Abstract
Purpose
Botulinum toxin-A (or Botox) is widely used for the management of equinus gait in children with cerebral palsy but few recent studies have included instrumented gait analysis.
Methods
This was a prospective cohort study. Gait analysis was performed four weeks before and four weeks after Botulinum toxin-A injection for spastic equinus to detect the maximum effects on gait kinematics. Outcome measures included the Gait Profile Score (GPS), the Gait Variable Score (GVS) for the ankle, maximal ankle dorsiflexion and maximal knee extension at midstance.
Results
In all, 37 children participated (20 boys); mean age five years seven months (4 years 1 month to 8 years 2 months); 19 with unilateral and 18 bilateral involvement. At a mean four weeks post-injection, the GPS and ankle GVS were unchanged. However maximum ankle dorsiflexion increased for the whole group; median 7.7° (confidence interval (CI) 4° to 10.6°) to 11.5° (CI 7.7° to 12.9°), p = 0.02. Maximum midstance knee extension was unchanged for the whole group, but median knee flexion increased in children with bilateral involvement; 10.9° (CI 7.4° to 20.8°) to 16.5° (CI 8.4° to 19.7°), p = 0.58.
Conclusion
Injections of the gastrocsoleus for spastic equinus did not result in objective improvements in overall gait. Improvements in ankle dorsiflexion for children with bilateral involvement may be offset by deterioration at the knee.
Level of Evidence
II - prospective cohort study, before and after intervention
Keywords: Botulinum toxin-A, Botox, cerebral palsy, kinematics, gait profile score
Introduction
Equinus is the most common gait abnormality affecting children with cerebral palsy (CP) and injections of Botulinum neurotoxin-A (BoNT-A) have become the standard of care for younger children with spastic equinus, despite modest and short-lived effects.1-4 In the early years of childhood, both gait function and gross motor function show spontaneous improvement as myelination of the corticospinal tracts proceeds.1,5 Given that both gait function and gross motor function are on an upward trajectory during early childhood, randomized clinical trials (RCTs) are needed as well as objective measure of gait and function because improvements as part of natural history are much larger than those related to intervention.3-6 However, objective, serial measurements of gait function in younger children with cerebral palsy can be demanding and difficult. For this reason, many studies have utilized surrogate measures for assessing the effects of BoNT-A injections for spastic equinus such as the Modified Ashworth Scale and the Modified Tardieu Scale.7-9 However, these surrogate measures have poor correlation with gait function and gross motor function and have both limited reliability and sensitivity.1,8,10 To overcome these difficulties, assessment of gait using 2D video in conjunction with gait scoring systems are often used.11 However, the benchmark remains 3D kinematics derived from instrumented gait analysis (IGA).1,6,12 A weakness of previous studies is the selection of isolated gait parameters biased towards detection of positive changes in gait, with limited or no reporting of gait parameters which may deteriorate, or overall gait function.13,14 Summary statistics of gait may balance some of these methodological problems in previous studies. The Gait Profile Score (GPS) is based on a mathematical synthesis of nine clinically relevant kinematic traces and can be broken down into Gait Variable Scores (GVS) for each of these nine variables.15 In addition, the minimal clinically important difference (MCID) of the GPS has been established, and used to contextualize the effects of interventions such as gait improvement surgery for children with CP.16,17 To the best of our knowledge, the effects of BoNT-A for spastic equinus have not been reported using the GPS. After a randomized clinical trial in which the frequency of BoNT-A injection for spastic equinus was investigated, part of the study protocol included a 3D gait analysis four weeks before and four weeks following injection to determine BoNT-A effects on gait function.18 The aim of this study was to quantify using summary and selected parameters, the effect of gastrocsoleus BoNT-A injections on lower limb function during gait in children with spastic equinus.
Patients and methods
Trial design, ethical approval, and study funding
This study was a prospective cohort design. Ethical approval was given by each institution’s Human Research and Ethics Committee (27062C and 07083C). Written, informed consent was obtained from the parents of all children before study inclusion. All children who participated and completed the preceding RCT investigating the frequency of BoNT-A injection for spastic equinus were included in this study.18 No pharmaceutical company sponsorship was received in support of this clinical trial.
