Abstract
AIM
To investigate the effect of interleukin-8 (IL-8) on neural retinal ganglion cells (RGCs) and whether it can be alleviated by G31P.
METHODS
RGC-5 cells were exposed to IL-8 with or without its specific receptor antagonist G31P for 24h, and the cell viability was assessed by Cell Counting Kit 8 (CCK-8). Apoptosis was measured by examining nuclear morphology and quantifying with flow cytometry. Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) and Western blot were used to investigate the expression of apoptosis-related genes.
RESULTS
CCK-8 assay showed that IL-8 significantly inhibits the viability of RGC-5 cells in a dose-dependent manner. Cell apoptosis assays exhibited higher apoptotic rate in IL-8 treatment group compared to control group. We further found that IL-8 could promote Bax and caspase-3 expressions, but decrease the level of Bcl-2 in the aspect of mRNA and protein. However, pre-treatment with G31P partly attenuated these effects in RGC-5 cells (P<0.05).
CONCLUSION
These results indicate that anti-proliferation effects of IL-8 through induction of cell apoptosis regulated by Bcl-2, Bax and caspase-3 expressions, can be ameliorated by G31P.
Keywords: glaucoma, inflammation, interleukin-8, retinal ganglion cell-5, apoptosis, G31P
INTRODUCTION
Glaucoma refers to a complex multivariate ocular disease, considered as a degenerative and progressive optic neuropathy that causes damage to the optic nerve and retinal ganglion cells (RGCs)[1]–[2]. Recent studies showed the prevalence of glaucoma globally is about 64.3 million, reaching 76.0 million in 2020 and 111.8 million in 2040[3]. Consist with the rapid development of the world, the incidence of glaucoma has been increasing continually. Glaucoma is initiated by several risk factors, including increased intraocular pressure, oxidative stress, mitochondrial dysfunction and glutamate neurotoxicity[4]–[8]. These factors lead to nerve cells loss, the common pathway for damage of optic nerve and visual field. It is urgent to find more potential injury mechanisms and protective methods because nerve cells are the sole critical output way in the retina[9]. In recent studies, the increased levels of antibodies and activation of glial cells in glaucoma patients, and antibody deposit in the retina revealed the involvement of the immune system in the pathology of glaucoma[10]–[11].
Cytokines are synthesized and secreted peptides or glycoproteins which main function is modulating immune reactions, but they can also participate in angiogenesis, cell proliferation and tumor metastasis[12]. Interleukin-8 (IL-8, also called CXCL8), a proinflammatory chemokine, functions as neutrophil chemotaxis and mediates lymphocyte infiltration[13]–[15]. It is also closely implicated in both the development and progression of various cancer. Additionally, previous research has shown that IL-8 is toxic to cultured neurons[16], and increased levels of IL-8 are detected in glaucomatous aqueous humors (AH) and patients with retinal detachment, proliferative vitreous retinopathy and proliferative diabetic retinopathy[17]–[18]. IL-8 usually exerts its function via binding to chemokine (C-X-C) receptor1 (CXCR1) and CXCR2, important therapeutic targets in many solid tumors and inflammatory diseases[19]–[20]. These receptors have been reported to be expressed in both human and rabbit retina[21].
We have reported before that G31P, an analogue of IL-8, is a selective CXCR1 and CXCR2 antagonist which includes Arg and Pro replacement for Lys11 and Gly31 respectively of human CXCL8 (3-72). It has also been shown that G31P can competitively block the binding of ELR-CXC chemokines to both receptors[22]. Based on this background, we chose RGC-5 cells which express tau, βIII-tubulin to simulate neurocyte[23], evaluated the impact of IL-8 on neural cells and whether the antagonist G31P can attenuate its effect, in order to explore alternative therapy in glaucoma and other nerve diseases.
MATERIALS AND METHODS
Main Reagents
The IL-8 used for the study was obtained from Peprotech (American), the culture media; Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM-F12) medium from Gibco, and fetal bovine serum (FBS) from PAN-Biotech. The RGC-5 cell was purchased from Shanghai Yubo Biological Science and Technology Company. Penicillin/streptomycin and trypsin were from Transgen (Beijing, China) and Gibco respectively. The antibodies used were mouse anti-β-actin (Bioworld, Beijing, China), mouse anti-Bax and Bcl-2 antibodies (Abcam), and mouse anti-caspase-3 (Cell Signaling Technology). The G31P used was prepared as described previously[22],[24].
