Abstract
Background
Proximal femoral growth disturbance (PFGD) can be the most devastating complication of the treatment of development dysplasia of the hip. The reported incidence ranges from 0% to 73%. The condition involves varying degrees of growth disturbances of the femoral capital epiphysis, the physeal plate or both.
Purpose
This manuscript will discuss normal growth and development of the hip, the blood supply to the upper end of the femur, pathological and radiographic changes, classifications used to describe PFGD and, most importantly, the potential causes of these growth disturbances and the authors’ strategies for avoiding PFGD.
Keywords: proximal femoral growth disturbance, developmental dysplasia of the hip, developmental hip dislocation, aseptic necrosis
Introduction
The most devastating complication after the treatment of developmental dysplasia of the hip (DDH) is what is generally termed avascular or aseptic necrosis (AVN). AVN often dooms the hip to early-onset osteoarthritis (OA). The global incidence of the condition in the literature varies from 0% to 73%.1–13 The variation in incidence encompasses results from all treatments; from the use of a Pavlik harness in the newborn period to the complex procedures often used in addressing late-diagnosed cases. The condition involves varying degrees of growth disturbance of either the femoral capital epiphysis, the physeal plate or both. As no pathological case of AVN after treatment of DDH has ever been reported, we prefer to use the term ‘proximal femoral growth disturbance’ (PFGD). In this manuscript, we will discuss normal growth and development of the hip, the blood supply to the upper end of the femur, the pathological and radiographic changes seen in untreated and treated DDH, classifications used to describe PFGD and, most importantly, discuss the potential causes of these growth disturbances and avoidance strategies.
Normal growth and development of the hip joint
Knowledge of the normal growth and development of the hip joint is essential to the understanding of PFGD.14 For the hip joint to grow and develop normally, there must be a genetically-determined balance of growth of the acetabular and triradiate cartilages and a well-located and centered femoral head. Embryologically, the components of the hip joint, the acetabulum and the femoral head, develop from the same primitive mesenchymal cells.15,16 A cleft develops in the pre-cartilaginous cells at about the seventh week of gestation and by week 11 the hip joint is fully formed. Although rare, this is also the earliest possible time that a dislocation could occur. Acetabular development continues throughout intrauterine life, particularly by means of growth and development of the labrum.17
In the infant, the entire proximal end of the femur, including the greater trochanter, the intertrochanteric zone and the proximal femur, is composed of cartilage. The proximal femoral ossification centre appears between the fourth and seventh months of life. This bony centrum and its cartilaginous anlage continue to enlarge until adult life, at which time only a thin layer of articular cartilage remains.
The proximal femur and the trochanter enlarge by appositional cartilage cell proliferation. The three main growth areas in the proximal femur are the physeal plate, the growth plate of the greater trochanter and the femoral neck isthmus18 (Fig. 1) Balance in the growth rates of these centres accounts for the normal configuration of the proximal femur, the relation between the proximal femur and the greater trochanter and the overall width of the femoral neck. The growth of the proximal femur is affected by muscle pull, the forces transmitted across the hip joint by weight-bearing, normal joint nutrition, circulation and muscle tone.18–20 Any alterations in these factors, by whatever mechanism, may cause profound changes in its development,21,22 even in untreated dislocations (Fig. 2). Hyperemia secondary to any of the various DDH surgeries may also stimulate growth in any or all of these growth plates and alter the shape of the proximal femur.18
Fig. 1.
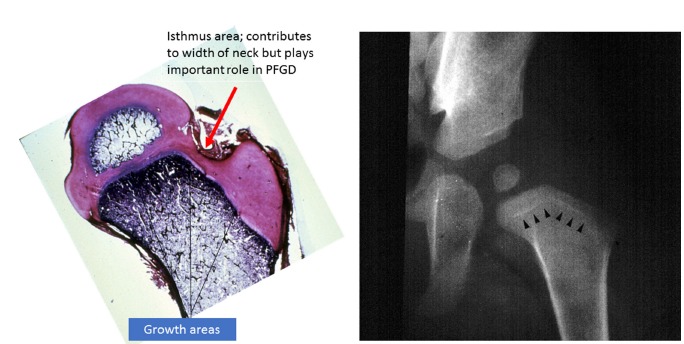
Left: growth zones in the proximal femur in a young child. Note the isthmus where the lateral ascending cervical traverses. Right: growth arrest lines (O’Brien’s lines) after closed reduction of developmental dysplasia of the hip (PFGD, proximal femoral growth disturbance).
Fig. 2.
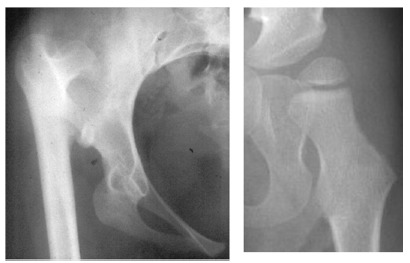
Left: adult with untreated developmental dysplasia of the hip. Note that the growth of proximal femur is abnormal. Right: hip dysplasia in a patient with neuromuscular disease.
During infancy, a small cartilaginous isthmus (Fig. 1) connects the trochanteric and femoral growth plates along the lateral border of the femoral neck (reflecting their previous common origin). The isthmus contributes to the lateral width of the femoral neck and remains active until maturity. Although small, the isthmic area plays an important role in the development of PFGD.
The proximal femoral physeal plate contributes to approximately 30% of the overall length of the femur and 13% to the entire limb. Any damage to, or disruption of, the blood supply to the plate disrupts the growth and results in a varus deformity as the trochanter and the growth plate along the femoral neck continue to develop normally.18,23 The relation between the growth of the trochanter and the physis of the proximal femur should remain constant. The greater trochanter is usually classified as a traction epiphysis, requiring the normal abductor pull for growth stimulation. The trochanter, like the proximal femur, grows appositionally. Partial physeal arrest patterns may be caused by damage to portions of the proximal femoral physeal plate.
On the acetabular side of the joint, the entire acetabular cartilage complex is composed of very cellular hyaline cartilage (Fig. 3). The lateral portion of the acetabular cartilage is homologous with other epiphyseal cartilages of the skeleton.24 The labrum, or fibrocartilaginous edge of the acetabulum, is at the margin of the acetabular cartilage.
Fig. 3.