Children were selected for injection of the gastrocsoleus on the basis of having the appearance of equinus gait on watching them walk (observational gait analysis, OGA) and the presence of gastrocsoleus spasticity on physical examination by the Modified Tardieu Test.8,11,13,19 Children who had both the appearance of jump gait and hamstring spasticity were also selected for concomitant injection of the hamstring muscles.19 Exclusion criteria were fixed contractures lever arm deformities such as femoral torsion, tibial torsion or severe pes valgus.19
Interventions
The selection of children for injection of the calf for spastic equinus was based on a thorough clinical assessment by an experienced team, which included a physiotherapist (TH-I) and an experienced injector (HKG, BR, MF). Indications included toe walking noted on OGA, symptoms from gait difficulties such as tripping and falling and a spastic catch using the Modified Tardieu Scale8, in the equinus range. In accordance with typical clinical practice, the baseline IGA was not used for the planning of injections. All children had spastic hypertonia, based on a clinical examination with reference to standard definitions.1
Following previously published guidelines, the calf muscle was injected with a fixed dose of BoNT-A (Botox; Allergan Pharmaceuticals Inc., Irvine, California) 6 U/kg body weight at a fixed dilution of 100 U in 4.0 ml of normal saline. Injections were guided by electrical stimulation under mask anaesthesia, to two sites in the medial and one site in the lateral bellies of the gastrocnemius.18,19 Children with spastic diplegia had injections to each head of gastrocnemius of both legs, children with hemiplegia had each head of gastrocnemius and soleus injected on the affected side. The maximum total dose was fixed at 18 U/kg body weight with the difference between the calf muscle dose and total dose available for use at other sites, according to clinical indication. IGA was collected by experienced assessors in a single gait laboratory four weeks before, and four weeks after BoNT-A injection. Each participant maintained their pre-injection use of ankle foot orthoses (six to eight hours) and community physiotherapy (usual care) during the study period.18
Primary outcome measure
The GPS from IGA was the primary outcome measure.15 This is a summary statistic of gait, derived from the root mean square of the difference between the participant’s kinematic curve and the corresponding typical curve for nine key kinematic gait variables. It summarizes both improvements and deterioration in each kinematic parameter at each time point in the gait cycle.1,15
Secondary outcome measures
Several secondary outcome measures were used:
The ankle GVS is one of the nine subcomponents of the GPS;15
maximal ankle dorsiflexion and maximum knee extension during stance;
the Plantarflexor-Knee Extension (PFKE) couple index is a scatter plot based on the difference between the subject’s ankle and knee kinematics at midstance, derived from normative data and allows quantitative identification of the sagittal gait pattern.20
Statistical analysis
As the outcome data were not normally distributed, non-parametric statistical tests were used, including the Kruskal-Wallis one-way analysis of variance, to analyze the difference between pre- and post-injection kinematic variables. All statistical tests were two-sided and the significance level set at p = 0.05. Statistical analyses were performed using Minitab statistical software, version 17 (Minitab Inc., State College, Pennsylvania). Computational algorithms to perform the PFKE couple index were completed using statistical software MATLAB version R2016b (The MathWorks Inc., Natick, Massachusetts).
Results
A total of 39 children (22 boys, 17 girls) underwent pre-injection IGA; 37 children completed both the pre- and post-injection gait studies. Two children who completed initial gait analysis did not return for follow-up IGA due to parental circumstances. These pre-injection data were excluded from analysis. The mean age was five years seven months (4 years 1 month to 8 years 2 months). In all, 19 children had unilateral spastic CP (hemiplegia) and 18 bilateral spastic CP (diplegia). Children functioned predominantly at Gross Motor Classification System levels I (n = 16) and II (n = 18), with three children at level III.21 Gait analysis was performed at a median of 16 days (interquartile range (IQR) 8 to 23) prior and 33 (IQR 29 to 42) days following, injection of BoNT-A.
In all, 18 children had received two prior injections of the calf muscles and 19 children had received six previous injections, as per the RCT protocol.18 A total of 12 children received injection of the medial hamstrings (five unilateral and seven bilateral) at the same time as injection of the calf muscle because of the presence of jump gait on OGA and in accordance with the RCT protocol.18
Physical examination measures
Prior to injection, mean ankle dorsiflexion was 17.9° (sd 12.6°) with the knee flexed and 6.2° (sd 11 .9°) with the knee extended. Dynamic ankle dorsiflexion was -14.2° (sd 12.7°) (equinus range). The mean popliteal angle was 41.6° (sd 11.8°) and mean knee extension was 0° (sd 6.3°).