Cell Culture
RGC-5 was cultured in complete DMEM-F12 medium containing 10% FBS and 1% penicillin/streptomycin at 37°C in humidified incubator containing 5% CO2. The cells were divided into three groups: control, IL-8, and G31P pretreatment. The concentration of G31P used was 100 ng/mL.
Cell Proliferation Assay
Cell Counting Kit 8 (CCK-8; Transgen, Beijing) was used in to determine the cell viability of RGCs. The cells were seeded at 5×103/well into 96-well plate and cultured for 12h in 10% FBS containing DMEM-F12 growth medium. Cells were then treated with IL-8 at different concentrations in 100 µL of basic DMEM-F12 medium. The control wells also received equivalent volume of the media without IL-8. After 24h of incubation, 10 µL CCK-8 reagent was added to each well, and incubated for additional 4h. Absorbance of each well was determined at 450 nm. The absorbance obtained were proportional to the number of viable cells. The experiments were repeated trice to confirm the reproducibility.
Hoechst 33258 Staining
To determine nuclear morphology by Hoechst 33258 staining, 5×103/well cells were plated on sterile cover glass which were placed in the 6-well plates before treatment. After stimulation for 24h, cells were fixed for 10min, rinsed twice in phosphate buffered saline (PBS) and incubated with 0.5 mL Hoechst 33258 in the dark for 5min at room temperature, and then washed with PBS again. Stained nuclei was observed under a fluorescent microscope (BX-51, TR32000 Olympus, Japan).
Annexin V-FITC/Propidium Iodide Assay
To investigate the effect of IL-8 on cell apoptosis in RGC-5 cells, a flow cytometry assay was performed. Briefly, 1×106 cells were harvested and washed twice with PBS. Cells were then resuspended in 500 µL 1×binding buffer and stained with AnnexinV-FITC and Propidium iodide (PI) for 30min in the dark. The proportion of cell distribution in each quadrant was analyzed using Accuri C6 software. The percentage of apoptotic cells was considered as whole stages of apoptotic cells presented in Lower Right (Annexin V-FITC+/PI−)+Upper Right (Annexin V-FITC+/PI+) of the histograms. Statistical analyses of flow cytometry were conducted by calculating the number of Annexin V positive cells. The experiments were performed three times with similar outcome.
Reverse Transcription Quantitative Real-time Polymerase Chain Reaction
Cells in each group were extracted for the total RNA using Trizol (Takara, Dalian, China) following the manufacture's protocols. cDNA was synthesized from 3 µL total RNA with oligo (dT) primer in reverse transcription system. Polymerase chain reaction (PCR) reaction was performed with the following primers: CXCR1 5′-AAGTCCTGGGTGAAGCCACAA-3′ (forward) and 5′-AGCCCGTAGCAGACCAGCATA-3′ (reverse); CXCR2 5′-CCTTGAATGCTACGGAGATT-3′ (forward) and 5′-AGGGTAGTAGAGGTGTTTGC-3′ (reverse). Quantitative real-time polymerase chain reaction (qPCR) was performed following the manufacture's instructions with the following primers: Bax 5′-CAGGATGCGTCCACCAAGAA-3′ (forward) and 5′-CGTGTCCACGTCAGCAATCA-3′ (reverse); Bcl-2 5′-AACATCGCCCTGTGGATGAC-3′ (forward) and 5′-GGTCTTCAGAGACAGCCAGGAG-3′ (reverse); caspase-3 5′-GGCCTGAAATACCAAGTCAGGAA-3′ (forward) and 5′-CCATGGCTTAGAATCACACACACA-3′ (reverse). qPCR mRNA expressions were normalized to the levels of house-keeping gene β-actin.
Western Blot Analyses
Cell cultures were treated as described above, washed two times with cold PBS and total protein was extracted from cells by lysis buffer in the presence of protease inhibitors. We employed the Bradford method to measure the protein concentration in each group. Equal amounts of denatured protein were resolved by 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membrane which was then incubated with 5% non-fat milk for 1h. The membranes were probed with the primary antibodies against Bax, Bcl-2 (Abcam, USA), or caspase-3 (Cell Signaling Technology, USA) at 4°C overnight. β-actin (Bioworld Technnology, Nanjing, China) was used as a loading control. After washed, membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Abbkine, USA) for 2h at room temperature. Immunoblotting products were visualized with an ECL Plus Western Blotting Detection kit (KeyGen, Nanjing, China). Bands intensities were quantified using ImageJ software. For quantitative assays, the gray values of target protein bands were normalized to the β-actin protein and compared with the control. The experiments were performed in triplicate.