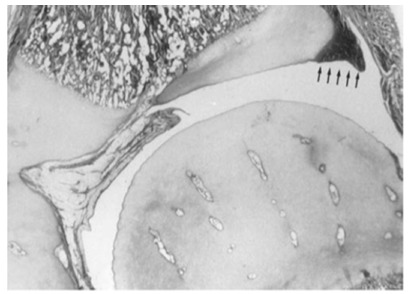
Hip joint in an infant. Note the vascular channels in the cartilaginous femoral head and the acetabular cartilage and labrum at the periphery (reproduced with permission from Weinstein SL, Flynn JJ, eds. Lovell and Winter’s Pediatric Orthopaedics. Vol. 2. Seventh ed. Philadelphia: Lippincott Williams & Wilkins, 2014).14
Articular cartilage covers the acetabular cartilage on the side that articulates with the femoral head. On the opposite side is a growth plate, with its degenerating cells facing toward the pelvic bone that it opposes. New bone formation occurs in the metaphysis adjacent to the degenerating cartilage cells. Growth of the acetabular cartilage occurs by means of interstitial growth within the cartilage and appositional growth under the perichondrium. Acetabular cartilage forms the outer two-thirds of the acetabular cavity, and the nonarticular medial wall of the acetabulum is formed by a portion of the ilium above, the ischium below and portions of the triradiate cartilage. Interstitial growth within the triradiate cartilage causes the hip joint to expand in diameter during growth. After birth, continued growth of the proximal femur and the acetabular cartilage complex is extremely important to the continuing development of the hip joint.16,24–26 The growth of these two components of the hip joint is interdependent and a key factor in outcomes of patients with a PFGD. Of particular concern in this context is the presence of persistent acetabular dysplasia, either primarily from the DDH or as a result of PFGD. Dysplasia is a major contributing factor to the development of OA.1,8,9,11,27–30
In DDH, the majority of pathological changes are seen on the acetabular side of the joint.31 The changes seen on the femoral side in untreated DDH include excessive anteversion and shape changes in the cartilaginous analogue as a result of the presence and duration of subluxation or dislocation. As in normal development of the proximal femur, the shape and growth of the untreated proximal femur in DDH is affected by muscle pull, the forces transmitted across the hip joint in its subluxated or dislocated position and by weight-bearing, normal joint nutrition, circulation and muscle tone (Fig. 2).
The key question in discussing outcomes in cases with PFGD is: is the PFGD seen at maturity caused by the damage or growth alteration incurred prior to treatment (as a result of the forces in the subluxated or dislocated position) or is the PFGD a result of treatment, or a combination of both?
While the manuscript addresses PFGD, the ultimate end result for each patient is also determined by the relationship of the femoral head and the acetabulum at maturity.32,33 The complexities surrounding the development of OA in these patients cannot only be viewed through the lens of the PFGD. We know that the shape of the acetabulum depends on the geometric pattern within it during growth.34 Hence if the acetabular side of the joint is normal it may accommodate alterations in shape of the proximal femur due to PFGD. This, however, is a big ‘if’, as the majority of the pathological changes in DDH are on the acetabular side of the joint.31 These abnormalities may limit the ability of the acetabulum to accommodate changes in the proximal femoral anatomy, and are intimately related to patient long-term outcomes.
Blood supply to the proximal femur
There are three main sources of blood supply to the proximal femur: an extracapsular arterial ring; the ascending cervical (retinacular branches) vessels; and the artery of the ligamentum teres35 (Fig. 4). The extracapsular ring is formed mostly by the medial and lateral femoral circumflex vessels. This ring gives rise to the ascending cervical branches, which are extracapsular, and these in turn give rise to the metaphyseal and epiphyseal branches. The anterior portion of the extracapsular ring is formed primarily by the lateral femoral circumflex artery. The posterior, lateral and medial aspects of the ring are formed by the medial femoral circumflex artery. Chung35 found that the greatest volume of blood flow to the femoral head comes through the lateral ascending cervical vessel (the termination of the medial femoral circumflex artery). This corresponds to the lateral epiphyseal artery described by Trueta36 which crosses the capsule in the posterior trochanteric fossa (Fig. 1). The all-important lateral ascending cervical artery passes through this fossa, which is extremely narrow in children under eight years of age, making it a potential source of disruption of proximal femoral blood flow.35 Before the appearance of the secondary ossification centre of the proximal femur, branches of the ascending cervical artery penetrate the head and terminate in sinusoidal expansions which will eventually supply the ossification centre(s) of the proximal femur35 (Fig. 1). Trueta36 and Chung35 demonstrated that the anterior vascular anastomotic network is much less extensive than the posterior anastomotic network, particularly in specimens taken from patients aged three to ten years. Ogden37 reported the presence of vessels crossing the physeal plate in some of his specimens, but Chung35 disagreed, suggesting instead that the vessels do not actually cross the plate, but pass through the peripheral perichondral fibrocartilaginous complex.
Fig. 4.
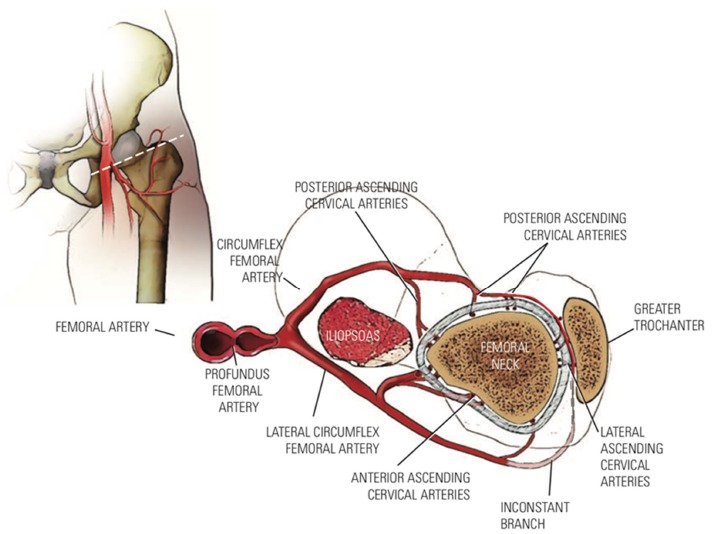
Blood supply to the proximal femur (reproduced with permission from Weinstein SL, Flynn JJ, eds. Lovell and Winter’s Pediatric Orthopaedics. Vol. 2. Seventh ed. Philadelphia: Lippincott Williams & Wilkins, 2014).14
The physeal plate is an absolute barrier to blood flow between the epiphysis and the metaphysis,38–40 with the epiphyseal and metaphyseal vessels originating from the same ascending cervical branches. There is an anastomosis between these two circulations on the bone surface but not within the bone. The metaphyseal area is well supplied by many small metaphyseal arteries while the epiphyseal side lacks this extensive network, making it more vulnerable to disruption.35 Trueta and Amato41 demonstrated experimentally that the epiphyseal circulation is responsible for the nourishment of the physeal plate cartilage while the metaphyseal circulation is responsible for calcification of the cartilaginous matrix, removal of degenerative cells and laying down of the boney matrix.
Incidence and classifications
The aetiology of PFGD is speculative. Abnormalities resembling those seen in humans with treated DDH and PFGD have been produced experimentally by creating vascular injuries in animals. These growth disturbances may be caused by vascular insults to the epiphysis or the physeal plate, or by pressure injury to the epiphyseal cartilage and/or the physeal plate.7,27,37,39,42–54 Interestingly, although uncommon, PFGD may also occur in the contralateral ‘normal hip’ in a patient being treated for DDH.55–58
The reported incidence of PFGD varies, perhaps because authors do not agree on what specific radiographic features constitute a growth disturbance.11 Thomas et al30 concluded that the reported incidence in a given series was due to the rigor with which the diagnosis had been sought. Three PFGD classifications are used most frequently in the literature: Salter et al,59 Bucholz and Ogden7 and Kalamchi and MacEwen.49 There are few studies of the intra- and interobserver reliability of these classifications60–62 or about how reliability (or lack thereof) may influence the relationship between PFGD and the long-term outcome. In addition, as many as 25% of hips may not fit into one of the above-mentioned classifications.