Kinematic outcomes
For the whole group, as well as for those with unilateral or bilateral involvement, there was no significant difference pre- and post-BoNT-A injection in the primary outcome measure of GPS, or in the secondary outcome measures, the ankle GVS and maximum knee extension (Table 1). In contrast, maximal ankle dorsiflexion increased from 7.7° (CI 4.0° to 10.6°) to 11.5° (CI 7.7° to 12.9°) (p = 0.02) for the whole cohort. Gait kinematics illustrating increased ankle dorsiflexion for a single participant are illustrated in Figure 1. In children with bilateral involvement, maximal ankle dorsiflexion increased from 8.8° (CI 3.2° to 11.9°) to 12.1° (CI 9.8° to 17.1°) (p = 0.04). No significant change was seen in maximal dorsiflexion for children with unilateral involvement. A similar result was found when children receiving simultaneous BoNT-A injections in proximal muscles (e.g. semitendinosus) were excluded. Maximal ankle dorsiflexion increased in all children who did not have proximal BoNT-A injections (n = 32, p = 0.015), in children with bilateral involvement (n = 15, p = 0.017), but not in those with unilateral involvement (n = 17, p = 0.118).
Table 1.
Kinematic outcome measures following Botulinum toxin-A injections for spastic equinus
| Pre-injection | Post-injection | Kruskal-Wallis | ||||
|---|---|---|---|---|---|---|
| Variable | Group | Median | CI | Median | CI | p-value |
| GPS | All | 11.3 | 10.3 to 14.8 | 12.5 | 10.3 to 13.2 | 0.96 |
| Unilateral | 8.9 | 7.9 to 9.6 | 9.3 | 8.0 to 10.1 | 0.849 | |
| Bilateral | 15.5 | 11.5 to 15.9 | 13.5 | 12.5 to 15.2 | 0.718 | |
| Ankle GVS | All | 11.8 | 8.9 to 16 | 10.2 | 8.5 to 12.1 | 0.34 |
| Unilateral | 8.7 | 5.9 to 13.6 | 8.9 | 6.8 to 10.6 | 0.988 | |
| Bilateral | 15.9 | 9.0 to 23.8 | 11.8 | 8.9 to 14.9 | 0.241 | |
| Max ankle dorsiflexion | All | 7.7 | 4.0 to 10.6 | 11.5 | 7.7 to 12.9 | 0.021 |
| Unilateral | 5.8 | 2.4 to 10.7 | 7.7 | 4.9 to 12.8 | 0.204 | |
| Bilateral | 8.8 | 3.2 to 11.9 | 12.1 | 9.8 to 17.1 | 0.044 | |
| Max knee extension | All | 8.1 | 5.3 to 11.4 | 8.5 | 6.7 to 16.8 | 0.331 |
| Unilateral | 3.5 | -2.6 to 7.6 | 5.1 | 2.4 to 8.4 | 0.194 | |
| Bilateral | 10.9 | 7.4 to 20.8 | 16.5 | 8.4 to 19.7 | 0.581 |
The Gait Profile Score (GPS) is a summary statistic of the distance between the subject’s and the typical gait pattern. The ankle Gait Variable Score (GVS) is a summary statistic of the distance between the subject’s and the typical ankle kinematic in the sagittal plane during gait. The maximum (Max) ankle dorsiflexion and knee extension were searched within the stance phase of gait
CI, confidence interval
Fig. 1.
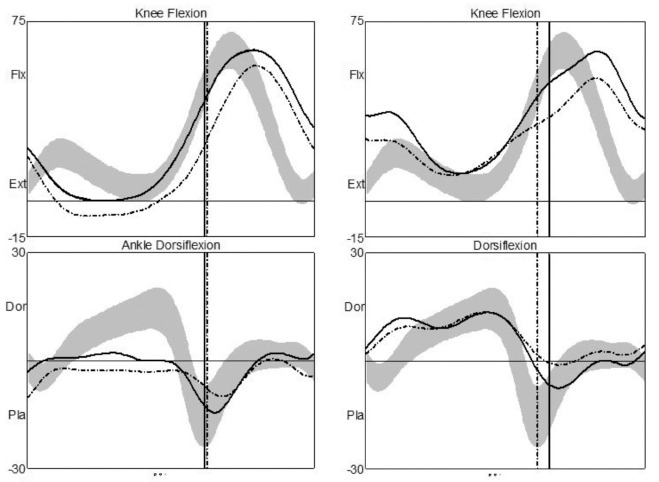
Ankle and knee kinematics before injection (left) and four weeks after injection (right) for a single participant with bilateral involvement (spastic diplegia). Knee kinematics show flexion (Flx) to the top and extension (Ext) towards the bottom of the horizontal line; ankle kinematics show dorsiflexion (Dor) above and plantarflexion (Pla) below the horizontal line on the graphs. Ankle kinematics show an improvement after injection, moving towards the normal range (grey band). However, there was a simultaneous deterioration in knee kinematics, with increased knee flexion.
