Abstract
Objective: The impact of hypertonic saline (HTS) on the control of increased intracranial pressure (ICP) in head-injured patients undergoing decompressive craniectomy (DC) has yet to be established. The current retrospective study was conducted to compare the effect of HTS and mannitol on lowering the ICP burden of these patients.
Methods: We reviewed data on patients who had sustained a traumatic brain injury (TBI) and were admitted to the First People's Hospital of Kunshan between January 1, 2012, and August 31, 2017. Patients who received only one type of hyperosmotic agent, 3% HTS or 20% mannitol, after DC were included. The daily ICP burden (h/day) and response to the hyperosmolar agent were used as primary outcome measures. The numbers of days in the intensive care unit and in the hospital, and the 2-weeks mortality rates were also compared between the groups.
Results: The 30 patients who received 3% HTS only and the 30 who received 20% mannitol only were identified for approximate matching and additional data analyses. The demographic characteristics of the patients in the two groups were comparable, but the daily ICP burden was significantly lower in the HTS group than in the mannitol group (0.89 ± 1.02 h/day vs. 2.11 ± 2.95 h/day, respectively; P = 0.038). The slope of the reduction in ICP in response to a bolus dose at baseline was higher with HTS than with mannitol (P = 0.001). However, the between-group difference in the 2-weeks mortality rates was not statistically significant (2 [HTS] vs. 1 [mannitol]; P = 0.554).
Conclusion: When used in equiosmolar doses, the reduction in the ICP of TBI patients achieved with 3% HTS was superior to that achieved with 20% mannitol after DC. However, this advantage did not seem to confer any additional benefit terms of short-term mortality.
Keywords: traumatic brain injury, intracranial pressure, decompressive craniectomy, hypertonic saline, mannitol
Introduction
Traumatic brain injury (TBI) is the major cause of death and severe disability among young people (1), and intracranial hypertension (ICH) accounts for about half of all deaths associated with TBI (2). Boluses of mannitol are often administered as part of the hyperosmolar treatment of ICH (3). This approach reduces intracranial pressure (ICP) and mortality rates after head injury and is superior to the use of pentobarbital for reducing the occurrence and severity of ICP elevation (4); however, its adverse effects include hypotension, electrolyte imbalance, and worsening of cerebral edema (5).
A growing body of evidence from pilot studies supports the efficacy of hypertonic saline (HTS), which has been used for hemodynamic resuscitation in shock secondary to trauma, gastrointestinal hemorrhage, burns, and sepsis (6). A number of studies have shown that decompressive craniectomy (DC) is an effective means of controlling ICH, especially in patients with traumatic lesions (7). However, very few studies have compared the efficacy of equiosmolar loads of HTS and mannitol in head-injured patients undergoing DC. Therefore, we used prospective data to compare the effect of equiosmolar doses of 3% HTS and 20% mannitol on the treatment of post-traumatic ICH in patients after DC.
Methods
Study population
Trained nurses collected clinical information about patients with severe TBI and entered it into the database for prospective analyses. This retrospective study was approved by the Institutional Ethics Board of the First People's Hospital of Kunshan. We reviewed data on TBI patients aged 16 years or older who were admitted to our institution between January 1, 2012, and August 31, 2017, and who underwent DC. Intraventricular ICP monitoring catheters (Codman Microsensors ICP Transducer, Codman & Shurtleff, Raynham, MA, USA) were placed during surgery. Initially elevated ICP was defined as the first recorded ICP >20 mmHg for >5 min. Additionally, patients were included if they received only one hyperosmotic agent, 3% HTS or 20% mannitol, for the treatment of intracranial hypertension. Patients were excluded if they met one of the following criteria: Glasgow Coma Scale (GCS) score of three with bilateral fixed and dilated pupils; death on Day 1; or arrival at the trauma center 24 h or more after injury. Pregnant women and patients with multiple systemic injuries were also excluded.
Treatment protocol
All patients were managed according to the Brain Trauma Foundation guidelines (8, 9). Patients were sedated with dexmedetomidine, Propofol, or diazepam to facilitate mechanical ventilation. The aim of mechanical ventilation was to maintain SpO2 of >95%. Glycemic levels were targeted to around 150 mg/dl by administering insulin. The head end of the patient's bed was elevated by 15–30°. The aim of the therapy was to maintain the ICP below 20 mmHg. If, despite adequate sedation, ventilation, and head positioning, the ICP spontaneously increased to >20 mmHg for >5 min, patients received a bolus of osmotic therapy. If the osmotic agent failed to decrease the ICP to below 20 mmHg, cerebral spinal fluid (CSF) was drained until it stopped flowing spontaneously. If the ICP remained elevated, propofol or moderate hyperventilation was instituted.