The most widely-used PFGD classification is that of Salter et al59 (Fig. 5). The inclusion of coxa magna as a sign of PFGD in this classification is questionable, because coxa magna is often seen after open reduction as a result of the stimulation of blood flow to the proximal femur.63–65 As noted above, it is also often difficult to ascertain whether some of the residual deformities seen after treatment are secondary to disturbances existing before the reduction, or the result of complications associated with the reduction. One of the most common deformities seen is the flattening of the medial aspect of the proximal femur, which may result from the pressure of the femoral head lying against the ilium prior to reduction.
Fig. 5.
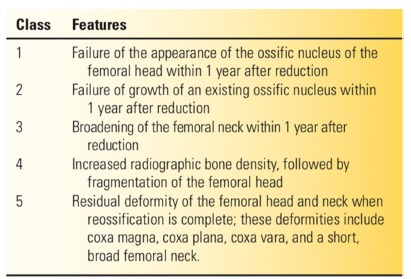
Salter classification of proximal femoral growth disturbance (reproduced with permission from Weinstein SL, Flynn JJ, eds. Lovell and Winter’s Pediatric Orthopaedics. Vol. 2. Seventh ed. Philadelphia: Lippincott Williams & Wilkins, 2014).14
The classification systems of Bucholz and Ogden7 and Kalamchi and MacEwen49 characterize growth disturbance in the capital femoral epiphysis after treatment. They suggested early recognition of growth disturbance patterns based on the degree of involvement of the physis rather than changes in the ossific nucleus alone. Attention to the physis may result in more accurate anticipation of subsequent problems and residual deformities of the proximal femur and thus can be helpful in planning additional treatment. Unfortunately, the deformity seen at maturity cannot be predicted during the early stages of PFGD in certain patterns of physeal arrest.30,46,49,66–68
Factors implicated in or contributing to the development of PFGD
Factors contributing to, or preventing, the development of PFGD include the use of pre-reduction traction,2,9,11,13,49,69,70 adductor tenotomy,43,59,71,72 open or closed reduction,9,19,27,42,45,49,73–80 the force applied during reduction,5,72,81 the position of postoperative immobilization,7,9,13,37,42,51,59,82–84 soft-tissue interposition45,85,86 and the age at reduction.9,51,83,87
The German Society for Orthopaedics and Traumatology did an extensive study on the development of PFGD.88,89 Conservatively- and operatively-treated hips were evaluated to determine the factors associated with the development of PFGD. The associated factors included: high dislocations and dislocations with an inverted labrum; narrowing of the introitus between the superior labrum and the transverse ligament in the position of reduction; inadequate depth of reduction of the femoral head (> 3 mm from the acetabular floor); the age of the patient (> 12 months); immobilization ≥ 60° of abduction because of joint instability; and use of adductor tenotomy.
Several of the above factors thought to be associated with an increased incidence of PFGD have been documented in the clinical setting as well as experimentally, including extreme positioning of the proximal femur in abduction and abduction with high degrees of medial rotation. Such positioning can cause compression of the medial femoral circumflex vessel as it passes between the iliopsoas tendon and the pectineus, and compression of the terminal branch between the lateral femoral neck and the acetabular margin.37,59,90 Anatomical and experimental investigations have consistently shown that forceful internal rotation with concomitant abduction, and extreme abduction alone (e.g. the Lorenz position), can compromise the blood flow to the capital femoral epiphysis. If the hip is maximally abducted against firm resistance, blood flow can be completely or almost completely arrested. The same is true in forced internal rotation. Blood vessels and the blood supply to the proximal femur can be occluded by compression, either outside the femoral head or as the vessels cross through the epiphyseal cartilage.39,52,59,91 Canine studies have shown a diminution of epiphyseal perfusion with increasing pressure, which was relieved after the external fixation device was removed.42,43,92 The extreme position of abduction, frequently called the frog-leg position, used in cases of unrelieved adduction contracture, uniformly results in severe growth disturbances of the epiphysis.59,92,93 Extreme positions can also cause pressure necrosis of the vulnerable epiphyseal cartilage and the physeal plate, as shown in experiments by Law et al43 and by Schoenecker et al.92 These studies and others demonstrate the severe effects of cartilage necrosis.52,89 Interference with growth in a rabbit model was directly proportionate to the damage caused by compression to the epiphyseal side of the growth plate, and, in general, to the duration of compression. Persistent compression affects the growth plate by interference with the blood flow on one or both sides of the growth cartilage.91
Severin advocated placing the femoral head in close apposition to the acetabulum to induce regression of the obstacles to reduction,74 forcing the labrum to develop a spherical contour by applying pressure with the femoral head. This manoeuvre can be used for obtaining reduction, but the price may be an increased incidence of PFGD.72,89 In our study of arthrograms and observations during open reduction after failed closed reductions94 we found the intra-articular obstacles to reduction to be the anteromedial joint capsule, enlarged ligamentum teres and the transverse acetabular ligament. In our opinion, PGFD can also be precipitated by circumscribed pressure, created by using the vulnerable femoral head as a ‘dilating sound’ (Fig. 6) and other such manoeuvres to overcome these obstacles.
Fig. 6.
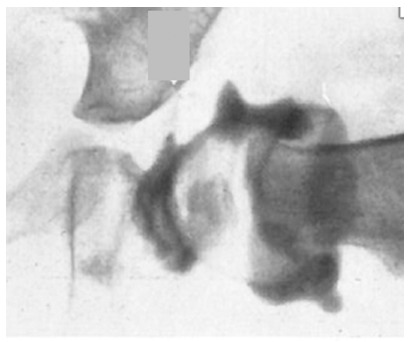
Arthrogram from an attempted closed reduction with the femoral head incompletely reduced. Note the distortion of the peripheral acetabular tissue, the infolded ligamentum teres and the large medial dye pool.
Pre-reduction traction has been used for decades to facilitate closed reduction, decrease the need for open reduction and to decrease the incidence of PFGD. Although there are several impressive reports of the positive effects of traction on reduction,95–97 there are no clinical or experimental studies of the direct effect of traction on the development of PFGD. Most clinical series poorly document even the variables associated with the use of traction or patient treatment including the relationship of traction weight versus the patients’ body weight; the inability to quantify the effect of traction on the various obstacles to reduction; the estimation of actual amounts of hip flexion, abduction and internal rotation; the subjectivity in determining end points as to when to discontinue traction; the subjective estimation of positioning in casts even when using the so-called human position; and the effect of treatments before the use of traction on the obstacles to reduction and on the blood supply to the proximal femur or on the nutrition of epiphyseal and physeal cartilage. Despite its widespread use, several studies and reviews have questioned the practice.27,30,44,73,88,98–100 Traction has rarely been used at our institution for the last 40 years.