The sagittal gait pattern (Table 2) was derived using the PFKE index, based on sagittal kinematics from IGA. Please refer to Sangeux et al20 for a complete description. Of 55 limbs pre-injection, 33 limbs (true equinus and normal gait) demonstrated effective PFKE coupling, maintaining full knee extension in midstance. However, post-injection, this number reduced to 27 limbs, indicating greater knee flexion and ineffective coupling for half of the limbs injected. Most children walked in true equinus pre-injection, with four limbs improving to within normal parameters post injection. However, six limbs classified as true equinus pre-injection, demonstrated reduced PFKE coupling, with four classified as jump and two as crouch gait. Those classified as being in crouch gait pre-injection did not improve, with an additional ten limbs walking in crouch post-injection. The scatter-plot PFKE couple index (Fig. 2) illustrates that for all limbs, as well as the unilateral and bilateral subgroups, greater ankle dorsiflexion occurs in addition to increased knee flexion post-injection.
Table 2.
Sagittal gait pattern classification pre- and post-Botulinum toxin-A injection
| Pre-injection | Post-injection | |||||
|---|---|---|---|---|---|---|
| True equinus | Jump | Apparent equinus | Crouch | Normal | ||
| True equinus | 27 | 17 | 4 | - | 2 | 4 |
| Jump | 14 | 3 | 8 | - | 3 | - |
| Apparent equinus | 4 | - | 1 | 1 | 2 | - |
| Crouch | 4 | - | - | - | 4 | - |
| Normal | 6 | - | - | - | 3 | 3 |
| Total limbs | 55 | 20 | 13 | 1 | 14 | 7 |
Fig. 2.

Ankle Plantarflexor-Knee Extension couple index.24 Sagittal gait pattern areas are represented by labelled boxes. Note that whilst all groups improve in ankle dorsiflexion post-injection, this is accompanied by greater knee flexion in midstance (AE, apparent equinus; WNL, within normal limits; Hemi, hemiplegia/unilateral involvement; Di, diplegia/bilateral involvement).
Discussion
Toe walking and equinus are the most common gait patterns in younger children with CP.1,2,22 However, with time the equinus gradually becomes fixed as muscle contracture develops, particularly in children with spastic hemiplegia.18,22 In children with diplegia, the evolution of gait is very different, with a high prevalence of disabling flexed-knee or crouch gait in older children and teenagers, often combined with excessive dorsiflexion at the ankle.1,23 Interventions for equinus gait must be understood and conducted with awareness of this natural history, especially the differences between children with spastic hemiplegia and those with spastic diplegia.1,23
In this study, injections of BoNT-A for spastic equinus did not result in objective improvements in overall gait function, as measured using the GPS, for the cohort as a whole and in no child did gait improvement exceed the MCID.15,16 Small improvements in ankle dorsiflexion for children with bilateral involvement were offset by deterioration in knee kinematics and elsewhere. In addition, sagittal gait patterns showed a movement toward crouch gait, as determined by the PFKE index. This is objective evidence of a potential harmful effect from injection of BoNT-A, similar to that described after isolated surgical lengthening of the gastrocsoleus.1,23 Effective PFKE coupling is vital for continued walking function into adulthood for individuals with bilateral involvement.1,23 The cumulative effect of multiple cycles of BoNT-A injection on PFKE coupling is not known.
This finding is similar to a recent longitudinal study investigating gait quality in children with bilateral CP who received repeated lower limb intramuscular injections of BoNT-A.11 Children were assessed using the Edinburgh Visual Gait Score, and despite statistically significant improvements in gait quality, these changes were not enough to reach the smallest real difference value of four points.