Outcome variables
The primary outcomes were daily ICP burden (h/day) and response to the hyperosmolar agent. The daily ICP burden was calculated as the mean daily duration of ICP >20 mmHg, expressed as the number of hours per day. Response to the hyperosmolar agent was documented as the ratio of the magnitude of the ICP reduction to the initial elevated ICP following the individual bolus of the hyperosmolar agent. Furthermore, we examined total number of ICU days, number of ICP monitoring days, and 2-weeks mortality. We used the recorded doses of HTS and mannitol to calculate cumulative doses.
Statistical analyses
The goal of this study was to compare the effects of HTS and mannitol on the outcome variables described above. Descriptive summaries of the data are presented as means ± standard deviations (SDs) for continuous variables and as frequencies for categorical variables. Differences between the two groups in the baseline variables were assessed using independent-samples t-tests for continuous variables and chi-square tests for categorical variables. We used a P-value of < 0.05 for evaluating baseline differences between groups. All statistical tests were two-sided, and a P < 0.05 was considered statistically significant. Analyses were performed using IBM SPSS Statistics 23.
Results
Demographic profile
We identified 30 patients who received 3% HTS only and 30 who received 20% mannitol only for the approximate matching and additional data analyses. Table 1 shows the demographic characteristics of the patients, which were comparable between groups.
Table 1.
Demographic characteristics.
| HTS (n = 30) | Mannitol (n = 30) | P-value | |
|---|---|---|---|
| Age, years | 42.27 ± 17.03 | 41.53 ± 15.27 | 0.861 |
| Female: male | 6:24 | 5:25 | 0.739 |
| Admission GCS score | 5.59 ± 1.78 | 5.75 ± 1.32 | 0.563 |
| Mechanism of head injury (n) | 0.834 | ||
| Traffic accident | 17 | 19 | |
| Fall | 10 | 9 | |
| Other | 3 | 2 | |
| Abnormal pupils | 22 | 18 | 0.273 |
| Predominant lesion on CT scan (n) | |||
| Extradural hematoma | 8 | 10 | 0.869 |
| Subdural hematoma | 12 | 9 | |
| Contusion | 7 | 8 | |
| Diffuse injury | 3 | 3 | |
| Mean days of ICP monitoring | 8.01 ± 2.67 | 8.87 ± 2.47 | 0.197 |
HTS, hypertonic saline; GCS, glasgow coma scale; CT, computed tomography; ICP, intracranial pressure.
Outcome variables
The mean durations of ICU and hospital stays for the two groups were comparable (Table 2). The daily ICP burden was significantly lower in the HTS group than in the mannitol group (0.89 ± 1.02 h/day vs. 2.11 ± 2.95 h/day, respectively; P = 0.038). Neither the cumulative mean doses of the hyperosmolar agent (535.61 ± 74.31 ml [HTS] vs. 519.18 ± 75.54 ml [mannitol]; P = 0.399) nor the 2-weeks mortality rates (2 [HTS] vs. 1 [mannitol]; P = 0.554) of the two groups differed significantly.
Table 2.
Study outcomes in the two groups.
| HTS (n = 30) | Mannitol (n = 30) | P-value | |
|---|---|---|---|
| Mean days in ICU | 11.57 ± 3.65 | 12.37 ± 2.95 | 0.355 |
| Mean days of hospital stay | 33.13 ± 17.54 | 32.53 ± 18.27 | 0.897 |
| Mean daily ICP burden (h/day) | 0.89 ± 1.02 | 2.11 ± 2.95 | 0.038 |
| Cumulative mean dose (ml) | 535.61 ± 74.31 | 519.18 ± 75.54 | 0.399 |
| 2-weeks deaths (n) | 2 | 1 | 0.554 |
| GCS score at discharge | 11.75 ± 3.11 | 11.90 ± 2.41 | 0.843 |
HTS, hypertonic saline; ICU, intensive care unit; ICP, intracranial pressure.
ICP response
The ICP responses to individual boluses of hyperosmolar agents are shown in Table 3. The effective doses were defined as those that returned the ICP to < 20 mmHg for >5 min after individual boluses of hyperosmolar agents were given. The number of effective doses was significantly higher in the HTS group than in the mannitol group (P = 0.009). When the initial ICP elevation was plotted against the reduction in the ICP after each dose of osmotic agent, the slope of the regression line was significantly steeper in the HTS than in the mannitol group (P = 0.001) (Figure 1).