Age at reduction is also thought to be a factor as the incidence of PFGD increases with delay in reduction and younger patients tend to have a lower rate of growth disturbance.1,7,9,59,74,79,83,87,92,101 Kalamchi and MacEwen,49 however, documented an increase in the incidence of the severe form of PFGD (Type IV) in younger patients. Salter et al59 and Ogden37 proposed that the femoral head in DDH is most vulnerable to ischemic changes during the first 12 to 18 months of life, when it is composed mostly of cartilage. According to some authors, the risk of total head involvement becomes somewhat less after the appearance of the femoral ossific nucleus,102,103 although, as noted earlier, this concept has been challenged.74,93,101,104,105
Outcomes of PFGD
The Type II pattern, lateral physeal arrest7,49 is the most common pattern of growth disturbance reported, seen in approximately 25% of cases.1,106 It may be difficult to identify this pattern in its early stages, and it may not be evident until a patient is older than 12 years of age (Fig. 7). Therefore, series with a shorter follow-up period may underestimate the prevalence of Type II.30,46,49,66,67
Fig. 7.
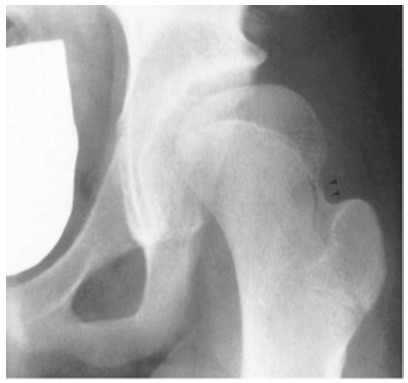
Example of a hip with a Type-II proximal femoral growth disturbance.
Type-II is characterized by retarded growth in the lateral aspect of the physis or by premature lateral fusion, resulting in the subsequent development of valgus deformity of the head on the neck. The pathogenesis of a Type-II growth disturbance is unknown, but several hypotheses suggest mechanical or ischemic insults. One possible explanation is a growth disturbance of the germinal layer of the lateral part of the femoral physis or an abnormally sustained compressive force transmitted through the epiphysis.46 Given that this type of growth disturbance is often not evident until a bar develops as the cartilage ossifies,18,46 and the fact that the ossification of the subcapital growth plate normally begins on the lateral side and progresses medially,18,107 may explain the late appearance of valgus tilt of the femoral head.
Associated problems with femoral coverage, as a result of progressive valgus deformity and subsequent poor acetabular development, are assumed to occur more frequently in these patients. We performed a retrospective study to evaluate acetabular development in patients with a Type-II growth disturbance after reduction for the treatment of DDH.68 We documented acetabular development over an average of 21 years (range, 10 to 55 years) in 48 patients (58 hips). Lateral tilting of the epiphysis was noted between four and ten years of age. Serial radiographs did not suggest any consistent, early patterns of change in the physis predicting development of growth arrest. Variable degrees of localized premature fusion or even irregularity in the lateral aspect of the physis and adjacent metaphysis were detected. In addition, substantial osseous bridging across the superior portion of the physeal plate could not be clearly identified early after reduction in many hips. Regarding epiphyseal changes, Bucholz and Ogden7 observed that the secondary ossification centre always demonstrates changes at some point following reduction. However, 12 (21%) of the hips in our series did not show any changes in the ossific nucleus, a finding that is consistent with other observations.46,49 In all, 17 hips (29%) showed complete irregular fragmentation after reduction. Whether this represented damage to the epiphyseal cartilage or merely multiple ossification centres that eventually coalesced could not be determined. Our conclusion that ossific nucleus changes alone have no prognostic importance supports earlier work.49,50,106,108 We also looked at the predictability of O’Brien’s lines.109 We observed hips with normal growth lines that nonetheless developed a Type-II growth disturbance. Moreover, the intensity of these lines was variable, perhaps because some radiographs were not taken with the hip in full internal rotation.68 At the last follow-up, 59% of the hips were classified as Severin I/II. By six to eight years of age (frequently before the development of lateral tilt), differences in the average acetabular angle, acetabular quotient, acetabular roof angle and percentage femoral head coverage were noted between the Severin I/II and Severin III/IV hips. The lower percentage of Severin III/IV classification seen in Type-II relative to Type-III PFGD (41% versus 90%) is most likely the result of the relatively late development of Type-II PFGD, by which time the majority of acetabular development is complete. Thus, the relationship between the femoral head and the acetabulum may be relatively normal, resulting in a better long-term prognosis.68 The residual acetabular dysplasia at the time of the final follow-up is more reflective of an already poorly-developed acetabulum prior to any evidence of lateral physeal arrest. Thus, monitoring acetabular development after closed or open reduction is as important, if not more important, to long-term outcome than searching for radiographic changes of physeal arrest, which are difficult to detect in young children.68 We found no association with reduction quality, older age at reduction or femoral head deformity with acetabular dysplasia. The fact that both satisfactory and unsatisfactory hips showed maintenance of reduction and equivalent acetabular development at two years after treatment ruled out early incongruence or residual dysplasia as the reason for the difference in later outcomes.
Acetabular dysplasia did develop in 41% of the hips in this series, but it appears that the dysplasia preceded the appearance of a Type-II PFGD. Acetabular development was already inadequate in the Severin III and IV hips by approximately seven years of age. This was prior to the appearance of the growth disturbance, which was noted at an average of ten years of age. At no time during the development of the acetabulum was the degree of valgus tilt of the femoral head prognostic of outcome. There is also the possibility of the hip classification changing over time. For example, involvement of the lateral portion Type-III or Type-IV growth disturbance.49,106
The long-term outcomes of Type-III PFGD are significantly different.12 According to Bucholz and Ogden7 and Kalamchi and MacEwen,49 Type-III hips (Kruczynski Type-V)88 sustain severe damage to the femoral head and the central part of the physis, characterized by symmetrical growth retardation of the femoral neck, relative over growth of the greater trochanter and abnormal growth of the entire epiphysis, coxa vara, limb length discrepancy and eventual OA (Fig. 8).88 The prevalence of Type-III has been estimated to range from 14% to 30%.1,66,88
Fig. 8.
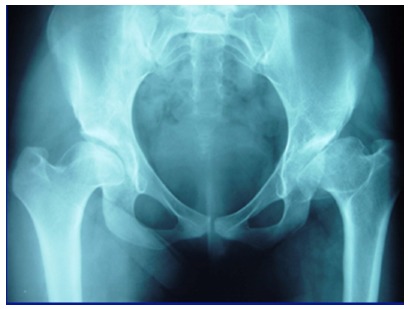
Example of a hip with a Type-III proximal femoral growth disturbance.
We evaluated the long-term outcome of 29 hips in 22 patients who developed Type-III PFGD, after treatment by either closed or open reduction. These hips were compared with similarly treated hips without a growth disturbance,12,110 focusing on acetabular development and the prevalence of OA. The growth disturbance was apparent five to 19 months after reduction. The odds of developing a Type-III PFGD were three times greater in high dislocations (Tönnis grade 4) relative to grades 2 or 3, independent of the treatment performed. We did not find a significant difference in the risk of Type-III due to the age at reduction or the presence or absence of the femoral ossific nucleus. Acetabular remodelling proceeded normally in these hips until approximately five years after reduction, when development slowed in the Type-III hips resulting in an upsloping or horizontal sourcil in 90%. OA is an almost certainty in Severin III/IV hips. At skeletal maturity, 90% of the hips were Severin III/IV compared with 35% of controls, and 24% had already developed OA. Type-III remains the most severe and devastating complication after treatment of DDH.