Statistical Analysis
All data are presented as mean value± standard error of the mean (SEM). To assess statistical significance, analysis of variance (ANOVA) was used to analyze variance among groups. SPSS was used for the analysis of data, and P value less than 0.05 was statistically significant.
RESULTS
Effect of IL-8 on the Expression of CXCR1/2 and Cell Viability in RGC-5
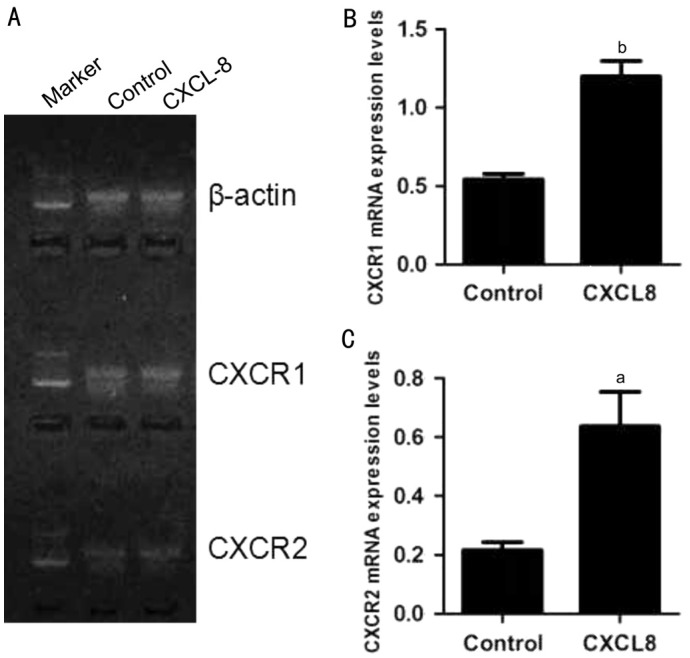
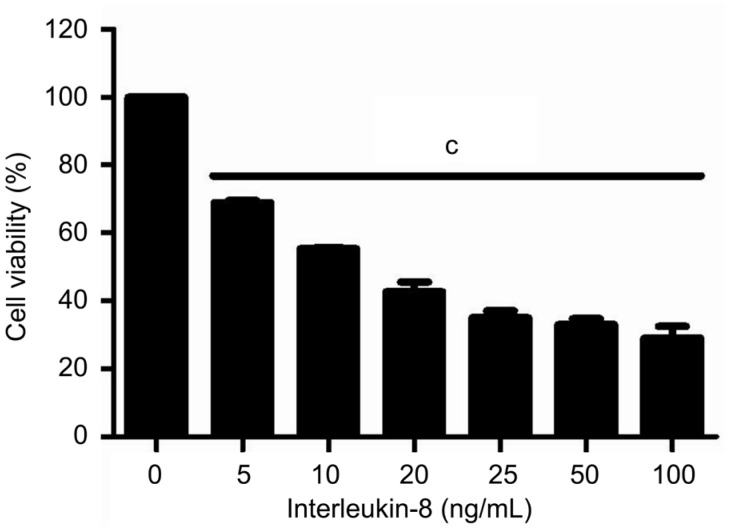
At the beginning of the experiments, we assessed CXCR1/2 mRNA expression levels in RGC-5 cells by reverse transcription polymerase chain reaction (RT-PCR). Our results showed that RGC-5 cells express CXCR1/2 either under normal condition or exposed to IL-8 for 24h. Additionally, IL-8 could increase both CXCR1 (1.2±0.1 vs 0.54±0.03, P<0.01) and CXCR2 (0.64±0.12 vs 0.22±0.03, P<0.05) mRNA levels by about three-fold (Figure 1). Following the presence of these receptors, we confirmed IL-8 could promote the expression of CXCR1/2 in RGC-5 cells, and then hypothesized IL-8 antagonists may intercept its associated effects on RGC-5 cells. Subsequently, we assessed the effect of IL-8 on RGC-5 cells viability by using CCK-8 assay. After treatment with different concentrations of IL-8 for 24h, the cell viability was reduced in each group. As shown in Figure 2, CCK-8 assay revealed that 20 ng/mL IL-8 could cause approximately 50% (51.28%±5.5%, P<0.01) decrease in cell viability compared with untreated control. This concentration used in subsequent treatments throughout the study.
Figure 1. Expression of CXCR1/2 in RGC-5.
A: CXCR1 and CXCR2 mRNA were detected in RGC-5 cells by PCR assay. Representative electrophoresis results showed that IL-8 promotes the expression of CXCR1/2; B-C: Quantification data for A were summarized from three independent experiments. All error bars in our pictures represent SEM. aP<0.05, bP<0.01.