We consider the group of children in this study to be typical of those with spastic equinus with little or no fixed contracture, who are currently managed in our centres and in many other centres by periodic injections of BoNT-A for spastic equinus.1-4,9,13,14 All children had toe walking when their gait was observed, with associated symptoms and gait impairment. All children had a spastic catch in the equinus range, on physical examination. The children reported in this study previously participated in a RCT investigating injection frequency of BoNT-A for spastic equinus.18 Children had been randomized to receive injections either 12-monthly or four-monthly over a two-year period. Given the mean age at study entry was three years five months (sd 1 month), IGA was not feasible for many children at the commencement or during the trial. As with many other trials, surrogate measures were required, with an instrumented measure of passive ankle dorsiflexion used.9,18,24 The trial found no significant difference between 12-monthly and four-monthly injection regimens on passive ankle dorsiflexion or secondary functional outcome measures.18 At the time of study exit, the mean age of children was five years seven months and most were familiar with clinical assessment and able to cooperate with IGA; 3D kinematics were therefore captured one month before and one month after the final injection of BoNT-A and the purpose of this study was to report changes in gait kinematics irrespective of prior injection frequency.
Despite IGA kinematic capture at the optimum time when the effects of BoNT-A would have been considered maximal, no change in overall gait function as determined by the GPS was found for the whole cohort or the subgroups of children with unilateral or bilateral involvement. In addition, dynamic ankle function as measured by the ankle GVS did not improve for the whole cohort or the CP subgroups. The ankle GVS is representative of dynamic ankle function throughout the gait cycle rather than at one time point. When selected kinematic variables were considered, as in previous studies, maximum ankle dorsiflexion increased, as expected, for the whole cohort and the bilateral group, but not for the group with unilateral involvement (Table 1).13,14 Previous studies have reported improvements in selected gait parameters, but no study to date has looked at overall gait function with the GPS as a summary statistic of gait.13,14,15 Not examining overall gait function represents a methodological weakness in prior studies, which we have addressed in this study. It should be noted that several children who met clinical criteria for calf injection might have been excluded if IGA had been used as a planning tool. The fact that experienced injectors could select children for calf injection who were not ideal candidates, raises further questions for routine clinical practice, in which access to IGA is very limited. We believe our findings to be novel and important to management protocols for children with spastic equinus.
Firstly, children with unilateral involvement had a very limited response with no significant change in GPS, ankle GVS or maximal ankle dorsiflexion in stance. During the RCT, children with unilateral involvement in both the 12-monthly and four-monthly injection groups lost passive dorsiflexion at the ankle despite injection of BoNT-A, the use of orthoses and physiotherapy.18 This is a disappointing outcome, and given the increasing information from animal studies demonstrating harmful effects of repeated intra-muscular BoNT-A injections, this poses serious questions as to the usefulness of this therapy.25 It is incumbent on those who practise injection of BoNT-A to support the practice in individual children by objective measures of outcome, taking into consideration the expected spontaneous improvements in gait function and gross motor function in early childhood.
The findings in children with bilateral involvement are concerning. In the subgroup of children with diplegia, although the ankle GVS did not improve, maximum ankle dorsiflexion showed a significant improvement from a median of 8.8° before injection (CI 3.2° to 11.9°) to a median of 12.1° after injections (CI 9.8° to 17.1°) combined with a trend for deterioration in maximum knee extension and a worsening of the PFKE index. The explanation of this novel finding is that the ‘improvements’ in ankle dorsiflexion were offset by a mild deterioration in sagittal knee function (Figs 1 and 2) and other gait parameters. Therefore, the ‘improvement’ in ankle dorsiflexion is likely to be at the expense of decreased ankle PFKE coupling i.e. as ankle dorsiflexion improved, midstance knee extension deteriorated (Fig. 3). The most common long-term gait problem in children with diplegia is not equinus gait, but crouch gait.23 Therefore, it is a concern that the improvements gained in ankle dorsiflexion in children with diplegia did not translate to improvements in overall gait function but a trend towards deterioration at other levels. Clinical examination, video gait analysis, observational gait scales and goal attainment scaling are insensitive for objective measurement of overall gait function or decline at proximal levels. Again, our findings demand an examination of BoNT-A protocols in children with bilateral involvement. Clinicians should be aware of the potential for deterioration in PFKE coupling, and the possibility that repeated injections for spastic equinus might increase the frequency and severity of crouch gait in later childhood.
Fig. 3.