Table 3.
Response to individual boluses in the two groups.
| HTS (n = 30) | Mannitol (n = 30) | P-value | |
|---|---|---|---|
| Initial elevated ICP (mmHg) | 28.43 ± 6.92 | 30.27 ± 6.12 | 0.282 |
| ICP reduction (mmHg) | 7.50 ± 2.45 | 6.13 ± 1.79 | 0.017 |
| Efficacy of individual doses | 0.009 | ||
| Effective doses (n) | 18 | 8 | |
| Ineffective doses (n) | 12 | 22 |
HTS, hypertonic saline; ICP, intracranial pressure.
Figure 1.
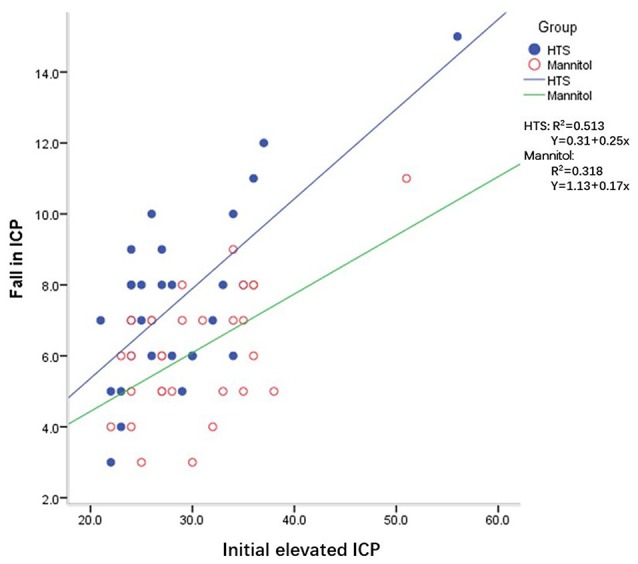
Intracranial pressure (ICP) reduction for a given ICP value with mannitol and hypertonic saline (HTS) in patients with traumatic brain injury after decompressive craniectomy.
Discussion
We examined the effects of HTS and mannitol on intracranial hypertension among patients with severe TBI who had undergone DC. Our findings indicated that HTS was superior to mannitol for reducing the daily ICP burden in patients with severe TBI who underwent DC. Additionally, the HTS bolus produced a more effective reduction in elevated ICP than did mannitol; however, the mortality rates of the two groups did not significantly differ.
HTS and mannitol share similar mechanisms of action for reducing elevated ICP. Both establish an osmotic gradient across the blood–brain barrier, leading to fluid shifts from the intercellular space into the microcirculation (10, 11). Indeed, several clinical studies have shown that HTS and mannitol reduce ICP and improve brain physiology to different extents. Previous studies have also demonstrated that HTS increases brain oxygenation and reduces ICP when it is used as a first-line agent and when it is used in patients with intracranial hypertension refractory to mannitol (12–14). HTS has a more pronounced and longer-lasting effect than does mannitol on elevated ICP and does not cause a rebound increase in ICP (15, 16). It causes rapid and sustained volume expansion and is effective for lowering elevated ICP when the latter is refractory to other therapies (15–18).
Three studies using equimolar doses of HTS and mannitol found that HTS was associated with either equal or greater reductions in ICP and a longer duration of effect; they also reported that mannitol produced significantly greater diuresis and volume loss (19, 20). However, conflicting results have also been reported by earlier studies that compared HTS with mannitol in patients with severe TBI (21–24). This variation appears to be related to differences in the concentrations and doses of HTS, the use of colloids in combination with HTS (21–24), and the different primary end points (6, 25).
Despite these previous studies, to our knowledge, no convincing evidence in support of the superiority of HTS over mannitol for the management of TBI patients undergoing DC has been presented. DC reduces medically refractory intracranial hypertension and is a valuable tool in the management of severe head injury (26, 27). The presence of high ICP after DC is also strongly related to unfavorable outcomes (7). We examined the daily ICP burden rather than the effects on the individual ICP spikes of repeated elevations in ICP. Sheth et al. used the “pressure time dose” (PTD) to demonstrate that the total PTD for patients with ICP > 20 mm Hg had strong predictive power for functional outcome and in-hospital mortality (28, 29). Therefore, the daily ICP burden is a meaningful outcome variable. The current study, which involved equiosmolar doses of HTS and mannitol, showed that the former had a more significant impact on the daily control of ICP after DC. Additionally, we also evaluated the responses of TBI patients who had undergone DC to an individual bolus of HTS or mannitol. The slope of the regression for a given ICP value with respect to the magnitude of the ICP reduction was higher with HTS (Figure 1). This indicates that an HTS bolus yields a more effective reduction in ICP than does mannitol when ICP is elevated, which is consistent with a previous study (30).