Figure 2. Cell viability of RGC-5 treated with varying concentrations of IL-8 for 24h and measured by CCK-8 assay at 450 nm.
Bars are mean±SEM. cP<0.001.
Effect of IL-8 on the Apoptosis in RGC-5
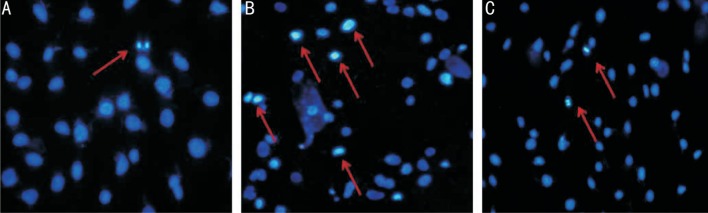
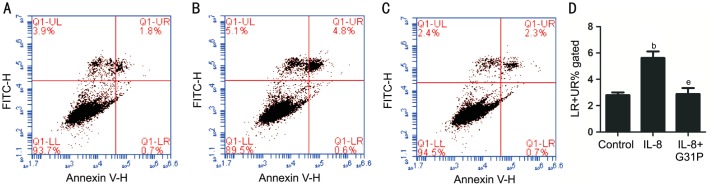
To establish whether IL-8 mediated growth inhibitory activity was associated with apoptosis in RGC-5 cells, we firstly observed changes in the cell nuclei using Hoechst 33258 (Figure 3). Relatively high numbers of apoptotic cells indicated by condensed and fragmented fluorescent nuclei, were observed in IL-8 treated group. However, the number of apoptotic cells was reduced in G31P pre-treated group, while the control cells exhibited almost no apoptotic nuclei. Additionally, we evaluated the effect of G31P on IL-8 induced cell apoptosis by detecting the apoptotic index using Annexin V-FITC/PI staining and flow cytometry. As shown in Figure 4, IL-8 increased the number of apoptotic cells from 2.8%±0.20% to 5.62%±0.50% (P<0.01). In the contrast, G31P group decreased the IL-8 induced apoptotic proportion (2.89%±0.45%, P<0.01). These findings suggested that IL-8 has a damage effect on RGC-5 cells and its specific receptor antagonist G31P could alleviate this condition.
Figure 3. Hoechst staining of RGC-5 (100×).
A: All the nuclei were uniformly weakly stained in control group; B: RGC-5 cells exposed to 20 ng/mL IL-8 for 24h. Apoptotic cells were identified by condensation of chromatin and fragmentation of their nuclei; C: RGC-5 cells exposed to 20 ng/mL IL-8 and pre-treatment with G31P for 24h. The amount of apoptotic cells was decreased with G31P pre-incubation. Three experiments were performed with similar results.
Figure 4. Effect of G31P on IL-8 induced apoptosis.
A-C: Flow cytometry analysis showing the effect of IL-8 and G31P pretreatment on RGC-5 cells. A: Control group; B: IL-8 group; C: IL-8+G31P group; D: The percentage of apoptotic cells by quantitative analysis. (LR+UR)% means the percentage of every period of apoptosis in total sample. bP<0.01 compared with control; eP<0.01 compared with G31P.