Avoidance strategies
With respect to avoidance strategies, opinions abound. The senior author’s strategy is based on our continuous cycle of evidence review, application to practice and outcomes assessment begun in 1915 with the establishment of our academic department.111 On the basis of our personal and institutional reviews and the information presented earlier in this manuscript, the following general principles are applied to the treatment of DDH in hopes of lessening the incidence of PFGD. The majority of children with DDH diagnosed at less than one year of age can be successfully treated by closed means. A Pavlik harness is used for patients less than six months of age. We perform closed reduction in the operating room when the hip remains unreduced despite a Pavlik harness, or for children between six and 12 months of age (when the harness has a low likelihood of success). Closed reduction is routinely accompanied by adductor tenotomy and release of the extra-articular obstacles to reduction (adductor longus and the iliopsoas) by sectioning through an anteromedial approach. If anatomical reduction is obtained (no dye pooling medially and restoration of normal coverage and shape of the peripheral acetabulum) and documented by arthrogram, a cast is applied in the ‘human position’. If anatomical reduction is not attained, we proceed with open reduction. At no time is the vulnerable femoral head used as a dilating sound to overcome the intra-articular obstacles to reduction. Over one year of age, the chance of successful closed treatment steadily decreases. Thus, the closer the child is to 18 months of age, the more likely open reduction will be required, which we do through the anteromedial approach, as this is the most direct approach to the obstacles to reduction.73,94 Patients with high dislocations (Tönnis grade 4) are treated using the anterior approach. Our series suggests that > 70% of hips treated with open reduction alone will undergo satisfactory acetabular remodelling, resulting in a Severin I or II classification at maturity; hence, no concurrent secondary procedures are included.66,110 We examine children every three to six months, watching for qualitative improvement in the teardrop, acetabular development through measures of the acetabular index and the acetabular floor thickness and the appearance of accessory centres of ossification in the acetabular cartilage. For children diagnosed around 24 months of age, we are more likely to accompany open reduction with an acetabular procedure and possibly femoral shortening, because the probability of persistent dysplasia in this age group is approximately 50%.110 In this situation, we accept the fact that we may be overtreating some hips, but believe the high probability of residual dysplasia and early development of OA justify additional surgery. There is good evidence that femoral anteversion will correct spontaneously after reduction, and therefore, we do not routinely add procedures on the femoral side. We never use traction prior to closed or open treatment, but in high dislocations, we consider adding femoral shortening, with anteversion correction, if we feel that pulling the femoral head down to the acetabulum would be difficult even with open treatment, in the hopes of decreasing the risk of PFGD. A certain number of hips, despite excellent early results, may still have biological failure of acetabular development. As Steindler et al112 observed, “We are dealing with a congenital deformity which has a strong tendency to persist”. Our data suggest the first two to three years post-reduction are critical to normalization of the hip. If the acetabular index is not decreasing into the normal range, we intervene with an acetabular osteotomy to hopefully prevent or delay the development of hip OA.
Conclusion
In patients with DDH, PFGD is considered the most disastrous complication of either closed or open treatment. While the reliability of classification systems may be problematic, it is clear that any disturbance of proximal femoral growth may jeopardize the long-term outcome even in the face of normal acetabular development. We still have much to learn about the complex growth interactions in a DDH hip compromised by a PFGD.
Compliance with ethical standards
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
OA Licence Text
This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) license (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Ethical statement
Ethical approval: No human subjects review or informed consent from human participants was required for this review article.
ICMJE Conflict of interest statement
The authors have no potential conflicts of interest related to this article.
References
- 1.Malvitz TA, Weinstein SL. Closed reduction for congenital dysplasia of the hip. Functional and radiographic results after an average of thirty years. J Bone Joint Surg [Am] 1994;76-A:1777–1792. [DOI] [PubMed] [Google Scholar]
- 2.Berkeley ME, Dickson JH, Cain TE, Donovan MM. Surgical therapy for congenital dislocation of the hip in patients who are twelve to thirty-six months old. J Bone Joint Surg [Am] 1984;66-A:412–420. [PubMed] [Google Scholar]
- 3.Rab GT. Preoperative roentgenographic evaluation for osteotomies about the hip in children. J Bone Joint Surg [Am] 1981;63-A:306–309. [PubMed] [Google Scholar]
- 4.Hansson LI, Olsson TH, Selvik G, Sundén G. A roentgen stereophotogrammetric investigation of innominate osteotomy (Salter). Acta Orthop Scand 1978;49:68–72. [DOI] [PubMed] [Google Scholar]
- 5.Crego CH Jr, Schwartzmann JR. Follow-up study of the early treatment of congenital dislocation of the hip. J Bone Joint Surg [Am] 1948;30-A:428–442. [PubMed] [Google Scholar]
- 6.Kalamchi A, Schmidt TL, MacEwen GD. Congenital dislocation of the hip. Open reduction by the medial approach. Clin Orthop Relat Res 1982;169:127–132. [PubMed] [Google Scholar]
- 7.Bucholz R, Ogden J, editors. Patterns of ischemic necrosis of the proximal femur in nonoperative treated congenital hip disease. Sixth Open Scientific Meeting of the Hip Society. 1978. St. Louis, MO: C. V. Mosby. [Google Scholar]
- 8.Cooperman DR, Wallensten R, Stulberg SD. Acetabular dysplasia in the adult. Clin Orthop Relat Res 1983;175:79–85. [PubMed] [Google Scholar]