This study did not address the benefits of HTS on the lengths of ICU stay and hospitalization. There was also no statistically significant tendency toward a lower 2-weeks mortality rate in the HTS group. A larger cohort is required to perform the additional cost-benefit analysis between the two groups.
Conclusion
When used in equiosmolar doses, 3% HTS is associated with a greater reduction in ICP than is 20% mannitol in TBI patients undergoing DC. A steeper ICP reduction in response to the HTS bolus was also observed. However, these advantages do not seem to confer any additional benefit terms of short-term mortality.
Author contributions
FC and ZW designed the experiments; MX and HL performed data analysis; WW provided scientific expertize; FC wrote the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by Clinical and basic researches of brain disease (KYC004), Kunshan science and technology fund (KS1718 and KS1644).
References
- 1.Liu H, Wang W, Cheng F, Yuan Q, Yang J, Hu J, et al. External ventricular drains versus intraparenchymal intracranial pressure monitors in traumatic brain injury: a prospective observational study. World Neurosurg. (2015) 83:794–800. 10.1016/j.wneu.2014.12.040 [DOI] [PubMed] [Google Scholar]
- 2.Celli P, Fruin A, Cervoni L. Severe head trauma. Review of the factors influencing the prognosis. Minerva Chir. (1997) 52:1467–80. [PubMed] [Google Scholar]
- 3.Bratton SL, Chestnut RM, Ghajar J, McConnell HFF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. II. Hyperosmolar therapy. J Neurotrauma (2007) 24:S14–20. 10.1089/neu.2007.9994 [DOI] [PubMed] [Google Scholar]
- 4.Marshall LF, Smith RW, Rauscher LA, Shapiro HM. Mannitol dose requirements in brain-injured patients. J Neurosurg. (1978) 48:169–72. 10.3171/jns.1978.48.2.0169 [DOI] [PubMed] [Google Scholar]
- 5.Kalita J, Ranjan P, Misra UK. Current status of osmotherapy in intracerebral hemorrhage. Neurol India (2003) 51:104–9. [PubMed] [Google Scholar]
- 6.Strandvik GF. Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure. Anaesthesia (2009) 64:990–1003. 10.1111/j.1365-2044.2009.05986.x [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Xu R, Yang J, Ren G, He S. Initial intracranial pressure as a prognosticator in head-injured patients undergoing decompressive craniectomy. Oncotarget (2016) 7:62657–63. 10.18632/oncotarget.11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bratton SL, Chestnut RM, Ghajar J, McConnell HFF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma (2007) 24:S55–8. 10.1089/neu.2007.9988 [DOI] [PubMed] [Google Scholar]
- 9.Bratton SL, Chestnut RM, Ghajar J, McConnell HFF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma (2007) 24:S59–64. 10.1089/neu.2007.9987 [DOI] [PubMed] [Google Scholar]
- 10.Berger S, Schürer L, Härtl R, Messmer K, Baethmann A. Reduction of post-traumatic intracranial hypertension by hypertonic/hyperoncotic saline/dextran and hypertonic mannitol. Neurosurgery (1995) 37:98–107; discussion: 107–8. 10.1227/00006123-199507000-00015 [DOI] [PubMed] [Google Scholar]
- 11.Mangat HS, Chiu YL, Gerber LM, Alimi M, Ghajar J, Härtl R. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. J Neurosurg. (2015) 122:202–10. 10.3171/2014.10.JNS132545 [DOI] [PubMed] [Google Scholar]
- 12.Oddo M, Levine JM, Frangos S, Carrera E, Maloney-Wilensky E, Pascual JL, et al. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J Neurol Neurosurg Psychiatry (2009) 80:916–20. 10.1136/jnnp.2008.156596 [DOI] [PubMed] [Google Scholar]
- 13.Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery (2009) 65:1035–41; discussion: 1041-2. 10.1227/01.NEU.0000359533.16214.04 [DOI] [PubMed] [Google Scholar]