Effect of IL-8 on the mRNA and Protein Expression of Bax, Bcl-2 and Caspase-3 in RGC-5
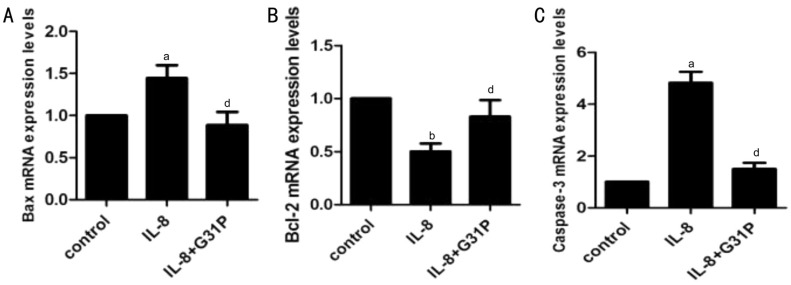
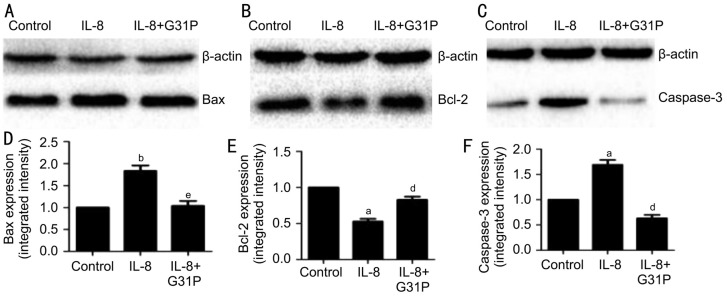
We next investigated selected apoptotic genes affected by IL-8, focusing on Bax, Bcl-2 and caspase-3. The mRNA expression of these genes were analyzed using RT-PCR techniques, and the variations observed has been presented in Figure 5. Treatment with IL-8 alone significantly increased the expression of the pro-apoptotic genes Bax (1.44±0.15, P<0.05), and at the same time increased caspase-3 (4.82±0.43, P<0.05) mRNA level by about five-fold. Additionally, IL-8 treated cells showed a double fold reduction in the anti-apoptotic gene Bcl-2 mRNA level (0.50±0.07, P<0.01). Contrary to these observations, pre-treatment with G31P attenuated the IL-8-mediated decrease in Bcl-2 mRNA expression (0.82±0.16, P<0.05). G31P also attenuated the increase in Bax and caspase-3 mRNAs. The expression of these genes were normalized with respect to β-actin. To confirm the translation of these genes, we further determined their protein expressions by Western blot (Figure 6). Consistent with the mRNA results, Bax and caspase-3 proteins in RGC-5 cells exposed to IL-8 alone were increased both by about two-fold, and Bcl-2 protein was decreased by two-fold. Pre-treatment with G31P attenuated the increase of pro-apoptotic proteins and enhanced the anti-apoptotic protein expression. These results suggested that G31P intercepts the activation of IL-8 induced apoptosis-related genes.
Figure 5. G31P attenuates the effects of IL-8 on Bax, Bcl-2 and caspase-3 mRNAs.
A: Bax; B: Bcl-2; C: Caspase-3. The levels of mRNAs were analyzed by qPCR following treatment of RGC-5 cells with IL-8±G31P, normalized to the levels of β-actin mRNA, and standardized to the control group. aP<0.05 compared with control; bP<0.01 compared with control; dP<0.05 compared with G31P.
Figure 6. G31P attenuates the effects of IL-8 on Bax, Bcl-2 and caspase-3 proteins.
A-C: Representative photographs of the Western blot analysis; D-F: Quantitative data obtained by densitometry. Data were normalized to β-actin protein levels and shown as mean±SEM of three independent experiments. aP<0.05 compared with control; bP<0.01 compared with control; dP<0.05 compared with G31P; eP<0.01 compared with G31P.
DISCUSSION
In this study, for the first time, we demonstrated the specific role of IL-8 on RGC-5 cells survival in vitro. It is well known that IL-8 is an inflammatory chemokine, playing important roles in immune reactions, neutrophil recruitment and tumor growth. But how specific this chemokine affects neuronal ability remains unclear[16].
Although some previous studies have stated IL-8 protects neurons, elevated levels of this chemokine in the brain are usually linked with negative events[25]–[26], particularly in neuroinflammation resulting from in severe traumatic brain injuries. It has been reported that treatment of cortical neurons with IL-8 induces neuronal cell death[16]. RGCs are typically elongated projections which are susceptible to aging and neurodegenerations[27], and can't be rejuvenated adequately during optic neuropathies[28]. They play a critical role in transmitting visual information from retina to the brain[27]. In the present study, we investigated the impact of IL-8 on RGC-5 cells, with some characteristics of RGCs and neural cells which we mostly wanted. The cell viability assay we performed indicated that IL-8 has dose-dependent repressive effects after treated for 24h.
Like Alzheimer's disease, glaucoma is increasingly recognized as neurodegenerative disorder, leading to deficient visual function due to the progressive loss of RGCs[29]. Available study suggested RGCs die during normal development and in diseases impacting the optic nerves via a genetically mediated form of cell death called apoptosis[30]. Emerging evidence obtained from some studies indicate chemokine plays important role in the survival of RGCs. An in vitro experiment has revealed that the survival rate of RGCs decreases in a time-dependent manner after TNF-α exposure[31]. We observed the condensed and fragmented fluorescent nuclei with Hoechst 33258 fluorescence dye presenting the apoptosis induced by IL-8 treatment. The induction of apoptosis by IL-8 was subsequently confirmed by flow cytometry using Annexin V-FITC/PI kit. The data indicated the apoptotic cells were elevated after exposure to IL-8 for 24h, suggesting that IL-8-caused apoptosis contributes to the viability inhibition of RGC-5 cells. These findings were similar to previous research obtained from cultured neurons[16].