The pattern of response before and after bilateral injections of BoNT-A for spastic equinus in a child with bilateral spastic cerebral palsy. Pre-injection there is a moderate degree of spastic equinus with the knees and hips extended. Post-injection, ankle dorsiflexion has increased with increased knee flexion and mild crouch gait. Illustration drawn by Bill Reid, H. Kerr Graham, Educational Resource Centre, The Royal Children’s Hospital, Parkville, Australia.
This study has several limitations. Children had participated in the preceding RCT and were of too young an age at RCT entry for IGA and therefore GPS to be undertaken. The effects of BoNT-A naivety or previous exposure may affect response and therefore kinematics pre- or post-injection. Kinematic changes were only assessed at the one-time interval after final injection, and it is possible that further changes may have occurred following the IGA at four weeks following injection, affecting stability of the GPS. This study has relevance for ambulatory children with CP only; the efficacy of BoNT-A injections to reduce spasticity in non-ambulatory children has not been addressed and would require different outcome measures. Gait-related outcome measures for other domains of the International Classification of Functioning, Disability and Health (ICF) were not collected and this limits the findings in relation to activity and participation.
We are, however, firmly in support of the use of 3D kinematics and summary statistics of gait to assess the limitations and potential harms of BoNT-A injections for spastic equinus. By relying on visual gait analysis and clinical examination, we mistakenly selected children with mild crouch gait for injection of their calf muscles (Fig. 2). Reliance on surrogate end points such as measures of spasticity or dynamic gait function using insensitive tools are not enough to resolve this important question. Injections of BoNT-A have been found in a recent study to offer little benefit in the areas of gross motor function, levels of physical activity or quality of life.26 If there is no improvement in gait function above the MCID, re-evaluation of current BoNT-A injection protocols is urgently required.11 This is not the first study which has shown the potential for gait deterioration after injection of BoNT-A in children with CP.27 In a large study of 110 children with CP, who were being considered for muscle-tendon lengthening surgery, 21% showed clinically significant deterioration in gait, using 3D gait analysis.27 Clearly, refining indications, improved patient selection and more conservative injection protocols should be urgently considered.
Acknowledgements
We thank the children and families who participated in the study, as well as staff involved in data collection in the Gait Laboratory. The study was funded by the Australian National Health and Medical Research Council, Grant Number: 454705. The funding body was not involved in study design, data collection, data analysis, manuscript preparation or publication decisions. No pharmaceutical company sponsorship was received in support of this clinical trial. We also acknowledge support provided by the National Health and Medical Research Centre; Centre of Research Excellence in Cerebral Palsy.
Compliance with ethical standards
Funding statement
This study was funded by the Australian National Health and Medical Research Council. Grant Number: 454705.
OA Licence Text
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) license (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Ethical statement
Ethical approval: Ethical approval was given by IRB Number 27062C and 07083C.
Informed consent: Written, informed consent was obtained from the parents of all children before study inclusion.
ICMJE Conflict of interest statement
BR reports research grants and consultant honoraria from Allergan, the manufacturer of Onabotulinum toxin-A and Ipsen Pharmaceuticals.
The remaining authors report no conflicts of interest or financial relationships relevant to this article. No pharmaceutical company sponsorship was received in support of this study.