- 14.Schatzmann C, Heissler HE, König K, Klinge-Xhemajli P, Rickels E, Mühling M, et al. Treatment of elevated intracranial pressure by infusions of 10% saline in severely head injured patients. Acta Neurochir Suppl. (1998) 71:31–3. 10.1007/978-3-7091-6475-4_9 [DOI] [PubMed] [Google Scholar]
- 15.Kerwin AJ, Schinco MA, Tepas JJ, Renfro WH, Vitarbo EA, Muehlberger M. The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma (2009) 67:277–82. 10.1097/TA.0b013e3181acc726 [DOI] [PubMed] [Google Scholar]
- 16.Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery (2005) 57:727–36; discussion: 727-36. 10.1227/01.NEU.0000175726.08903.0A [DOI] [PubMed] [Google Scholar]
- 17.Cottenceau V, Masson F, Mahamid E, Petit L, Shik V, Sztark F, et al. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma (2011) 28:2003–12. 10.1089/neu.2011.1929 [DOI] [PubMed] [Google Scholar]
- 18.Eskandari R, Filtz MR, Davis GE, Hoesch RE. Effective treatment of refractory intracranial hypertension after traumatic brain injury with repeated boluses of 14.6% hypertonic saline. J Neurosurg. (2013) 119:338–46. 10.3171/2013.4.JNS121541 [DOI] [PubMed] [Google Scholar]
- 19.Battison C, Andrews PJ, Graham C, Petty T. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med. (2005) 33:196–202; discussion 257-8. 10.1097/01.CCM.0000150269.65485.A6 [DOI] [PubMed] [Google Scholar]
- 20.Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. (2008) 36:795–800. 10.1097/CCM.0B013E3181643B41 [DOI] [PubMed] [Google Scholar]
- 21.Sakellaridis N, Pavlou E, Karatzas S, Chroni D, Vlachos K, Chatzopoulos K, et al. Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. J Neurosurg. (2011) 114:545–8. 10.3171/2010.5.JNS091685 [DOI] [PubMed] [Google Scholar]
- 22.Ichai C, Armando G, Orban JC, Berthier F, Rami L, Samat-Long C, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med. (2009) 35:471–9. 10.1007/s00134-008-1283-5 [DOI] [PubMed] [Google Scholar]
- 23.Vialet R, Albanèse J, Thomachot L, Antonini F, Bourgouin A, Alliez B, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. (2003) 31:1683–7. 10.1097/01.CCM.0000063268.91710.DF [DOI] [PubMed] [Google Scholar]
- 24.Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients–a randomized clinical trial [ISRCTN62699180]. Crit Care (2005) 9:R530–40. 10.1186/cc3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogden AT, Mayer SA, Connolly ES. Hyperosmolar agents in neurosurgical practice: the evolving role of hypertonic saline. Neurosurgery (2005) 57:207–15; discussion: 207-15. 10.1227/01.NEU.0000166533.79031.D8 [DOI] [PubMed] [Google Scholar]
- 26.Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. (2006) 104:469–79. 10.3171/jns.2006.104.4.469 [DOI] [PubMed] [Google Scholar]
- 27.Ucar T, Akyuz M, Kazan S, Tuncer R. Role of decompressive surgery in the management of severe head injuries: prognostic factors and patient selection. J Neurotrauma (2005) 22:1311–8. 10.1089/neu.2005.22.1311 [DOI] [PubMed] [Google Scholar]
- 28.Kahraman S, Dutton RP, Hu P, Xiao Y, Aarabi B, Stein DM, et al. Automated measurement of “pressure times time dose” of intracranial hypertension best predicts outcome after severe traumatic brain injury. J Trauma (2010) 69:110–8. 10.1097/TA.0b013e3181c99853 [DOI] [PubMed] [Google Scholar]
- 29.Sheth KN, Stein DM, Aarabi B, Hu P, Kufera JA, Scalea TM, et al. Intracranial pressure dose and outcome in traumatic brain injury. Neurocrit Care (2013) 18:26–32. 10.1007/s12028-012-9780-3 [DOI] [PubMed] [Google Scholar]
- 30.Jagannatha AT, Sriganesh K, Devi BI, Rao GS. An equiosmolar study on early intracranial physiology and long term outcome in severe traumatic brain injury comparing mannitol and hypertonic saline. J Clin Neurosci. (2016) 27:68–73. 10.1016/j.jocn.2015.08.035 [DOI] [PubMed] [Google Scholar]