The IL-8 induced apoptosis may occur via a great variety of mechanisms, we therefore chose some classic apoptosis-related genes including Bcl-2, Bax and caspase-3, and detected their expressions of mRNAs and proteins. It's well known that Bcl-2 has strong antiapoptotic activity in multifarious cells, and Bax, structurally related to Bcl-2, has the contrary function of promoting cell death[30],[32]. Caspase-3, a member of the cysteine-aspartic acid protease (caspase) family, is essential in the execution-phase of cell apoptosis[33]. Our finding established that IL-8-induces RGC-5 apoptosis by reducing the level of Bcl-2, and enhancing the levels of Bax and caspase-3.
The action of IL-8 is always mediated by the activation of its high-affinity receptors, CXCR1 and CXCR2[34]. Previous research reported the presence of CXCR1 and CXCR2 in neurons and glial cells in the retinas of both human and rabbit. Additionally, CXCR1 and CXCR2 immunoreactions were observed in the nerve fibers of ganglion cells and somata respectively. Moreover, the expression of theses receptors in cultured Müller and glial cells of proliferative vitreoretinopathy suggests an involvement of them in the process of gliosis[21]. We examined the expression of CXCR1 and CXCR2 in RGC-5 cells by RT-PCR. Consistently, RGC-5 cells express both receptors under normal condition. At the same time, we found increased levels of CXCR1 and CXCR2 mRNAs after exposure of IL-8, indicating possible pathological functions of these receptors beyond their well-known role in inflammatory procedure.
Numerous studies have demonstrated the upregulation of IL-8 under suboptimal oxygenation conditions[35]–[36], in patients retinas with proliferative vitreoretinopathy or proliferative diabetic retinopathy[17],[37]. Clinically, IL-8 has been reported to significantly increased in the aqueous humor of pseudophakic glaucomatous eyes and turns to increase with aging[38]. Reseacher found that the concentration of IL-8 was elevated in aqueous humor of glaucomatous eyes eyes[18], especially in asymmetric glaucoma patients with severe visual field defects. Similarly, the report of a positive correlation between IL-8 and intraocular pressure suggests the effect of IL-8 in the active regulation of aqueous humor outflow resistance[24]. Moreover, it has been suggested that IL-8 could be produced by ocular tissue[18]. Strategies to block/neutralize IL-8 may be a vital therapeutic mechanism for optic neuropathies. G31P is an engineered analogue of CXCL8, which effectively antagonizes ELR-CXC chemokines and possesses higher binding affinity for CXCR1 and CXCR2[39]. Our findings demonstrate that pretreatment with G31P attenuates the apoptotic effect of IL-8. Additionally, G31P increased the expression of anti-apoptotic gene Bcl-2 and downregulated the expression of pro-apoptotic genes Bax and caspase-3.
In summary, this report demonstrates that IL-8 is detrimental to RGC-5 cells in vitro via regulating the expression of apoptotic genes and binding with CXCR1/2, but pre-treatment with G31P could attenuate its damaging effect. In view of the fact that RGC-5 cells do not fully simulate RGCs in vivo, we need to further clarify and study the toxicity of IL-8 in vivo with better model. However, this study provides a novel molecular research approach for nerve cell injury. By exploring the potential risk factors to neurocytes survival, the results of our study may provide original therapeutic target for protecting neurons and preventing further visual ability loss. At the same time, we couldn't ignore the role of IL-8 in other ocular and neurodegenerative diseases.
Acknowledgments
Conflicts of Interest: Wang JJ, None; Williams W, None; Wang B, None; Wei J, None; Lu X, None; Cheng JW, None; Gordon JG, None; Li JM, None; Li F, None.
