dollinger J. Die Druckentlastung der Augenhöhle durch Entfernung der äußeren Orbitalwand bei hochgradigem Exophthalmus (Morbus Basedowii) und konsekutiver Hornhauterkrankung DMW-Deutsche Medizinische Wochenschrift. Dtsch Med Wochenschr. 1911;37(4):1888–1890. [Google Scholar]
- 12.Braun TL, Bhadkamkar MA, Jubbal KT, Weber AC, Marx DP. Orbital decompression for thyroid eye disease. Semin Plast Surg. 2017;31(1):40–45. doi: 10.1055/s-0037-1598192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Victores AJ, Takashima M. Thyroid eye disease: optic neuropathy and orbital decompression. Int Ophthalmol Clin. 2016;56(1):69–79. doi: 10.1097/IIO.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 14.McKeag D, Lane C, Lazarus JH, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves' Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91(4):455–458. doi: 10.1136/bjo.2006.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neigel JM, Rootman J, Belkin RI, Nugent RA, Drance SM, Beattie CW, Spinelli JA. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988;95(11):1515–1521. doi: 10.1016/s0161-6420(88)32978-7. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein A, Schittkowski M, Esser J. Surgical treatment of Graves' ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):339–358. doi: 10.1016/j.beem.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Fichter N, Guthoff RF, Schittkowski MP. Orbital decompression in thyroid eye disease. ISRN Ophthalmol. 2012;2012:739236. doi: 10.5402/2012/739236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCord CD., Jr Current trends in orbital decompression. Ophthalmology. 1985;92(1):21–33. doi: 10.1016/s0161-6420(85)34079-4. [DOI] [PubMed] [Google Scholar]
- 19.Kennerdell JS, Maroon JC. An orbital decompression for severe dysthyroid exophthalmos. Ophthalmology. 1982;89(5):467–472. doi: 10.1016/s0161-6420(82)34776-4. [DOI] [PubMed] [Google Scholar]
- 20.Choi SU, Kim KW, Lee JK. Surgical outcomes of balanced deep lateral and medial orbital wall decompression in Korean population: clinical and computed tomography-based analysis. Korean J Ophthalmol. 2016;30(2):85–91. doi: 10.3341/kjo.2016.30.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham SM, Brown CL, Carter KD, Song A, Nerad JA. Medial and lateral orbital wall surgery for balanced decompression in thyroid eye disease. Laryngoscope. 2003;113(7):1206–1209. doi: 10.1097/00005537-200307000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Garrity JA, Fatourechi V, Bergstralh EJ, Bartley GB, Beatty CW, DeSanto LW, Gorman CA. Results of transantral orbital decompression in 428 patients with severe Graves' ophthalmopathy. Am J Ophthalmol. 1993;116(5):533–547. doi: 10.1016/s0002-9394(14)73194-0. [DOI] [PubMed] [Google Scholar]
- 23.Carter KD, Frueh BR, Hessburg TP, Musch DC. Long-term efficacy of orbital decompression for compressive optic neuropathy of Graves' eye disease. Ophthalmology. 1991;98(9):1435–1442. doi: 10.1016/s0161-6420(91)32115-8. [DOI] [PubMed] [Google Scholar]
- 24.Soares-Welch CV, Fatourechi V, Bartley GB, Beatty CW, Gorman CA, Bahn RS, Bergstralh EJ, Schleck CD, Garrity JA. Optic neuropathy of Graves' disease: results of transantral orbital decompression and long-term follow-up in 215 patients. Am J Ophthamol. 2003;136(3):433–441. doi: 10.1016/s0002-9394(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 25.Paridaens DA, Verhoeff K, Bouwens D, van Den Bosch WA. Transconjunctival orbital decompression in Graves' ophthalmopathy: lateral wall approach ab interno. Br J Ophthalmol. 2000;84(7):775–781. doi: 10.1136/bjo.84.7.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey KL, Tower RN, Dailey RA. Customized, single-incision, three-wall orbital decompression. Ophthalmic Plast Redonstr Surg. 2005;21(1):1–9. discussion 9–10. doi: 10.1097/01.iop.0000150410.30992.c3. [DOI] [PubMed] [Google Scholar]
- 27.Cruz AA, Leme VR. Orbital decompression: a comparison between trans-fornix/transcaruncular inferomedial and coronal inferomedial plus lateral approaches. Ophthalmic Plast Redonstr Surg. 2003;19(6):440–445. doi: 10.1097/01.IOP.0000092796.43025.B1. [DOI] [PubMed] [Google Scholar]
- 28.Perry JD, Kadakia A, Foster JA. Transcaruncular orbital decompression for dysthyroid optic neuropathy. Ophthalmic Plast Redonstr Surg. 2003;19(5):353–358. doi: 10.1097/01.IOP.0000083645.19368.99. [DOI] [PubMed] [Google Scholar]
- 29.Lipski A, Eckstein A, Esser J, Loesch C, Mann K, Mohr C, Jurklies B. Course of pattern-reversed visual evoked cortical potentials in 30 eyes after bony orbital decompression in dysthyroid optic neuropathy. Br J Ophthalmol. 2011;95(2):222–226. doi: 10.1136/bjo.2009.173658. [DOI] [PubMed] [Google Scholar]
- 30.Choe CH, Cho RI, Elner VM. Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthalmic Plast Redonstr Surg. 2011;27(1):4–11. doi: 10.1097/IOP.0b013e3181df6a87. [DOI] [PubMed] [Google Scholar]