- 9.Gage JR, Winter RB. Avascular necrosis of the capital femoral epiphysis as a complication of closed reduction of congenital dislocation of the hip. A critical review of twenty years’ experience at Gillette Children’s Hospital. J Bone Joint Surg [Am] 1972;54-A:373–388. [PubMed] [Google Scholar]
- 10.Brougham DI, Broughton NS, Cole WG, Menelaus MB. The predictability of acetabular development after closed reduction for congenital dislocation of the hip. J Bone Joint Surg [Br] 1988;70-B:733–736. [DOI] [PubMed] [Google Scholar]
- 11.Westin GW, Ilfeld FW, Provost J. Total avascular necrosis of the capital femoral epiphysis in congenital dislocated hips. Clin Orthop Relat Res 1976;119:93–98. [PubMed] [Google Scholar]
- 12.Fernandez CA, Dolan LA, Weinstein SL, Morcuende JA. Natural history of type III growth disturbance after treatment of developmental dislocation of the hip. Iowa Orthop J 2008;28:27–35. [PMC free article] [PubMed] [Google Scholar]
- 13.Buchanan JR, Greer RB 3rd. Prevention of avascular necrosis during treatment of congenital dislocation of the hip. Surg Forum 1978;29:546–549. [PubMed] [Google Scholar]
- 14.Weinstein SL. Developmental hip dysplasia and dislocation. In: Weinstein SL, Flynn JJ, eds Lovell and Winter’s Pediatric Orthopaedics. Vol. 2 Seventh ed. Philadelphia: Lippincott Williams & Wilkins, 2014:983–1111. [Google Scholar]
- 15.Strayer LM. The embryology of the human hip joint. Yale J Biol Med 1943;16:13–26.6. [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe RS. Embryology of the human hip. Clin Orthop Relat Res 1974;98:8–26. [DOI] [PubMed] [Google Scholar]
- 17.Strayer LM., Jr Embryology of the human hip joint. Clin Orthop Relat Res 1971;74:221–240. [PubMed] [Google Scholar]
- 18.Siffert RS. Patterns of deformity of the developing hip. Clin Orthop Relat Res 1981;160:14–29. [PubMed] [Google Scholar]
- 19.Gage JR, Cary JM. The effects of trochanteric epiphyseodesis on growth of the proximal end of the femur following necrosis of the capital femoral epiphysis. J Bone Joint Surg [Am] 1980;62-A:785–794. [PubMed] [Google Scholar]
- 20.Osborne D, Effmann E, Broda K, Harrelson J. The development of the upper end of the femur, with special reference to its internal architecture. Radiology 1980;137:71–76. [DOI] [PubMed] [Google Scholar]
- 21.Schofield CB, Smibert JG. Trochanteric growth disturbance after upper femoral osteotomy for congenital dislocation of the hip. J Bone Joint Surg [Br] 1990;72-B:32–36. [DOI] [PubMed] [Google Scholar]
- 22.Sugano N, Noble PC, Kamaric E, et al. The morphology of the femur in developmental dysplasia of the hip. J Bone Joint Surg [Br] 1998;80-B:711–719. [DOI] [PubMed] [Google Scholar]
- 23.Iwersen LJ, Kalen V, Eberle C. Relative trochanteric overgrowth after ischemic necrosis in congenital dislocation of the hip. J Pediatr Orthop 1989;9:381–385. [PubMed] [Google Scholar]
- 24.Harrison TJ. An experimental study of pelvic growth in the rat. J Anat 1958;92: 483–488. [PMC free article] [PubMed] [Google Scholar]
- 25.Ponseti IV. Growth and development of the acetabulum in the normal child. Anatomical, histological, and roentgenographic studies. J Bone Joint Surg [Am] 1978;60-A:575–585. [PubMed] [Google Scholar]
- 26.Lee MC, Eberson CP. Growth and development of the child’s hip [v.]. Orthop Clin North Am 2006;37:119–132. [DOI] [PubMed] [Google Scholar]
- 27.Brougham DI, Broughton NS, Cole WG, Menelaus MB. Avascular necrosis following closed reduction of congenital dislocation of the hip. Review of influencing factors and long-term follow-up. J Bone Joint Surg [Br] 1990;72-B:557–62. [DOI] [PubMed] [Google Scholar]
- 28.Gibson PH, Benson MK. Congenital dislocation of the hip. Review at maturity of 147 hips treated by excision of the limbus and derotation osteotomy. J Bone Joint Surg [Br] 1982;64-B:169–175. [DOI] [PubMed] [Google Scholar]
- 29.Harris NH, Lloyd-Roberts GC, Gallien R. Acetabular development in congenital dislocation of the hip. With special reference to the indications for acetabuloplasty and pelvic or femoral realignment osteotomy. J Bone Joint Surg [Br] 1975;57-B:46–52. [PubMed] [Google Scholar]
- 30.Thomas IH, Dunin AJ, Cole WG, Menelaus MB. Avascular necrosis after open reduction for congenital dislocation of the hip: analysis of causative factors and natural history. J Pediatr Orthop 1989;9:525–531. [DOI] [PubMed] [Google Scholar]
- 31.Ponseti IV. Morphology of the acetabulum in congenital dislocation of the hip. Gross, histological and roentgenographic studies. J Bone Joint Surg [Am] 1978;60-A:586–599. [PubMed] [Google Scholar]
- 32.Roposch A, Ridout D, Protopapa E, Nicolaou N, Gelfer Y. Osteonecrosis complicating developmental dysplasia of the hip compromises subsequent acetabular remodeling. Clin Orthop Relat Res 2013;471:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roposch A, Liu LQ, Offiah AC, Wedge JH. Functional outcomes in children with osteonecrosis secondary to treatment of developmental dysplasia of the hip. J Bone Joint Surg [Am] 2011;93-A:e145. [DOI] [PubMed] [Google Scholar]
- 34.Coleman CR, Slager RF, Smith WS. The effect of environmental influence on acetabular development. Surg Forum 1958;9:775–780. [PubMed] [Google Scholar]
- 35.Chung SM. The arterial supply of the developing proximal end of the human femur. J Bone Joint Surg [Am] 1976;58-A:961–970. [PubMed] [Google Scholar]
- 36.Trueta J. The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg [Br] 1957;39-B:358–394. [DOI] [PubMed] [Google Scholar]
- 37.Ogden JA. Changing patterns of proximal femoral vascularity. J Bone Joint Surg [Am] 1974;56-A:941–950. [PubMed] [Google Scholar]
- 38.Crock HV. A revision of the anatomy of the arteries supplying the upper end of the human femur. J Anat 1965;99:77–88. [PMC free article] [PubMed] [Google Scholar]
- 39.Trueta J. Studies of the development and decay of the human frame. Philadelphia: W.B. Saunders, 1968. [Google Scholar]
- 40.Brookes M. The Blood Supply of Bone: An Approach to Bone Biology. New York: Appleton-Centry-Crofts, 1971. [Google Scholar]
- 41.Trueta J, Amato VP. The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischaemia. J Bone Joint Surg [Br] 1960;42-B:571–587. [DOI] [PubMed] [Google Scholar]