REFERENCES
- 1.Majsterek I, Malinowska K, Stanczyk M, Kowalski M, Blaszczyk J, Kurowska AK, Kaminska A, Szaflik J, Szaflik JP. Evaluation of oxidative stress markers in pathogenesis of primary open-angle glaucoma. Exp Mol Pathol. 2011;90(2):231–237. doi: 10.1016/j.yexmp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 2.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 3.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Dusting GJ, Triggle C. Are we over oxidized? Oxidative stress, cardiovascular disease, and the future of intervention studies with antioxidants. Vasc Health Risk Manag. 2005;1(2):93–97. doi: 10.2147/vhrm.1.2.93.64080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Himori N, Kunikata H, Shiga Y, Omodaka K, Maruyama K, Takahashi H, Nakazawa T. The association between systemic oxidative stress and ocular blood flow in patients with normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 2016;254(2):333–341. doi: 10.1007/s00417-015-3203-z. [DOI] [PubMed] [Google Scholar]
- 6.Liang Y, Friedman DS, Zhou Q, Yang XH, Sun LP, Guo L, Chang DS, Lian L, Wang NL, Handan Eye Study Group Prevalence and characteristics of primary angle-closure diseases in a rural adult Chinese population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2011;52(12):8672–8679. doi: 10.1167/iovs.11-7480. [DOI] [PubMed] [Google Scholar]
- 7.Girard MJ, Beotra MR, Chin KS, Sandhu A, Clemo M, Nikita E, Kamal DS, Papadopoulos M, Mari JM, Aung T, Strouthidis NG. In vivo 3-dimensional strain mapping of the optic nerve head following intraocular pressure lowering by trabeculectomy. Ophthalmology. 2016;123(6):1190–1200. doi: 10.1016/j.ophtha.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Doucette LP, Rasnitsyn A, Seifi M, Walter MA. The interactions of genes, age, and environment in glaucoma pathogenesis. Surv Ophthalmol. 2015;60(4):310–326. doi: 10.1016/j.survophthal.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Xiang M, Zhou H, Nathans J. Molecular biology of retinal ganglion cells. Proc Natl Acad Sci U S A. 1996;93(2):596–601. doi: 10.1073/pnas.93.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joachim SC, Mondon C, Gramlich OW, Grus FH, Dick HB. Apoptotic retinal ganglion cell death in an autoimmune glaucoma model is accompanied by antibody depositions. J Mol Neurosci. 2014;52(2):216–224. doi: 10.1007/s12031-013-0125-2. [DOI] [PubMed] [Google Scholar]
- 11.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Cui S, Zhu Y, Du J, Khan MN, Wang B, Wei J, Cheng JW, Gordon JR, Mu Y, Li F. CXCL8 antagonist improves diabetic nephropathy in male mice with diabetes and attenuates high glucose-induced mesangial injury. Endocrinology. 2017;158(6):1671–1684. doi: 10.1210/en.2016-1781. [DOI] [PubMed] [Google Scholar]
- 13.Carrero Y, Mosquera J, Callejas D, Alvarez-Mon M. In situ increased chemokine expression in human cervical intraepithelial neoplasia. Pathol Res Pract. 2015;211(4):281–285. doi: 10.1016/j.prp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Solari R, Pease JE, Begg M. Chemokine receptors as therapeutic targets: Why aren't there more drugs? Eur J Pharmacol. 2015;746:363–367. doi: 10.1016/j.ejphar.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 15.Berger C, Rossaint J, Van Aken H, Westphal M, Hahnenkamp K, Zarbock A. Lidocaine reduces neutrophil recruitment by abolishing chemokine-induced arrest and transendothelial migration in septic patients. J Immunol. 2014;192(1):367–376. doi: 10.4049/jimmunol.1301363. [DOI] [PubMed] [Google Scholar]
- 16.Thirumangalakudi L, Yin L, Rao HV, Grammas P. IL-8 induces expression of matrix metalloproteinases, cell cycle and pro-apoptotic proteins, and cell death in cultured neurons. J Alzheimers Dis. 2007;11(3):305–311. doi: 10.3233/jad-2007-11307. [DOI] [PubMed] [Google Scholar]
- 17.Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14(11):1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- 18.Kuchtey J, Rezaei KA, Jaru-Ampornpan P, Sternberg P, Jr, Kuchtey RW. Multiplex cytokine analysis reveals elevated concentration of Interleukin-8 in glaucomatous aqueous humor. Invest Ophthalmol Vis Sci. 2010;51(12):6441–6447. doi: 10.1167/iovs.10-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Horuk R, Rice GC, Bennett GL, Camerato T, Wood WI. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992;267(23):16283–16287. [PubMed] [Google Scholar]