- 31.Mehta P, Durrani OM. Outcome of deep lateral wall rim-sparing orbital decompression in thyroid-associated orbitopathy: a new technique and results of a case series. Orbit. 2011;30(6):265–268. doi: 10.3109/01676830.2011.603456. [DOI] [PubMed] [Google Scholar]
- 32.Chang EL, Piva AP. Temporal fossa orbital decompression for treatment of disfiguring thyroid-related orbitopathy. Ophthalmology. 2008;115(9):1613–1619. doi: 10.1016/j.ophtha.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Liao SL, Shih MJ, Chang TC, Lin LL. Transforniceal lateral deep bone decompression-a modified technique to prevent postoperative diplopia in patients with disfiguring exophthalmos due to dysthyroid orbitopathy. J Formos Med Assoc. 2006;105(8):611–616. doi: 10.1016/S0929-6646(09)60159-5. [DOI] [PubMed] [Google Scholar]
- 34.Ben Simon GJ, Wang L, McCann JD, Goldberg RA. Primary-gaze diplopia in patients with thyroid-related orbitopathy undergoing deep lateral orbital decompression with intraconal fat debulking: a retrospective analysis of treatment outcome. Thyroid. 2004;14(5):379–383. doi: 10.1089/105072504774193221. [DOI] [PubMed] [Google Scholar]
- 35.Eing F, Abbud CM, Velasco Cruz AA. Cosmetic orbital inferomedial decompression: quantifying the risk of diplopia associated with extraocular muscle dimensions. Ophthalmic Plast Redonstr Surg. 2012;28(3):204–207. doi: 10.1097/IOP.0b013e31824dd8a0. [DOI] [PubMed] [Google Scholar]
- 36.Boboridis KG, Uddin J, Mikropoulos DG, Bunce C, Mangouritsas G, Voudouragkaki IC, Konstas AG. Critical appraisal on orbital decompression for thyroid eye disease: a systematic review and literature search. Adv Ther. 2015;32(7):595–611. doi: 10.1007/s12325-015-0228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mainville NP, Jordan DR. Effect of orbital decompression on diplopia in thyroid-related orbitopathy. Ophthalmic Plast Redonstr Surg. 2014;30(2):137–140. doi: 10.1097/IOP.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 38.Metson R, Samaha M. Reduction of diplopia following endoscopic orbital decompression: the orbital sling technique. Laryngoscope. 2002;112(10):1753–1757. doi: 10.1097/00005537-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Jimenez-Chobillon MA, Lopez-Oliver RD. Transnasal endoscopic approach in the treatment of Graves' ophthalmopathy: the value of a medial periorbital strip. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(3):97–103. doi: 10.1016/j.anorl.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Sellari-Franceschini S, Berrettini S, Santoro A, Nardi M, Mazzeo S, Bartalena L, Mazzi B, Tanda ML, Marcocci C, Pinchera A. Orbital decompression in Graves' ophthalmopathy by medial and lateral wall removal. Otolaryngol Head Neck Surg. 2005;133(2):185–189. doi: 10.1016/j.otohns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg RA, Perry JD, Hortaleza V, Tong JT. Strabismus after balanced medial plus lateral wall versus lateral wall only orbital decompression on for dysthyroid orbitopathy. Ophthalmic Plast Redonstr Surg. 2000;16(4):271–277. doi: 10.1097/00002341-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Leone CR, Jr, Piest KL, Newman RJ. Medial and lateral wall decompression for thyroid ophthalmopathy. Am J Ophthalmol. 1989;108(2):160–166. doi: 10.1016/0002-9394(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 43.Shepard KG, Levin PS, Terris DJ. Balanced orbital decompression for Graves' ophthalmopathy. Laryngoscope. 1998;108(11 Pt 1):1648–1653. doi: 10.1097/00005537-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Unal M, Leri F, Konuk O, Hasanreisoğlu B. Balanced orbital decompression combined with fat removal in Graves' ophthalmopathy: do we really need to remove the third wall? Ophthalmic Plast Redonstr Surg. 2003;19(2):112–118. doi: 10.1097/01.IOP.0000056145.71641.F5. [DOI] [PubMed] [Google Scholar]
- 45.Chu EA, Miller NR, Lane AP. Selective endoscopic decompression of orbital apex for dysthyroid optic neuropathy. Laryngoscope. 2009;119(6):1236–1240. doi: 10.1002/lary.20240. [DOI] [PubMed] [Google Scholar]
- 46.Fatourechi V, Bartley GB, Garrity JA, Bergstralh EJ, Ebersold MJ, Gorman CA. Transfrontal orbital decompression after failure of transantral decompression in optic neuropathy of Graves' disease. Mayo Clin Proc. 1993;68(6):552–555. doi: 10.1016/s0025-6196(12)60368-1. [DOI] [PubMed] [Google Scholar]
- 47.Ben Simon GJ, Syed HM, Douglas R, Scbwartz R, Goldberg RA, McCann JD. Clinical manifestations and treatment outcome of optic neuropathy in thyroid-related orbitopathy. Ophthal Surg Las Im. 2006;37:284–290. doi: 10.3928/15428877-20060701-04. [DOI] [PubMed] [Google Scholar]
- 48.Korkmaz S, Konuk O. Surgical treatment of dysthyroid optic neuropathy: long-term visual outcomes with comparison of 2-wall versus 3-wall orbital decompression. Curr Eye Res. 2016;41(2):159–164. doi: 10.3109/02713683.2015.1008641. [DOI] [PubMed] [Google Scholar]