- 42.Schoenecker PL, Lesker PA, Ogata K. A dynamic canine model of experimental hip dysplasia. Gross and histological pathology, and the effect of position of immobilization on capital femoral epiphyseal blood flow. J Bone Joint Surg [Am] 1984;66-A:1281–1288. [PubMed] [Google Scholar]
- 43.Law EG, Heistad DD, Marcus ML, Mickelson MR. Effect of hip position on blood flow to the femur in puppies. J Pediatr Orthop 1982;2:133–137. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein SL. Traction in developmental dislocation of the hip. Is its use justified? Clin Orthop Relat Res 1997;338:79–85. [DOI] [PubMed] [Google Scholar]
- 45.Cooperman DR, Wallensten R, Stulberg SD. Post-reduction avascular necrosis in congenital dislocation of the hip. J Bone Joint Surg [Am] 1980;62-A:247–258. [PubMed] [Google Scholar]
- 46.Campbell P, Tarlow SD. Lateral tethering of the proximal femoral physis complicating the treatment of congenital hip dysplasia. J Pediatr Orthop 1990;10:6–8. [PubMed] [Google Scholar]
- 47.Gotoh E, Ando M. The pathogenesis of femoral head deformity in congenital dislocation of the hip. Experimental study of the effects of articular interpositions in pigs. Clin Orthop Relat Res 1993;288:303–309. [PubMed] [Google Scholar]
- 48.Gregosiewicz A, Wośko I. Risk factors of avascular necrosis in the treatment of congenital dislocation of the hip. J Pediatr Orthop 1988;8:17–19. [DOI] [PubMed] [Google Scholar]
- 49.Kalamchi A, MacEwen GD. Avascular necrosis following treatment of congenital dislocation of the hip. J Bone Joint Surg [Am] 1980;62-A:876–888. [PubMed] [Google Scholar]
- 50.Keret D, MacEwen GD. Growth disturbance of the proximal part of the femur after treatment for congenital dislocation of the hip. J Bone Joint Surg [Am] 1991;73-A:410–423. [PubMed] [Google Scholar]
- 51.Nicholson JT, Kopell HP, Mattei FA. Regional stress angiography of the hip; a preliminary report. J Bone Joint Surg [Am] 1954;36-A:503–510. [PubMed] [Google Scholar]
- 52.Salter RB, Field P. The effects of continuous compression on living articular cartilage: an experimental investigation. J Bone Joint Surg [Am] 1960;42-A:31–90. [Google Scholar]
- 53.Ogden JA, Southwick WO. A possible cause of avascular necrosis complicating the treatment of congenital dislocation of the hip. J Bone Joint Surg [Am] 1973;55-A:1770. [Google Scholar]
- 54.Ogden JA. Normal and abnormal circulation. In: Tachdjian MO, ed Congenital dislocation of the hip. New York: Churchill Livingstone, 1982:59. [Google Scholar]
- 55.Gore DR. Iatrogenic avascular necrosis of the hip in young children. A review of six cases. J Bone Joint Surg [Am] 1974;56-A:493–502. [PubMed] [Google Scholar]
- 56.Gore DR. Iatrogenic avascular necrosis of the hip in young children: a long-term follow-up. J Pediatr Orthop 1999;19:635–640. [PubMed] [Google Scholar]
- 57.Herold HZ. Avascular necrosis of the femoral head in congenital dislocation of the hip. Isr J Med Sci 1980;16:295–300. [PubMed] [Google Scholar]
- 58.Herold HZ. Avascular necrosis of the femoral head in children under the age of three. Clin Orthop Relat Res 1977;126:193–195. [PubMed] [Google Scholar]
- 59.Salter RB, Kostuik J, Dallas S. Avascular necrosis of the femoral head as a complication of treatment for congenital dislocation of the hip in young children: a clinical and experimental investigation. Can J Surg 1969;12:44–61. [PubMed] [Google Scholar]
- 60.Roposch A, Wedge JH, Riedl G. Reliability of Bucholz and Ogden classification for osteonecrosis secondary to developmental dysplasia of the hip. Clin Orthop Relat Res 2012;470:3499–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinstein SL. CORR insights: reliability of Bucholz and Ogden classification for osteonecrosis secondary to developmental dysplasia of the hip. Clin Orthop Relat Res 2012;470:3506–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Firth GB, Robertson AJ, Schepers A, Fatti L. Developmental dysplasia of the hip: open reduction as a risk factor for substantial osteonecrosis. Clin Orthop Relat Res 2010;468:2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gamble JG, Mochizuki C, Bleck EE, Rinsky LA. Coxa magna following surgical treatment of congenital hip dislocation. J Pediatr Orthop 1985;5:528–533. [DOI] [PubMed] [Google Scholar]
- 64.O’Brien T, Salter RB. Femoral head size in congenital dislocation of the hip. J Pediatr Orthop 1985;5:299–301. [DOI] [PubMed] [Google Scholar]
- 65.Imatani J, Miyake Y, Nakatsuka Y, Akazawa H, Mitani S. Coxa magna after open reduction for developmental dislocation of the hip. J Pediatr Orthop 1995;15:337–341. [DOI] [PubMed] [Google Scholar]
- 66.Morcuende JA, Meyer MD, Dolan LA, Weinstein SL. Long-term outcome after open reduction through an anteromedial approach for congenital dislocation of the hip. J Bone Joint Surg [Am] 1997;79-A:810–817. [DOI] [PubMed] [Google Scholar]
- 67.Robinson HJ Jr, Shannon MA. Avascular necrosis in congenital hip dysplasia: the effect of treatment. J Pediatr Orthop 1989;9:293–303. [PubMed] [Google Scholar]
- 68.Kim HW, Morcuende JA, Dolan LA, Weinstein SL. Acetabular development in developmental dysplasia of the hip complicated by lateral growth disturbance of the capital femoral epiphysis. J Bone Joint Surg [Am] 2000;82-A:1692–1700. [DOI] [PubMed] [Google Scholar]
- 69.Visser JD. Functional treatment of congenital dislocation of the hip [Suppl]. Acta Orthop Scand Suppl 1984;206:1–109. [DOI] [PubMed] [Google Scholar]
- 70.Kutlu A, Ayata C, Ogün TC, Kapicioglu MI, Mutlu M. Preliminary traction as a single determinant of avascular necrosis in developmental dislocation of the hip. J Pediatr Orthop 2000;20:579–584. [DOI] [PubMed] [Google Scholar]
- 71.Gabuzda GM, Renshaw TS. Reduction of congenital dislocation of the hip. J Bone Joint Surg [Am] 1992;74-A:624–631. [PubMed] [Google Scholar]
- 72.Race C, Herring JA. Congenital dislocation of the hip: an evaluation of closed reduction. J Pediatr Orthop 1983;3:166–172. [DOI] [PubMed] [Google Scholar]
- 73.Weinstein SL, Ponseti IV. Congenital dislocation of the hip. J Bone Joint Surg [Am] 1979;61-A:119–124. [PubMed] [Google Scholar]
- 74.Severin E. Contribution to the knowledge of congenital dislocation of the hip joint. Late results of closed reduction and arthrographic studies of recent cases. Acta Chir Scandinavica 1941;Supplementum 63.