References
- 1.Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primers 2016;2:15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ade-Hall RA, Moore AP. Botulinum toxin type A in the treatment of lower limb spasticity in cerebral palsy [Review]. Cochrane Database Syst Rev 2000;CD001408. [DOI] [PubMed] [Google Scholar]
- 3.Simpson DM, Gracies JM, Graham HK, et al. Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008;70:1691-1698. [DOI] [PubMed] [Google Scholar]
- 4.Ryll U, Bastiaenen C, De Bie R, Staal B.. Effects of leg muscle botulinum toxin A injections on walking in children with spasticity-related cerebral palsy: a systematic review. Dev Med Child Neurol 2011;53:210-216. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum PL, Walter SD, Hanna SE, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA 2002;288:1357-1363. [DOI] [PubMed] [Google Scholar]
- 6.Eames NW, Baker R, Hill N, et al. The effect of botulinum toxin A on gastrocnemius length: magnitude and duration of response. Dev Med Child Neurol 1999;41:226-232. [DOI] [PubMed] [Google Scholar]
- 7.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206-207. [DOI] [PubMed] [Google Scholar]
- 8.Gracies JM, Burke K, Clegg NJ, et al. Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Arch Phys Med Rehabil 2010;91:421-428. [DOI] [PubMed] [Google Scholar]
- 9.Delgado MR, Tilton A, Russman B, et al. Abobotulinumtoxin A for equinus foot deformity in cerebral palsy: a randomized controlled trial. Pediatrics 2016;137:e20152830. [DOI] [PubMed] [Google Scholar]
- 10.Yam WKL, Leung MSM. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol 2006;21:1031-1035. [DOI] [PubMed] [Google Scholar]
- 11.Read FA, Boyd RN, Barber LA. Longitudinal assessment of gait quality in children with bilateral cerebral palsy following repeated lower limb intramuscular Botulinum toxin-A injections. Res Dev Disabil 2017;68:35-41. [DOI] [PubMed] [Google Scholar]
- 12.Baker R. Gait analysis methods in rehabilitation. J Neuroeng Rehabil 2006;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corry IS, Cosgrove AP, Duffy CM, et al. Botulinum toxin A compared with stretching casts in the treatment of spastic equinus: a randomised prospective trial. J Pediatr Orthop 1998;18:304-311. [PubMed] [Google Scholar]
- 14.Sutherland DH, Kaufman KR, Wyatt MP, Chambers HG, Mubarak SJ. Double-blind study of botulinum A toxin injections into the gastrocnemius muscle in patients with cerebral palsy. Gait Posture 1999;10:1-9. [DOI] [PubMed] [Google Scholar]
- 15.Baker R, McGinley JL, Schwartz MH, et al. The gait profile score and movement analysis profile. Gait Posture 2009;30:265-269. [DOI] [PubMed] [Google Scholar]
- 16.Baker R, McGinley JL, Schwartz M, et al. The minimal clinically important difference for the Gait Profile Score. Gait Posture 2012;35:612-615. [DOI] [PubMed] [Google Scholar]
- 17.Thomason P, Baker R, Dodd K, et al. Single-event multilevel surgery in children with spastic diplegia: a pilot randomized controlled trial. J Bone Joint Surg [Am] 2011;93-A:451-460. [DOI] [PubMed] [Google Scholar]
- 18.Hastings-Ison T, Blackburn C, Rawicki B, et al.. Injection frequency of botulinum toxin A for spastic equinus: a randomized clinical trial. Dev Med Child Neurol 2016;58:750-757. [DOI] [PubMed] [Google Scholar]
- 19.Graham HK, Aoki KR, Autti-Rämö I, et al.. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture 2000;11:67-79. [DOI] [PubMed] [Google Scholar]
- 20.Sangeux M, Rodda J, Graham HK. Sagittal gait patterns in cerebral palsy: the plantarflexor-knee extension couple index. Gait Posture 2015;41:586-591. [DOI] [PubMed] [Google Scholar]
- 21.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214-223. [DOI] [PubMed] [Google Scholar]
- 22.Hägglund G, Wagner P.. Development of spasticity with age in a total population of children with cerebral palsy. BMC Musculoskelet Disord 2008;9:150-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borton DC, Walker K, Pirpiris M, Nattrass GR, Graham HK. Isolated calf lengthening in cerebral palsy. Outcome analysis of risk factors. J Bone Joint Surg [Br] 2001;83-B:364-370. [DOI] [PubMed] [Google Scholar]
- 24.Hastings-Ison T, Blackburn C, Opie NL, et al.. Reproducibility of an instrumented measure for passive ankle dorsiflexion in conscious and anaesthetized children with cerebral palsy. Dev Med Child Neurol 2014;56:378-385. [DOI] [PubMed] [Google Scholar]
- 25.Catsman-Berrevoets CE, Bussmann JBJ, Pangalila RF, et al.. Treatment with botulinum toxin of children with cerebral palsy has no added therapeutical value or cost effectiveness for gross motor function, everyday physical activity levels or quality of life when combined with intensive functional physiotherapy. Eur J Paediatr Neurol 2015;19:S11. [Google Scholar]
- 26.Minamoto VB, Suzuki KP, Bremner SN, Lieber RL, Ward SR. Dramatic changes in muscle contractile and structural properties after 2 botulinum toxin injections. Muscle Nerve 2015;52:649-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutz E, Hofmann E, Brunner R. Preoperative botulinum toxin test injections before muscle lengthening in cerebral palsy. J Orthop Sci 2010;15:647-653. [DOI] [PubMed] [Google Scholar]