- 20.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61(13):4961–4965. [PubMed] [Google Scholar]
- 21.Goczalik I, Ulbricht E, Hollborn M, Raap M, Uhlmann S, Weick M, Pannicke T, Wiedemann P, Bringmann A, Reichenbach A, Francke M. Expression of CXCL8, CXCR1, and CXCR2 in neurons and glial cells of the human and rabbit retina. Invest Ophthalmol Vis Sci. 2008;49(10):4578–4589. doi: 10.1167/iovs.08-1887. [DOI] [PubMed] [Google Scholar]
- 22.Gordon JR, Li F, Zhang X, Wang W, Zhao X, Nayyar A. The combined CXCR1/CXCR2 antagonist CXCL8 (3-74) K11R/G31P blocks neutrophil infiltration, pyrexia, and pulmonary vascular pathology in endotoxemic animals. J Leukoc Biol. 2005;78(6):1265–1272. doi: 10.1189/jlb.0805458. [DOI] [PubMed] [Google Scholar]
- 23.Wood JP, Chidlow G, Tran T, Crowston JG, Casson RJ. A comparison of differentiation protocols for RGC-5 cells. Invest Ophthalmol Vis Sci. 2010;51(7):3774–3783. doi: 10.1167/iovs.09-4305. [DOI] [PubMed] [Google Scholar]
- 24.Takai Y, Tanito M, Ohira A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Invest Ophthalmol Vis Sci. 2012;53(1):241–247. doi: 10.1167/iovs.11-8434. [DOI] [PubMed] [Google Scholar]
- 25.Kushi H, Saito T, Makino K, Hayashi N. IL-8 is a key mediator of neuroinflammation in severe traumatic brain injuries. Acta Neurochir Suppl. 2003;86:347–350. doi: 10.1007/978-3-7091-0651-8_74. [DOI] [PubMed] [Google Scholar]
- 26.Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GM, Jr, Brachova L, Yan SD, Walker DG, Shen Y, Rogers J. Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia. 2001;35(1):72–79. doi: 10.1002/glia.1072. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Suyeoka G, Papa S, Franzoso G, Neufeld AH. Growth arrest and DNA damage protein 45b (Gadd45b) protects retinal ganglion cells from injuries. Neurobiol Dis. 2009;33(1):104–110. doi: 10.1016/j.nbd.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborne NN, Chidlow G, Layton CJ, Wood JP, Casson RJ, Melena J. Optic nerve and neuroprotection strategies. Eye (Lond) 2004;18(11):1075–1084. doi: 10.1038/sj.eye.6701588. [DOI] [PubMed] [Google Scholar]
- 29.Fahy ET, Chrysostomou V, Crowston JG. Mini-review: impaired axonal transport and glaucoma. Curr Eye Res. 2016;41(3):273–283. doi: 10.3109/02713683.2015.1037924. [DOI] [PubMed] [Google Scholar]
- 30.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Migallón MC, Valiente-Soriano FJ, Nadal-Nicolás FM, Vidal-Sanz M, Agudo-Barriuso M. Apoptotic retinal ganglion cell death after optic nerve transection or crush in mice: delayed RGC loss with BDNF or a caspase 3 inhibitor. Invest Ophthalmol Vis Sci. 2016;57(1):81–93. doi: 10.1167/iovs.15-17841. [DOI] [PubMed] [Google Scholar]
- 32.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 33.Grootaert MO, Schrijvers DM, Hermans M, Van Hoof VO, De Meyer GR, Martinet W. Caspase-3 deletion promotes necrosis in atherosclerotic plaques of ApoE knockout mice. Oxid Med Cell Longev. 2016;2016:3087469. doi: 10.1155/2016/3087469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52(1):145–176. [PubMed] [Google Scholar]
- 35.Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot study. Schlaf & Atmung. 2005;9(3):119–126. doi: 10.1007/s11325-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 36.Lutz J, Luong le A, Strobl M, Deng M, Huang H, Anton M, Zakkar M, Enesa K, Chaudhury H, Haskard DO, Baumann M, Boyle J, Harten S, Maxwell PH, Pusey C, Heemann U, Evans PC. The A20 gene protects kidneys from ischaemia/reperfusion injury by suppressing pro-inflammatory activation. J Mol Med. 2008;86(12):1329–1339. doi: 10.1007/s00109-008-0405-4. [DOI] [PubMed] [Google Scholar]
- 37.Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM. Roles of IL-8 in ocular inflammations: a review. Ocul Immunol Inflamm. 2011;19(6):401–412. doi: 10.3109/09273948.2011.618902. [DOI] [PubMed] [Google Scholar]
- 38.Inoue T, Kawaji T, Inatani M, Kameda T, Yoshimura N, Tanihara H. Simultaneous increases in multiple proinflammatory cytokines in the aqueous humor in pseudophakic glaucomatous eyes. J Cataract Refract Surg. 2012;38(8):1389–1397. doi: 10.1016/j.jcrs.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Town JR, Li F, Zhang X, Cockcroft DW, Gordon JR. ELR-CXC chemokine receptor antagonism targets inflammatory responses at multiple levels. J Immunol. 2009;182(5):3213–3222. doi: 10.4049/jimmunol.0800551. [DOI] [PubMed] [Google Scholar]