- 75.Simons GW. A comparative evaluation of the current methods for open reduction of the congenitally displaced hip. Orthop Clin North Am 1980;11:161–181. [PubMed] [Google Scholar]
- 76.Massie WK. Vascular epiphyseal changes in congenital dislocation of the hip; results in adults compared with results in coxa plana and in congenital dislocation without vascular changes. J Bone Joint Surg [Am] 1951;33-A:284–306. [PubMed] [Google Scholar]
- 77.Powell EN, Gerratana FJ, Gage JR. Open reduction for congenital hip dislocation: the risk of avascular necrosis with three different approaches. J Pediatr Orthop 1986;6:127–132. [DOI] [PubMed] [Google Scholar]
- 78.Scaglietti O, Calandriello B. Open reduction of congenital dislocation of the hip. J Bone Joint Surg [Br] 1962;44-B:257–283. [DOI] [PubMed] [Google Scholar]
- 79.Mau H, Dörr WM, Henkel L, Lutsche J. Open reduction of congenital dislocation of the hip by Ludloff’s method. J Bone Joint Surg [Am] 1971;53-A:1281–1288. [PubMed] [Google Scholar]
- 80.Ferguson AB., Jr Primary open reduction of congenital dislocation of the hip using a median adductor approach. J Bone Joint Surg [Am] 1973;55-A:671–689. [PubMed] [Google Scholar]
- 81.Esteve R. Congenital dislocation of the hip. A review and assessment of results of treatment with special reference to frame reduction as compared with manipulative reduction. J Bone Joint Surg [Br] 1960;42-B:253–263. [DOI] [PubMed] [Google Scholar]
- 82.Almby B, Hjelmstedt A, Lönnerholm T. Neonatal hip instability. Reason for failure of early abduction treatment. Acta Orthop Scand 1979;50:315–327. [DOI] [PubMed] [Google Scholar]
- 83.Buchanan JR, Greer RB 3rd, Cotler JM. Management strategy for prevention of avascular necrosis during treatment of congenital dislocation of the hip. J Bone Joint Surg [Am] 1981;63-A:140–146. [PubMed] [Google Scholar]
- 84.Ponseti IV, Frigerio ER. Results of treatment of congenital dislocation of the hip. J Bone Joint Surg [Am] 1959;41-A:823–846. [PubMed] [Google Scholar]
- 85.Lindstrom JR, Ponseti IV, Wenger DR. Acetabular development after reduction in congenital dislocation of the hip. J Bone Joint Surg [Am] 1979;61-A:112–118. [PubMed] [Google Scholar]
- 86.Tönnis D. Ischemic necrosis as a complication of treatment of C.D.H. Acta Orthop Belg 1990;56:195–206. [PubMed] [Google Scholar]
- 87.Weiner DS, Hoyt WA Jr, O’dell HW. Congenital dislocation of the hip. The relationship of premanipulation traction and age to avascular necrosis of the femoral head. J Bone Joint Surg [Am] 1977;59-A:306–311. [PubMed] [Google Scholar]
- 88.Kruczynski J. Avascular necrosis of the proximal femur in developmental dislocation of the hip. Incidence, risk factors, sequelae and MR imaging for diagnosis and prognosis [Suppl]. Acta Orthop Scand Suppl 1996;268:1–48. [PubMed] [Google Scholar]
- 89.Tönnis D. Congenital hip dislocation. Avascular necrosis. New York: Thieme-Stratton Inc, 1982. [Google Scholar]
- 90.Ogden JA. Anatomic and histologic study of factors affecting development and evolution of avascular necrosis in congenital hip dislocation. The Hip: Proceedings of the 2nd Annual Meeting of the Hip Society. St. Louis: Mosby; 1974. p. 125. [Google Scholar]
- 91.Trueta J, Trias A. The vascular contribution to osteogenesis. IV. The effect of pressure upon the epiphysial cartilage of the rabbit. J Bone Joint Surg [Br] 1961;43-B: 800–813. [DOI] [PubMed] [Google Scholar]
- 92.Schoenecker PL, Bitz M, Witeside LA. The acute effect of position of immobilization on capital femoral epiphyseal blood flow. A quantitative study using the hydrogen washout technique. J Bone Joint Surg [Am] 1978;60-A:899–904. [PubMed] [Google Scholar]
- 93.Fogarty EE, Accardo NJ Jr. Incidence of avascular necrosis of the femoral head in congenital hip dislocation related to the degree of abduction during preliminary traction. J Pediatr Orthop 1981;1:307–311. [DOI] [PubMed] [Google Scholar]
- 94.Ishii Y, Weinstein SL, Ponseti IV. Correlation between arthrograms and operative findings in congenital dislocation of the hip. Clin Orthop Relat Res 1980;153:138–145. [PubMed] [Google Scholar]
- 95.Morel G. The treatment of congenital dislocation and subluxation of the hip in the older child. Acta Orthop Scand 1975;46:364–399. [PubMed] [Google Scholar]
- 96.Petit P, Queneau P, Borde J. Traitement des luxation et subluxation congénitaled de la hanche dans la première enfance. Rev Chir Orthop Repar Appar Mot 1962;48:148–186. [PubMed] [Google Scholar]
- 97.Petit P, Queneau P, Dubousset J. Less défauts de centrage de la tête fémorale aprés traitement des luxations ou subluxations congénitales de la hanche. 10ème Congrès International de Chirurgie Orthopaedique 1966:504–512.
- 98.Coleman S. A critical analysis of the value of preliminary traction in the treatment of CDH. Orthop Trans 1987;13:180. [Google Scholar]
- 99.Kahle WK, Anderson MB, Alpert J, Stevens PM, Coleman SS. The value of preliminary traction in the treatment of congenital dislocation of the hip. J Bone Joint Surg [Am] 1990;72-A:1043–1047. [PubMed] [Google Scholar]
- 100.Quinn RH, Renshaw TS, DeLuca PA. Preliminary traction in the treatment of developmental dislocation of the hip. J Pediatr Orthop 1994;14:636–642. [DOI] [PubMed] [Google Scholar]
- 101.Fisher RI, Cary JM. Avascular necrosis complicating congenital dislocation of the hip. Course, prognosis and orthopaedic management. Int Orthop 1978;2:229–240. [Google Scholar]
- 102.Segal LS, Boal DK, Borthwick L, et al. Avascular necrosis after treatment of DDH: the protective influence of the ossific nucleus. J Pediatr Orthop 1999;19:177–184. [DOI] [PubMed] [Google Scholar]
- 103.Segal LS, Berlin JM, Schneider DJ, Moulton MJ, Frauenhoffer EE. Chondronecrosis of the hip. The protective role of the ossific nucleus in an animal model. Clin Orthop Relat Res 2000;377:265–271. [PubMed] [Google Scholar]
- 104.Niziol R, Elvey M, Protopapa E, Roposch A. Association between the ossific nucleus and osteonecrosis in treating developmental dysplasia of the Hip: updated meta-analysis. BMC Musculoskelet Disord 2017;18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luhmann SJ, Schoenecker PL, Anderson AM, Bassett GS. The prognostic importance of the ossific nucleus in the treatment of congenital dysplasia of the hip. J Bone Joint Surg [Am] 1998;80-A:1719–1727. [DOI] [PubMed] [Google Scholar]
- 106.Thomas CL, Gage JR, Ogden JA. Treatment concepts for proximal femoral ischemic necrosis complicating congenital hip disease. J Bone Joint Surg [Am] 1982;64-A:817–828. [PubMed] [Google Scholar]
- 107.Dvonch VM, Bunch WH. Pattern of closure of the proximal femoral and tibial epiphyses in man. J Pediatr Orthop 1983;3:498–501. [DOI] [PubMed] [Google Scholar]
- 108.O’Brien T, Millis MB, Griffin PP. The early identification and classification of growth disturbances of the proximal end of the femur. J Bone Joint Surg [Am] 1986;68-A:970–980. [PubMed] [Google Scholar]
- 109.O’Brien T. Growth-disturbance lines in congenital dislocation of the hip. J Bone Joint Surg [Am] 1985;67-A:626–632. [PubMed] [Google Scholar]
- 110.Albinana J, Dolan LA, Spratt KF, et al. Acetabular dysplasia after treatment for developmental dysplasia of the hip. Implications for secondary procedures. J Bone Joint Surg [Br] 2004;86-B:876–886. [DOI] [PubMed] [Google Scholar]
- 111.Weinstein SL, Dolan LA, Morcuende JA. The 2018 Nicholas Andry Award: the evidence base for the treatment of developmental dysplasia of the hip: the Iowa contribution. Clin Orthop Relat Res 2018;476:1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steindler A, Kulowski J, Freund E. Congenital dislocation of the hip. Statistical analysis. JAMA 1935;104:302–307. [Google Scholar]


